Abstract
Background
T cell tolerance of allergic cutaneous contact sensitivity (CS) induced in mice by high doses of reactive hapten is mediated by suppressor cells that release antigen-specific suppressive nanovesicles.
Objective
To determine the mechanism(s) of immune suppression mediated by the nanovesicles.
Methods
T cell tolerance was induced by i.v. injections of hapten conjugated to self antigens of syngeneic erythrocytes and subsequent contact immunization with the same hapten. Lymph node and spleen cells from tolerized or control donors were harvested and cultured to produce a supernatant containing suppressive nanovesicles that were isolated for testing in active and adoptive cell transfer models of CS.
Results
Tolerance was shown due to exosome-like nanovesicles in the supernatant of CD8+ suppressor T cells that were not Treg. Antigen specificity of the suppressive nanovesicles was conferred by a surface coat of antibody light chains, or possibly whole antibody, allowing targeted delivery of selected inhibitory miRNA-150 to CS effector T cells. Nanovesicles also inhibited CS in actively sensitized mice after systemic injection at the peak of the responses. The role of antibody and miRNA-150 was established by tolerizing either panimmunoglobulin deficient JH-/- or miRNA-150-/- mice that produced non-suppressive nanovesicles. These nanovesicles could be made suppressive by adding antigen-specific antibody light chains or miRNA-150, respectively.
Conclusions
This is the first example of T cell regulation via systemic transit of exosome-like nanovesicles delivering a chosen inhibitory miRNA to target effector T cells in an antigen-specific manner by a surface coating of antibody light chains.
Keywords: Exosomes, exosome-like nanovesicles, nanovesicles, T Cell Suppression, miRNA, miRNA-150, Antibody Light Chains, Allergic Cutaneous Contact Dermatitis, Contact Sensitivity
Introduction
Exosomes are nanovesicles generated intracellularly by budding from the terminal endosomal membranes of multivesicular bodies (MVB) where they accumulate and are released from the cell during exocytosis of the MVB1,2. Exosomes, or related vesicles, are produced by all cell types in virtually all species, and have been found in all fluids studied. Their outstanding property is that they contain a cargo of donor cell proteins, mRNA and miRNA that are delivered extracellularly to acceptor cells, where they can function 3-6. Thus, the vesicular transport of proteins may drive or inhibit signaling pathways5,6,7, mRNA can translate donor cell proteins3,4 and delivered miRNA can bind acceptor cell mRNA to regulate protein translation3,4,8,9.
Contact sensitivity (CS) in mice is a major model of the clinical allergic skin diseases: contact dermatitis and atopic dermatitis. Additionally, CS is a model of delayed-type hypersensitivity (DTH) mechanisms that participate in other T cell mediated processes; such as in T cell aspects of autoimmunity, transplantation, infection resistance and cancer. Further, the effector phase of CS recently has been shown to have unanticipated complexity. The new findings established that sensitization involves TLRs10, initiation of elicitation involves B-1 B cells, iNKT cells, IL-4, mast cells, platelets, endothelial cells and complement11, and responses can be mediated by CD4, CD812, or Th1713 T cells, and even NK cells14. Finally, there is now recognition of regulation of CS by either Treg15 or myeloid suppressor cells16. The present study presents evidence of yet another regulatory pathway involving suppressor T cells producing antigen-specific exosome-like nanovesicles that deliver inhibitory miRNA.
Such exosomal transport of functional miRNA passing genetic information between donor and acceptor cells has been confirmed in diverse instances3,4,8,9 and has provided insight into new levels of regulation between cells in the immune system10-12. Exosome targeting usually is paracrine5,6,17-23, but there also is endocrine transport of the exosome-like nanovesicles via the bloodstream, enabling regulation of distant acceptor cell function24-26, as is true here. The current study presents new evidence of a cell to cell suppressive pathway involving CD8+ suppressor T cell-derived exosome-like nanovesicles that antigen-specifically target the effector T cell mixture of CS by delivering inhibitory miRNA. Selection of the particular antigen-specificity and of the inhibitory miRNA shown here opens up significant translational possibilities for treatment of a variety of human diseases.
Materials and Methods
Description of the Materials and Methods can be found in the Online Repository supplemental section at jacionline.org.
Results
High dose antigen tolerance induces suppressor T cells (Ts) that produce a suppressive supernatant (Ts Sup)
We found that high TNP Ag dose tolerance induced suppressor T cells (Ts) whose culture supernatant (Ts Sup) contained all their suppressive activity for CS-effector cells (Fig. 1a, Group C vs B & D). We suspected that vesicles in the Ts Sup might be responsible for the suppression. Therefore, putative vesicles were enriched by progressive ultrafiltrations and differential centrifugations, culminating with pelleting by two 100,000g ultracentrifugations1,2. The final pellet contained 130nm-sized nanovesicles resembling exosomes by electron microscopy (Fig. 1b, right) and nanoparticle tracking analysis27 (NTA) (Fig. 1c). Like exosomes, these nanovesicles expressed tetraspanins such as CD9 by immunoblotting (Fig. 1d), and CD3 and TCRβ by flow cytometry (not shown), confirming their T cell origin.
Fig. 1. High dose Ag tolerization of the CS immune response induces suppressor T cells (Ts) producing suppressive supernatant (Ts Sup) containing exosome-like nanovesicles.
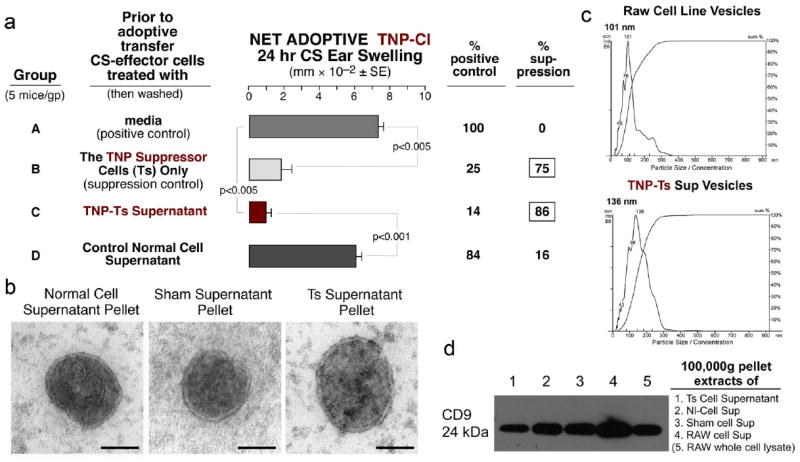
a. Ts cells (Group B) or their culture supernatant (Ts Sup, Group C) suppressed adoptive transfer of CS. b. Electron microscopy revealed that Nl Sup, Sham Sup and TNP-Ts Sup pellets contained nanovesicles resembling exosomes at 80,000 times magnification (the bar is 65nm). c. Nanoparticle tracking analysis (NTA) showed homogenous sized nanovesicles from control RAW cell line supernatant and from TNP-Ts Sup pellets, indicating particle size/concentration. d. Western immunoblotting showed CD9 tetraspanin expression by extracts of pellets from TNP-Ts Sup, Nl Cell Sup, Sham Sup and RAW cell line Sup, compared to control RAW cell lysate.
