Abstract
Large bone defects are a considerable challenge to reconstructive surgeons. Numerous traditional Chinese herbal medicines have been used to repair and regenerate bone tissue. This study investigated the bone regeneration potential of Danggui Buxue Tang (DBT), a Chinese herbal decoction prepared from Radix Astragali (RA) and Radix Angelicae Sinensis (RAS), from a molecular biology perspective. The optimal ratio of RA and RAS used in DBT for osteoblast culture was obtained by colorimetric and alkaline phosphatase (ALP) activity assays. Moreover, the optimal concentration of DBT for bone cell culture was also determined by colorimetric, ALP activity, nodule formation, Western blotting, wound-healing, and tartrate-resistant acid phosphatase activity assays. Consequently, the most appropriate weight ratio of RA to RAS for the proliferation and differentiation of osteoblasts was 5 : 1. Moreover, the most effective concentration of DBT was 1,000 μg/mL, which significantly increased the number of osteoblasts, intracellular ALP levels, and nodule numbers, while inhibiting osteoclast activity. Additionally, 1,000 μg/mL of DBT was able to stimulate p-ERK and p-JNK signal pathway. Therefore, DBT is highly promising for use in accelerating fracture healing in the middle or late healing periods.
1. Introduction
Bone injuries are commonly caused by trauma, infection, diseases, or tumor removal. Clinically, bone begins to repair itself within weeks following injury and lasts for months. The healing process includes three stages: inflammation, repair, and remodeling. Bone remodeling is dynamically equilibrated by bone-forming osteoblasts and bone-resorbing osteoclasts for several months up to 1 year. Bone mineralization generally allows more time to proceed with healing in order to comply with changing skeletal growth for mechanical requirements. Many clinical and animal studies have demonstrated that traditional Chinese medicines have beneficial therapeutic effects on bone fracture healing [1–4]. Therefore, the biochemical effects of traditional Chinese medicines using an in vitro bone cell culture model have received considerable attention [5–7].
Danggui Buxue Tang (DBT), a Chinese herbal decoction consisting of Huangqi (Radix Astragali, RA) and Danggui (Radix Angelicae Sinensis, RAS) with a weight ratio of 5 : 1, is widely used for menopausal women to nourish qi and blood. According to recent pharmacological studies, DBT can enhance cardiovascular circulation, prevent osteoporosis, increase antioxidant activity, and stimulate and regulate immune functions [8, 9]. Additionally, RA and RAS can promote the proliferation of bone cells, induce bone formation, inhibit bone resorption in patients [10], and increase the proliferation and differentiation of the osteoblasts [11, 12].
This study examined the biological effects of different ratios of RA to RAS in DBT and various DBT concentrations on bone cell activities via in vitro cell culture. The possible pharmacological mechanism of the DBT to facilitate bone regeneration was also investigated.
2. Materials and Methods
2.1. Plant Materials and DBT Preparation
Fresh roots, RA (A. membranaceus var. mongholicus) and RAS (A. sinensis), were purchased from Chuang Song Zong Pharmaceutical Co. (Kaohsiung, Taiwan). Their identity was confirmed by experts in pharmacognosy. DBT was prepared using a method described previously [13]. The extraction process of the crude drugs was performed under strict quality control. Briefly, RA and RAS were boiled separately in 6 volumes of water for 1 h. The residue from first extraction was then boiled in 8 volumes of water for 1.5 h. The aqueous extracts were combined, filtered to remove insoluble debris, and stored at −20°C. The biological activities of DBT extracts were evaluated by preparing RA and RAS at ratios of 1 : 5, 2 : 1, 5 : 1, and 10 : 1. Finally, various concentrations of DBT were prepared and stored at 4°C until the in vitro assays. The culture medium without DBT was used as a control.
