Summary
Background
Present mechanical devices are unable to achieve recanalisation in up to 20–40% of large vessel occlusion strokes. We compared efficacy and safety of the Trevo Retriever, a new stent-like device, with its US Food and Drug Administration-cleared predecessor, the Merci Retriever.
Methods
In this open-label randomised controlled trial, we recruited patients at 26 sites in the USA and one in Spain. We included adults aged 18–85 years with angiographically confirmed large vessel occlusion strokes and US National Institutes of Health Stroke Scale (NIHSS) scores of 8–29 within 8 h of symptom onset. We randomly assigned patients (1:1) with sequentially numbered sealed envelopes to thrombectomy with Trevo or Merci devices. Randomisation was stratified by age (≤68 years vs 69–85 years) and NIHSS scores (≤18 vs 19–29) with alternating blocks of various sizes. The primary efficacy endpoint, assessed by an unmasked core laboratory, was thrombolysis in cerebral infarction (TICI) scores of 2 or greater reperfusion with the assigned device alone. The primary safety endpoint was a composite of procedure-related adverse events. Analyses were done by intention to treat. This study is registered with ClinicalTrials.gov, number NCT01270867.
Findings
Between Feb 3, 2011, and Dec 1, 2011, we randomly assigned 88 patients to the Trevo Retriever group and 90 patients to Merci Retriever group. 76 (86%) patients in the Trevo group and 54 (60%) in the Merci group met the primary endpoint after the assigned device was used (odds ratio 4·22, 95% CI 1·92–9·69; psuperiority<0·0001). Incidence of the primary safety endpoint did not differ between groups (13 [15%] patients in the Trevo group vs 21 [23%] in the Merci group; p=0·1826).
Interpretation
Patients who have had large vessel occlusion strokes but are ineligible for (or refractory to) intravenous tissue plasminogen activator should be treated with the Trevo Retriever in preference to the Merci Retriever.
Funding
Stryker Neurovascular.
Introduction
Intravenous recombinant tissue plasminogen activator (rt-PA) is the standard of care for treatment of acute ischaemic stroke. However, rt-PA has limitations, including a short therapeutic window that restricts more widespread adoption and poor reperfusion rates in the setting of extensive clot burden.1–6 Use of mechanical clot retrieval devices in acute ischaemic stroke might result in increased reperfusion rates compared with intravenous or intra-arterial thrombolysis.7 However, mechanical devices are unable to achieve recanalisation in as many as 20–40% of large vessel occlusion strokes.8–10
Presently available thombectomy devices work through application of different degrees of retrieval force on the thrombus with either a proximal approach (eg, thromboaspiration devices) or distal approach (eg, snare-like devices). The Merci Retriever (Stryker Neurovascular, Mountain View, CA, USA; figure 1) is a flexible nitinol wire with distal corkscrew-shaped coil loops with attached filaments. The device is deployed distally to the clot through a microcatheter and is used to ensnare and remove the thrombus into a balloon-guide catheter placed in the cervical internal carotid or vertebral arteries. The Trevo Retriever (Stryker Neurovascular; figure 1) is a novel thrombectomy device belonging to a category increasingly known as stent retrievers because of their resemblance to intracranial stents. Unlike their predecessors, stent retrievers apply a radial retrieval force in the centre of the thrombus and along its whole length. A microcatheter is placed distal to the thrombus and the closed-cell stent-like nitinol device is delivered via the microcatheter. The retriever is deployed by unsheathing the microcatheter, resulting in opening of the stent and radial displacement of the thrombus against the blood vessel wall with incorporation of the clot material into the stent struts. The device is subsequently retrieved into a catheter placed in the internal carotid or vertebral arteries. In animals, Trevo was very effective at achieving immediate reperfusion of occluded arteries without causing any clinically significant disruption of the vascular integrity.11 In the multicentre, prospective, single-group TREVO trial12 of 60 patients with stroke at seven European centres, treatment with the Trevo Retriever had a recanalisation rate of 92% with a rate of independent functional outcome (90 day modified Rankin scale [mRS] score of ≤2) of 55%.
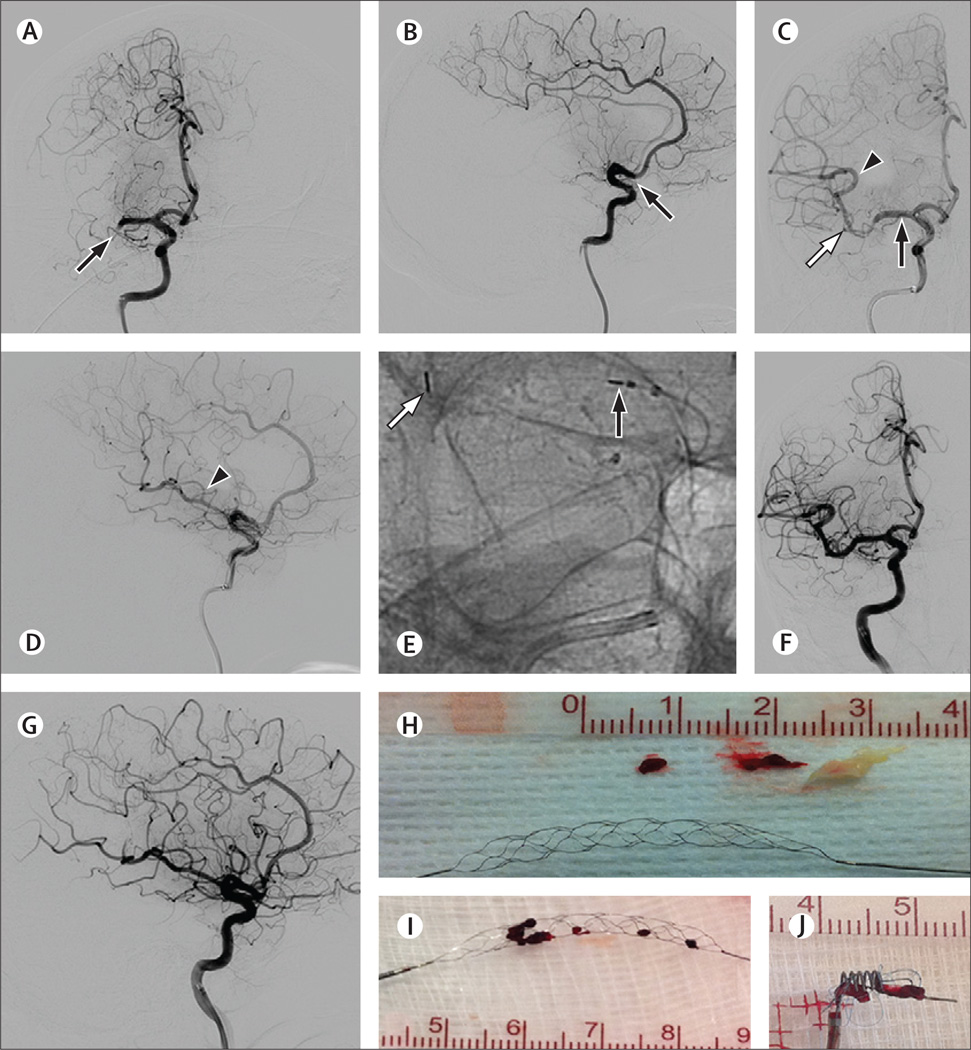
Figure 1. Example angiography and devices.
