Abstract
Background
This is an updated version of the original Cochrane review published in Issue 4, 2001.
Worldwide, phenytoin and phenobarbitone are commonly used antiepileptic drugs. They are more likely to be used in the developing world than the developed world, primarily because they are inexpensive. The aim of this review is to summarize data from existing trials comparing phenytoin and phenobarbitone.
Objectives
To review the effects of phenobarbitone compared to phenytoin when used as monotherapy in patients with partial onset seizures or generalized tonic-clonic seizures with or without other generalized seizure types.
Search methods
We searched the Cochrane Epilepsy Group trials register (20 October 2009), the Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 4, 2009) and MEDLINE (1950 to October week 2, 2009). In addition, we handsearched relevant journals, and contacted pharmaceutical companies and researchers in the field to seek any ongoing or unpublished studies.
Selection criteria
Randomized controlled trials in children or adults with partial onset seizures or generalized onset tonic-clonic seizures. Trials must have included a comparison of phenobarbitone monotherapy with phenytoin monotherapy.
Data collection and analysis
This was an individual patient data review. Outcomes were time to (a) withdrawal of allocated treatment, (b) 12-month remission and (c) first seizure post randomization. Data were analyzed using a stratified logrank analysis with results expressed as hazard ratios (HR) and 95% confidence intervals (95% CI), where a HR > 1 indicates an event is more likely to occur earlier on phenobarbitone than phenytoin.
Main results
To date, data have been obtained for four of ten studies meeting the inclusion criteria, amounting to 599 individuals, or approximately 65% of the potential data. The main overall results (HR) were (a) time to treatment withdrawal 1.62 (95% confidence interval 1.22 to 2.14); (b) time to 12-month remission 0.93 (95% confidence interval 0.70 to 1.23) and (c) time to first seizure 0.84 (95% confidence interval 0.68 to 1.05). These results indicate a statistically significant clinical advantage for phenytoin in terms of treatment withdrawal and a non-significant advantage in terms of 12-month remission. Results for time to first seizure suggest a non-significant clinical advantage for phenobarbitone.
Authors’ conclusions
The results of this review favour phenytoin over phenobarbitone, as phenobarbitone was significantly more likely to be withdrawn than phenytoin. Given that no significant differences for seizure outcomes were found, the higher withdrawal rate with phenobarbitone may be due to adverse effects.
Medical Subject Headings (MeSH): Anticonvulsants [* therapeutic use]; Epilepsies, Partial [* drug therapy]; Epilepsy, Generalized [* drug therapy]; Phenobarbital [* therapeutic use]; Phenytoin [* therapeutic use]; Randomized Controlled Trials as Topic; Seizures [* drug therapy]
MeSH check words: Humans
BACKGROUND
This review is an update of a previously published review in The Cochrane Database of Systematic Reviews (Issue 4, 2001) on ’Phenobarbitone versus phenytoin monotherapy for partial onset seizures and generalized onset tonic-clonic seizures’.
Worldwide, phenytoin and phenobarbitone are commonly used antiepileptic drugs (AEDs). They are more likely to be used in the developing world than the developed world, primarily because they are inexpensive. In the USA and much of Europe, phenytoin and phenobarbitone are no longer considered first-line agents due to worries over adverse effects. Phenobarbitone more so than phenytoin is associated with connective tissue abnormalities such as Dupuytrens contracture, and frozen shoulder, whilst phenytoin is associated with coarsening of facial features and gum hypertrophy. In addition, both drugs are teratogenic and are associated with low folic acid levels and megaloblastic anaemia.
In the largest reported randomized controlled trial investigating phenobarbitone as monotherapy in adults (Mattson 1985), no difference was found with respect to seizure control when compared with phenytoin and carbamazepine. However, for the outcome time to treatment withdrawal, phenobarbitone fared significantly worse, implying that it was less well tolerated. In children, there is concern about behavioural disturbances caused by phenobarbitone. In one paediatric study in the UK (de Silva 1996) the phenobarbitone arm of the trial was withdrawn due to concerns about adverse effects. However, another study based in rural India (Pal 1998) comparing phenobarbitone with phenytoin found no such problem, and the authors concluded that phenobarbitone was a suitable first-line agent in this setting.
Phenytoin and phenobarbitone are thought to be effective for both partial and generalized seizure types. No single trial has found convincing differences between phenytoin and phenobarbitone with respect to seizure control. However, confidence intervals around estimates have been wide and equivalence cannot be inferred. The aim of this review is to summarize data from existing trials comparing phenobarbitone and phenytoin.