2.2. Cell Culture
The human osteoblast-like cell line MG-63 (BCRC number 60279) was obtained from the Food Industry Research and Development Institute (FIRDI, Hsinchu, Taiwan). Cells were grown in Modified Eagle's medium (MEM, Gibco-BRL, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA) and 1% penicillin/streptomycin (Gibco) in a humidified 5% CO2 incubator at 37°C. Cells were tested after growth to 80% confluence. Cultured MG-63 cells were seeded in 24-well tissue culture plates (Corning, NY, USA) at a density of 1 × 104 cells/well. After 1 day of culture, the culture medium was replaced with DBT extract. After culturing for 2 days, the proliferation and differentiation of osteoblasts were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich, St. Louis, MO, USA) assay and alkaline phosphatase (ALP) activity assay, respectively, as described below [5].
Murine monocyte/macrophage RAW 264.7 cells (BCRC number 60001) were obtained from FIRDI. 2 × 103 cells/well RAW 264.7 cells were cultured in Dulbecco's Modified Eagle's medium (DMEM, Gibco) supplemented with 5% FBS and 1% penicillin/streptomycin in a humidified 5% CO2 incubator at 37°C. After 1 day of culture, osteoclast differentiation from RAW 264.7 cells was induced with 50 ng/mL RANKL (Alexis Biochemicals, Lausen, Switzerland) in α-minimal essential medium (α-MEM, Gibco) with 2% FBS for 6 days. The cells were also treated with various concentrations of DBT added at different periods. DBT was added to the cells from the start of the culture to day 6 (group 1) or from day 7 to day 8 (group 2) [6]. The culture medium was refreshed every 2 days. The proliferation and differentiation of osteoclasts were examined by MTT assay and tartrate-resistant acid phosphatase (TRAP) activity assay, respectively.
2.3. MTT Assay for Cell Viability
The proliferation of bone cells was evaluated by MTT assay. After culture, cells were incubated with 10 μL MTT solution (5 mg/mL) and 100 μL culture medium for 4 h at 37°C to form insoluble formazan crystals. The formazan crystals were then dissolved by adding 100 μL of acid isopropyl alcohol (0.04 M HCl in isopropyl alcohol). The concentration of formazan crystals formed in the viable cells was estimated by measuring the absorbance at 570 nm on a multiwell scanning spectrophotometer (MRX Microplate Reader, Dynatech Laboratories Inc., Chantilly, USA) [14]. All experiments were performed in triplicate.
2.4. Analysis of ALP for Osteoblast Differentiation
The differentiation of osteoblasts was determined by ALP activity assay as described elsewhere [15]. Briefly, the cells were treated with 20 μL/well 0.1% Triton X-100 (Sigma) for 5 min at room temperature for cell lysis. 100 μL/well of the ALP assay kit (procedure number DG1245-K, Sigma-Aldrich) was then added to produce p-nitrophenol from the hydrolysis of p-nitrophenyl phosphate. The ALP activity of cell lysates was determined by measurement of absorbance at 405 nm caused by p-nitrophenol using a MRX Microplate Reader. Each experimental condition was repeated three times.
2.5. Quantifying Bone Nodules via von Kossa Stain
The formation of the mineralized nodules was confirmed using the von Kossa stain [16]. Briefly, 5 × 104 cells/well cultured MG-63 cells were added to the culture medium supplemented with 50 μg/mL L-ascorbic acid (Sigma), 10 mM β-glycerol phosphate (Sigma), and 10 nM dexamethasone (Sigma). The medium was mixed with various DBT concentrations. The medium was changed every 3 days. After 14 days of culture, cultures were fixed in 2% glutaraldehyde for 20 min. The fixed plates were stained with 5% silver nitrate (Union Chemical Works, Ltd., Hsinchu, Taiwan) for 30 min in darkness, exposed to ultraviolet light for 1 h, and then treated with 5% sodium thiosulfate (Union Chemical Works, Ltd.) for 2 min. After washing, the cells are counterstained with 0.1% nuclear fast red (Sigma) dissolved in 5% aluminum sulfate (JT Baker, Phillipsburg, NJ, USA) for 5 min. The number of mineralized bone nodules was counted under an inverted optical microscope (Axiovert 25, Carl Zeiss, Inc., Goettingen, Germany).
