Abstract
Background
This is an updated version of the original Cochrane review published in Issue 2, 2000. Dihydrocodeine is a synthetic opioid analgesic developed in the early 1900s. Its structure and pharmacokinetics are similar to that of codeine and it is used for the treatment of postoperative pain or as an antitussive. It is becoming increasingly important to assess the relative efficacy and harm caused by different treatments. Relative efficacy can be determined when an analgesic is compared with control under similar clinical circumstances.
Objectives
To quantitatively assess the analgesic efficacy and adverse effects of single‐dose dihydrocodeine compared with placebo in randomised trials in moderate to severe postoperative pain.
Search methods
Published reports were identified from electronic databases (MEDLINE, EMBASE, CENTRAL, the Oxford Pain Relief Database in December 2007, the original search was conducted in October 1999). Additional studies were identified from the reference lists of retrieved reports.
Selection criteria
Inclusion criteria: full journal publication, clinical trial, random allocation of participants to treatment groups, double blind design, adult participants, baseline pain of moderate to severe intensity, postoperative administration of study drugs, treatment arms which included dihydrocodeine and placebo and either oral or injected (intramuscular or intravenous) administration of study drugs.
Data collection and analysis
Data collection and analysis: summed pain intensity and pain relief data over four to six hours were extracted and converted into dichotomous information to yield the number of participants obtaining at least 50% pain relief. This was used to calculate relative benefit and number‐needed‐to‐treat‐to‐benefit (NNT) for one participant to obtain at least 50% pain relief. Single‐dose adverse effect data were collected and used to calculate relative risk and number‐needed‐to‐treat‐to‐harm (NNH).
Main results
Fifty‐two reports were identified in the original review as possible randomised trials which assessed dihydrocodeine in postoperative pain. Four reports met the inclusion criteria; all assessed oral dihydrocodeine. Three reports (194 participants) compared dihydrocodeine with placebo and one (120 participants) compared dihydrocodeine (30 mg or 60 mg) with ibuprofen 400 mg. For a single dose of dihydrocodeine 30 mg in moderate to severe postoperative pain the NNT for at least 50% pain relief was 8.1 (95% confidence interval 4.1 to 540) when compared with placebo over a period of four to six hours. Pooled data showed significantly more participants to have reported adverse effects with dihydrocodeine 30 mg than with placebo. When compared to ibuprofen 400 mg both dihydrocodeine 30 mg and 60 mg were significantly inferior. No additional studies were found for this update.
Authors' conclusions
A single 30 mg dose of dihydrocodeine is not sufficient to provide adequate pain relief in postoperative pain. Statistical superiority of ibuprofen 400 mg over dihydrocodeine (30 mg or 60 mg) was shown. Since the last version of this review no new relevant studies have been identified.
Plain language summary
Dihydrocodeine in a single dose in the treatment of acute postoperative pain
This review assessed the efficacy of single‐dose dihydrocodeine in adults with moderate/severe postoperative pain using information from randomised placebo controlled trials. There was a lack of data that could be included in the analyses; all assessed the oral form of the drug and none assessed dihydrocodeine 60 mg. The results were not robust. The implication was that single‐dose oral dihydrocodeine 30 mg was more effective than placebo, but was inferior to ibuprofen 400 mg. Dizziness, drowsiness and confusion were commonly reported.
Background
This is an update of a previously published review in the Cochrane Database of Systematic Reviews (Issue 2, 2000) on 'Single dose dihydrocodeine for the treatment of acute postoperative pain'.
Opioids are used extensively in the management of pain and are believed capable of relieving severe pain more effectively than non‐steroidal anti‐inflammatory drugs (NSAIDs) (Alexander 1987). The aim of this systematic review was to assess the efficacy and safety of a single dose of dihydrocodeine in the management of postoperative pain of moderate to severe intensity.
Dihydrocodeine is a synthetic opioid analgesic developed in the early 1900s. Its structure and pharmacokinetics are similar to that of codeine (Rowell 1983) and it is used for the treatment of postoperative pain or as an antitussive. In England, 2.2 million prescriptions were written for dihydrocodeine tablets in 2006, and 4.2 million prescriptions for dihydrocodeine in combination with paracetamol (PACT 2006). This is only about one‐fifth of the level of use a decade earlier. The amount of dihydrocodeine used for the treatment of postoperative pain is not known, but it is not now commonly used to treat acute pain.
It is becoming increasingly important to assess the relative efficacy and harm caused by different treatments. Relative efficacy can be determined when an analgesic is compared with control under similar clinical circumstances. Mean pain outcome values from categorical pain intensity and pain relief scales (percent of maximum possible pain intensity or pain relief; %maxSPID and %maxTOTPAR) can be converted into dichotomous information (number of participants with at least 50% pain relief) (Moore 1996; Moore 1997a; Moore 1997b). This can then be used to derive the number‐needed‐to‐treat‐to‐benefit (NNT) for at least 50% pain relief. Comparison of the NNTs against placebo for different analgesics allows a rank order of relative efficacy to be established.
Objectives
To quantitatively evaluate the analgesic efficacy and adverse effects of dihydrocodeine in moderate to severe postoperative pain. To compare its efficacy and safety to that of other analgesics assessed in the same way.
Methods
Criteria for considering studies for this review
Types of studies
Studies were included if they were full journal publications of single‐dose, double blind, randomised controlled (placebo or active) trials (RCTs) of dihydrocodeine over four to six hours in postoperative pain. Multiple dose studies were included if they provided single‐dose data. Study drugs needed to have been administered (by injection or orally) postoperatively to adult participants with moderate or severe pain at baseline.
Studies were excluded if they did not state clearly that study medication had been randomly allocated or the method of randomisation was considered inappropriate (e.g. date of birth), or they examined other pain conditions (postfracture, postpartum uterine cramps, chronic pain, muscle strain, post‐trauma pain).
Abstracts, review articles, case reports, clinical observations, and unpublished data were not sought. Neither pharmaceutical companies nor authors were contacted for unpublished reports.