(A–H) Angiography images of a 67-year-old woman presenting with left hemiplegia and dysarthria (NIHSS 12). Intravenous recombinant tissue plasminogen activator was given 115 min after symptom onset without improvement. (A–B) Angiography before treatment showing complete occlusion of the M1 segment of the right MCA (arrows). (C–E) Angiography after deployment of the Trevo Retriever across the occluded segment showing a perfusion channel with contrast opacification of the distal MCA territory (arrowheads). Black arrows in panels C–E show the proximal Trevo markers and white arrows show the distal Trevo markers. (E) Magnified native image of panel C. (F–G) Angiography after treatment showing near complete reperfusion of the right MCA territory (TICI 2b). At 90 days, the patient’s NIHSS was 0 and modified Rankin scale score was 1. (H) Trevo device and retrieved complex thromboembolic material. (I–J) Thrombus incorporation by the Trevo (I) and Merci (J) retrievers. NIHSS=National Institutes of Health Stroke Scale. MCA=middle cerebral artery. TICI=thrombolysis in cerebral infarction grading scale score.
In this trial, TREVO 2, we aimed to compare the efficacy and safety of mechanical thrombectomy with the Trevo Retriever with that of the Merci Retriever in the arterial revascularisation of patients with acute ischaemic stroke.
Methods
Study design and patients
TREVO 2 was a randomised, prospective, controlled, multicentre, open-label, adaptive, non-inferiority trial. The study used an executive committee, an independent clinical events committee, a CT and MRI imaging core laboratory, a separate angiography core laboratory, and an independent data safety and monitoring board (DSMB) to ensure appropriate oversight and analysis of the study data. The independent DSMB, which was made up of investigators who were not actively participating in enrolment in the study, established stopping rules on the basis of safety and futility analyses and regularly reviewed the ongoing trial data. The study sponsor, Stryker Neurovascular, monitored and managed the data. Statistical analyses were done by two independent external statisticians. The executive committee oversaw the trial design and operations and vouches for the completeness and accuracy of the data and the analysis. The clinical events committee adjudicated all safety endpoints including procedure-related adverse events, intracranial haemorrhages, and deaths. Haemorrhages were classified as asymptomatic or symptomatic and were defined as in the European Cooperative Acute Stroke Study (ECASS) III trial.2
Adults aged 18–85 years presenting with acute onset of stroke symptoms leading to significant clinical deficit in the setting of an angiographically proven occlusion of a proximal intracranial artery (eg, internal carotid, middle cerebral M1 and/or M2 segments, basilar and/or vertebral arteries) who could have endovascular therapy (defined as the first pass with the assigned study device) started within 8 h from time last assessed at baseline were eligible for the study. We enrolled patients at 26 centres in the USA and one centre in Spain. Eligible patients had to have baseline National Institutes of Health Stroke Scale scores of 8–29, failure of treatment with intravenous rt-PA (defined as absence of recanalisation on baseline conventional cerebral angiography) or ineligibility for intravenous rt-PA, no significant pre-stroke disability (mRS score ≤1), and life expectancy of at least 6 months. We used standard exclusion criteria, including severe sustained hypertension (systolic blood pressure >185 mm Hg or diastolic blood pressure >110 mm Hg), baseline glucose concentrations of less than 2·78 mmol/L (50 mg/dL) or higher than 22·20 mmol/L (400 mg/dL), known haemorrhagic diathesis, coagulation factor deficiency, or oral anti coagulant therapy with an international normalised ratio of more than 3·0, treatment with heparin within 48 h with a partial thromboplastin time more than two times the laboratory normal, baseline platelet count of less than 30×109/L, history of severe allergy (worse than rash) to contrast medium or nitinol, and pregnancy.8–10 Key imaging exclusion criteria included baseline CT or MRI evidence of ischaemic changes involving more than a third of the middle cerebral artery territory or more than 100 mL of tissue, intracranial haemorrhage, significant mass effect with midline shift, intracranial tumour (apart from small meningioma), stenosis in a proximal vessel requiring treatment or preventing device access to the thrombus, and excessive arterial tortuosity precluding the device from reaching the thrombus. All patients or their representatives provided written informed consent before enrolment.
Randomisation and masking
We enrolled eligible patients immediately after the first angiographic run confirming the appropriateness of the occlusive lesion and randomly allocated them in a 1:1 ratio to mechanical thrombectomy with Trevo or Merci devices. Enrolled patients were stratified on the basis of age (≤68 years vs 69–85 years) and US National Institutes of Health Stroke Scale (NIHSS) scores (≤18 vs 19–29). Within each stratum at each study site, we did the randomisation with alternating blocks of various sizes, with the first block chosen at random by use of sealed, opaque, sequentially numbered envelopes coded by the four different age and NIHSS strata combinations. An independent study statistician prepared the envelopes and the local sites were unaware of the randomisation algorithm or the block sizes. Immediate availability of the randomisation envelopes to the investigators prevented any treatment delays. Enrolment in the study was defined as the moment when the randomisation was completed and the assigned study device revealed.
Procedures
CT or MRI scans undertaken before the procedure were sent to an independent imaging core laboratory for confirmation of the imaging inclusion and exclusion criteria. CT or MRI scans, done 24 h (with a tolerance of 18–36 h) after the procedure, were systematically reviewed for haemorrhagic complications by a core laboratory reader who was masked to treatment assignment and clinical data. We categorised haemorrhages according to the method used by Berger and colleagues in the ECASS trials.13 The complete set of angiographic images was reviewed by an independent angiography core laboratory and graded with the thrombolysis in cerebral infarction (TICI) grading scale.14 TICI grade 0 was defined as no perfusion; grade 1 was defined as perfusion past the initial obstruction but limited distal branch filling with little or slow distal perfusion; grade 2a was defined as perfusion of less than two-thirds of the vascular distribution of the occluded artery; grade 2b was defined as perfusion of two-thirds or more of the vascular distribution of the occluded artery; and grade 3 was defined as full perfusion with filling of all distal branches. We adjudicated revascularisation outcomes immediately after use of the assigned thrombectomy device and after the complete procedure if additional adjunctive or rescue treatments were done. The angiography core laboratory was not masked to study device allocation because independent review of all images for potential angiographic complications including perforation, dissection, vasospasm, and contrast extravasation was necessary. However, the core laboratory was masked to all clinical data and CT and MRI imaging data. Individual site investigators were also asked to report revascularisation success. The investigating sites were asked to provide clinical outcome assessments from masked investigators (certified on NIHSS and mRS grading but not part of the treating team).