2.6. Western Blot Analysis
4 × 105 cells/well cultured MG-63 cells were seeded to osteogenic medium with various concentrations of DBT in a 6-well culture plate. The medium was replaced every 3 days. After culturing for 7 days, adherent cells were washed and immersed in ice-cold lysis buffer containing 50 mM Tris (pH 7.5), 1 mM EDTA (pH 7.5), 500 mM NaCl, 10% glycerol, 1 mM β-mercaptoethanol, 1% IGEPAL-630/Nonidet P-40, and proteinase inhibitor cocktail (Roche, Basel, Switzerland) [17]. After 30 min of immersion, the cellular lysates were centrifuged at 12000 g for 20 min. The concentration of protein was measured using a BCA protein assay kit (Pierce, Rockford, IL, USA). Equal amounts of protein were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Nonspecific protein binding was blocked with 5% nonfat milk in PBS for 1 h and then incubated with primary antibodies at 1 : 1000 dilutions for 2 days. The membranes were washed to remove unbound antibodies and then incubated with the secondary antibody diluted at 1 : 1000 for 90 min. The blots were visualized by chemiluminescence using the ECL kit (Pierce) with X-ray film (Konica Minolta, Japan).
2.7. Cell Migration in a Wound-Healing Assay
Wound-healing assay was employed to detect the migration effect of DBT on osteoblasts. Briefly, transparent adhesive tape with 0.1 cm of wide (3M, St. Paul, MN, USA) was applied on the 12-well tissue culture plates and exposed to UV light for 1 h. After washing three times with PBS, 3 × 105 cells/well of cultured MG-63 cells were seeded in the culture plate. After 1 day of culture, the tape was removed to produce 1 mm gap (wound). After rinsing three times with α-MEM, the cells were cultured with various concentrations of DBT for 2 days. The cell layers were rinsed with PBS, fixed in 2% glutaraldehyde, and stained with Liu's stain solution (Chin Pao Co., Ltd., Taipei, Taiwan). The degree of cells migration was examined using an inverted optical microscope.
2.8. TRAP Analysis and TRAP Stain for Osteoclast Differentiation
Several studies have demonstrated that the formation of mature osteoclasts requires 6 days [18, 19]. After 6 days (group 1) or 8 days (group 2) of culture, TRAP activity was assessed by measuring the amount of TRAP released from osteoclasts using a TRAP assay kit (procedure number 435, Sigma). Briefly, 30 μL culture media was mixed with 100 μL TRAP reagent. Absorbance at 405 nm corresponded to the formation of p-nitrophenol that was observed using a MRX Microplate Reader. Each experimental condition was repeated three times.
Osteoclasts in the culture were also observed by using TRAP stain [20]. Briefly, cells were fixed using citrate/acetone fixative solution for 30 s, followed by rinsing twice with deionized water. The cells were then incubated in the dark using a 300 μL of TRAP stain reagent (procedure number 387A, Sigma) at 37°C for 1 h. After washing twice, cells were counterstained by hematoxylin solution and observed using an inverted optical microscope.
2.9. Statistical Analysis
All quantitative data were expressed as means ± standard deviations. Statistical analysis was done using one-way analysis of variance followed by post hoc Fisher's LSD test for multiple comparisons. P values lower than 0.05 were considered of statistical significance.
3. Results
3.1. Effects of DBT Concentration on Osteoblast
The proliferation of osteoblasts induced by different ratios of RA to RAS in DBT and various concentrations of DBT was quantified by MTT assay. DBT extracted from RA and RAS in ratios of 1 : 5, 2 : 1, and 10 : 1 did not significantly influence the proliferation of osteoblasts at all concentrations, 0.1–1,000 μg/mL, except that 1,000 μg/mL of DBT extracted from RA and RAS at a ratio of 2 : 1 significantly decreased the number of osteoblasts (Figure 1(a)). However, DBT prepared from RA and RAS at a ratio of 5 : 1 significantly affected the proliferation of osteoblasts in a dose-dependent manner (Figure 2(a)). DBT significantly increased the number of osteoblastic cells at the concentrations between 1,000 and 2,000 μg/mL (P < 0.05). However, DBT significantly inhibited osteoblast growth when the concentration of DBT was >5,000 μg/mL (P < 0.01).