Full details of the individual studies are provided in the 'Characteristics of included studies' and 'Characteristics of excluded studies' sections.
Types of participants
Adult participants with established postoperative pain of moderate to severe intensity.
Types of interventions
Reports were included if they assessed participants who had been randomised to either dihydrocodeine (oral or injected) or placebo.
Types of outcome measures
Pain outcomes used were TOTPAR, SPID, VAS TOTPAR or VAS SPID over four to six hours or sufficient data provided to allow their calculation. Pain measures allowed for the calculation of TOTPAR were a standard five point pain relief scale (none, slight, moderate, good, complete) and for SPID a standard four point pain intensity scale (none, mild, moderate, severe).
Search methods for identification of studies
The following electronic databases were searched:
Cochrane CENTRAL (Issue 3, 1999 for the original review and Issue 4, 2007 for the update);
MEDLINE and Pre‐MEDLINE from 1966 to October 1999 for the original review, and MEDLINE from January 1999 to December 2007 for the update;
EMBASE from 1989 to October 1999 for the original review and January 1999 to December 2007 for the update;
the Oxford Pain Relief database (handsearch records for the years 1954 to 1995 (Jadad 1996a).
The MEDLINE search strategy can be seen in Appendix 1 and was adapted for the other databases searched.
Reference lists of retrieved reports were also manually searched.
Data collection and analysis
From each study we extracted: (i) the number of participants treated, (ii) mean TOTPAR, SPID, VAS TOTPAR or VAS SPID, (iii) study duration, (iv) the dose of dihydrocodeine, (v) the dose of active comparator (if relevant), (vi) information on adverse effects, and (vii) information on remedication.
Mean TOTPAR, SPID, VAS TOTPAR and VAS SPID values were converted to %maxTOTPAR or %maxSPID by division into the calculated maximum value (Moore 1996). Verified equations were used to estimate the proportion of participants achieving at least 50% maxTOTPAR (Moore 1997a; Moore 1997b). This was then converted in to the number of participants achieving at least 50% maxTOTPAR by multiplying the figure by the total number of participants in the treatment group. The number of participants with at least 50% maxTOTPAR was then used to calculate estimates of relative benefit and NNT.
Relative benefit/risk estimates with 95% confidence intervals (CI) were calculated using the fixed‐effect model (Morris 1995). Homogeneity was assumed when P > 0.1 and was tested for using a chi‐squared test. A statistically significant benefit of active treatment over placebo was assumed when the lower limit of the 95% CI of the relative benefit was >1. A statistically significant benefit of placebo over active treatment was assumed when the upper limit of the 95% CI of the relative benefit was <1. Number‐needed‐to‐treat‐to‐harm (NNH) and NNT with 95% CIs were calculated (Cook 1995). The CI includes no benefit of one treatment over the other when the upper limit is represented as infinity. Calculations were performed with the help of Excel 5.0 on a Macintosh Performa 6320.
There was no attempt at anonymisation of the studies prior to assessment. Two review authors (JE, HM) independently carried out data abstraction and quality assessments. A consensus meeting with all review authors was held to agree on the data abstracted, the quality scores, the data to be used in the analyses, and the studies for inclusion in the review.
Results
Description of studies
Fifty‐two published reports of dihydrocodeine in postoperative pain (359 participants in total) were identified; one could not be obtained from the British Library (Hummel 1995). Of the retrieved studies, 18 studies were not randomised or were abstracts and were excluded. Thirty‐two studies were randomised. Of the randomised studies eight were not double blind (two were single blind), five did not specify baseline pain of moderate to severe intensity, five had no extractable pain outcome data, six did not assess the analgesic properties of dihydrocodeine, three included other pain conditions (e.g. chronic/cold‐induced/trauma pain) and two did not use a placebo group. These studies were excluded (see 'Characteristics of excluded studies' table).
Four studies met the inclusion criteria from the original searches. No further studies were identified in the updated searches.
Risk of bias in included studies
Each study was scored for quality using a three‐item scale (see below) (Jadad 1996b); a consensus score was agreed for each study. The quality scores for individual studies are reported in the 'Characteristics of included studies' table. These scores were not used to weight the results in any way.
The three item scale is as follows: Is the study randomised? If yes then one point Is the randomisation procedure reported and is it appropriate? If yes then add one point, if not deduct one Is the study double blind? If yes then one point Is the double blind method reported and is it appropriate? If yes then add one point, if not deduct one Are withdrawals and dropouts described? If yes add one point
Effects of interventions
Four studies (359 participants) met the inclusion criteria: three were placebo‐controlled and one used ibuprofen 400 mg as an active control. All four studies examined the effects of oral dihydrocodeine. Three of the studies (Frame 1989; Galasko 1989; McQuay 1985) compared dihydrocodeine 30 mg with placebo, and one (McQuay 1993) compared dihydrocodeine (30 mg or 60 mg) with ibuprofen 400 mg. No additional studies were identified in the updated searches. Oral dihydrocodeine versus placebo No studies comparing dihydrocodeine 60 mg with placebo met the inclusion criteria. Three studies compared dihydrocodeine tartrate 30 mg (97 participants) with placebo (97 participants). One study investigated dental pain (Frame 1989), one orthopaedic pain (Galasko 1989), and one pain following minor day‐case surgery (McQuay 1985).
The proportion of participants experiencing at least 50% pain relief with dihydrocodeine varied between 14% and 50% (mean 32%). The proportion of participants experiencing at least 50% pain relief with placebo varied between 5% and 50% (mean 20%). Dihydrocodeine 30 mg was significantly different from placebo, relative benefit 1.6 (1.01 to 2.5). For a single dose of dihydrocodeine 30 mg compared with placebo the NNT was 8.1 (4.1 to 540) for at least 50% pain relief over four to six hours in postoperative pain of moderate to severe intensity.
Adverse effects The most frequently reported adverse effects were nausea, vomiting, headache, and central nervous system effects (dizziness/drowsiness/confusion). All were mild, transient in nature and no participants withdrew as a result.