There are difficulties in undertaking a systematic review of epilepsy monotherapy trials as the important efficacy outcomes require analysis of time to event data (for example, time to one-year remission). Although methods have been developed to synthesize survival type data using summary information (Parmar 1998), it is unlikely that all trials will have reported appropriate statistics. We have therefore performed a review using individual patient data.
The use of individual patient data will help overcome a number of other problems. First, although seizure data have been collected in most epilepsy monotherapy trials, there has been no uniformity in the reporting of outcomes. For example, trials may report time to 12-month remission but not time to first seizure or vice versa. Second, trialists have adopted differing approaches to the analysis, particularly with respect to the censoring of time to event data. This review is one in a series investigating pairwise monotherapy comparisons.
OBJECTIVES
To review the effects of phenobarbitone compared to phenytoin when used as monotherapy in people with partial onset seizures or generalized tonic-clonic seizures with or without other generalized seizure types.
METHODS
Criteria for considering studies for this review
Types of studies
We included studies that were:
randomized monotherapy studies comparing phenobarbitone and phenytoin;
double, single or unblinded;
adequately randomized or quasi randomized (eg. allocation by date of birth).
Types of participants
Children or adults with partial onset seizures (simple partial, complex partial or secondary generalized tonic-clonic), or generalized tonic-clonic seizures (with or without other generalized seizure types).
Individuals with a new diagnosis of epilepsy, or who had had a relapse following antiepileptic monotherapy withdrawal.
Types of interventions
Phenobarbitone or phenytoin as monotherapy.
Types of outcome measures
Time on allocated treatment (retention time). This is a combined outcome reflecting both efficacy and tolerability as treatment may be withdrawn due to continued seizures, adverse effects or a combination of both. This is an outcome to which the individual makes a contribution, and is the primary outcome measure recommended by the Commission on Antiepileptic Drugs of the International League Against Epilepsy (Commission 1998).
Time to achieve 12-month seizure free period (remission).
Time to first seizure post randomization.
Search methods for identification of studies
Electronic searches
We searched the following databases. There were no language restrictions.
The Cochrane Epilepsy Group trials register (20 October 2009) using the search terms ’phenobarbital or phenobarbitone’ and ’phenytoin’.
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 4, 2009) using the strategy outlined in Appendix 1.
MEDLINE (Ovid) (1950 to October week 2, 2009) was searched using the strategy outlined in Appendix 2.
Searching other resources
In addition, we handsearched relevant journals, and contacted pharmaceutical companies and researchers in the field to seek any ongoing or unpublished studies.
Data collection and analysis
Data Collection
Trials have been independently assessed for inclusion by two investigators (Steve Taylor and Tony Marson).
We approached original trialists with a view to obtaining their co-operation in providing individual patient data. We asked each group to provide data on the following:
date of randomization;
drug allocated and dose;
age;
sex;
presence of neurological signs;
seizure types;
number of seizures pre-randomization;
EEG results;
CT/MRI results;
dates of follow-up;
dates of dose changes;
dates of seizures post-randomization or seizure frequency data;
date of treatment withdrawal and reasons for treatment withdrawal.
For each trial for which individual patient data were not obtained, an assessment was carried out to see whether any relevant aggregate level data had been reported.
Data Checking
For each trial that we obtained individual patient data we carried out range and consistency checks. Results from the trial reports were reproduced where possible. Inconsistencies were chased up with a nominated individual. The chronological randomization sequence was checked for each trial.
Data Analysis
We carried out our analysis on an intention to treat basis (that is, participants were analyzed in the group to which they were randomized).
We used a logrank analysis (stratified by trial to preserve the withintrial randomization) to obtain trial-specific and pooled estimates of hazard ratios (with 95% confidence intervals), and to test for no overall treatment effect and homogeneity across trials (Early 1990).
In one study (Mattson 1985), summary seizure data at each follow-up were provided, rather than specific dates of seizures. To allow combination with data from the other trials, we used linear interpolation to obtain estimates of the dates of seizures between follow-up visits. This allowed an estimate of the time to 12-month remission and the time to first seizure to be computed.
The analysis was conducted such that a hazard ratio (HR) greater than 1 indicates an event is more likely to occur earlier on phenobarbitone than phenytoin. Hence, for treatment withdrawal and time to first seizure, a HR > 1 indicates an advantage for phenytoin. For time to 12-month remission, a HR > 1 indicates an advantage for phenobarbitone.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Ten studies met our inclusion criteria (Table 1). Seven of the studies recruited adults, and three recruited children (de Silva 1996; Pal 1998; Thilothammal 1996). Four studies recruited individuals with partial onset and generalized onset seizures (Cereghino 1974; de Silva 1996; Heller 1995; Pal 1998), three recruited individuals with partial onset seizures only (Czapinski 1997; Mattson 1985; Meador 1990), one recruited individuals with generalized onset seizures only (Thilothammal 1996) and two studies gave insufficient information on seizure types (Bird 1966; Gruber 1962).