Figure 1.
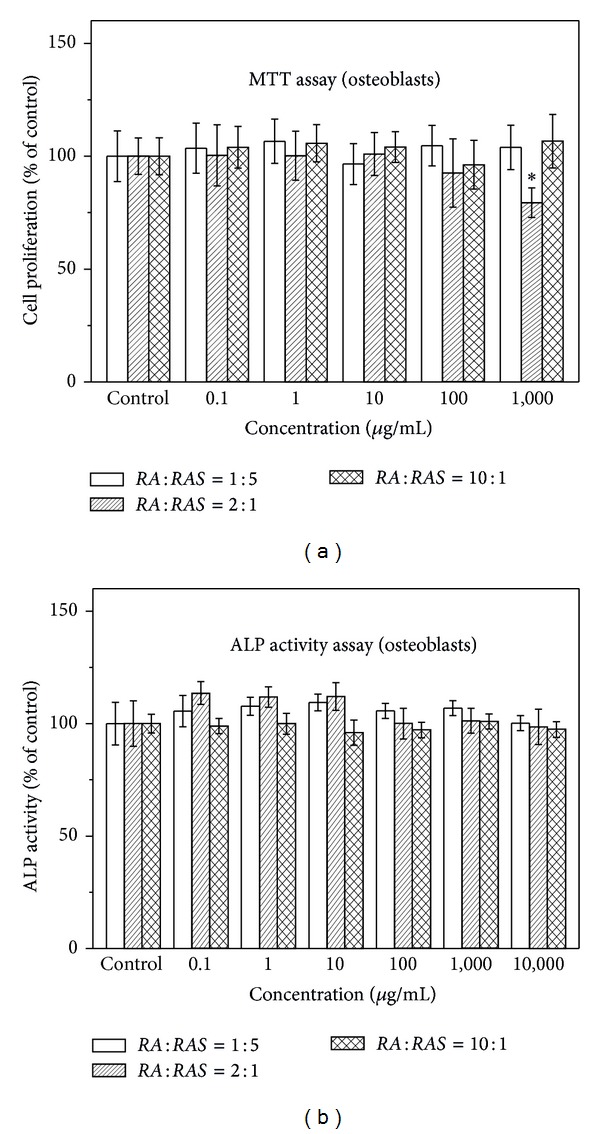
Effect of DBT extract prepared at various ratios of Radix Astragali (RA) and Radix Angelicae Sinensis (RAS) (1 : 5, 2 : 1, and 10 : 1) on osteoblast proliferation and differentiation by (a) MTT assay and (b) ALP activity assay, respectively. Results are expressed as percentage of control (*P < 0.05 versus control).
Figure 2.