Participants reporting any adverse effect Information on the number of participants reporting any adverse effect was pooled. The proportion of participants who reported adverse effects was 13/67 with dihydrocodeine 30 mg and 4/69 with placebo. The relative risk was 3.4 (1.2 to 9.8) and the NNH was 7.4 (4.1 to 38).
Particular adverse effects There was no significant difference in the reported incidence of particular (e.g. drowsiness) adverse effects with dihydrocodeine 30 mg than with placebo. Oral dihydrocodeine versus ibuprofen One study (McQuay 1993) compared the efficacy and safety of either dihydrocodeine tartrate 30 mg (40 participants) or 60 mg (40 participants) with ibuprofen 400 mg (40 participants) in dental pain.
The proportion of participants experiencing at least 50% pain relief with dihydrocodeine 30 mg was 8%, with dihydrocodeine 60 mg it was 15%, and with ibuprofen 400 mg (active control) it was 45%. A statistical superiority of ibuprofen 400 mg over dihydrocodeine 30 mg and dihydrocodeine 60 mg was shown, relative benefit 0.2 (0.05 to 0.5) and 0.3 (0.2 to 0.8) respectively.
Ibuprofen 400 mg was significantly more effective than dihydrocodeine 30 mg or dihydrocodeine 60 mg. The NNT for a single dose of ibuprofen 400 mg compared to dihydrocodeine 30 mg was 2.7 (1.8 to 5.0) for at least 50% pain relief over a period of four to six hours in postoperative pain of moderate to severe intensity. For a single dose of ibuprofen 400 mg compared to dihydrocodeine 60 mg the NNT was 3.3 (2.0 to 9.1) for at least 50% pain relief over a period of four to six hours.
Adverse effects No single dose adverse effect data were presented (McQuay 1993).
Discussion
We found no studies which investigated injected dihydrocodeine in the evaluation of postoperative pain with standard analgesic measurement methods.
For a single dose of oral dihydrocodeine tartrate 30 mg compared with placebo the NNT was 8.1 (4.1 to 540) for at least 50% pain relief over four to six hours in postoperative pain of moderate to severe intensity. This means that one in every eight participants with moderate to severe postoperative pain would experience at least 50% pain relief with dihydrocodeine 30 mg who would not have done with placebo.
A rank order of single dose analgesic efficacy in postoperative pain of moderate to severe intensity has been established by comparing orally administered analgesics from methodologically similar studies. This rank order has been published previously, both in its entirety (McQuay 1998), for third molar extraction studies only (Barden 2004), and as individual reports (Collins 1998a; Collins 1998b; Moore 1997c; Moore 1997d). It is also available on the World Wide Web (http://www.jr2.ox.ac.uk/bandolier/painres/painpag/Acutrev/Analgesics/Leagtab.html) or is available from the authors. The rank order shows the results of a number of meta‐analyses which compare analgesic with placebo in acute postoperative pain. The point estimates of the NNTs of many of these analgesics are lower (better) than that of dihydrocodeine 30 mg (for example: paracetamol, paracetamol plus codeine and dextropropoxyphene plus paracetamol) although the CIs overlap. The CIs of the NNTs for ibuprofen 400 mg, tramadol 150 mg and ibuprofen 200 mg do not overlap with those of dihydrocodeine 30 mg indicating greater analgesic efficacy.
This rank order of relative efficacy against placebo is supported by a head‐to‐head comparison with ibuprofen (McQuay 1993). The analgesic efficacy of a single dose of oral ibuprofen 400 mg was significantly better than dihydrocodeine (30 mg or 60 mg). The NNT was 2.7 (1.8 to 5.0) for at least 50% pain relief over a period of four to six hours in postoperative pain of moderate to severe intensity. This means that for every three participants with moderate to severe postoperative pain treated with ibuprofen 400 mg one will experience at least 50% pain relief who would not have done if given dihydrocodeine 30 mg. Similarly, for a single dose of ibuprofen 400 mg compared with dihydrocodeine 60 mg the NNT was 3.3 (2.1 to 9.0) over a period of four to six hours.
Nausea, vomiting, headache, dizziness, drowsiness and confusion were the most commonly reported adverse effects with a single dose of oral dihydrocodeine 30 mg compared with placebo. Significantly more participants reported at least one adverse effect with dihydrocodeine 30 mg than with placebo; the NNH was 7.4 (4.1 to 38). This means that for every seven participants treated with dihydrocodeine 30 mg one would experience an adverse effect who would not have done with placebo.
Our results suggest dihydrocodeine to be less effective than other analgesics when administered as a single oral dose. Few of the retrieved reports investigating oral dihydrocodeine met the criteria for inclusion in this quantitative systematic review. This resulted in little participant data being available for analysis, particularly for dihydrocodeine 60 mg which is often the preferred dose. Administering dihydrocodeine in multiple doses may improve its analgesic efficacy, but may also increase the incidence of adverse effects (McQuay 1993).
Authors' conclusions
Implications for practice.
The update of this review has not identified any further information to provide evidence for or against the use of single dose dihydrocodeine for acute postoperative pain. Based on the limited amount of information in the available studies, a 30 mg dose of dihydrocodeine is not sufficient to provide good pain relief and higher doses are required. When compared with ibuprofen 400 mg, fewer participants benefited with dihydrocodeine (30 mg and 60 mg).
Implications for research.
The annual prescriptions for dihydrocodeine in the UK alone indicate it to be a commonly used drug. To date, there is not enough high quality data available, especially for dihydrocodeine 60 mg, on which to make policy decisions.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 5 June 2008 | Review declared as stable | The review authors consider that additional relevant studies are unlikely to be conducted, and that further updates of this review are unnecessary. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 8 February 2011 | Amended | Contact details updated. |
| 24 September 2010 | Amended | Contact details updated. |
| 4 July 2010 | Amended | Contact details updated. |
| 13 May 2009 | Amended | Contact details updated. |
| 28 May 2008 | Amended | Converted to new review format. |
| 15 January 2008 | New search has been performed | Review updated ‐ studies sought but none found |
| 15 January 2008 | New citation required but conclusions have not changed | Further studies satisfying our inclusion criteria were sought in MEDLINE (via Ovid), EMBASE (via Ovid) and Cochrane CENTRAL to December 2007. No further studies were identified, so the conclusions of the review are unchanged. |
| 30 December 1999 | New citation required and conclusions have changed | Substantive amendment |
Notes
The review authors consider that additional relevant studies are unlikely to be conducted in the future, and that further updates of this review are unnecessary.