Appropriate anaesthesia (intravenous sedation or general anaesthesia) and arterial access was obtained as per standard practices at the treating institution. A diagnostic angiogram was done before final enrolment in the study. Patients who underwent angiography but did not meet the angiographic criteria for inclusion were followed up separately. Time of arterial access was defined as the beginning of the procedure. There were no lead-in cases, but all operators in the trial were active Merci users with extensive device experience and well-established stroke treatment protocols at their respective institutions. Each operator was trained in the use of the Trevo device by use of a bench model of the human cerebrovasculature. The assigned device was the initial and primary device in the thrombectomy procedure. No more than six retrieval attempts were allowed in the same target vascular territory. The use of a distal access catheter for added support or a balloon guide catheter for flow arrest during the retrieval process was considered optional. If TICI 2 or greater recanalisation was achieved with the assigned device, the thrombectomy procedure was stopped and no further interventions were done. If recanalisation was not successful and at least three passes had been made with the assigned device, adjunctive treatment (rescue therapy) with an approved or cleared thrombectomy device could be started if deemed appropriate by the treating doctor. The use of intra-arterial thrombolytics or extracranial carotid artery stenting or angioplasty automatically categorised the patient as a treatment failure irrespective of revascularisation status achieved with the assigned study device. Patients were followed up to 90 days after the procedure.
Our primary efficacy endpoint was revascularisation success, defined as TICI 2 or greater flow in the territory of the occlusion assessed by the independent angiography core laboratory. The primary safety endpoint was a composite of procedure-related adverse events 24 (18–36) h after the procedure and defined as the following events: any vascular perforation or intramural dissection, symptomatic intracranial haemorrhage, embolisation to a previously uninvolved territory, access site complication requiring surgical repair or blood trans fusion, periprocedural mortality, device failure (in-vivo breakage), or any other complications regarded by the clinical events committee to be related to the procedure.
Prespecified secondary endpoints were time to revascularisation (mean time from initial guide catheter placement to achievement of TICI 2 or greater reperfusion or end of procedure in non-reperfused patients), good clinical outcomes at 90 days (defined as an mRS score or ≤2), all-cause mortality at 90 days, incidence of any asymptomatic intracranial haemorrhage within 24 (18–36) h of the procedure, and neurological deterioration (≥4 point increase in NIHSS score) at 24 h. Study entry criteria and endpoints did not change during the trial.
Statistical analysis
The primary study hypothesis was that the proportion of patients revascularised with the Trevo Retriever would be non-inferior to the proportion of patients revascularised with the Merci Retriever. The non-inferiority hypothesis was tested with Blackwelder’s method,15 assuming a one-sided α=0·025 and a clinically relevant non-inferiority margin of 10%. In mathematical terms, the null hypothesis (H0) and alternative hypotheses (HA) can be stated as follows:
where RM and RTrevo are the revascularisation rates noted after use of the Merci Retriever (RM) and Trevo Retriever (RTrevo). The hypothesis test consisted of construction of the one-sided 97·5% CI around the difference in revascularisation rate (RM−RTrevo). If the upper bound of the interval was less than 0·10, the null hypothesis was rejected and non-inferiority was established. If non-inferiority of the Trevo device was shown, we planned to test superiority with a one-sided Wald test with the following rules:
To establish the sample size, we retrospectively applied the TREVO 2 exclusion criteria to the Multi MERCI trial9 cohort to generate an initial estimate for the Merci group in this trial. Based on these calculations, the Trevo device was assumed to have a revascularisation rate of 70% and the Merci device 60%. With these assumptions, the number of patients needed to have 80% power to detect non-inferiority (one-sided α=0·025) was 89 per group, or 178 overall. The study was powered to show success of revascularisation only (primary efficacy endpoint), with an interim analysis planned after enrolment of 120 patients to adjust the sample size with a prespecified adaptive algorithm. We assessed primary safety outcomes and secondary endpoints with two-sided tests. All patients randomly allocated to either group were included in the intention-to-treat population, and were included in all analyses. We analysed continuous variables with the Wilcoxon rank sum test or two-sample t test as appropriate. We assessed categorical variables with Fisher’s exact test. We calculated odds ratios and 95% CIs for the primary and secondary endpoints. We did a Kaplan-Meier survival analysis, using the Greenwood method for standard error, and the log-rank test to compare the two treatment groups. Study data are presented with descriptive statistics. For all endpoints or measurements, results are summarised for all enrolled patients in their assigned groups. Statistical analyses were done with SAS version 9·1.
A preplanned interim analysis was done after 120 patients were enrolled and had revascularisation endpoint data from the core laboratory. This analysis was used to adjust the sample size or stop the trial if evidence of efficacy or futility was established. An independent study statistician undertook this analysis, which suggested that the trial could be stopped for efficacy, and presented it to the DSMB. Without revealing any results, the DSMB recommended to the executive committee and sponsor that the trial continue to the original preplanned sample size. The basis for this recommendation was that, because of the rapid enrolment at the time of the interim analysis, there was insufficient 90 day data to assess the secondary endpoints, although there were no safety concerns. The executive committee and sponsor accepted the DSMB recommendation to continue enrolment, but remained masked to all study results. Participating centres were not informed of the interim analysis or resulting decision until after the trial was completed.
This study is registered with ClinicalTrials.gov, number NCT01270867.
Role of the funding source
The executive committee, made up of HLL and WSS (academic stroke neurologists) and RGN (an academic neurointerventionalist), supervised the trial design and operations. The sponsor managed the conduct and monitoring of the study according to good clinical practice and US Food and Drug Administration (FDA) regulations and was masked to the results until after the study was completed and the database was locked. The sponsor had no role in the data analysis or interpretation or writing of the report. Independent statisticians and academic clinical researchers did the statistical analysis and adjudication of the endpoints. The executive committee had full access to all data in the study, independently interpreted the data and wrote the report, and had final responsibility for the decision to submit for publication.