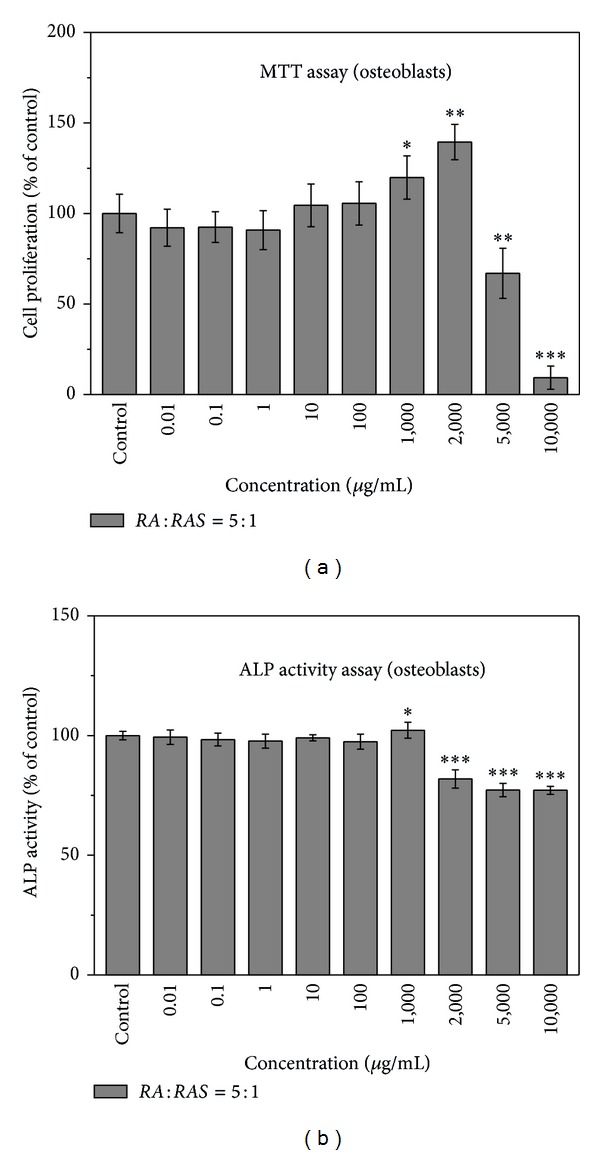
Effect of DBT extract prepared from Radix Astragali and Radix Angelicae Sinensis at a ratio of 5 : 1 on osteoblast proliferation and differentiation by (a) MTT assay and (b) ALP activity assay, respectively. Results are expressed as percentage of control (*P < 0.05, **P < 0.01, and ***P < 0.001 versus control).
ALP localized on the cell membrane of osteogenic cells was assessed by ALP activity assay. Figure 1(b) shows that DBT prepared from RA and RAS in ratios of 1 : 5, 2 : 1, and 10 : 1 had no statistical difference in the ALP activity. However, various concentrations of DBT prepared from RA and RAS at a ratio of 5 : 1 had different effects on the ALP activity of MG-63 cells (Figure 2(b)). Compared with the control, 1,000 μg/mL of DBT significantly increased osteoblastic cell differentiation (P < 0.05). However, the ALP activity significantly reduced when the concentration of DBT was > 2,000 μg/mL (P < 0.001). Therefore, DBT prepared from RA and RAS in ratios of 1 : 5, 2 : 1, and 10 : 1 was not evaluated in the following study. Moreover, concentrations higher than 1,000 μg/mL for DBT prepared from RA and RAS at a ratio of 5 : 1 were also not investigated in the following study except Western blot analysis.
Figure 3 demonstrates the effect of various concentrations of DBT prepared from RA and RAS at a ratio of 5 : 1 on calcium deposition stained with von Kossa stain. 1,000 μg/mL of DBT had higher percentage of areas of calcium nodules to total area than all of the other concentrations, 0–100 μg/mL (Figure 3(a)). Moreover, compared with control, DBT significantly increased the number of total nodules formed when the concentration of DBT was > 10 μg/mL (P < 0.05). In particular, 1,000 μg/mL of DBT significantly raised the number of total calcified nodules by 380% (Figure 3(b)).
Figure 3.