The final 100,000g pellet from OX-Ts-Sup from mice tolerized with oxazolone-labeled mouse red blood cells (OX-mRBC) compared to the supernatant above the pellet, contained all the Ts Sup ability to suppress adoptive transfer of OX CS-effector T cells (Fig. 2a, Group D vs C). Identical results were obtained in the trinitrphenol (TNP) CS system (not shown). Further, a dose response experiment in the TNP CS system was done to test the potency and validity of the suppressive nanovesicles and showed a declining suppression of adoptive CS by the resuspended serially diluted Ts Sup pellet nanovesicles (See Fig. E1 Groups D, E, and F in this article’s Online Repository at jacionline.org), whereas those from the normal (Nl) Cell Sup pellet at the high dose were not suppressive (Group C). Finally, resuspension of the 100,000g pellet and repeated ultracentrifugation on a sucrose gradient resulted in buoyant fractions. Only the fraction that showed buoyancy identical to that of exosomes1,2 suppressed adoptive transfer of CS (Fig. 2b, Group E), like the starting TNP Ts-Sup nanovesicles (Group D). Considering all the above characteristics, we henceforth called these suppressive CD8+ T cell-derived vesicles exosome-like nanovesicles.
Fig. 2. Ts Sup function is entirely in the 100,000g pellet and in the buoyant fraction of a discontinuous sucrose gradient.
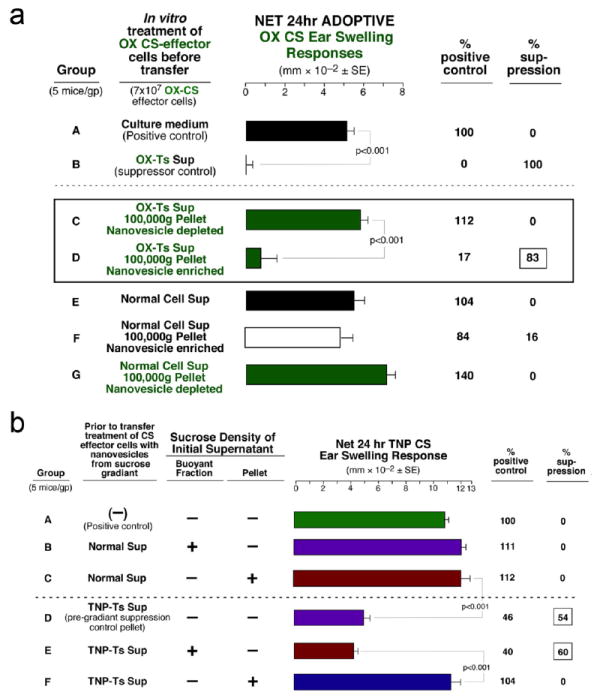
a. The OX-Ts Sup pellet was suppressive (D), whereas OX-Ts Sup depleted of nanovesicles (C), Starting Nl Sup (E), Nl Sup pellet (F), and Nl Sup depleted of nanovesicles (G) all were non-suppressive. b. Vesicles from the TNP-Ts Sup 1.86/1.08 buoyant fraction (E), and vesicles from the original Ts Sup pellet (D) were suppressive, whereas the pellet depleted of buoyant material (F) and Nl Cell Sup fractions were not inhibitory (B & C).
An in vitro non-Ag specific assay confirms the in vivo suppressive function of the Ts Sup-derived nanovesicles
To further confirm the above, an Ag-non-specific in vitro assay was used to test the inhibition of the HT-2 T cell line responsiveness to IL-2 by the exosome-like nanovesicles. The end point was the lowest numbers of serially diluted nanovesicles that resulted in at least 50% HT-2 cell viability. This assay confirmed the suppressive activity of the Ts Sup exosome-like nanovesicles (Fig. 3c; B).
Fig 3. Determination that the Ts Sup suppressive exosome-like nanovesicles are derived from CD8+ cells, are present in plasma of Ts donors, and are not produced by Treg cells.
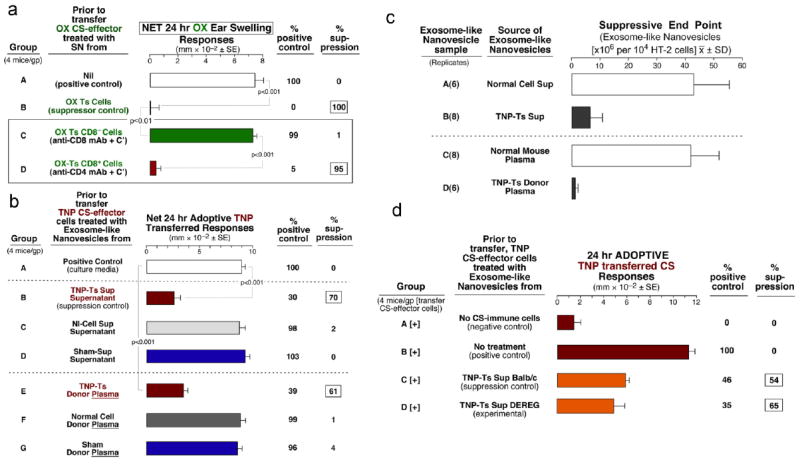
a. Treatment of Ts cells from tolerized mice with anti-CD8 mAb + C’ prior to culture to derive Ts Sup eliminated suppression of adoptivelly transferred CS (C). Similar anti-CD4 mAb treatment of the OX-Ts had no effect (D). b. Tolerized Ts donor plasma nanovesicles were suppressive (E), whereas nanovesicles from other sources (C, D, F, G) were non-inhibitory. c. Only TNP-Ts Sup and Ts donor plasma exosome-like nanovesicles inhibited the in vitro HT-2 cell response to IL-2 (B & D). d. DEREG mice depleted of Treg and wild type mice were tolerized with high Ag dose and showed similar suppressive ability (C & D).
Another in vitro, but Ag specific assay confirmed suppressive activity of Ts Sup exosome-like nanovesicles
Here, immunobead isolated CD4+ CS-effector T cells responded in vitro to TNP-linked DC by producing IFNγ. Shown are four separate experiments confirming that the 100,000g pellet-derived exosome-like nanovesicles from Ts Sup suppressed the IFNγ production, whereas similar Nl Cell Sup nanovesicles did not (See Fig. E2 in this article’s Online Repository at jacionline.org).
The suppressive exosome-like nanovesicles are derived from CD8+ T cells, are present in the plasma of the Ts donors, and are not derived from Treg cells
Depletion of CD8+ cells from the Ts cell culture Sup with anti-CD8 mAb plus complement (Fig. 3a Group C), or with anti-CD8 vs anti-CD4-conjugated beads (not shown) removed the ability to generate suppressive supernatant. Further, blood plasma from the antigen high dose tolerized donors of Ts cells processed for exosomes to the 100,000g pellet also contained suppressive nanovesicles (Fig. 3b; E), whereas similar Nl Cell and Sham plasma-derived nanovesicles had none (Fig. 3b; F & G). In support of these findings, the in vitro IL-2 dependent HT-2 cell Ag-non-specific assay showed strong suppressive activity of plasma exosome-like nanovesicles from tolerized mice vs normal mice (Fig. 3c D vs C). Finally, we tested if Treg were involved using DEREG mice28. High antigen dose tolerance resulted in exosome-like nanovesicles that had equivalent suppressive ability when derived from the Treg depleted mice compared to wild type mice (Fig. 3d).
Suppressor T cell exosome-like nanovesicles inhibit active cutaneous CS responses in vivo
We tested if the nanovesicles could act in vivo when directly injected into actively sensitized mice that were already expressing a CS response. Nanovesicles were administered i.p. at the 24h peak response (Fig. 4, open circles). Then, the subsequent time-course of ear swelling was compared to actively sensitized untreated and ear challenged mice (squares), and to recipients of control vesicles from Sham tolerized mice (triangles). Ts Sup exosome-like nanovesicles strongly suppressed subsequent ear swelling at 48h and 72h by 53% and 60%, respectively (Fig. 4), whereas Sham Sup nanovesicles did not. Further, similar in vivo treatment with the nanovesicles showed that suppression could last up to 120h after a single injection (See Fig. E3a triangles, in this article’s Online Repository at jacionline.org), and significant inhibition even occurred when nanovesicles were given orally (See Fig. E3b Group D in this article’s Online Repository at jacionline.org).