Table 1. Studies meeting inclusion criteria.
| Trial | N random’d PHB | N random’d PHT | 1st sz/rem PHB | 1st sz/rem PHT | Withdrawal PHB | Withdrawal PHT | Follow-up median | Mean age (range) | % male |
| IPD available | |||||||||
| Mattson 1985 | 155 | 165 | 151 | 162 | 155 | 165 | 20 months (range 0-66) | 40 (18-81) | 88 |
| Heller 1995 | 58 | 63 | 58 | 63 | 55 | 61 | 61 months (1-156) | 34 (14-77) | 49 |
| de Silva 1996 | 10 | 54 | 10 | 54 | 10 | 53 | 103 months (30-164) | 9 (3-16) | 59 |
| Pal 1998 | 47 | 47 | 47/30 | 47/27 | - | - | 9 months (0-12) | 11 (2-18) | 52 |
| No IPD: | |||||||||
| Czapinski 1997 | 30 | 30 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported (18-40) | Not reported |
| Thilothammal 1996 | 51 | 52 | Not reported | Not reported | Not reported | Not reported | Not reported (22-36) | Not reported (4-12) | 54 |
| Meador 1990 | 15 | 15 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported (19-62) | 60 |
| Cereghino 1974 | 45 | 45 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported (18-51) | 62 |
| Bird 1966 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 43 |
| Gruber 1962 | 48 | 48 | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
To date, we have obtained individual patient data (IPD) for four of the ten studies (de Silva 1996; Heller 1995; Mattson 1985; Pal 1998), amounting to 65% of the potential data. However, IPD for ’time to treatment withdrawal’ have not been provided for the Pal trial (Pal 1998). In these studies, data were available for the following individual participant factors: type of epilepsy (100%); sex (99.5%); age at randomization (99.5%); time between first seizure and randomization (99%) and number of seizures pre-randomization (92.2%). EEG and CT data were computerized for one of the studies (Mattson 1985), but have not been computerized for the other three studies providing IPD. Similar problems occurred in the assessment of neurological signs.
No IPD have yet been obtained for the remaining six studies (Bird 1966; Cereghino 1974; Czapinski 1997; Gruber 1962; Meador 1990; Thilothammal 1996), none of which reported the outcomes chosen for this review. We were therefore unable to make use of aggregate level data from these studies. We are hopeful that further data may become available for the Pal (Pal 1998) and Czapinski (Czapinski 1997) trials for the next update of the review.
Due to drug-related adverse effects, de Silva et al (de Silva 1996) discontinued the phenobarbitone arm in their trial after randomizing only 10 children to this drug. Randomization continued between phenytoin, carbamazepine and sodium valproate. The initial analysis included all participants in this study randomized to either phenobarbitone or phenytoin. A sensitivity analysis was undertaken by repeating the analysis without those participants in this study who were randomized to phenytoin after the removal of phenobarbitone.
Three studies (Cereghino 1974; Gruber 1962; Meador 1990) used crossover designs, each with differing lengths of drug treatment period, ranging from one week to three months. These treatment periods are relatively short in terms of the outcome measures chosen for this review.
For the most recent update of this review (October 2009), the search of MEDLINE retrieved 20 hits, and the search of CENTRAL retrieved six hits. None of these was judged to be relevant for this review. The search of the Cochrane Epilepsy Group’s Specialised Register returned no new hits.
Risk of bias in included studies
(1) Trials for which individual patient data were provided
All four trials used adequate methods of concealment of randomization; one used minimization (Pal 1998) and three used sealed opaque envelopes (Heller 1995; Mattson 1985; de Silva 1996). Two trials were double blind (Mattson 1985; Pal 1998) with Mattson using a ’double dummy technique’ (participants received phenobarbitone and placebo phenytoin, or phenytoin and placebo phenobarbitone). The Heller (Heller 1995) and de Silva (de Silva 1996) trials were unblinded.
(2) Trials for which no individual patient data were available
One study (Bird 1966) used sealed envelopes to conceal randomization. The method of randomization concealment was not stated in the other five reports. All of these trials were double blind, except for Czapinski (Czapinski 1997) which was unblinded.