Results
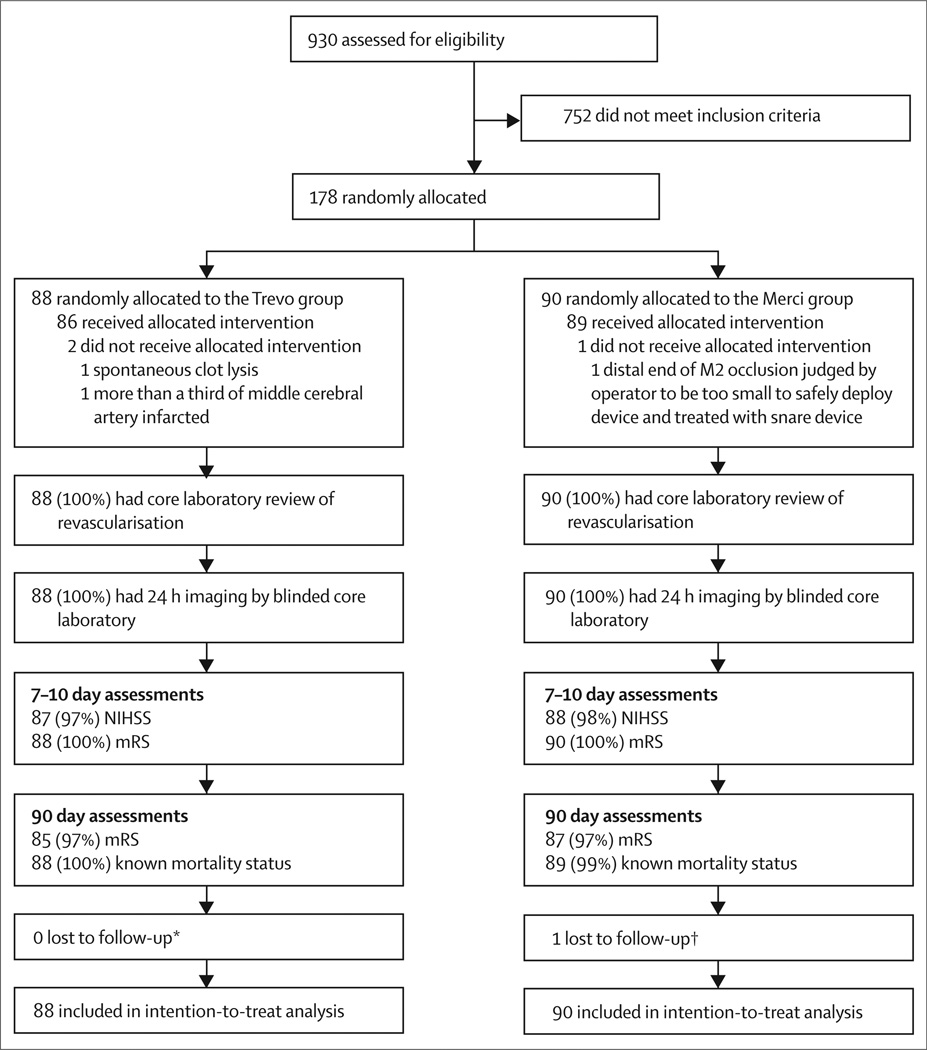
Figure 2 shows the screening, enrolment, and follow-up profile of the trial. No patients crossed over or were incorrectly randomised. 178 (19%) of 930 patients presenting with stroke-like symptoms were screened within 8 h of symptom onset between Feb 3, 2011, and Dec 1, 2011. Table 1 shows baseline characteristics of the 88 patients randomly assigned to the Trevo group and 90 patients to the Merci group. The mean age was 67·2 years (SD 14·2), 43% of patients were men, median baseline NIHSS score was 19 (IQR 15–21), and median time from symptom onset to groin puncture was 4·4 h. Patients in the Trevo group had higher body-mass indexes and diastolic blood pressures before treatment than did patients in the Merci group (table 1). Other baseline characteristics and time from symptom onset to arterial puncture were much the same between treatment groups.
Figure 2. Trial profile.
NIHSS=National Institutes of Health Stroke Scale. mRS=modified Rankin scale. *Modified Rankin scores were not obtained for three patients at 90 days, but survival status was known for all patients. †Modified Rankin scores were not obtained for three patients at 90 days, but survival status was known for all but one patient, who was lost to follow-up after discharge.
Table 1.
Baseline characteristics
| Trevo group (n=88) | Merci group (n=90) | |
|---|---|---|
| Age, years | ||
| Mean | 67·4 (13·9) | 67·0 (14·7) |
| Median | 70·2 (60·8–77·0) | 70·8 (58·0–79·4) |
| NIHSS score | ||
| Mean | 18·3 (5·3) | 17·9 (4·8) |
| Median | 19 (14·0–21·3) | 18 (15·0–21·0) |
| Male sex | 40 (45%) | 36 (40%) |
| Glucose concentration, mg/dL | 127·0 (105·0–158·0) | 117·0 (102·0–143·0) |
| Intravenous t-PA failure | 51 (58%) | 45 (50%) |
| Modified Rankin score | ||
| 0 | 67 (76%) | 67 (74%) |
| 1 | 21 (24%) | 21 (23%) |
| 2 | 0 | 1 (1%)* |
| 3 | 0 | 1 (1%)* |
| Systolic blood pressure, mm Hg† | 153·5 (135·0–171·3) | 143·0 (124·0–168·5) |
| Diastolic blood pressure, mm Hg | 80·5 (72·0–94·3) | 77·0 (65·0–87·8) |
| Body-mass index | 30·0 (25·7– 33·5) | 27·8 (23·7–32·1) |
| Most proximal occlusion site‡ | ||
| Vertebrobasilar | 7 (8%) | 5 (6%) |
| Intracranial internal carotid artery | 14 (16%) | 17 (19%) |
| M1 | 53 (60%) | 55 (61%) |
| M2 | 14 (16%) | 13 (14%) |
| Suspected stroke cause | ||
| Large artery atherosclerosis | 6 (7%) | 11 (12%) |
| Cardioembolic | 63 (72%) | 60 (67%) |
| Unknown | 14 (16%) | 14 (16%) |
| Other | 5 (6%) | 5 (6%) |
| Hemispheric occlusion side, right | 38 (47%) | 44 (52%) |
| Medical history | ||
| Diabetes mellitus | 33 (38%) | 23 (26%) |
| Congestive heart failure | 20 (23%) | 22 (24%) |
| Patent foramen ovale | 3 (3%) | 5 (6%) |
| Coronary artery disease | 29 (33%) | 29 (32%) |
| Hypertension | 67 (76%) | 74 (82%) |
| Atrial fibrillation | 42 (48%) | 38 (42%) |
| Previous ischaemic stroke | 15 (17%) | 12 (13%) |
| Previous transient ischaemic attack | 10 (11%) | 10 (11%) |
| Previous intracerebral haemorrhage | 1 (1%) | 2 (2%) |
| Dyslipidaemia | 55 (63%) | 49 (54%) |
| Smoking | 37 (42%) | 35 (39%) |
| Peripheral vascular disease | 8 (9%) | 4 (4%) |
| Time from symptom onset to arterial puncture, h | ||
| Mean | 4·6 (1·5) | 4·5 (14) |
| Median | 4·7 (3·5–5·7) | 4·2 (3·4–5·4) |
| Intubation | 72 (82%) | 69 (77%) |
Data are mean (SD), median (IQR), or n (%). NIHSS=National Institutes of Health Stroke Scale. t-PA=tissue plasminogen activator.
Protocol deviation.
Blood pressures were measured on arrival to angiography suite.
For the internal carotid artery, M1, and M2, only the most proximal clot location is included.
More patients in the Trevo group than the Merci group met the primary efficacy endpoint (absolute difference 26·4%; table 2). Adjunctive treatment of any kind was less often necessary with Trevo devices than with Merci devices, and after adjunctive interventions, the rate of TICI 2 or greater reperfusion was higher in the Trevo group than the Merci group (table 2). Only eight patients (4%) received adjuvant intra-arterial thrombolytic drugs. Two patients in the Trevo group and three patients in the Merci group who had achieved TICI 2a received intra-arterial rt-PA in an attempt to improve reperfusion further and were thus regarded as failures for the primary endpoint analysis. Three other patients in the Merci group who had recanalisation failure received intra-arterial rt-PA and two of these patients reperfused.
Table 2.