Fig. 4. In vivo treatment with suppressive exosome-like nanovesicles inhibits established CS responses in actively sensitized mice.
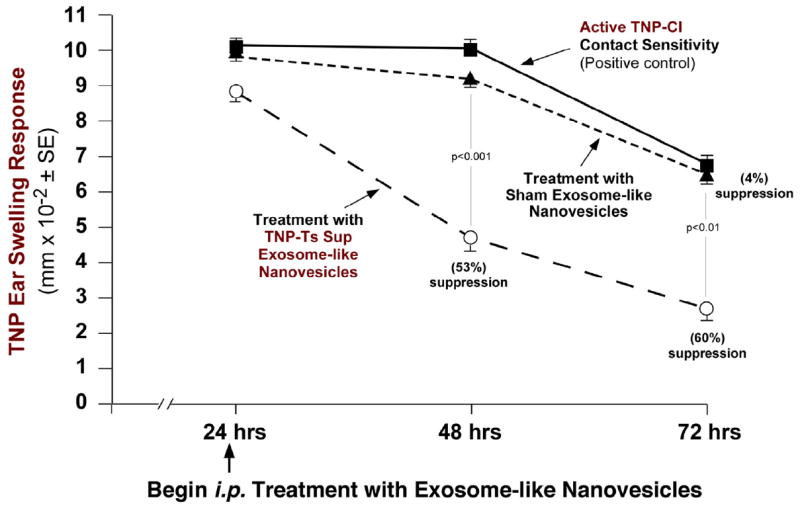
Ts Sup vs. control Sham Sup nanovesicles were injected i.p. at 24h of an ongoing CS response in actively sensitized and ear challenged mice. The Ts Sup exosomes suppressed CS at 48h and 72 wher eas the Sham Sup vesicles were non-inhibitory.
Suppression by exosome-like nanovesicles is Ag-specific via a dual reciprocal Ag-specificity test
Preliminary results suggested functional Ag-specificity of the suppressive nanovesicles. This was confirmed by a dual reciprocal antigen criss-cross experiment which demonstrated that nanovesicles from TNP tolerized mice only suppressed TNP CS-effector cells (Fig. 5a; Group B) and not CS responses to OX, another hapten antigen (Fig. 5a; E). Similarly, exosome-like nanovesicles from OX hapten tolerized mice suppressed OX CS-effector cells (Fig. 5a; F), but not TNP CS-effector T cells (Fig. 5a; C).To possibly account for Ag-specificity, flow cytometry showed antibody kappa light chains (Ab kappa LC) on the surface of the nanovesicles from tolerized mice (right, red peak vs gray isotype control), compared to the control macrophage cell line (Fig. 5b; left, blue peak vs gray isotype control). This suggested that Ag-specific Ab or Ab LC on the nanovesicle surface could provide a mechanism for their Ag-specificity.
Fig. 5. The suppressive exosome-like nanovesicles are Ag-specific.
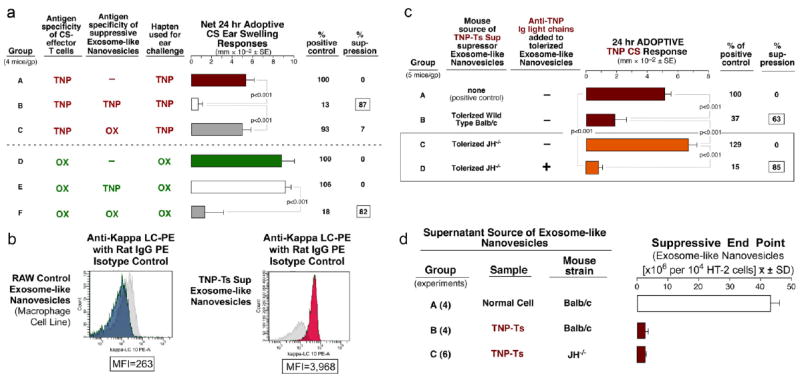
a. Dual Reciprocal Ag-Specificity of Ts Sup Exosome-like nanovesicles. TNP-CS effector cells only, positive control (A), suppression in the TNP homologous system (B), no suppression in the TNP heterologous system (C), OX-CS effector cells only, positive control (D), no suppression in the OX heterologous system (E), suppression in the OX homologous system (F). b. Flow cytometry analysis of kappa light chain expression on TNP Ts Sup (right, red) and RAW cell-derived (left, blue) nanovesicles. Isotype controls are shown in gray. c. Nanovesicles from TNP-tolerized JH-/- mice did not mediate suppression (C). In vitro addition of monoclonal anti-TNP Ab LC to nanovesicles from tolerized JH-/- reconstituted suppression (D). d. The Non-Ag-specific assay of HT-2 T cell responsiveness to IL-2 showed that nanovesicles from tolerized JH-/- mice had strong non-Ag specific suppressive activity (Group C vs. A), equivalent to that of wild type Ts Sup nanovesicles (B vs. A).
Tolerization of pan Ig deficient JH-/- mice confirms that Ag-specificity was due to antibody on the surface of the exosomes-like nanovesicles
We found that nanovesicles from tolerized pan Ig deficient JH-/- mice29 were non-suppressive (Fig. 5c; C). Further, after the first 100,000g pelleting we incubated the exosome-like nanovesicles with monoclonal anti-TNP Ab LC30 in vitro for 30 min at 37°C and then washed away free Ab LC by a second ultracentrifugation step. Very importantly, these likely Ab LC sensitized nanovesicles now were suppressive (Fig. 5c; D, and see Fig. E4 Group C in this article’s Online Repository at jacionline.org), whereas comparable Ab heavy chain exposed vesicles were not (See Fig. E4 Groups D & E in this article’s Online Repository at jacionline.org).
Lack of inhibition of cell transfers by nanovesicles from the tolerized JH-/- donors29 may have been due to a lack of surface antibody on intrinsically suppressive exosome-like nanovesicles. Thus, we tested the nanovesicles in the Ag-non-specific assay for inhibition of the HT-2 T cell line responsiveness to IL-2. Interestingly, despite their inability to suppress CS-effector cell adoptive transfer in vivo, JH-/- Ts Sup exosome-like nanovesicles were suppressive in this Ag-non-specific assay, like the wild type TNP Ts Sup nanovesicles (Fig. 5d; B & C vs A).
Antigen affinity chromatography isolates a minor subpopulation that has all the suppressive activity
We considered that if the exosomes had Ag-specific Ab LC or Ab on their surface then they might be Ag-binding. Thus, we attempted Ag-affinity chromatography of the suppressive nanovesicles (Fig. 6a) and recovered an Ag binding subfraction (12% of the total) with all the suppressive activity in the TNP CS model (Fig. 6b; D). Further, Fig. 6c shows that there was suppression of the HT-2 T cell response to IL-2 by the Ag-binding nanovesicles eluted from the column (Group C), while the flow through and column wash fractions were non-suppressive (Groups A & B), confirming the findings from the CS model.
Fig. 6. Isolation of a small suppressive nanovesicle subpopulation by Antigen affinity chromatography.