Effects of interventions
(1) Time to treatment withdrawal
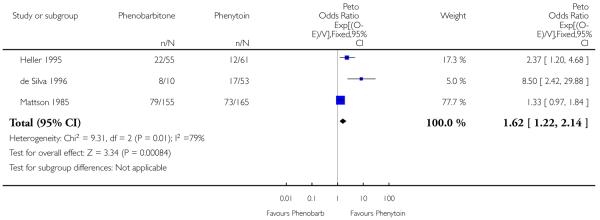
Withdrawal information was available for 499 individuals in three trials supplying IPD. Phenobarbitone was significantly more likely to be withdrawn than phenytoin, with an estimated common hazard ratio of 1.62 (95% confidence interval 1.22 to 2.14). However, there was evidence of quantitative heterogeneity between the trials (chi squared = 9.34, p = 0.009).
Due to drug-related adverse effects, de Silva et al (de Silva 1996) discontinued the phenobarbitone arm in their trial after randomizing only 10 children to this drug. In that study, randomization continued between phenytoin, carbamazepine and sodium valproate. In order to assess whether this had any effect on the phenobarbitone/phenytoin comparison, we repeated the analysis without those participants who were randomized to phenytoin after the removal of phenobarbitone in this study. Phenytoin was still favoured, with a hazard ratio of 1.57 (95% confidence interval 1.18 to 2.08) and there was still some evidence of heterogeneity (chi squared = 5.93, p = 0.052).
(2) Time to 12-month remission
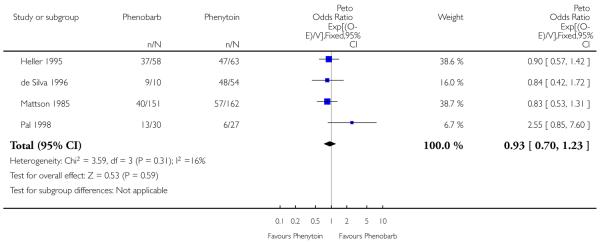
Time to 12-month remission data were available for 555 individuals. There was no evidence of heterogeneity between studies for this outcome (chi squared = 3.58, p = 0.31). The common estimated hazard ratio was 0.93 (95% confidence interval 0.70 to 1.23), favouring phenytoin but without demonstrating statistical significance.
The sensitivity analysis relating to the de Silva (de Silva 1996) study gave similar results, with an estimated pooled hazard ratio of 0.88 (95% confidence interval 0.65 to 1.19). However, there is slight evidence of heterogeneity (chi squared = 6.59, p = 0.086).
(3) Time to first seizure
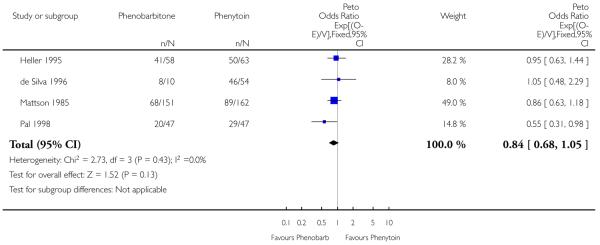
Data for this outcome were available for 592 individuals. There was no evidence of heterogeneity between trials (chi squared = 2.73, p = 0.43). The estimated common hazard ratio was 0.84 (95% confidence interval 0.68 to 1.05), favouring phenobarbitone but without statistical significance.
The sensitivity analysis produced a similar result, with a common hazard ratio of 0.85 (95% confidence interval 0.68 to 1.07) and no evidence of heterogeneity between the four trials (chi squared = 3.89, p = 0.27).
DISCUSSION
Ten trials met our inclusion criteria for this review, in that individuals with partial onset seizures or generalized onset tonic-clonic seizures were randomly allocated to monotherapy with either phenytoin or phenobarbitone. We were able to obtain individual patient data for only four of these trials for at least one of the outcomes of interest, which represents 65% of the potentially available data. The trials for which individual patient data were made available were of good quality and used adequate methods of allocation concealment. All four trials provided data for the outcome time to first seizure post randomization (592 individuals) and time to 12-month remission (555 individuals), whilst three (499 individuals) provided data on time to treatment withdrawal.
Of the six trials for which no individual patient data were made available, none reported the outcomes of interest in this review and as a result, we were unable to use aggregate data from trial reports in this review. Of these six trials, one was recently completed (Czapinski 1997) and is yet to be reported in full. We hope to receive individual patient data for this trial in the near future. Of the remaining five trials, only one described an adequate method of allocation concealment (Bird 1966), bringing the quality of the remainder into question. Also, three of the five used crossover designs with relatively short treatment periods and hence were not designed to examine the outcomes of interest in this review.
Although we have individual patient data for only four of ten trials, these four trials were of high quality. We therefore thought it reasonable to proceed with a meta-analysis.