Acknowledgements
We would like to thank Claire Abbott at the Cairns Library for helping to obtain reports.
Appendices
Appendix 1. MEDLINE search strategy
Search strategy in MEDLINE 1. dihydrocodeine [single term MeSH] 2. dihydrocodeine 3. 1 OR 2 4. PAIN, POSTOPERATIVE [single term MeSH] 5. ((postoperative adj4 pain$) or (post‐operative adj4 pain$) or post‐operative‐pain$ or (post$ NEAR pain$) or (postoperative adj4 analgesi$) or (post‐operative adj4 analgesi$) or ("post‐operative analgesi$")) [in title, abstract or keywords] 6. ((post‐surgical adj4 pain$) or ("post surgical" adj4 pain$) or (post‐surgery adj4 pain$)) [in title, abstract or keywords] 7. (("pain‐relief after surg$") or ("pain following surg$") or ("pain control after")) [in title, abstract or keywords] 8. (("post surg$" or post‐surg$) AND (pain$ or discomfort)) [in title, abstract or keywords] 9. ((pain$ adj4 "after surg$") or (pain$ adj4 "after operat$") or (pain$ adj4 "follow$ operat$") or (pain$ adj4 follow$ surg$")) [in title, abstract or keywords] 10. ((analgesi$ adj4 "after surg$") or (analgesi$ adj4 "after operat$") or (analgesi$ adj4 "follow$ operat$") or (analgesi$ adj4 follow$ surg$")) 11. OR/4‐10 12. randomized controlled trial.pt. 13. controlled clinical trial.pt. 14. randomized controlled trials.sh. 15. random allocation.sh. 16. double‐blind method.sh. 17. single blind method.sh. 18. clinical trial.pt. 19. exp clinical trials/ 20. (clin$ adj25 trial$).ti,ab. 21. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 22. placebos.sh. 23. placebo$.ti,ab. 24. random$.ti,ab. 25. research design.sh. 26. OR/12‐25 26. 3 AND 11 AND 26
Data and analyses
Comparison 1. Dihydrocodeine 30 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with at least 50% pain relief | 3 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.01, 2.50] |
1.1. Analysis.
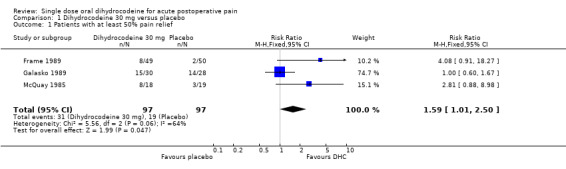
Comparison 1 Dihydrocodeine 30 mg versus placebo, Outcome 1 Patients with at least 50% pain relief.
Comparison 2. Dihydrocodeine 30 mg versus ibuprofen 400 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with at least 50% pain relief | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.52] |
2.1. Analysis.
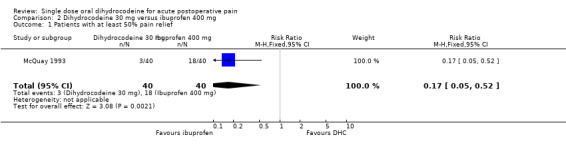
Comparison 2 Dihydrocodeine 30 mg versus ibuprofen 400 mg, Outcome 1 Patients with at least 50% pain relief.
Comparison 3. Dihydrocodeine 60 mg versus ibuprofen 400 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with at least 50% pain relief | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.15, 0.75] |
3.1. Analysis.
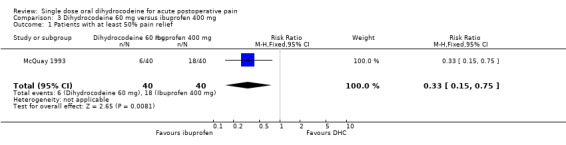
Comparison 3 Dihydrocodeine 60 mg versus ibuprofen 400 mg, Outcome 1 Patients with at least 50% pain relief.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Frame 1989.
| Methods | RCT, DB, single oral dose, parallel groups. Assessed at t = 1/2, 1 hour and then hourly for 5 hrs. Medication taken when pain of moderate to severe intensity |
|
| Participants | Impacted third molar removal n = 148 Age: Adults |
|
| Interventions | DHC 30 mg, n = 49 Placebo, n = 50 |
|
| Outcomes | Measures:
PI (9 point scale ) nonstandard
PR (5 point scale) standard Analgesic outcome: DHC 30 mg was not significantly different to placebo. 5 hr TOTPAR: DHC 30 mg: 4.2 Placebo: 2.6 |
|
| Notes | Remedication allowed at t > 2 hrs. If remedicated patients were withdrawn and their PR set to zero for all further time points. Withdrawals: 18 withdrew: 9 insufficient pain, 7 did not return assessment forms, 1 did not complete assessment forms, 1 postoperative complications Adverse effects: No serious adverse effects were reported and no patients withdrew as a result. DHC 30 mg: 1/49 with 1 AE Placebo: 1/50 with 3 AE Quality score: 3 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Galasko 1989.