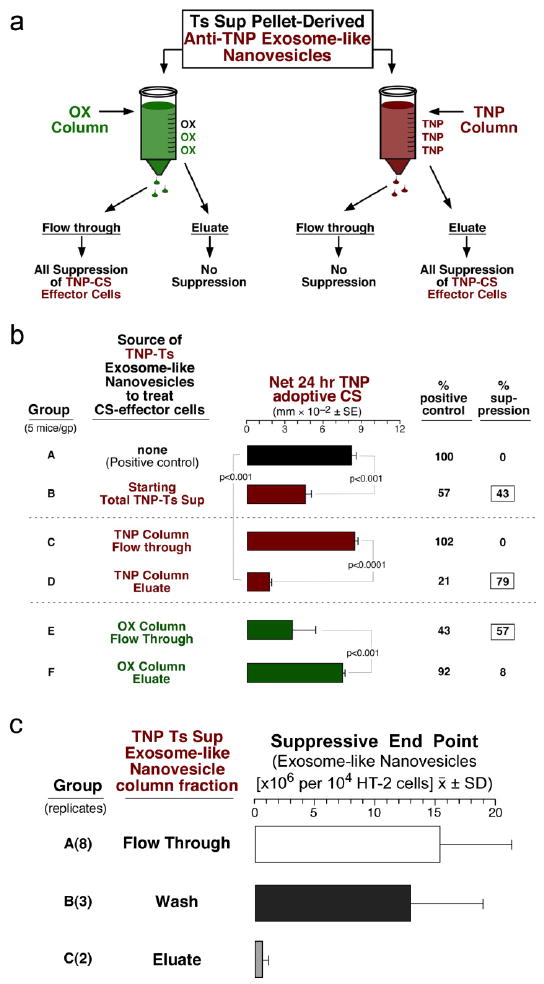
a. Suppressive TNP-Ts Sup nanovesicles were applied to either a column conjugated with TNP or OX. Only 12% of applied nanovesicles adhered to the TNP column and were eluted with dilute guanidine. b. TNP-Ts Sup vesicles from the TNP column flow through (FT) mediated no suppression (C), whereas the TNP-nanovesicles from the eluate had all the activity (D). The OX column FT, but not eluate had all the suppressive activity (E vs F). c. The eluate fraction from the TNP column strongly inhibited HT-2 cell viability (Group C), whereas the column wash (Group B), and the flow through (Group A) were not suppressive.
Cloning, sequencing, and bioinformatic comparison of reads from the exosome-like nanovesicle populations separated by the TNP Ag-affinity column
Comparison and ranking of frequency of sequences between the two nanovesicle fractions from the TNP-column; (i.e. the Ag binding and suppressive vs the non-binding and non-suppressive), was performed (See Table E1 in this article’s Online Repository at jacionline.org). This suggested that miRNA-150 (line 7), previously associated with T cell regulation31-37, might be a candidate for mediating suppression by the T cell-derived exosome-like nanovesicles. In contrast, among the sequences more frequent in the opposite FT vs the eluate sequences was miRNA-155 (See Table E2 line 12 in this article’s Online Repository at jacionline.org) that therefore was depleted from the Ts nanovesicles. This miRNA is strongly associated with Treg and guides expression of Foxp338.This data supports our findings that Treg were not involved in the CD8+ T cell suppression described here.
Anti-miRs confirm potential involvement of miRNA-150 in the suppression by high Ag dose tolerization
Fig. 7a, Group C shows that anti-miR antagonistic to miR-150 reversed suppression mediated by the exosome-like nanovesicles in adoptive transfer of CS. In contrast, a set of five anti-miR controls, aimed at the other prominent miRNAs more frequent in the column eluate compared to flow through vesicles (See Table E1 in this article’s Online Repository at jacionline.org), or mimic controls, did not reverse suppression. Similarly, besides these in vivo data, the miR-150 antagonist reversed Ts Sup nanovesicle suppression of the HT-2 cell response to IL-2, again compared to these controls (Fig. 7b; B), suggesting that miR-150 also was involved in the in vitro inhibition of HT-2 cell responses to IL-2.
Fig. 7. Use of specific anti-miRs to test for candidate suppressive cargo of the Ts Sup exosome-like nanovesicles.
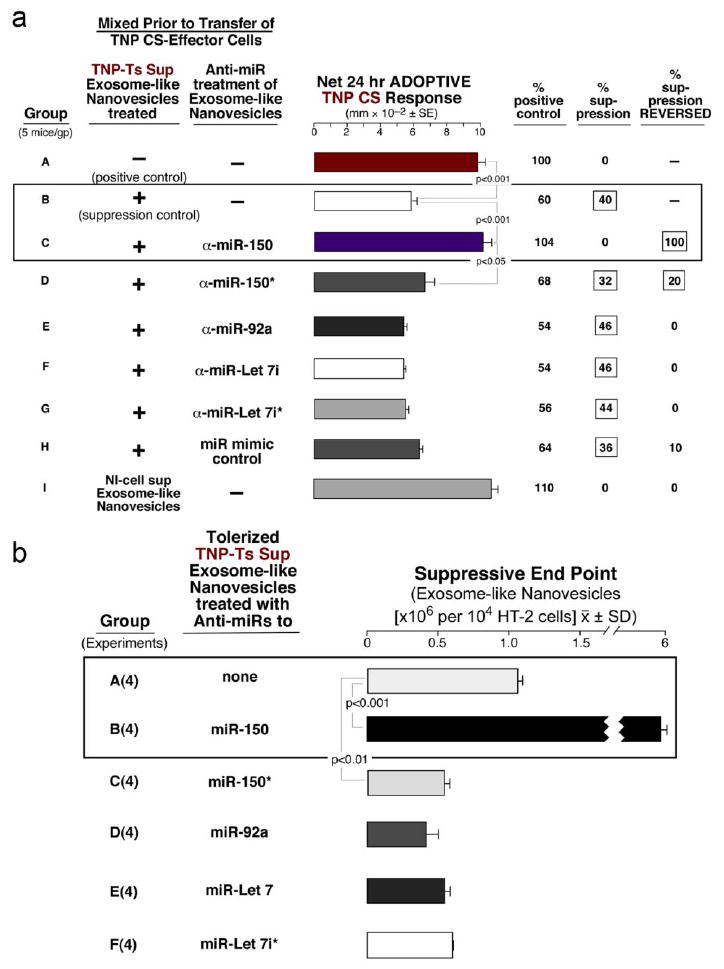
a. TNP Ts Sup nanovesicles pre-incubated with anti-miR-150 completely inhibited the suppression of CS (C). Similar treatment with anti-miR-150*, (the anti-sense passenger strand), only resulted in 20% reversed suppression (D). Treatment with anti-miR to miR-92 (E), Let 7i (F), Let7i (G)*, or the mimic control (H), all did not reverse suppression. b. The same anti-miRs were tested in the in HT-2 assay. Only the anti-miR to miR-150 reversed suppression (B).
Experiments with mice deficient in miR-15031 definitively identified miR-150 as crucial to in vivo suppression by the exosome-like nanovesicles
The miR-150-/- mice could not be tolerized (Fig. 8a; D) compared to wild type controls (Group B), and after attempted tolerization their exosome-like nanovesicles were not suppressive compared to wild type (Fig. 8b; C vs B). Very importantly, these non-suppressive exosome-like nanovesicles could be transfected for reconstitution of suppression by mere in vitro incubation with miR-150 alone (Fig. 8c; D). Again, this procedure was performed between the two 100,000gpelleting steps.
Fig. 8. miRNA-150 deficient mice show definitively that miR-150 is the suppressive entity in the exosome-like nanovesicles from tolerized mice.