For our primary outcome, we found that people taking phenobarbitone were statistically significantly more likely to have treatment withdrawn, with a hazard ratio of 1.62 (95% confidence interval 1.22 to 2.14). The fact that a clear advantage for phenytoin was not seen for the seizure outcomes reported would imply that treatment was withdrawn primarily because of adverse effects. There was however, significant heterogeneity for this outcome, which was not present for time to 12-month remission or time to first seizure. It is interesting to note that the two trials with the higher withdrawal rates for phenobarbitone compared to phenytoin were undertaken in the UK and were unblinded, whereas the trial with the lower withdrawal rate for phenobarbitone was double blinded and undertaken in the USA. This would suggest two potential explanations for the heterogeneity observed. Firstly, clinicians are biased to expect adverse effects from phenobarbitone and would be more likely to withdraw people from this drug in an unblinded trial, in which they know what drug a person is taking. Secondly, there could be a higher expectation of adverse effects from phenobarbitone amongst clinicians in the UK compared to those working elsewhere. In support of the latter point, it is interesting to note that the UK trial recruiting children (de Silva 1996) suspended randomization to phenobarbitone, due to adverse effects, after 10 children had been randomized to that drug, whereas this problem was not encountered in a trial conducted in India. Unfortunately, there are too few studies to investigate these hypotheses further. Regression models investigating participant factors such as age and seizure type are planned, and may provide further explanations for the heterogeneity observed. These regression models will be reported in a later version of this review.
For our seizure outcomes we found no difference between phenobarbitone and phenytoin. However the confidence intervals around summary estimates for hazard ratios are wide and do not suggest equivalence. For example, for time to 12-month remission, the summary hazard ratio was 0.93 (95% confidence interval 0.70 to 1.23). In other words, although the point estimate suggests an advantage for phenytoin, these results cannot exclude a hazard ratio of 0.70 in favour of phenytoin or a hazard ratio of 1.23 in favour of phenobarbitone.
AUTHORS’ CONCLUSIONS
Implications for practice
The results of this review do not provide evidence on which a choice can be made between phenytoin and phenobarbitone with respect to seizure control. Phenytoin is significantly less likely to be withdrawn however, presumably due to adverse effects, making it the preferred choice of the two drugs compared in this review.
Implications for research
Finding overall differences between these two antiepileptic drugs has proved elusive. If overall differences do exist across heterogeneous populations of individuals such as those studied here, those differences are likely to be small, and in order to be clinically useful, future comparative antiepileptic drug studies will need to be powered accordingly. It has been argued that future comparative antiepileptic drug studies be powered to establish equivalence (Jones 1996), and therefore be capable of detecting what is considered the smallest important clinical difference.
The International League Against Epilepsy currently recommends time to treatment withdrawal as the primary outcome in comparative monotherapy trials. One explanation for the heterogeneity observed for this outcome in this review is that clinicians were biased towards withdrawing phenobarbitone, leading to a higher withdrawal rate in unblinded studies. The issue of blinding will need to be considered in future pragmatic trials. Blinding significantly increases the cost of a trial and results in a departure from the everyday clinical practice that pragmatic trials try to mirror. However where there is prejudice against a particular treatment, failure to blind may result in a significant bias for this outcome.
PLAIN LANGUAGE SUMMARY.
Phenobarbitone versus phenytoin monotherapy for partial onset seizures and generalized onset tonic-clonic seizures
No evidence that phenytoin is better than phenobarbitone at controlling seizures.
Epilepsy is a disorder where recurrent seizures are caused by abnormal electrical discharges from the brain. Worldwide, phenobarbitone and phenytoin are commonly used antiepileptic drugs. This review found no evidence to suggest a difference between phenobarbitone and phenytoin for the control of the seizure types investigated. Phenobarbitone was more likely to be withdrawn, presumably due to adverse effects.
ACKNOWLEDGEMENTS
The MRC Clinical Trials Unit, meta-analysis group, for the use of, and advice on, their SCHARP meta-analysis software application, which was developed in collaboration with the Istituto “Mario Negri”, Milan.
Professor John Duncan, The National Hospital for Neurology and Neurosurgery, London, for refereeing the review.
SOURCES OF SUPPORT
Internal sources
University of Liverpool, UK.
Royal Liverpool and Broadgreen Hospital Trust, UK.