| Methods | RCT, DB, multiple oral dose, parallel groups. Assessed at t = 1/2, 1 hour and then hourly for 6 hrs. Medication taken when pain of moderate to severe intensity |
|
| Participants | Orthopaedic surgery n = 89 Age: 18 ‐ 80 years |
|
| Interventions | DHC 30 mg, n = 30 Placebo, n = 28 |
|
| Outcomes | Measures:
PI (5 point scale ) nonstandard PR (5 point scale) standard VAS 100 mm (no pain ‐ worst pain I have ever felt) Analgesic outcomes: DHC was not significantly different to placebo. Mean TOTPAR at 6 hours: DHC: 11.3 Placebo: 11.1 |
|
| Notes | Remedication:
Multiple dose study, 2nd dose given as required. If remedicated patients were excluded from the analysis. Withdrawals: 9 withdrew because of inadequate analgesia after the first dose. DHC 30 mg, n = 3 Placebo, n = 6 Adverse effects: No patients experienced adverse effects in the single dose analysis. Quality score: 4 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
McQuay 1985.
| Methods | RCT, DB, multiple oral dose, parallel groups. Assessed at t = 1/2, 1 hour and then hourly for 4 hrs. Medication taken when pain of moderate to severe intensity |
|
| Participants | Minor day‐case surgery (general) n = 54 Age: Adults |
|
| Interventions | DHC 30 mg, n = 18 Placebo, n = 19 |
|
| Outcomes | Outcome measures:
PI (4 point scale) standard
PR (5 point scale) standard
VAS 100 mm Analgesic outcomes: 4 hr SPID and TOTPAR presented. TOTPAR: DHC was significantly better than placebo (P<0.05) DHC30 mg: 6.5 Placebo: 3.2 |
|
| Notes | Remedication:
Allowed after 1 hr. If remedicated patients initial PI and PR scores were used for all further time points. Withdrawals and adverse effects: Single dose analysis: All adverse effects were mild and no patients withdrew as a result. No significant difference was found between DHC and placebo . DHC 30 mg: 6/18 with 6 AE Placebo: 3/19 with 3 AE Quality score: 4 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
McQuay 1993.
| Methods | RCT, DB, multiple oral dose, crossover design. Self‐assessed at t = 1/2. 1 hr and then hourly for 6 hrs. Medication allowed when pain of moderate to severe intensity |
|
| Participants | Lower third molar removal. n = 68 Age = Adults |
|
| Interventions | DHC 30 mg, n = 40 DHC 60 mg, n = 40 Placebo, n = 40 |
|
| Outcomes | Outcome measures:
PI (4 point scale ) standard PR (5 point scale) standard Global rating (5 point scale) standard Analgesic outcomes: TOTPAR at 6 hrs: DHC 30 mg : 3.3 DHC 60 mg: 4.7 Ibuprofen 400 mg: 10.0 Ibuprofen was significantly better than dihydrocodeine 30 mg or 60 mg (P < 0.01) |
|
| Notes | Remedication:
If remedicated at t< 6 hrs the initial PI score and PR score of zero were used for all further time points. Withdrawals: 3 patients withdrew. Adverse effects: Single dose adverse effects data was not presented. Quality score: 4 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Abbreviations: DHC = dihydrocodeine; R = randomised; DB = double blind; PI = pain intensity; PR = pain relief; AE = adverse effect.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aitken 1990 | No data to extract |
| Beecher 1957 | Abstract |
| Brittain 1959 | Not randomised |
| Brown 1993 | Abstract |
| Cahill 1987 | No data to extract: no pain outcomes |
| Carapeti 1998 | Dihydrocodeine used as a rescue analgesic only |
| Daniel 1971 | Included chronic pain/trauma and fracture |
| Eddy 1934 | Not randomised controlled trial |
| Fassoulaki 1995 | Did not assess analgesic properties of DHC. Assessed morphine consumption not pain outcomes |
| Fenton‐Lee 1994 | Not double blind. No pain outcome data to extract |
| Fricke 1993 | Abstract |
| Galasko 1988 | Single blind |
| Grace 1994 | Did not assess analgesic properties of DHC. No placebo control (used suprofen) |
| Grainger 1977 | Baseline pain not moderate or severe |
| Gravenstein 1956 | Not randomised |
| Habib 1990 | Single blind |
| Heath 1989 | Baseline pain not moderate or severe |
| Henderson 1999 | Assessed dextromethorphan not DHC. Combination of dihydrocodeine with another drug |
| Howard 1953 | Not randomised controlled trial |
| Hummel 1995 | Could not be obtained: no UK location |
| Jorgensen 1985 | Not double blind |
| Kay 1988 | No data to extract |
| Keats 1950 | Not randomised controlled trial. No placebo control (used floctafenine) |
| Keats 1957a | Abstract. No placebo control (used nalbuphine) |
| Keats 1957b | Not double blind. No control group |
| Kerrick 1993 | Did not assess analgesic properties of DHC |
| Lipton 1975 | Not randomised. No placebo control (used MST + DF118) |
| Lomas 1976 | Chronic pain |
| Lund 1959 | Not randomised. No placebo control (used buprenorphine) |
| Masson 1981 | Not double blind. No placebo control (used seconal). Included children (i.e. <16 yrs) |
| Moore 1983 | No data to extract: placebo data not presented |
| Morrison 1971 | Did not state randomised. Not assessing analgesic response of DHC |
| Myers 1958 | Not double blind |
| Riethmuller 1987 | Not randomised controlled trial. Not double blind. No placebo control |
| Ruch 1957 | Not randomised. Single blind |
| Seymour 1981 | Abstract |
| Seymour 1982 | Baseline pain not moderate or severe |
| Seymour 1983a | Baseline pain not moderate or severe |
| Seymour 1983b | Baseline pain not moderate or severe. Not double blind. |
| Sliom 1970 | Not randomised. Not double blind. No placebo control; baseline pain not moderate or severe |
| Squirrell 1998 | Did not assess analgesic properties of dihydrocodeine. Method of surgery randomised not drug. Drugs administered on demand |
| Traykova 1996 | No placebo group |
| Walker 1977 | No data to extract (non standard PI and PR scales) |
| Walters 1984 | Abstract |
| Walters 1985 | Baseline pain intensity not moderate or severe |
| Williams 1995 | Not double blind. No pain outcome data |
| Wotherspoon 1991 | Not postoperative pain (cold induced pain). No placebo control (naproxen sodium). Baseline pain not moderate or severe |
| Yadav 1984 | Not double blind |
Contributions of authors
Original review JR: was involved with searching, data extraction, analysis and writing. AM and HJM were involved with analysis and writing.