Angiographic and clinical efficacy endpoints
| Trevo group | Merci group | Odds ratio (95% CI) | p value | |
|---|---|---|---|---|
| Primary efficacy endpoint | ||||
| Assigned device TICI ≥2 reperfusion as per core laboratory (intra-arterial lytic equals failure) |
76/88 (86%) | 54/90 (60%) | 4·22 (1·92–9·69) | <0·0001*; <0·0001† |
| Angiographic efficacy endpoints | ||||
| Assigned device TICI ≥2 as per site investigator | 73/86 (85%) | 58/88 (66%) | 2·90 (1·32–6·61) | 0·0047‡ |
| Adjuvant therapy after assigned device | 16/88 (18%) | 28/90 (31%) | 049 (0·23–1·05) | 0·0851‡ |
| Final TICI ≥2 reperfusion as per core laboratory | 81/88 (92%) | 69/90 (77%) | 3·52 (1·33–10·35) | 0·0068‡ |
| Assigned device TICI§ reperfusion as per core laboratory | 0·0001¶ | |||
| 0 | 8% | 17% | 0·44 (0·14–1·22) | |
| 1 | 2% | 20% | 0·09 (0·01–0·42) | |
| 2a | 22% | 20% | 1·12 (0·51–2·47) | |
| 2b | 54% | 38% | 1·93 (1·02–3·68) | |
| 3 | 14% | 6% | 2·68 (0·83–10·13) | |
| Mean time to TICI ≥2 reperfusion or end of procedure | 47·8 (44·2) | 47·3 (38·8) | NA | 0·53† |
| Clinical efficacy endpoint | ||||
| 90 day good outcome (modified Rankin score 0–2) | 34/85 (40%) | 19/87 (22%) | 2·39 (1·16–4·95) | 0·0130‡ |
TICI=thrombolysis in cerebral infarction grading scale score. NA=not applicable.
Non-inferiority hypothesis with Blackwelder’s method and a non-inferiority margin of 10%.
One-sided Wald test of superiority.
Fisher’s exact test.
TICI grade 0 is no perfusion; grade 1 is perfusion past the initial obstruction but limited distal branch filling with little or slow distal perfusion; grade 2a is perfusion of less than two-thirds of the vascular distribution of the occluded artery; grade 2b is perfusion of two-thirds or more of the vascular distribution of the occluded artery; grade 3 is full perfusion with filling of all distal branches.
Wilcoxon rank sum.
Time to revascularisation was much the same in both groups (table 2). A perfusion channel was noted angiographically in 72% of patients in the Trevo group immediately after device deployment (figure 1). The median number of passes with the assigned study device was 2 (IQR 1–3) in the Trevo group (mean 2·4 [SD 1·4]) and 2 (1–3) in the Merci group (2·5 [1·2]).
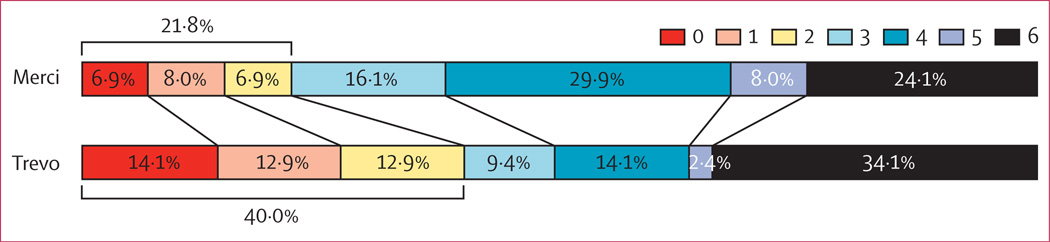
Compared with the Merci group, more patients in the Trevo group had good long-term functional outcomes (absolute difference 18·2%; table 2). Figure 3 shows the overall distribution of the mRS scores at 90 days in the two treatment groups. We noted improved outcomes in patients treated with the Trevo device compared with the Merci device (p=0·0168, two-sided Wilcoxon test). The median NIHSS score at 24 h was 12 (IQR 6–20) in the Trevo group compared with 18 (12–21·75) in the Merci group. Median duration of hospital stay was 7 days (IQR 4–10) in the Trevo group and 8·5 days (6–14) in the Merci group.
Figure 3. 90 day modified Rankin scale scores.
Data were available for 85 (97%) of 88 patients in the Trevo group and 87 (97%) of 90 patients in the Merci group.
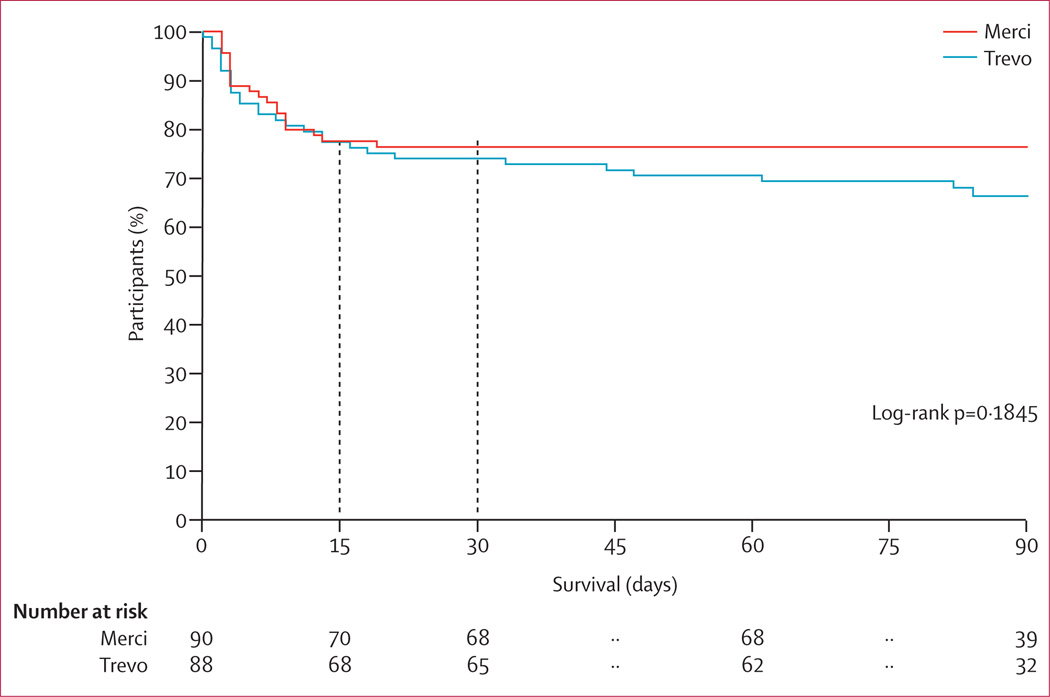
We noted no significant differences between groups in terms of the primary safety endpoint (table 3). However, vessel perforations were more common with the Merci device than the Trevo device. Symptomatic intracranial haemorrhage occurred in six (7%) of 88 patients in the Trevo group and eight (9%) of 90 patients in the Merci group (p=0·7820; table 3), with three of these events in the Trevo group and four in the Merci group (both 50%) regarded as device-related by the clinical events committee. Other prespecified procedure-related adverse events did not differ between groups. Table 3 lists procedure-related adverse events by assigned device. For secondary safety endpoints, rates of neurological deterioration at 24 h and 90 day mortality did not differ between groups. 36 patients (41%) in the Trevo group and 48 (53%) in the Merci group had asymptomatic intracranial haemorrhage at 24 h (p=0·1017; table 3). Mortality in the two groups was much the same in the first 30 days, and although no deaths occurred beyond 30 days in the Merci group, the rates at 90 days did not differ significantly (p=0·1845; figure 4).