External sources
Medical Research Council, UK.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Parallel trial. Double blind. Participants allocated by statistician into control and treatment groups. Control groups remain on current therapy. Treatment groups allocated new therapy. Allocation concealed by plastic envelopes. |
|
| Participants | Institutionalized adult patients with uncontrolled epilepsy. Number randomized = 46 Number of people randomized to PHB and PHT not stated. Percentage of people with partial epilepsy not stated. 43% of participants were male. Mean age (range) not reported. Median follow-up not reported. |
|
| Interventions | Monotherapy with PHB or PHT. Median daily dose achieved not stated. |
|
| Outcomes | Mean number of days of attack (attack rate). | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | A crossover trial with 21 day treatment periods. Double blind. Randomization method not stated. Method of allocation concealment not stated. |
|
| Participants | Institutionalized adult patients with uncontrolled epilepsy. 91% of participants had partial epilepsy. Number randomized = PHB group = 45 people, PHT group = 45 people. 62% of participants were male. Age range 18-51 years. |
|
| Interventions | Monotherapy with PHB or PHT. Median daily dose achieved not stated. |
|
| Outcomes | Seizure frequency. Time to treatment withdrawal. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | An unblinded parallel trial. Method of generation of randomization list and allocation concealment not stated |
|
| Participants | Adults with newly diagnosed epilepsy. Number randomized = PHB group = 30 people; PHT group = 30 people. 100% of participants had partial epilepsy. Range of follow-up not mentioned. Percentage of participants that were male was not stated. Age range: 18-40 years. |
|
| Interventions | Monotherapy with PHB or PHT. Dose achieved not stated. | |
| Outcomes | Proportion of completers achieving 24-month remission at 3 years. Proportion of post randomization exclusions due to adverse effects or no efficacy |
|
| Notes | Abstract only. Outcomes chosen for this review were not reported. IPD pledged, but not yet received. |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Parallel trial. Randomization concealed using sealed opaque envelopes. Random list generated using random permuted blocks. Unblinded study. |
|
| Participants | People with newly diagnosed epilepsy. Number randomized = PHB group = 10 people, PHT group = 54 people. 55% partial epilepsy. 59% male. Mean age (range), 9 (3 to 16) years. Follow-up in months: median (range), 103 (30-64). |
|
| Interventions | Monotherapy with PHB or PHT. Median daily dose achieved PHB = not stated PHT = 175 mg/day. |
|
| Outcomes | Time to 12-month remission. Time to first seizure. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | A crossover trial with 1 week treatment periods. Double blind. Randomization method not stated. Allocation concealed by ’identical’ capsules. |
|
| Participants | Institutionalized adult patients. Number randomized = PHB group = 48 people, PHT group = 48 people. Percentage of participants with partial epilepsy not stated. Mean age (range) not reported. Percentage of participants that were male not reported. |
|
| Interventions | Monotherapy with PHB or PHT. Drug doses increased daily across period of treatment. |
|
| Outcomes | Daily seizure frequency. Seizure ’score’ (by the method of ridit transformation). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Parallel trial. Randomization concealed using sealed opaque envelopes. Random list generated using random permuted blocks. Unblinded. |
|
| Participants | People with newly diagnosed epilepsy. Number randomized = PHB group = 58 people, PHT group = 63 people. 44% of participants had partial epilepsy. 49% of participants were male. Mean age (range) 34 (14-77) years. Follow-up in months: median (range) 61 (1-156). |
|
| Interventions | Monotherapy with PHB or PHT. Median daily dose achieved PHB = 105 mg/day PHT = 300 mg/day. |
|
| Outcomes | Time to 12-month remission. Time to first seizure. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
| Methods | Parallel trial. Method of randomization and allocation concealment not stated. A double blind study using a dummy placebo |
|
| Participants | Participants had previously untreated or ’under-treated’ epilepsy. Number randomized = PHB group = 155 people, PHT group = 165 people. 100% of participants had partial epilepsy. 88% of participants were male. Mean age (range) 40 (18-81) years. Follow-up in months: median (range) 20 (0-66). |
|
| Interventions | Monotherapy with PHB or PHT. Median daily dose achieved not stated. |
|