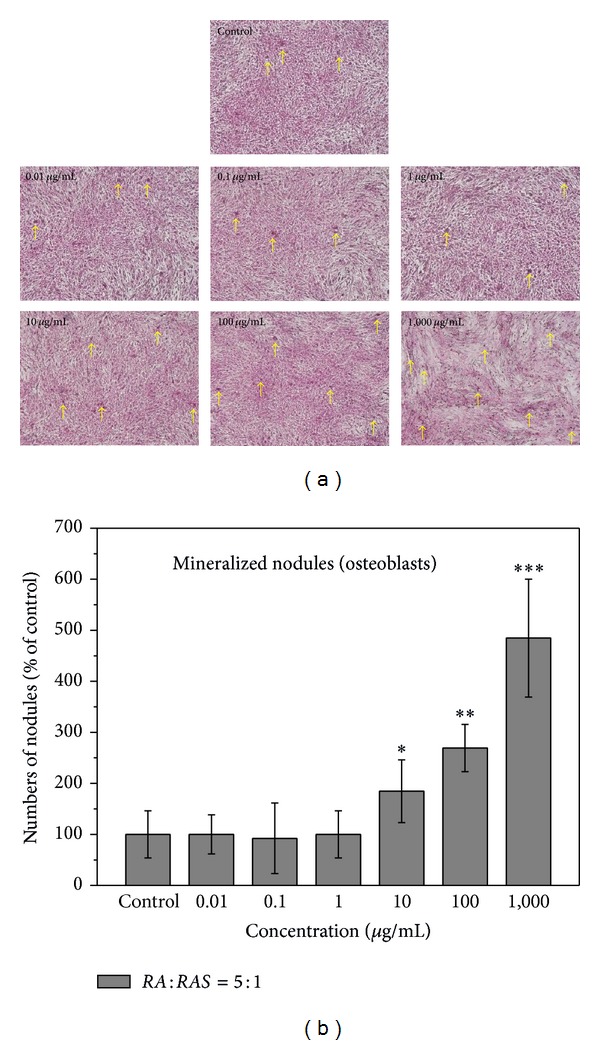
Effect of DBT extract prepared from Radix Astragali and Radix Angelicae Sinensis at a ratio of 5 : 1 on (a) matrix calcium deposition and (b) numbers of total calcified nodules formed in the osteoblast cultures at various concentrations of DBT, as determined by von Kossa stain. Results are expressed as percentage of control (*P < 0.05, **P < 0.01, and ***P < 0.001 versus control). Arrows demonstrate deposition of mineralized matrix.
To determine the effect of DBT prepared from RA and RAS at a ratio of 5 : 1 on osteoblast differentiation, MG-63 cells were treated with various concentrations of DBT (0.01–2,000 μg/mL) for 7 days. The expression levels of osteogenic-related proteins, ALP and osteopontin, were then evaluated by Western blot analysis. Figure 4(a) displays that all ALP, osteopontin, and γ-tubulin expression levels on DBT-treated osteoblasts were higher than those of the control group. However, the ALP activity assay showed that 2,000 μg/mL of DBT inhibited the differentiation of osteoblasts (Figure 2(b)). The difference in the results might be due to different culture periods (2 days versus 7 days) and media compositions used before the ALP activity assay and Western blot analysis were performed.
Figure 4.
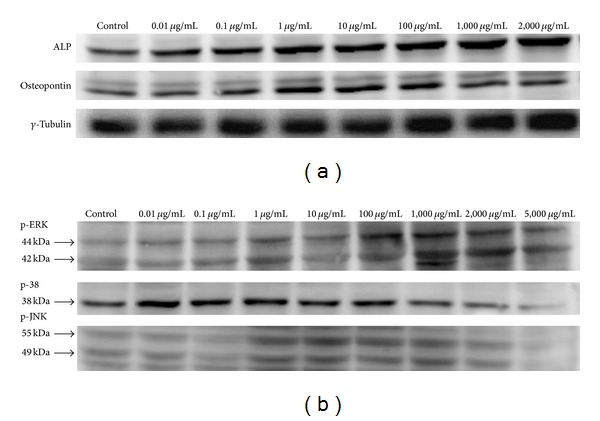
Effect of DBT extract prepared from Radix Astragali and Radix Angelicae Sinensis at a ratio of 5 : 1 on protein expression of (a) alkaline phosphatase, osteopontin, and γ-tubulin and (b) p-ERK, p-38, and p-JNK by Western blot analysis.