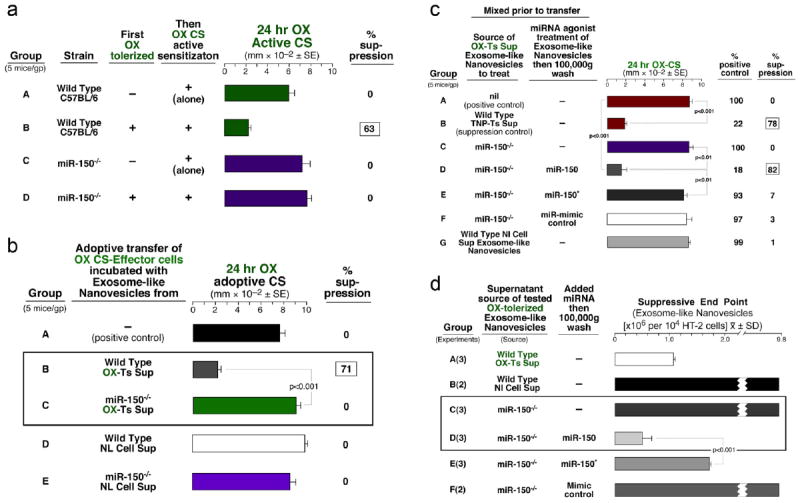
a. Wild type (WT) C57BL/6 and miR-150-/- mice were actively contact sensitized and had normal responses at 24h (A & C). WT mice first tolerized and then actively sensitized showed strong suppression (B), in contrast to miR-150-/- mice that showed no suppression after tolerance induction (D). b. Treatment of OX CS-effector cells with miR-150-/- OX Ts Sup nanovesicles (C) as well as with WT Nl Cell Sup (D) and miR-150-/- Nl Cell Sup (E) nanovesicles did not cause suppression of CS. c. Nanovesicles from OX tolerized miR-150-/- mice transfected with miR-150 mediated suppression, whereas transfection with miR-150* (E), or miR-mimic control (F), did not results in suppression. d. The Non-Ag-specific assay of HT-2 T cell responsiveness to IL-2 showed that nanovesicles from miR-150-/- OX tolerized mice (C) were non-inhibitory but transfection with miR-150 rendered them suppressive (D).
Performance of the HT-2 assay showed that miR-150 was also crucial to this in vitro assay
The tolerized miR-150-/- exosome-like nanovesicles were non-suppressive, whereas miR-150 transfection reconstituted their suppression in vitro (Fig. 8d; C vs D). This finding confirmed that nanovesicle inhibition of the in vitro correlative HT-2 T cell assay also was dependent on delivery of miR-150. Overall, we have shown that activated exosome-like nanovesicles from tolerized suppressor T cells were responsible for the in vivo and in vitro suppression and differed greatly from normal nanovesicles. The results suggest that the acquired ability of activated nanovesicles to permit antibody coating of choice and loading with selected miRNA might be used to generate very specific therapeutic exosome-likenanovesicles.
Discussion
Synopsis of new findings
This study of T cell-derived immunosuppressive exosome-like nanovesicles in allergic cutaneous CS generated two important discoveries. First, the nanovesicles were Ag-specific, which enabled them to suppress Ag-specific CS-effector T cells and bind to specific Ag-linked affinity columns. This Ag specificity resulted from a coating of Ab LC or Ab that we believe was produced by B cells activated during the tolerogenic procedure. The second discovery was that these nanovesicles could easily be transfected with selected miRNA, to therefore deliver a chosen regulatory dsRNA cargo to genetically affect particular functions of Ag-specifically targeted cells. Thus, suppressive function depended on the exosome-like nanovesicles from the Ts cells and B cell-produced Ag-specific Ab LC or Ab. Employment of unprecedented techniques like Ag-specific affinity chromatography of the nanovesicles led to isolation of a suppressive subset we subjected to molecular cloning. Deep sequencing and comparative bioinformatics of suppressive vs non-suppressive-associated miRNAs led to preliminary identification of the inhibitory miRNA as the previously T cell associated miRNA-15031-37.
Postulated pathway of effector T cell suppression by the exosome-like nanovesicles
We hypothesize that the described procedure of tolerance induction results in the activation of two essential cell types. One is the T CD8+ suppressor cell population which produces the exosome-like nanovesicles containing inhibitory miRNA-150. The other collaborating cells likely are B1 B cells, probably Ag-specifically activated in the peritoneal cavity by contact skin immunization during tolerogenesis11. After migration to the spleen, B1 B cells were shown to produce Ag-specific IgM and Ab LC into the circulation11. Therefore, the suppressive nanovesicles produced during tolerogenesis could be coated with Ab LC or Ab in vivo as shown by flow cytometry (Fig. 5b). Moreover, coating of exosome-like nanovesicles with Ab LC could be performed in vitro (Fig. 5c).
Treatment of the CS-effector cell mixture with the nanovesicles was effective in vitro, as shown in adoptive transfer experiments. Moreover, the exosome-like nanovesicles injected systemically at the peak response into actively sensitized mice were able to suppress CS resonses, likely by targeting the activated CS-effector T cells in vivo at the CS elicitation site.
The exact mechanism of tolerance and the targeted cell type are subject to ongoing research. The CS-effector T cells themselves may be the direct target of the suppressive nanovesicles action. However, preliminary results suggest that the regulatory signal could also be transmitted to CS-effector cells by targeted Ag-presenting cells, such as DC or macrophages, whose functions are altered by the suppressive exosome-like nanovesicles (unpublished data).
Ag-specificity of the suppressive exosome-like nanovesicles
The Ag-specificity we identified is an important new property of exosome-like nanovesicles. Not only is this the first demonstration of Ag-specific nanovesicles, but also the first demonstration that such vesicles with biological activity can be separated into at least two functional subpopulations; a minor Ag-binding fraction having all the activity and a major non-Ag binding fraction that was non-suppressive. The potential surface Ag-specificity of the suppressive exosome-like nanovesicles was based on four findings: 1. flow cytometry showing Ab kappa LC on their surface, 2. dual reciprocal Ag-specific suppressive function, 3. specific Ag binding for Ag affinity chromatography, and finally 4. reconstitution of suppression in non-suppressive nanovesicles from JH-/- pan immunoglobulin deficient tolerized mice by coating with Ag-specific monoclonal Ab LC.
Our data show that nanovesicle Ag-specificity and suppressive activity can be conferred by a coating with free Ab LC and not Ab HC, that therefore likely bind an unknown site on the activated exosome-like nanovesicles. An alternative possibility is that intact, antigen-specific IgM or IgG are responsible. Despite the usual low affinity Ag binding of isolated Ab LC, they can mediate Ag-specificity39,11. Further, when Ab LC are multiply displayed on the nanovesicle surface, the overall avidity for Ag likely increases, particularly in this hapten system where there is only one Ag determinant. Free Ig LC previously have been implicated in a variety of immune and allergic inflammatory diseases40-43 and may be one mechanism for the beneficial effect of anti-CD20 B cell therapy with Rituximab44,45. Effects of Ab LC in diverse responses first were ascribed to binding and activating mast cells11,30,40-43,46, but binding of Ab LC to human T cells, B cells and monocytes has been recently demonstrated47.
Suppression by antigen-specific exosome-like nanovesicles
Suppressive nanovesicles can be compared to immunosuppressive extracellular vesicles described previously in allergy and immunity48-51, and other conditions, such as: pregnancy52-54, breast feeding55 and especially in cancers where tumor-derived exosomes subvert a variety of host responses56-62. However, in none of these other systems have exosomes been shown to exhibit Ag-specificity. This was suggested previously, but without Ag-binding or dual reciprocal testing59. Our system of high Ag-dose-induced suppressive Ts and Ts Sup previously was elegantly characterized biologically and noted to be Ag-specific63-65. However, neither the mechanisms for Ag-specificity (here shown to be antibody), nor elucidation of how suppression was mediated (here shown due to miRNA-150 contained in exosome-like nanovesicles), was determined.