Table 3.
Safety endpoints and intracranial haemorrhage subtypes
| Trevo group (n=88) | Merci group (n=90) | Odds ratio (95% CI) | p value* | |
|---|---|---|---|---|
| Primary safety endpoint | ||||
| Composite events | 13 (15%) | 21 (23%) | 0·57 (0·26–1·22) | 0·1826 |
| Device-related (potential or definite) | 8 (9%) | 16 (18%) | 0·46 (0·19–1·14) | 0·1238 |
| Device-related (potential or definite): neurologically significant | 3 (3%) | 4 (4%) | 0·76 (0·16–3·49) | 1·0000 |
| Device-related (definite) | 2 (2%) | 6 (7%) | 0·33 (0·06–1·66) | 0·2780 |
| Device-related (definite): neurologically significant | 0 | 1 (1%) | 0·0 (NA) | 1·0000 |
| Vessel perforation | 1 (1%) | 9 (10%) | 0·10 (0·01–0·83) | 0·0182 |
| Intramural arterial dissection | 0 | 1 (1%) | 0·0 (NA) | 1·0000 |
| Symptomatic intracranial haemorrhage (ECASS III definition)† | 6 (7%) | 8 (9%) | 0·75 (0·25–2·26) | 0·7820 |
| Embolisation to previously uninvolved territory | 6 (7%) | 4 (4%) | 1·57 (0·43–578) | 0·5334 |
| Access-site complication requiring surgical repair or blood transfusion | 2 (2%) | 1 (1%) | 2·07 (0·18–23·25) | 0·6186 |
| Death within 24 h | 2 (2%) | 0 | ∞ (NA) | 0·2430 |
| In-vivo device failure | 0 | 0 | NA | 1·0000 |
| Other PRAE | 0 | 0 | NA | 1·0000 |
| Secondary safety endpoints | ||||
| Death by day 90 | 29 (33%) | 21 (24%) | 1·61 (0·83–3·13) | 0·1845 |
| Neurological deterioration at 24 h‡ | 14 (16%) | 20 (22%) | 0·66 (0·31–1·41) | 0·3418 |
| Haemorrhage categories | ||||
| Asymptomatic intracranial haemorrhage at 24 h | 36 (41%) | 48 (53%) | 0·61 (0·33–1·10) | 0·1017 |
| Symptomatic intracranial haemorrhage (SITS-MOST criteria)§ | 4 (4%) | 2 (2%) | 2·09 (0·37–1174) | 0·4411 |
| PH-1 (any) | 13 (15%) | 19 (21%) | 0·65 (0·30–1·40) | 0·3304 |
| PH-1 (device-related) | 0 | 1 (1%) | 0·00 (0·00–19·43) | 1·0000 |
| PH-2 (any) | 7 (8%) | 5 (6%) | 1·47 (0·45–4·82) | 0·5642 |
| PH-2 (device-related) | 1 (1%) | 1 (1%) | 1·02 (0·01–81·19) | 1·0000 |
| SAH (any) | 11 (12%) | 21 (23%) | 0·47 (0·21–1·04) | 0·0786 |
| SAH (device-related) | 7 (8%) | 17 (19%) | 0·37 (0·12–1·01) | 0·0469 |
ECASS=European Cooperative Acute Stroke Study. NA=not applicable. PRAE=procedure-related adverse event. SITS-MOST=Safe Implementation of Thrombolysis in Stroke Monitoring Study. PH=parenchymal haemorrhage. SAH=subarachnoid hemorrhage. NIHSS=National Institutes of Health Stroke Scale.
Fisher’s exact test.
Any apparently extravascular blood in the brain or within the cranium that was associated with clinical deterioration, as defined by an increase of 4 points or more in the NIHSS, or that led to death and was identified as the predominant cause of the neurological deterioration.
≥4 point increase compared with baseline in the NIHSS at 24 h.
Local or remote PH-2 on the imaging scan obtained at 24 (18–36) h, plus neurological deterioration as indicated by a score on the NIHSS that was higher by ≥4 points than the baseline value, or the lowest value between baseline and 24 h, or haemorrhage leading to death.
Figure 4.
Kaplan-Meier survival analysis
Discussion
Our trial showed that the Trevo Retriever was superior to the Merci Retriever for arterial revascularisation in terms of reperfusion to TICI 2 or greater in the setting of acute ischaemic stroke. Notably, increased frequency and extent of reperfusion was associated with improved clinical outcomes at 24 h, shorter hospital stays, and improved independence.
Overall safety profiles (composite events) were much the same with both devices (table 3); however, vessel perforations were almost ten times more common with the Merci retriever (10%) than they were with the Trevo retriever (1%; p=0·0182). Several possible reasons exist for this difference. First, because the Merci retriever was less effective at recanalisation of the artery, operators frequently resorted to more aggressive adjunctive treatment. Second, although the Merci deployment typically needs some active pushing of the device out of the microcatheter, Trevo is usually deployed by unsheathing the microcatheter. Thus, the more passive Trevo deployment probably attenuates any potential vascular injury that could be caused by the initial exposure of the device tip. Additionally, the radial force of the Trevo device is more evenly distributed throughout the multiple stent struts across the device length. By contrast, higher outward forces are concentrated on the smaller amount of device loops in the Merci retriever. The increased rates of vessel perforation reported with Merci device in this study compared with previous studies (including MERCI and Multi MERCI trials)8,9 was probably because this trial was the first Merci study to use an independent core laboratory that provided a detailed angiographic assessment of the entire procedure. Notably, these perforations do not seem to have a high clinical relevance, as we noted much the same rates of symptomatic intracranial haemorrhage and periprocedural mortality in the two groups.
Our trial differs from previous thrombectomy studies in terms of the rigour of its approach, including its use of several independent committees participating in the design, monitoring, and adjudication of the trial and outcome measures (panel). The randomisation scheme led to a well-balanced distribution of baseline characteristics, apart from higher diastolic blood pressures and body-mass indexes before treatment in the Trevo group. Increased diastolic blood pressure has been linked to reduced recanalisation rates19 and higher body-mass index can theoretically lead to delays in reperfusion owing to potential difficulties with vascular access. Despite these potential imbalances, the Trevo retriever achieved improved angiographic and clinical responses. Although the randomisation scheme makes this possibility less likely, imbalances might still have existed for other important baseline variables that have not been accounted for including the strength of collateral flow and the amount of infarcted and penumbral tissue. Another important consideration is that most patients in our study had cardioembolic events as their suspected stroke cause. This feature was related to the FDA mandate that excludes any proximal vessel stenosis that might require treatment from stroke device trials to avoid potential interferences of other therapies such as angioplasty and stenting with the outcomes of the study device. Although this might affect the generalisability of our findings, previous analyses suggest that endovascular treatment of carotid embolic strokes might actually result in increased recanalisation rates compared with cardio-embolic strokes.20 Other potential limitations of our study include the fact that the study interventionalists (because of difficulties in execution) and the angiographic core laboratory (because of the need to review all images to identify potential adverse events) were not masked to device assignment.