| Outcomes | Times to treatment withdrawal and first seizure. Proportion with ’seizure control’. Rating scale of seizure frequency. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | A crossover trial with a 3 month treatment period. Double blind. Method of randomization and allocation concealment not stated |
|
| Participants | Participants had partial complex epilepsy. Number randomized: PHB group = 21 people, PHT group = 21 people. 100% of participants had partial epilepsy. 60% of participants were male. Age range 19-62 years. |
|
| Interventions | Monotherapy with PHB or PHT. Mean/median daily dose achieved not stated. |
|
| Outcomes | Main outcome measures were cognitive. Seizure frequency recorded. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Allocation concealment not stated. Randomized by minimization. Double blind. |
|
| Participants | Participants were untreated during the 3 months prior to trial entry. Number randomized = PHB group = 47 people, PHT group = 47 people. 62% of participants had partial epilepsy. 52% of participants were male. Mean age (range), 11 (2-18) years. Follow-up in months: median (range) 9 (0-12). |
|
| Interventions | Monotherapy with PHB or PHT. Daily dose achieved not stated. |
|
| Outcomes | Time to first seizure. Proportion of adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | B - Unclear |
| Methods | Random list generated using computer generated random numbers. Concealment by placebo dummies. Double blind. |
|
| Participants | People with previously untreated epilepsy. Number randomized = PHB group = 51 people, PHT group = 52 people. None of the participants had partial epilepsy. 54% of the participants were male. Age range: 4-12 years. Range of follow-up (months), 22-36. |
|
| Interventions | Monotherapy with PHB or PHT. Dose achieved not stated. |
|
| Outcomes | Proportion with recurrence of seizures. Adverse effects. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | A - Adequate |
IPD: individual patient data
PHT: phenytoin
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cereghino 1975 | Polytherapy comparisons. |
| White 1966 | Polytherapy comparisons. |
DATA AND ANALYSES
Comparison 1. Phenobarbitone versus phenytoin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to treatment withdrawal | 3 | 499 | Peto Odds Ratio (95% CI) | 1.62 [1.22, 2.14] |
| 2 Time to 12-month remission from seizures | 4 | 555 | Peto Odds Ratio (95% CI) | 0.93 [0.70, 1.23] |
| 3 Time to first seizure post randomization | 4 | 592 | Peto Odds Ratio (95% CI) | 0.84 [0.68, 1.05] |
Analysis 1.1. Comparison 1 Phenobarbitone versus phenytoin, Outcome 1 Time to treatment withdrawal.
Review: Phenobarbitone versus phenytoin monotherapy for partial onset seizures and generalized onset tonic-clonic seizures
Comparison: 1 Phenobarbitone versus phenytoin
Outcome: 1 Time to treatment withdrawal
 |
Analysis 1.2. Comparison 1 Phenobarbitone versus phenytoin, Outcome 2 Time to 12-month remission from seizures.
Review: Phenobarbitone versus phenytoin monotherapy for partial onset seizures and generalized onset tonic-clonic seizures
Comparison: 1 Phenobarbitone versus phenytoin
Outcome: 2 Time to 12-month remission from seizures
 |
Analysis 1.3. Comparison 1 Phenobarbitone versus phenytoin, Outcome 3 Time to first seizure post randomization.
Review: Phenobarbitone versus phenytoin monotherapy for partial onset seizures and generalized onset tonic-clonic seizures
Comparison: 1 Phenobarbitone versus phenytoin
Outcome: 3 Time to first seizure post randomization
 |
Appendix 1. CENTRAL search strategy
phenytoin OR epanutin)
MeSH descriptor Phenytoin explode all trees
phenobarb*
MeSH descriptor Phenobarbital explode all trees
((#1 OR #2) AND (#3 OR #4))
MeSH descriptor Epilepsy explode all trees
MeSH descriptor Seizures explode all trees
epilep* or seizure* or convulsion*
(#6 OR #7 OR #8)
(#5 AND #9)
Appendix 2. MEDLINE search strategy
The following search was combined with phases 1 and 2 of the Cochrane highly sensitive search strategy for MEDLINE as set out in Appendix 5b of the Cochrane Handbook for Systematic Reviews of Interventions (version 4.2.4, updated March 2005) (Higgins 2005).
phenytoin/ OR (phenytoin or diphenylhydantoin or epanutin).tw.
phenobarbital/ OR phenobarbit*.tw.
exp epilepsy/ OR epilep$.tw.
exp seizures/ OR seizure$.tw.
convulsion$.tw.
1 AND 2
3 OR 4 OR 5
6 AND 7
WHAT’S NEW
Last assessed as up-to-date: 10 November 2009.
| Date | Event | Description |
|---|---|---|
| 11 November 2009 | New search has been performed | Searches updated 20 October 2009; no new trials identified. |
HISTORY
Protocol first published: Issue 3, 2000
Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 22 December 2006 | New search has been performed | Searches updated 22 December 2006; no new trials identified. |
Footnotes
DECLARATIONS OF INTEREST: None known.