Suppression is mediated by miRNA-150 in exosome-like nanovesicles from tolerized CD8+ suppressor T cells
A crucial step leading to identification of miR-150 as mediating suppression was isolation by Ag-affinity column chromatography of a suppressive Ag-binding subpopulation of nanovesicles representing only 12% of the total. Comparing miRNA sequences of this subpopulation to those of the non suppressive non-Ag-binding nanovesicles, led to miR-150 as a candidate, and inhibition of suppressive activity by miR-150 antagonist confirmed this idea. Finally, experiments with miR-150-/- mice definitively established miR-150 as the major suppressive small RNA carried by the exosome-like nanovesicles to Ag-specifically inhibit targeted cells in the CS-effector cell mixture. Of further importance, the miRNA-150 in the exosome-like nanovesicles also could act Ag-non-specifically to inhibited HT-2 cell responsiveness to IL-2; an in vitro assay66 that turned out to be truly correlative with the in vivo nanovesicle suppression of CS. This should prove to be an excellent system to determine the molecular mechanisms of the effects of miR-150 on targeted cells, here possibly taken up by non-specific mechanisms like pinocytosis instead of by an Ab-dependent Ag-specific mechanism. miR-150 was described originally in positive T cell mediation of B cell, T cell and myeloid/erythroid development31,33, and more recently development of T, NK and NKT cells35,37. As would be expected for regulation by an miRNA, activating vs. suppressive effects may depend on particular targeted transcription factors other than the strongly miR-150-associated c-Myb8,31,34,35. Accordingly, miR-150 also can inhibit B cell development depending on timing32, and is considered a tumor suppressor67. The intracellular target of miR-150 in nanovesicle-mediated suppression of CS will be the subject of future investigations.
How these studies of T cell suppression may relate to human patients with contact dermatitis and other inflammatory diseases
The present study demonstrates a mechanism of T cell tolerance in mice mediated by exosome-like nanovesicles carrying miRNA-150 produced by suppressor T CD8+ cells and possibly delivered at the cutaneous site of CS.
Human studies have noted Treg cells defined by Foxp3 and/or cytokine associations at skin lesions of patients with contact dermatitis68. Besides CD4+ Tregs, there are many clinical instances in which a role for regulatory CD8+ T cells has been described. These include regulation of IgE-mediated allergy69, autoimmunity70,71, viral diseases72-74 cancer75,76 and transplantation alloimmunity77. CD8+ Treg are being appreciated to play a role in a variety of regulatory processes78-80. However, in the current study, we ruled out Foxp3+ Treg cell participation in this tolerogenesis.
Therefore, it is important to consider whether similar Ag-specific CD8+ suppressor T cells or analogous tolerance mechanisms also exist in humans, and possibly modulate clinical diseases via the release of comparable exosome-like nanovesicles. On the other hand, cell populations mediating regulatory mechanisms observed in murine models may not always be directly translated to humans. However, even when different regulatory cell types are involved in mediating tolerance, still the same clinical effect can be observed. This was demonstrated in a clinical trial studying induction of suppression of the autoimmune response in multiple sclerosis where the same tolerogenic procedure was preformed in both species, but the resulting suppressive T cells had distinctly different phenotypes81,82. Since the activity of the suppressive nanovesicles could also be modulated by easy transfection with miRNA and surface coating with Ab LC, this newly described mechanism of suppression may have important potential in regulation of immune responses.
Translation potentials
Discovery of Ag-specific exosome-like nanovesicles suggests they could be targeted to specific cells by sensitizing their surface with a coat of chosen antibody light chains against a marker of the desired target cells, and loaded with selected miRNA cargo for specific intracellular genetic therapy. This might enable suppression of specific effector cells in allergic, autoimmune and inflammatory diseases. Alternatively, suppressive function of Treg in cancer, or small RNA derived from oncogenes in leukemia may be antagonized by nanovesicle-derived cargo. Although our findings pertain to hapten induced skin allergy, we are extending them to protein antigens in DTH and allergic asthma (Groot Kormelink et al, unpublished).
The ability of activated exosome-like nanovesicles to be transfected with selected small RNA cargo to bind surface antigens preferentially expressed on targeted cells and alter specific target cell functions, could achieve a high therapeutic index as a new physiological and specific delivery vehicle. Finally, our data suggest that therapeutic exosome-like vesicles are able to act at great distances via the blood for prolonged times after a single dose, and may even work when given orally. Further, as therapy, they seem to be able to suppress active disease via cooperating immunological and genetic mechanisms. In summary, the unique and very important potential translational properties of the suppressive exosome-like nanovesicles that we have described are their easy transfection with miRNA, and above all their maniputable antigen-specificity.
Supplementary Material
Key Messages.
Antigen specific exosome-like nanovesicles delivering selected inhibitory miRNA is a new form of regulation shown here to inhibit allergic contact dermatitis.
The described mechanism of tolerance enables antigen specific targeting of particular cell function via miRNA interference.
This process may potentially lead to establishment of a new form of natural immunologic and genetic therapy of many human diseases.
Acknowledgments
The authors thank Madeleine Michaud for her secretarial and administrative skills, David Fedson, Ivana Kawikova, Timur Yarovinsky and Avrion Mitchison for their advise on the manuscript, Jordan Pober for his continuing sage advise on the project, Jerry Domian for his tireless adjustments of the figures, Esther Nolte-t Hoen, and Marca Wauben of Utrecht University for helpful preliminary sucrose gradient experiments. We would like to especially thank Markus Hafner and Thomas Tuschl of the Rockerfeller University for the preparation and sequencing of small RNA cDNA libraries from provided samples. This was essential to the eventual identification of miR-150 as crucial.
Declaration of funding:
KB was supported by grants from the Polish Ministry of Science and Higher Education: N401 092 31/2176 and K/ZDS/001429, TGK by a grant from Lung Foundation Netherlands; 3.2.11.09FE, and PWA by grants from the NIH: AI-076366, AI-07174, and AI-1053786.
Abbreviations
- CS
contact sensitivity
- DC
dendritic cells
- FT
flow through
- Ab LC
free antibody light chains
- OX
oxazolone
- TNP
trinitrophenyl
- MVB
multivesicular body of the terminal endosomal pathway
- Nl Cell Sup
supernatant from culture of lymph and spleen cells from normal (non-immunized) mice
- mRBC
mouse red blood cells
- NTA
nanoparticle tracking analysis
- Ts
suppressor T cells from antigen tolerized mice
- Ts Sup
supernatant from culture of lymph and spleen suppressor T cells from tolerized mice
- Treg
T regulatory cells
- WT
wild type
Footnotes
Author contributions
K.B. Conducted the in vivo work and molecular biology. W.P. Guided K.B. A.J. Prepared the TNP-DC and CS-effector T cells for IFNγ assay. K.P. Performed the flow cytometry. A.C. Performed the initial differential centrifugation and was supervised by M.C. J.L. supervised B.A. B.A. Performed the PCRs. E.S. Performed the HT-2 in vitro tests. K.N. Performed in vivo tests. S.M. Performed some of the bioinformatics. S.K. Supervised S.M. P.S. Performed the Western analysis and was supervised by Y.I. E.D. Performed the gel electrophoreses. F.R. Supplied the anti-TNP Ig LC and HC, advised on their use and supervised BB in their producton. J.W. Provided a part of ultracentrifugation procedures and was supervised by A.W.D. T.G.K. Supplied and advised on the anti-TNP Ab LC and performed some of the important in vivo animal experiments P.W.A. Coordinated the work of the group and mostly wrote the manuscript.
Disclosure of conflicts of interest.