Although numerically lower, the number of Merci passes in our trial (mean 2·5 [SD 1·2]) did not differ significantly from the Merci Registry (2·7 [1·7]; p=0·278; 764 procedures) and was only slightly lower than in the MERCI (2·9 [1·5]; p=0·032; 151 procedures) and Multi MERCI (2·9 [1·6]; p=0·039; 164 procedures) trials. This reduction was probably because other approved therapies have become available since the MERCI and Multi MERCI trials and because of the recent suggestion that administration of more than three Merci passes does not improve the chances of revascularisation and might be linked to a higher complication risk.21 Although a higher number of Merci passes might lead to higher recanalisation rates, the number of passes made with Merci and Trevo devices (mean 2·4 [SD 1·4]) was much the same in our study. The revascularisation rate obtained in the Merci group of this study (60%) is consistent with the rates observed in previous pivotal studies of the Merci device (48% in MERCI and 55% in Multi MERCI after device revascularisation before any adjuvant treatment), although marginally different definitions of revascularisation were used in the previous studies (revascularisation was defined as thrombolysis in myocardial infarction 2 or 3 in all treatable vessels). 90 day results show a more complicated comparison. Although mortality in patients treated with the Merci device in this study was lower (24%) than that noted in MERCI (43·5%) and Multi MERCI (34·0%) trials, the rate of good outcomes (mRS scores 0–2) was lower for the Merci retriever in TREVO 2 (22%) than it was in MERCI (27·7%) and Multi MERCI (36·0%). However, this difference was not tested statistically and was probably due to the small sample sizes.
Only a few prospective clinical trials have assessed endovascular therapy for acute ischaemic stroke. Mechanical thrombectomy trials have historically been single-group studies that aim to show safety of recanalisation of intracranial thromboembolic occlusions for device regulatory approval purposes. Despite being completed almost 15 years ago, the Prolyse in Acute Cerebral Thrombo embolism (PROACT) II trial18 is the only completed randomised controlled trial to compare endovascular revascularisation with medical therapy. Recent FDA regulatory requirements for approval of new devices include their comparison to approved predecessors and present a unique opportunity to further substantiate the concept of endovascular therapy in acute ischaemic stroke. Indeed, the reperfusion rates in our study are among the highest ever published, with TICI 2 or greater in 86% of patients treated with the Trevo device and more than two-thirds of patients achieving TICI 2b or greater reperfusion. These results are in line with two recent uncontrolled clinical series of Trevo treatment in patients after an acute ischaemic stroke.22,23 Even after use of rescue treatment with intra-arterial thrombolytics and other devices, the reperfusion rates of Trevo were significantly higher than Merci (92% vs 77%, p=0·0068). Although US investigators in this trial had had no previous clinical experience with the Trevo Retriever and did not have the benefit of lead-in cases, they achieved very similar rates of reperfusion compared with the more experienced Trevo device operators in the European TREVO trial (90% reperfusion rates in patients treated with the Trevo device). Notably, the same core laboratory was used in both trials.
Independent functional outcomes (defined as mRS of ≤2) at 90 days is regarded as the gold standard in the assessment of clinical outcomes after endovascular therapy for acute ischaemic stroke. Notably, TREVO 2 is only the second published randomised trial of intra-arterial therapy to show superiority of one treatment over another in terms of independent functional outcomes. Unlike its predecessor, PROACT II, which used a medical treatment group as control, TREVO 2 compared two endovascular strategies. Accordingly, no definite conclusions can be made about the superiority of Trevo thrombectomy to medical therapy alone. However, patients in TREVO 2 treated with the Trevo Retriever achieved the same rate of good clinical outcomes reported in the pro-urokinase group of PROACT II despite older age (67·4 years [SD 13·9] in our study vs 64 years [14·0] in PROACT II), higher baseline NIHSS (median 19 vs 17), more proximal occlusions (vertebro basilar 8% vs 0%; intracranial internal carotid artery 15·9% vs 0%; middle cerebral artery-M1 60·2% vs 61%; middle cerebral artery-M2 15·9% vs 35%), and more refractory occlusions (intravenous rt-PA failure 58% vs 0%). Our results are therefore encouraging and support the use of the Trevo Retriever in a prospective randomised trial of endovascular therapy against medical treatment alone.
Panel: Research in context.
Systematic review
A comprehensive review7 of all prospective studies and trials on endovascular treatment (pharmacological, mechanical, or both) of acute ischaemic stroke published in 2009, concluded that “the preponderance of the data indicates that reperfusion should represent the near-term treatment goal in stroke”. Although a meta-analysis of randomised controlled trials of intra-arterial fibrinolysis for acute ischaemic stroke published in 2010, including five trials with 395 participants, concluded that “intra-arterial fibrinolysis substantially increases recanalisation rates and good and excellent clinical outcomes in acute ischaemic stroke”,16 a Medline search of publications up to July 26, 2012, with no language restrictions and the search terms “endovascular stroke therapy”, “intra-arterial stroke therapy”, and “randomized controlled trial” identified no randomised trials of stroke thrombectomy devices. A review on neurothrombectomy devices concluded that “future trials should use a randomized design, with adequate power to show equivalency or non-inferiority between competing strategies or devices, and strive to identify populations that are most likely to benefit from use of neurothrombectomy devices”.17
Interpretation
Only a few prospective clinical trials have assessed endovascular therapy for acute ischaemic stroke. Mechanical thrombectomy trials have historically been single-group studies that aim to show safety of recanalisation of intracranial thromboembolic occlusions for device regulatory approval purposes. Despite being completed almost 15 years ago, the Prolyse in Acute Cerebral Thromboembolism (PROACT) II trial18 is the only completed randomised controlled trial to compare endovascular revascularisation with medical therapy. This study (TREVO 2) is the second published randomised trial of intra-arterial treatment to show superiority of one treatment over another in terms of long-term independent functional outcomes. Our data suggest superiority of the novel Trevo technology over its predecessor, the Merci Retriever, with achievement of better reperfusion and higher rates of long-term functional independence, and supports the use of the Trevo Retriever in a prospective randomised trial of endovascular therapy against medical treatment alone.
Acknowledgments
We thank the patients and their families for their participation, Kirsten Valley, Cindy Jahans, and Patricia McMahon (Stryker Neurovascular, Mountain View, CA, USA) for their excellent job getting all of the sites ready to enrol patients and for monitoring of the conduct of the trial, and to Bin Xiang (San Jose, CA, USA) and Richard Chiacchierini (Rockville, MD, USA) for excellent statistical programming and analysis.
Footnotes
Contributors
RGN wrote the report. RGN, HLL, and WSS interpreted the data and designed and oversaw the study as part of the Executive Committee. RG and TGJ were study investigators. GAW designed the study and monitored and managed the data. HLL, RG, TGJ, GWA, GAW, DSL, and WSS critically revised the report.