References to studies included in this review
- Bird 1966 {published data only} .Bird CAK, Griffin PB, Miklasewska JM, Galbraith AW. Tegretol (carbamazepine): a controlled trial of a new anticonvulsant. British Journal of Psychiatry. 1966;112:737–42. [Google Scholar]
- Cereghino 1974 {published data only} .Cereghino JJ, Brock JT, Van Meter JC, Penry JK, Smith LD, White BG. Carbamazepine for epilepsy: a controlled prospective evaluation. Neurology. 1974;24(5):401–10. doi: 10.1212/wnl.24.5.401. [DOI] [PubMed] [Google Scholar]
- Czapinski 1997 {published data only} .Czapinski P, Terczynski A, Czapinska E. Randomised 36-month comparative study of valproic acid (VPA), phenytoin (PHT), phenobarbital (PHB) and carbamazepine (CBZ) efficacy in patients with newly diagnosed epilepsy with partial complex seizures. Journal of the Neurological Sciences. 1997;150:162–3. [Google Scholar]
- de Silva 1996 {published data only} .de Silva M, MacArdle B, McGowan M, Hughes E, Stewart K, Neville BG. Randomised comparative monotherapy trial of phenobarbitone, phenytoin, carbamazepine, or sodium valproate for newly diagnosed childhood epilepsy. Lancet. 1996;347:709–13. doi: 10.1016/s0140-6736(96)90074-4. [DOI] [PubMed] [Google Scholar]
- Gruber 1962 {published data only} .Gruber CM, Brock JT. Comparison of the effectiveness of phenobarbital, mephobarbital, primidone, diphenylhydantoin, ethotoin, metharbital and methylphenylhydantoin in motor seizures. Clinical Pharmacology and Therapeutics. 1962;3(1):23–8. doi: 10.1002/cpt19623123. [DOI] [PubMed] [Google Scholar]
- Heller 1995 {published data only} .Heller AJ, Chesterman P, Elwes RD, Crawford P, Chadwick D, Johnson AL, et al. Phenobarbitone, phenytoin, carbamazepine, or sodium valproate for newly diagnosed adult epilepsy: a randomised comparative monotherapy trial. Journal of Neurology, Neurosurgery, and Psychiatry. 1995;58(1):44–50. doi: 10.1136/jnnp.58.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson 1985 {published data only} .Mattson RH, Cramer JA, Collins JF, Smith DB, Delgado-Escueta AV, Browne TR, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic-clonic seizures. New England Journal of Medicine. 1985;313(3):145–51. doi: 10.1056/NEJM198507183130303. [DOI] [PubMed] [Google Scholar]
- Meador 1990 {published data only} .Meador K, Loring D, Huh K, Gallagher B, King D. Comparative cognitive effects of anticonvulsants. Neurology. 1990;40(3 Pt 1):391–4. doi: 10.1212/wnl.40.3_part_1.391. [DOI] [PubMed] [Google Scholar]
- Pal 1998 {published data only} .Pal DK, Das T, Chaudhury G, Johnson AL, Neville BGR. Randomised controlled trial to assess acceptability of phenobarbital for childhood epilepsy in rural India. Lancet. 1998;351:19–23. doi: 10.1016/S0140-6736(97)06250-8. [DOI] [PubMed] [Google Scholar]
- Thilothammal 1996 {published data only} .Thilothammal N, Banu K, Ratnam RS. Comparison of phenobarbitone, phenytoin with sodium valproate: randomised, double-blind study. Indian Pediatrics. 1996;33(7):549–55. [PubMed] [Google Scholar]
References to studies excluded from this review
- Cereghino 1975 {published data only} .Cereghino JJ, Brock JT, Van Meter JC, Penry JK, Smith LD, White BG. The efficacy of carbamazepine combinations in epilepsy. Clinical Pharmacology and Therapeutics. 1975;18(6):733–41. doi: 10.1002/cpt1975186733. [DOI] [PubMed] [Google Scholar]
- White 1966 {published data only} .White TW, Plott D, Norton J. Relative anticonvulsant potency of primidone. Archives of Neurology. 1966;14(1):31–5. doi: 10.1001/archneur.1966.00470070035004. [DOI] [PubMed] [Google Scholar]
Additional references
- Commission 1998 .ILAE commission on antiepileptic drugs Considerations on designing clinical trials to evaluate the place of new antiepileptic drugs in the treatment of newly diagnosed and chronic patients with epilepsy. Epilepsia. 1998;39(7):799–803. doi: 10.1111/j.1528-1157.1998.tb01167.x. [DOI] [PubMed] [Google Scholar]
- Early 1990 .Early Breast Cancer Trialists . Treatment of early breast cancer, worldwide evidence 1985-1990. Vol. 1. Oxford University Press; Oxford: 1990. [Google Scholar]
- Higgins 2005 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.4 [updated March 2005] 2. The Cochrane Library; [Google Scholar]
- Jones 1996 .Jones B, Jarvis P, Lewis JA, Ebutt AF. Trials to assess equivalence; the importance of rigorous methods. British Medical Journal. 1996;313:36–9. doi: 10.1136/bmj.313.7048.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar 1998 .Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine. 1998;17(24):2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study