The authors declare that there are no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 2.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current Protocols in Cell Biology. 2006;Chapter 3:3–22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 3.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 4.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korkut C, Ataman B, Ramachandran P, Korkut C, Ashley J, Barria R, Budnik V, et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, et al. Mechanism of evenness interrupted (evi)-exosome release at synaptic moutons. J Biol Chem. 2012;287:16820–34. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of betacatenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079–91. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Luo N, Luo Y, Peng Z, Zhang T, Li S. microRNA-150 inhibits human CD133-positive liver cancer stem cells through negative regulation of the transcription factor c- Myb. Int J Oncol. 2012;40:747–56. doi: 10.3892/ijo.2011.1242. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Hou D, Chen X, Li D, Zhu L, Zhang Y, et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2012;22:107–26. doi: 10.1038/cr.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin SF, Dudda JC, Bachtanian E, Lembo A, Liller S, Durr C, et al. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J Exp Med. 2008;205:2151–62. doi: 10.1084/jem.20070509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Askenase PW, Szczepanik M, Itakura A, Kiener C, Campos RA. Extravascular T cell recruitment requires initiation begun by V14+ NKT cells and B-1 B cells. Trends Immunol. 2004;25:441–9. doi: 10.1016/j.it.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Vocanson M, Hennino A, Cluzel-Tailhardat M, Saint-Mezard P, Benetiere J, Chavagnac C, et al. CD8+ T cells are effector cells of contact dermatitis to common skin allergens in mice. J Invest Dermatol. 2006;126:815–20. doi: 10.1038/sj.jid.5700174. [DOI] [PubMed] [Google Scholar]
- 13.Lee DS, Gulati N, Martiniuk F, Levis WR. CD70 and Th17 are involved in human contact sensitivity. J Drugs Dermatol. 2011;10:1192–4. [PMC free article] [PubMed] [Google Scholar]
- 14.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–35. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonetta F, Chiali A, Cordier C, Urrutia A, Girault I, Bloquet S, et al. Increased CD127 expression on activated FOXP3+CD4+ regulatory cells. Eur J Immunol. 2010;40:2528–38. doi: 10.1002/eji.201040531. [DOI] [PubMed] [Google Scholar]
- 16.Ilkovitch D. Role of immune-regulatory cells in skin pathology. J Leukoc Biol. 2011;89:41–9. doi: 10.1189/jlb.0410229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhuri K, Llodra J, Kam L, Stokes D, Dustin M. Antigen-induced release and retroviral subversion of TCR-enriched microvesicles at the CD4+ T cell immunological synapse. J Immunol. 2012;188:58–5. [Google Scholar]
- 18.Mittelbrunn M, Gutierrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González MÁ, et al. Unidirectional transfer of microRNA- loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282–91. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–66. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724–8. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJ, Zeiher AM, et al. Atheroprotective communication between endothelia cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–56. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 22.Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46:409–18. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447–58. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 24.Eldh M, Ekström K, Valadi H, Sjöstrand M, Olsson B, Jernas M, et al. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PloS ONE. 2010;5:e15353. doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meckes DG, jr, Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci USA. 2010;107:20370–5. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107:6328–33. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soo CY, Song Y, Zheng Y, Campbell EC, Riches AC, Gun-Moore E, et al. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology. 2012;136:192–7. doi: 10.1111/j.1365-2567.2012.03569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.KaLahl K, Sparwasser T. In Vivo Depletion of FoxP3+Tregs Using the DEREG Mouse Model. Methods Mol Biol. 2011;707:157–72. doi: 10.1007/978-1-61737-979-6_10. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Trounstine M, Alt FW, Young F, Kurahara C, Loring JF, et al. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993;5:647–56. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 30.Redegeld FA, van der Heijden MW, Kool M, Heijdra BM, Garssen J, Kraneveld AD, et al. Immunoglobulin-free light chains elici immediate hypersensitivity-like responses. Nat Med. 2002;8:694–701. doi: 10.1038/nm722. [DOI] [PubMed] [Google Scholar]
- 31.Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, et al. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131:146–59. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 32.Zhou B, Wang S, Mayr C, Bartel DP, Lodish HF. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci USA. 2007;104:7080–5. doi: 10.1073/pnas.0702409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruchova-Votavova H, Yoon D, Prchal JT. MiR-451 enhances erythroid differentiation in K562 cells. Leuk Lymph. 2010;51:686–93. doi: 10.3109/10428191003629362. [DOI] [PubMed] [Google Scholar]
- 34.Hussein K, Theophile K, Büsche G, Schlegelberger B, Göhring G, Kreipe H, et al. Significant inverse correlation of microRNA-150/MYB and microRNA-222/p27 in myelodysplastic syndrome. Leuk Res. 2010;34:328–34. doi: 10.1016/j.leukres.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Bezman NA, Chakraborty T, Bender T, Lanier LL. MiR-150 regulates the development of NK and iNKT cells. J Exp Med. 2011;208:2717–31. doi: 10.1084/jem.20111386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghisi M, Corradin A, Basso K, Frasson C, Serafin V, Mukherjee S, et al. Modulation of microRNA expression in human T-cell development: targeting of NOTCH3 by miR-150. Blood. 2011;117:7053–62. doi: 10.1182/blood-2010-12-326629. [DOI] [PubMed] [Google Scholar]
- 37.Zheng Q, Zhou L, Mi QS. MicroRNA miR-150 is involved in Valpha14 invariant NKT cell development and function. J Immunol. 2012;188:2118–26. doi: 10.4049/jimmunol.1103342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K. Foxp3-Dependent MicroRNA155 Confers Competitive Fitness to Regulatory T Cells by Targeting SOCS1 Protein. Immunity. 2009;30:80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Housden NG, Harrison S, Housden HR, Thomas KA, Beckingham JA, Roberts SE, et al. Observation and characterization of the interaction between a single immunoglobulin binding domain of protein L and two equivalents of human kappa light chains. J Biol Chem. 2004;279:9370–8. doi: 10.1074/jbc.M312938200. [DOI] [PubMed] [Google Scholar]
- 40.Powe DG, Groot Kormelink T, Sisson M, Blokhuis BJ, Kramer MF, Jones NS, et al. Evidence for the involvement of free light chain immunoglobulins in allergic and nonallergic rhinitis. J Allergy Clin Immunol. 2010;125:139–45. doi: 10.1016/j.jaci.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 41.Groot Kormelink T, Calus L, De Ruyck N, Holtappels G, Bachert C, Redegeld FA, et al. Local free light chain expression is increased in chronic rhinosinusitis with nasal polyps. Allergy. 2012;67:1165–72. doi: 10.1111/j.1398-9995.2012.02866.x. [DOI] [PubMed] [Google Scholar]
- 42.Thio M, Groot Kormelink T, Fischer MJ, Blokhuis BR, Nijkamp F, Redegeld FA. Characteristics of Immunoglobulin Free Light Chains: Crosslinking by Antigen is Essential to Induce Allergic Inflammation. Plos ONE. 2012;7:e40986. doi: 10.1371/journal.pone.004098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groot Kormelink T, Askenase PW, Redegeld FA. Immunobiology of antigens pecific immunoglobulin free light chains in chronic inflammatory diseases. Curr Pharm Des. 2012;18:2278–89. doi: 10.2174/138161212800166059. [DOI] [PubMed] [Google Scholar]
- 44.Groot Kormelink T, Tekstra J, Thurlings RM, Boumans MH, Vos K, Tak PP, et al. Decrease in immunoglobulin free light chains in patients with rheumatoid arthritis upon rituximab (anti-CD20) treatment correlates with decrease in disease activity. Ann Rheum Dis. 2010;69:2137–44. doi: 10.1136/ard.2009.126441. [DOI] [PubMed] [Google Scholar]