Update 2008 SD and AM carried out the searching and writing.
Sources of support
Internal sources
Pain Research Funds, Oxford, UK.
External sources
Biotechnology and Biological Sciences Research Council, UK.
SmithKline Beecham Consumer Healthcare, Not specified.
European Union Biomed2 (#BMH4‐CT95‐0172), Not specified.
NHS R&D Health Technology Evaluation Programmes, UK.
NHS Cochrane Collaboration Programme Grant Scheme, UK.
Declarations of interest
SD has no interests to declare. RAM has been a consultant for MSD. RAM and HJM have consulted for various pharmaceutical companies. RAM, HJM and JR have received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. All authors have received research support from charities, government and industry sources at various times: no such support was received for this work.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Frame 1989 {published data only}
- Frame JW, Evans RH, Flaum GR, Langford R, Rout PGJ. A comparison of ibuprofen and dihydrocodeine in relieving pain following wisdom teeth removal. British Dental Journal 1989;166:121‐4. [DOI] [PubMed] [Google Scholar]
Galasko 1989 {published data only}
- Galasko CSB, Russel S, Lloyd J. Double‐blind investigation of the efficacy of multiple doses of ketorolac tromethamine compared with dihydrocodeine and placebo. Current Therapeutic Research 1989;45:844‐52. [Google Scholar]
McQuay 1985 {published data only}
- McQuay HJ, Bullingham RES, Moore RA, et al. Zomepirac, dihydrocodeine and placebo compared in postoperative pain after day‐case surgery. The relationship between the effects of single and multiple doses. British Journal of Anaesthesia 1985;57:412‐9. [DOI] [PubMed] [Google Scholar]
McQuay 1993 {published data only}
- McQuay HJ, Carroll D, Guest PG, Robson S, Wiffen PJ, Juniper RP. A multiple dose comparison of ibuprofen and dihydrocodeine after third molar surgery. The British Journal of Oral and Maxillofacial Surgery 1993;31:95‐100. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aitken 1990 {published data only}
- Aitken HA, Clark EC, McArdle CS, Dimitri W, Kenny GN. Evaluation of two formulations of dihydrocodeine using patient controlled analgesia. Anaesthesia 1990;45(7):535‐7. [DOI] [PubMed] [Google Scholar]
Beecher 1957 {published data only}
- Beecher HK, Gravenstein JS, Pederson DP, Smith GM. Analgesic efects and side effect liability of dihydrocodeine and morphine in man. Federation Proceedings 1957;16:281. [Google Scholar]
Brittain 1959 {published data only}
- Brittain GJC. Dihydrohydroxycodeinone pectinate. The Lancet 1959;(no volume):544‐6. [DOI] [PubMed] [Google Scholar]
Brown 1993 {published data only}
- Brown P, Mehlisch DR, Kiersch T, Allan G, Sims I. A single dose study evaluating the analgesic efficacy of hydocodone / acetaminophen, oxycodone / acetaminophen and placebo following oral surgery. American Society for Clinical Pharmacology and Therapeutics 1993;53:172. [Google Scholar]
Cahill 1987 {published data only}
- Cahill DJ, McFaul PB. Local anaesthesia with bupivacaine following laparoscopy: a double blind controlled trial. Journal of Obstetrics and Gynecology 1987;7(4):277‐8. [Google Scholar]
Carapeti 1998 {published data only}
- Carapeti EA, Kamm MA, McDonald PJ, Phillips RK. Double‐blind randomised controlled trial of effect of metronidazole on pain after day‐case haemorrhoidectomy. Lancet 1998;351:169‐72. [DOI] [PubMed] [Google Scholar]
Daniel 1971 {published data only}
- Daniel JR. Two comparisons of the analgesic activity of orally administered pentazocine, dihydrocodeine and placebo. British Journal of Anaesthesia 1971;42:392‐9. [DOI] [PubMed] [Google Scholar]
Eddy 1934 {published data only}
- Eddy NB. Studies of morphine, codeine and their derivatives. IV. Hydrogenated codeine isomers. The Journal of Pharmacology and Experimental Therapeutics 1934;51:35‐44. [Google Scholar]
Fassoulaki 1995 {published data only}
- Fassoulaki A, Sarantopoulos C, Zotou M, Papoulia D. Preemptive opioid analgesia does not influence pain after abdominal hysterectomy. Canadian Journal of Anaesthesia 1995;42(2):109‐13. [DOI] [PubMed] [Google Scholar]
Fenton‐Lee 1994 {published data only}
- FentonLee D, Riach E, Cooke T. The use of a local anaesthetic wound perfusion device versus oral analgesia. A comparison in day case inguinal herniorrhaphy. British Journal of Intensive Care 1994;4(5):152, 154‐6. [Google Scholar]
Fricke 1993 {published data only}
- Fricke J, Halladay SC. Comparison of keterolac, hydrocodone plus acetaminophen and placebo for the relief of dental surgery pain. Clinical Pharmacology and Therapeutics 1993;53:222. [PubMed] [Google Scholar]
Galasko 1988 {published data only}
- Galasko CS, Courtney P, Jayne M, Coxhead PF, Russell S. Comparison of the efficacy of naproxen sodium and dihydrocodeine tartrate in the treatment of post operative pain. Current Medical Research and Opinion 1988;10(10):656‐62. [DOI] [PubMed] [Google Scholar]
Grace 1994 {published data only}
- Grace D, Milligan KR, Morrow BJ, Fee JPH. Co‐administration of pethidine and clonidine: A spinal anaesthetic technique for total hip replacement. British Journal of Anaesthesia 1994;73(5):628‐33. [DOI] [PubMed] [Google Scholar]
Grainger 1977 {published data only}