TREVO 2 Trialists
Executive Committee—H L Lutsep, R G Nogueira, and W S Smith. Data and Safety Monitoring Board—G Albers (Chair), P Meyers, and R Roberts. Clinical Events Committee—J English, A Flint, and A Rai. Imaging Core Laboratory—D S Liebeskind (chair) and N Sanossian. Sponsor Study Coordinating Centre—C Jahans, P McMahon, K Valley, and G Walker. Statistical analysis—R P Chiacchierini and B Xiang. Site principal investigators—N Akhtar, J Barr, B Baxter, M Berlet, P Blom, R Budzik, T Devlin, C Given, R Gupta, F Hellinger, T Jovin, E Levy, I Linfante, D Lopes, H Lutsep, J Macho, R Malek, M Mawad, P Ng, A Nohara, R Rosenwasser, N Rutledge, M Rymer, Q Shah, H Shownkeen, A Siddiqui, S Starkman, E Veznedaroglu, A Xavier, and C Zylak.
Conflicts of interest
RGN has served on scientific advisory boards for Stryker/Concentric Medical, Covidien/ev3 Neurovascular, CoAxia, Penumbra, Rapid Medical, Reverse Medical, and Neurointervention. HLL has served on scientific advisory boards for Stryker/Concentric Medical and CoAxia. RG has served on scientific advisory boards for Stryker/Concentric Medical, Covidien/ev3 Neurovascular, CoAxia, Rapid Medical, Reverse Medical, and Neurointervention. TGJ has served on scientific advisory boards for Stryker/Concentric Medical, Covidien/ev3 Neurovascular, CoAxia, and Neurointervention. GWA has served on scientific advisory boards for Stryker/Concentric Medical, consulted for Covidien, and has an equity interest in iSchemaView. GAW is an employee of Stryker/Concentric Medical. DSL has served on scientific advisory boards for Stryker/ Concentric Medical, Covidien/ev3 Neurovascular, and CoAxia. WSS has served on scientific advisory boards for Stryker/Concentric Medical.
Contributor Information
Raul G Nogueira, Departments of Neurology, Neurosurgery, and Radiology, Marcus Stroke and Neuroscience Center, Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA, USA.
Helmi L Lutsep, Department of Neurology, Oregon Health and Science University, Portland, OR, USA.
Rishi Gupta, Departments of Neurology, Neurosurgery, and Radiology, Marcus Stroke and Neuroscience Center, Grady Memorial Hospital, Emory University School of Medicine, Atlanta, GA, USA.
Tudor G Jovin, Department of Neurology, UPMC Stroke Center, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
Gregory W Albers, Department of Neurology, Stanford Stroke Center, Stanford University Medical Center, Palo Alto, CA, USA.
Gary A Walker, Department of Clinical Research, Stryker Neurovascular, Mountain View, CA, USA.
David S Liebeskind, Department of Neurology, UCLA Stroke Center, University of California Los Angeles, Los Angeles, CA, USA.
Wade S Smith, Department of Neurology, University of California San Francisco, San Francisco, CA, USA.
References
- 1.Katzan IL, Hammer MD, Hixson ED, Furlan AJ, Abou-Chebl A, Nadzam DM. Utilization of intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol. 2004;61:346–350. doi: 10.1001/archneur.61.3.346. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4·5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology. 2000;55:1649–1655. doi: 10.1212/wnl.55.11.1649. [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41:2254–2258. doi: 10.1161/STROKEAHA.110.592535. [DOI] [PubMed] [Google Scholar]
- 6.Riedel CH, Zimmermann P, Jensen-Kondering U, Stingele R, Deuschl G, Jansen O. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011;42:1775–1777. doi: 10.1161/STROKEAHA.110.609693. [DOI] [PubMed] [Google Scholar]
- 7.Nogueira RG, Yoo AJ, Buonanno FS, Hirsch JA. Endovascular approaches to acute stroke, part 2: a comprehensive review of studies and trials. AJNR Am J Neuroradiol. 2009;30:859–875. doi: 10.3174/ajnr.A1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 9.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the multi MERCI trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 10.Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–2768. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 11.Nogueira RG, Levy EI, Gounis M, Siddiqui AH. The Trevo device: preclinical data of a novel stroke thrombectomy device in two different animal models of arterial thrombo-occlusive disease. J Neurointerv Surg. 2012;4:295–300. doi: 10.1136/neurintsurg-2011-010053. [DOI] [PubMed] [Google Scholar]
- 12.Wahlgren N, Macho J, Killer M, Liebeskind DS, Jansen O. Final results from the Trevo Study (Thrombectomy RE vascularization of large Vessel Occlusions in acute ischemic stroke); International Stroke Conference; Feb 1–2, 2012; New Orleans, LA, USA. [Google Scholar]
- 13.Berger C, Fiorelli M, Steiner T, et al. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke. 2001;32:1330–1335. doi: 10.1161/01.str.32.6.1330. [DOI] [PubMed] [Google Scholar]
- 14.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 15.Blackwelder WC. “Proving the null hypothesis“ in clinical trials. Control Clin Trials. 1982;3:345–353. doi: 10.1016/0197-2456(82)90024-1. [DOI] [PubMed] [Google Scholar]
- 16.Lee M, Hong KS, Saver JL. Efficacy of intra-arterial fibrinolysis for acute ischemic stroke: meta-analysis of randomized controlled trials. Stroke. 2010;41:932–937. doi: 10.1161/STROKEAHA.109.574335. [DOI] [PubMed] [Google Scholar]
- 17.Baker WL, Colby JA, Tongbram V, et al. Neurothrombectomy devices for the treatment of acute ischemic stroke: state of the evidence. Ann Intern Med. 2011;154:243–252. doi: 10.7326/0003-4819-154-4-201102150-00306. [DOI] [PubMed] [Google Scholar]
- 18.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 19.Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the Mechanic al Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI Trials. Stroke. 2009;40:3777–3783. doi: 10.1161/STROKEAHA.109.561431. [DOI] [PubMed] [Google Scholar]
- 20.Hussain MS, Lin R, Cheng-Ching E, et al. Endovascular treatment of carotid embolic occlusions has a higher recanalization rate compared with cardioembolic occlusions. J Neurointerv Surg. 2010;2:71–73. doi: 10.1136/jnis.2009.001081. [DOI] [PubMed] [Google Scholar]
- 21.Loh Y, Jahan R, McArthur DL, et al. Recanalization rates decrease with increasing thrombectomy attempts. AJNR Am J Neuroradiol. 2010;31:935–939. doi: 10.3174/ajnr.A1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.San Román L, Obach V, Blasco J, et al. Single-center experience of cerebral artery thrombectomy using the TREVO device in 60 patients with acute ischemic stroke. Stroke. 2012;43:1657–1659. doi: 10.1161/STROKEAHA.111.640011. [DOI] [PubMed] [Google Scholar]
- 23.Mendonça N, Flores A, Pagola J, et al. Trevo system: single-center experience with a novel mechanical thrombectomy device. J Neuroimaging. 2011 doi: 10.1111/j.1552-6569.2011.00666.x. published online Dec 30. [DOI] [PubMed] [Google Scholar]