- 45.Tekstra J, van Roon J, Groot Kormelink T, Redegeld F. Immunoglobulin free lightchain levels and rituximab response in rheumatoid arthritis: comment on the article by Sellam et al. Arthritis Rheum. 2011;63:4034–5. doi: 10.1002/art.30632. [DOI] [PubMed] [Google Scholar]
- 46.Paliwal V, Tsuji RF, Szczepanik M, Kawikova I, Campos RA, Kneilling M, et al. Subunits of IgM reconstitute defective contact sensitivity in B-1 cell-deficient xid mice: kappa light chains recruit T cells independent of complement. J Immunol. 2002;169:4113–23. doi: 10.4049/jimmunol.169.8.4113. [DOI] [PubMed] [Google Scholar]
- 47.Hutchinson AT, Jones DR, Raison RL. The ability to interact with cell membranes suggests possible biological roles for free light chain. Immunol Lett. 2012;142:75–7. doi: 10.1016/j.imlet.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 48.Almqvist N, Lonnqvist A, Hultkrantz S, Rask C, Telemo E. Serum-derived exosomes from antigen-fed mice prevent allergic sensitization in a model of allergic asthma. Immunology. 2008;125:21–7. doi: 10.1111/j.1365-2567.2008.02812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim SH, Bianco N, Menon R, Lechman ER, Shufesky WJ, Morelli AE, et al. Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol Ther. 2006;13:289–300. doi: 10.1016/j.ymthe.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. MHC class II+ exosomes in plasma suppress inflammation in an antigen-specific and Fas ligand/Fas-dependent manner. J Immunol. 2007;179:2235–41. doi: 10.4049/jimmunol.179.4.2235. [DOI] [PubMed] [Google Scholar]
- 51.Prado N, Marazuela EG, Segura E, Fernández-García H, Villalba M, Théry C, et al. Exosomes from bronchoalveolar fluid of tolerized mice prevent allergic reaction. J Immunol. 2008;181:1519–25. doi: 10.4049/jimmunol.181.2.1519. [DOI] [PubMed] [Google Scholar]
- 52.Hedlund M, Stenqvist AC, Nagaeva O, Kjellberg L, Wulff M, Baranov V, et al. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol. 2009;183:340–51. doi: 10.4049/jimmunol.0803477. [DOI] [PubMed] [Google Scholar]
- 53.Sabapatha A, Gercel-Taylor C, Taylor DD. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol. 2006;56:345–55. doi: 10.1111/j.1600-0897.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 54.Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol. 2006;176:1534–42. doi: 10.4049/jimmunol.176.3.1534. [DOI] [PubMed] [Google Scholar]
- 55.Admyre C, Johansson SM, Qazi KR, Filén JJ, Lahesmaa R, Norman M, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179:1969–78. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 56.Hao S, Ye Z, Li F, Meng Q, Qureshi M, Yang J, et al. Epigenetic transfer of metastatic activity by uptake of highly metastatic B16 melanoma cell-released exosomes. Exp Oncol. 2006;28:126–31. [PubMed] [Google Scholar]
- 57.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down- modulate NKG2D expression. J Immunol. 2008;180:7249–58. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 58.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent Immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–71. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang C, Kim SH, Bianco NR, Robbins PD. Tumor-derived exosomes confer antigen-specific immunosuppression in a murine delayed-type hypersensitivity model. PloS ONE. 2011;6:e22517. doi: 10.1371/journal.pone.0022517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676–83. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 61.Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang Q, et al. Activated T Cell Exosomes Promote Tumor Invasion via Fas SignalingPathway. J Immunol. 2012;188:5954–61. doi: 10.4049/jimmunol.1103466. [DOI] [PubMed] [Google Scholar]
- 62.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ptak W, Rozycka D, Rewicka M. Induction of suppressor cells and cells producing antigen-specific suppressor factors by haptens bound to self-carriers. Immunobiology. 1980;156:400–9. doi: 10.1016/S0171-2985(80)80073-8. [DOI] [PubMed] [Google Scholar]
- 64.Ptak W, Rosenstein RW, Gershon RK. Interactions between molecules (subfatcors) released by different T cell sets that yield a complete factor with biological (suppressive) activity. Proc Natl Acad Sci USA. 1982;79:2375–8. doi: 10.1073/pnas.79.7.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ptak W, Gershon RK, Rosenstein RW, Murray JH, Cone RE. Purification and characterization of TNP- specific immunoregulatory molecules produced by T cells sensitized by picrylchloride (PC1F) J Immunol. 1983;131:2859–63. [PubMed] [Google Scholar]
- 66.Ferreri NR, Herzog WR, Askenase PW. Inhibition of IL-2-dependent proliferation by a prostaglandin- dependent suppressor factor. J Immunol. 1993;150:2102–11. [PubMed] [Google Scholar]
- 67.Watanabe A, Tagawa H, Yamashita J, Teshima K, Nara M, Iwamoto K, et al. The role of microRNA-150 as a tumor suppressor in malignant lymphoma. Leukemia. 2011;25:1324–34. doi: 10.1038/leu.2011.81. [DOI] [PubMed] [Google Scholar]
- 68.Matsushima H, Takashima A. Bidirectional homing of Tregs between the skin and lymph nodes. The Journal of clinical investigation. 2010;120:653–656. doi: 10.1172/JCI42280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kemeny DM, NOble A, Holmes BJ, Diaz-Sanchez D, Lee TH. The role of CD8+ T cells in immunoglobulin E regulation. Allergy. 1995;50:9–14. doi: 10.1111/j.1398-9995.1995.tb04268.x. [DOI] [PubMed] [Google Scholar]
- 70.Suzuki M, Konya C, Goronzy JJ, Weyand CM. Inhibitory CD8+ T cells in autoimmune disease. Hum Immunol. 2008;69:781–9. doi: 10.1016/j.humimm.2008.08.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang H, Canfield SM, Gallagher MP, Jiang HH, Jiang Y, Zheng Z, et al. HLA-E-restricted regulatory CD8(+) T cells are involved in development and control of human autoimmune type 1 diabetes. J Clin Invest. 2010;120:3641–50. doi: 10.1172/JCI43522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, et al. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963–72. doi: 10.1172/JCI20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buckheit RW, 3rd, Salgado M, Silciano RF, Blankson JN. Inhibitory potential of subpopulations of CD8+ T cells in HIV-1-infected elite suppressors. J Virol. 2012;86:13679–88. doi: 10.1128/JVI.02439-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tumne A, Prasad VS, Chen Y, Stolz DB, Saha K, Ratner DM, et al. Noncytotoxic suppression of human immunodeficiency virus type 1 transcription by exosomes secreted from CD8+ T cells. J Virol. 2009;83:4354–64. doi: 10.1128/JVI.02629-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, et al. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. 2004;91:1711–7. doi: 10.1038/sj.bjc.6602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olson BM, Jankowska-Gan E, Becker JT, Vignali DA, Burlingham WJ, McNeel DG. Human prostate tumor antigen-specific CD8+ regulatory T cells are inhibited by CTLA-4 or IL-35 blockade. J Immunol. 2012;189:5590–601. doi: 10.4049/jimmunol.1201744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ciubotariu R, Colovai AI, Pennesi G, Liu Z, Smith D, Berlocco P, et al. Specific suppression of human CD4+ Th cell responses to pig MHC antigens by CD8+CD28- regulatory T cells. J Immunol. 1998;161:5193–202. [PubMed] [Google Scholar]
- 78.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang CC, Zhang QY, Liu Z, Clynes RA, Suciu-Foca N, Vlad G. Downregulation of inflammatory microRNAs by Ig-like transcript 3 is essential for the differentiation of human CD8(+) T suppressor cells. J Immunol. 2012;188:3042–52. doi: 10.4049/jimmunol.1102899. [DOI] [PubMed] [Google Scholar]
- 80.Van Kaer L. Comeback kids: CD8(+) suppressor T cells are back in the game. J Clin Invest. 2010;120:3432–4. doi: 10.1172/JCI44395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tutaj M, Szczepanik M. Epicutaneous (EC) immunization with myelin basic protein (MBP) induces TCRalphabeta+ CD4+ CD8+ double positive suppressor cells that protect from experimental autoimmune encephalomyelitis (EAE) J Autoimmun. 2007;28:208–15. doi: 10.1016/j.jaut.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 82.Jurynczyk M, Walczak A, Jurewicz A, Jesionek-Kupnicka D, Szczepanik M, Selmaj K. Immune regulation of multiple sclerosis by transdermally applied myelin peptides. Ann Neurol. 2010;68:593–601. doi: 10.1002/ana.22219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