- Grainger DJ, Gawley TH, Dundee JW. Anidoxime: a clinical trial of an oral analgesic agent. British Journal of Anaesthesia 1977;49(3):257‐8. [DOI] [PubMed] [Google Scholar]
Gravenstein 1956 {published data only}
- Gravenstein JS, Smith GM, Sphire RD, Isaacs JP, Beecher HK. Dihydrocodeine. Further development in measurement of analgesic power and appraisal of psychologic side efects of analgesic agents. New England Journal of Medicine 1956;254:877. [DOI] [PubMed] [Google Scholar]
Habib 1990 {published data only}
- Habib S, Matthews RW, Scully C, Levers BG, Shepherd JP. A study of the comparative efficacy of four common analgesics in the control of postsurgical dental pain. Oral surgery, Oral Medicine, and Oral Pathology 1990;70(5):559‐63. [DOI] [PubMed] [Google Scholar]
Heath 1989 {published data only}
- Heath PJ, Ogg TW. Prophylactic analgesia for daycase termination of pregnancy. A double blind study with controlled release dihydrocodeine. Anaesthesia 1989;44(12):991‐4. [DOI] [PubMed] [Google Scholar]
Henderson 1999 {published data only}
- Henderson DJ, Withington BS, Wilson JA, Morrison LM. Perioperative dextromethorphan reduces postoperative pain after hysterectomy. Anesthesia and Analgesia 1999;89:399‐402. [DOI] [PubMed] [Google Scholar]
Howard 1953 {published data only}
- Howard BK, et al. [No title]. Journal of Obstetrics and Gynecology 1953;1:371. [PubMed] [Google Scholar]
Hummel 1995 {published data only}
- Hummel T, Kraetsch HG, Loetsch J, Hepper M, Liefhold J, Kobal G. Analgesic Effects of Dihydrocodeine and Tramadol when Administered Either in the Morning or Evening. Chronobiology International 1995;12:62‐72. [DOI] [PubMed] [Google Scholar]
Jorgensen 1985 {published data only}
- Jorgensen BC, Schmidt JF, Risbo A, Pedersen J, Kolby P. Regular interval preventive pain relief compared with in demand treatment after hysterectomy. Pain 1985;21:137‐42. [DOI] [PubMed] [Google Scholar]
Kay 1988 {published data only}
- Kay B, Lindsay RG, Mason CJ, Healy TEJ. Oral nalbuphine for the treatment of pain after dental extractions. British Journal of Anaesthesia 1988;61(3):313‐7. [DOI] [PubMed] [Google Scholar]
Keats 1950 {published data only}
- Keats AS, Beecher HK, Mosteller FC. Measurement of pathological pain in distinction to experimental pain. [Journal title unknown] 1950;3:35‐44. [DOI] [PubMed] [Google Scholar]
Keats 1957a {published data only}
- Keats AS, Telford J, Kurosu Y. Studies of two new potent analgesics: Anileridine and dihydrocodeine. Anesthesiology 1957;18:168. [DOI] [PubMed] [Google Scholar]
Keats 1957b {published data only}
- Keats AS. Studies of analgesic drugs: dihydrocodeine. The Journal of Pharmacology and Experimental Therapeutics 1957;120:354‐60. [PubMed] [Google Scholar]
Kerrick 1993 {published data only}
- Kerrick JM, Fine PG, Lipman AG, Love G. Low‐dose amitriptyline as an adjunct to opioids for postoperative orthopedic pain: A placebo‐controlled trial. Pain 1993;52(3):325‐30. [DOI] [PubMed] [Google Scholar]
Lipton 1975 {published data only}
- Lipton S, Conway M, Akbar FA. An analgesic comparison of floctafenine (Idarac) and dihydrocodeine in post‐operative pain. The Journal of International Medical Research 1975;3:172‐5. [DOI] [PubMed] [Google Scholar]
Lomas 1976 {published data only}
- Lomas DM, Gay J, Midha RN, Postlethwaite DL. A double‐blind comparative clinical trial of floctafenine and four other analgesics conducted in general practice. The Journal of International Medical Research 1976;4(3):179‐82. [DOI] [PubMed] [Google Scholar]
Lund 1959 {published data only}
- Lund I, Lind B. A comparison of pethidine and dihydrocodeine for the relief of labour pain. Acta Anaesthesiologica Scandinavica 1959;3:41‐8. [Google Scholar]
Masson 1981 {published data only}
- Masson AH. Sublingual buprenorphine versus oral dihydrocodeine in post operative pain. The Journal of International Medical Research 1981;9(6):506‐10. [DOI] [PubMed] [Google Scholar]
Moore 1983 {published data only}
- Moore RA, Bullingham RE, Simpson S, O'Sullivan G, Evans PJ, McQuay HJ, Lloyd JW. Comparison of flupirtine maleate and dihydrocodeine in patients following surgery. British Journal of Anaesthesia 1983;55(5):429‐32. [DOI] [PubMed] [Google Scholar]
Morrison 1971 {published data only}
- Morrison JD, Loan WB, Dundee JW. Controlled comparison of the efficacy of fourteen preparations in the relief of postoperative pain. British Medical Journal 1971;3:287‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 1958 {published data only}
- Myers JD. A preliminary clinical evaluation of dihydrocodeine‐bitartrate in normal parturition. American Journal of Obstetrics and Gynecology 1958;75:1096‐100. [DOI] [PubMed] [Google Scholar]
Riethmuller 1987 {published data only}
- Riethmuller Winzen H. Flupirtine in the treatment of post‐operative pain. Postgraduate Medical Journal 1987;63(S3):61‐6. [PubMed] [Google Scholar]
Ruch 1957 {published data only}
- Ruch WA. A preliminary report of dihydrocodeine‐scopolamine in obstetrics. American Journal of Obstetrics and Gynecology 1957;74:1125‐27. [DOI] [PubMed] [Google Scholar]
Seymour 1981 {published data only}
- Seymour RA, Rawlins MD. Paracetamol (acetaminophen), aspirin, dihydrocodeine tartrate and post‐operative dental pain. Pain 1981;5:S248. [Google Scholar]
Seymour 1982 {published data only}
- Seymour RA, Rawlins MD, Rowell FJ. Dihydrocodeine‐induced hyperalgesia in postoperative dental pain. Lancet 1982;1(8287):1425‐6. [DOI] [PubMed] [Google Scholar]
Seymour 1983a {published data only}
- Seymour RA. Analgesic efficacy and plasma concentration of three analgesics in pain after lower third molar removal. SAAD Digest 1983;5:172‐88. [PubMed] [Google Scholar]
Seymour 1983b {published data only}
- Seymour RA, et al. Postoperative dental pain and analgesic efficacy, part II. Analgesic usage and efficacy after dental surgery. The British Journal of Oral Surgery 1983;21:298‐303. [DOI] [PubMed] [Google Scholar]
Sliom 1970 {published data only}
- Sliom CM. Analgesia during labour: a comparison between dihydrocodeine and pethidine. South African Medical Journal 1970;44(11):317‐9. [PubMed] [Google Scholar]
Squirrell 1998 {published data only}
- Squirrell DM, Majeed AW, Troy G, Peacock JE, Nicholl JP, Johnson AG. A randomized, prospective, blinded comparison of postoperative pain, metabolic response, and perceived health after laparoscopic and small incision cholecystectomy. Surgery 1998;123:485‐95. [DOI] [PubMed] [Google Scholar]
Traykova 1996 {published data only}
- Traykova V, et al. Dihydrocodeine‐continus for postoperative pain relief in gynecology. Akusherstvo i Ginekologiia 1996;35:24‐6. [Google Scholar]
Walker 1977 {published data only}
- Walker JE, Kay LW. Postoperative jaw pain: floctafenine versus dihydrocodeine. International Journal of Oral Surgery 1977;6(5):256‐9. [DOI] [PubMed] [Google Scholar]
Walters 1984 {published data only}
- Walters BNJ, Smith VA, Swiet M. Comparative Trial of Suprofen and Dihydrocodeine in Post‐Episiotomy Pain. Pain 1984;91:S236. [Google Scholar]
Walters 1985 {published data only}
- Walters BN, Smith VA, De SwietM, Mustill TA. Pain relief after episiotomy a comparative study of suprofen and dihydrocodeine. British Journal of Obstetrics and Gynaecology 1985;92(11):1160‐3. [DOI] [PubMed] [Google Scholar]
Williams 1995 {published data only}
- Williams JE, Ainley TC, Shepherd JE. Economic impact of a patient‐controlled oral analgesia system for post‐operative pain. British Journal of Medical Economics 1995;9(1):41‐3. [Google Scholar]
Wotherspoon 1991 {published data only}
- Wotherspoon HA, Kenny GNC, McArdle CS. Analgesic efficacy of controlled‐release dihydrocodeine. A comparison of 60, 90 and 120 mg tablets in cold‐induced pain. Anaesthesia 1991;46:915‐7. [DOI] [PubMed] [Google Scholar]
Yadav 1984 {published data only}
- Yadav S, McLatchie GR, Mackenzie I. Evaluation of proctofoam as a post‐operative analgesic in patients undergoing haemorrhoidectomy. The British Journal of Clinical Practice 1984;38(11‐12):414‐5. [PubMed] [Google Scholar]
Additional references
Alexander 1987
- Alexander JI, Hill RG. Choices in the management of postoperative pain. Postoperative Pain. London: Blackwell Scientific Publications, 1987:130‐1. [Google Scholar]
Barden 2004
- Barden J, Edwards JE, McQuay HJ, Wiffen PJ, Moore RA. Relative efficacy of oral analgesics after third molar extraction. British Dental Journal 2004;197(7):407‐11. [DOI] [PubMed] [Google Scholar]
Collins 1998a
- Collins SL, Edwards JE, Moore RA, McQuay HJ. Dextropropoxyphene in postoperative pain: A quantitative systematic review. European Journal of Clinical Pharmacology 1998;54:107‐12. [DOI] [PubMed] [Google Scholar]
Collins 1998b
- Collins SL, Moore RA, Wiffen PJ, McQuay HJ. Oral ibuprofen and diclofenac in postoperative pain: a quantitative systematic review. European Journal of Pain 1998;2:285‐91. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. British Medical Journal 1995;310:452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore A, McQuay HJ. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66:239‐46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
McQuay 1998
- McQuay HJ, Moore RA. An Evidence‐Based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [Google Scholar]
Moore 1996
- Moore RA, McQuay HJ, Gavaghan DJ. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66:229‐37. [DOI] [PubMed] [Google Scholar]
Moore 1997a
- Moore RA, McQuay HJ, Gavaghan DJ. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: verification from independent data. Pain 1997;69:127‐30. [DOI] [PubMed] [Google Scholar]
Moore 1997b
- Moore RA, Moore O, McQuay HJ, Gavaghan DJ. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: use of pain intensity and visual analogue scales. Pain 1997;69:311‐15. [DOI] [PubMed] [Google Scholar]
Moore 1997c
- Moore RA, Collins SA, McQuay HJ. Paracetamol with and without codeine: A quantitative systematic review. Pain 1997;70:193‐201. [DOI] [PubMed] [Google Scholar]
Moore 1997d
- Moore RA, McQuay HJ. Single‐patient data meta‐analysis of 3453 postoperative patients: oral tramadol versus placebo, codeine and combination analgesics. Pain 1997;69:287‐94. [DOI] [PubMed] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics With Confidence ‐ Confidence Intervals and Statistical Guidelines. London: British Medical Journal, 1995:50‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
PACT 2006
- Anonymous. Prescription Cost Analysis, England 2006. ISBN: 1‐84636‐053‐6 2006.
Rowell 1983
- Rowell FJ, Seymour RA, Rawlins MD. Pharmacokinetics of intravenous and oral dihydrocodeine and its metabolites. European Journal of Clinical Pharmacology 1983;25:419‐24. [DOI] [PubMed] [Google Scholar]