The mitogen-activated protein kinases (MARKs) regulate cell proliferation, differentiation, motility, and survival in coordination with each other [21]. This study also observed the proliferative effect of DBT prepared from RA and RAS at a ratio of 5 : 1 on the regenerative ability of MG-63 cells cultured with various concentrations of DBT (0.01–5,000 μg/mL) for 12 h. Figure 4(b) reveals that DBT had a dose-dependent effect on the expression of MARKs such as p-ERK (about 42 and 44 kDa) and p-JNK (about 49 and 55 kDa). 1,000 μg/mL of DBT induced the highest p-ERK expression and higher p-JNK levels. No effects occurred at lower doses, while some declined at higher concentrations. Moreover, the decrease in p-38 phosphorylation was found as p-ERK and p-JNK activity increased. We believe that DBT can activate the phosphorylation of p-ERK and p-JNK signal pathway to stimulate the proliferation and differentiation of human osteosarcoma cell line MG-63.
The ability of osteoblastic cell to migrate along the growth direction was examined by an in vitro wound-healing experiment. Compared with the control, 0.01–2,000 μg/mL of DBT prepared from RA and RAS at a ratio of 5 : 1 markedly enhanced the mobility of MG-63 cells (Figure 5). Moreover, DBT induced osteoblastic cell proliferation. These results indicate that DBT could enhance bone cell regeneration.
Figure 5.
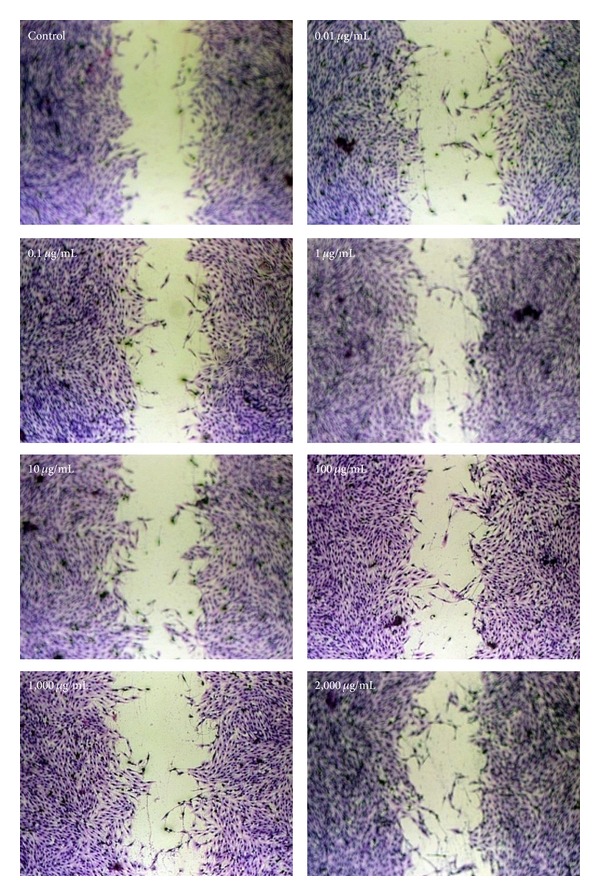
Effect of DBT extract prepared from Radix Astragali and Radix Angelicae Sinensis at a ratio of 5 : 1 on the migratory ability of osteoblasts, as determined by wound-healing assay.
3.2. Effects of DBT Concentration on Osteoclast
The RAW 264.7 cells were used to evaluate the osteoclastogenic effect of DBT prepared from RA and RAS at a ratio of 5 : 1. In group 1 (proliferative and differentiation phases), various concentrations of DBT and 50 ng/mL of soluble RANKL were applied onto the cultured RAW 264.7 cells for 6 days to induce the differentiation of monocytes/macrophages into osteoclasts. Figure 6(a) displays how various doses (0.01–1,000 μg/mL) affect the proliferation of osteoclasts measured by MTT assay. Consequently, no statistically significant difference from the control group was observed at the lower concentration of 0.01–100 μg/mL. Conversely, DBT significantly lowered the proliferation of osteoclasts at 1,000 μg/mL (P < 0.05). Moreover, the TRAP activity of osteoclasts decreased when adding DBT at concentrations of 1–1,000 μg/mL (P < 0.05) (Figure 6(b)). When DBT inhibited TRAP activity, the number of osteoclasts was lower than the control group (Figure 8(a)).
Figure 6.
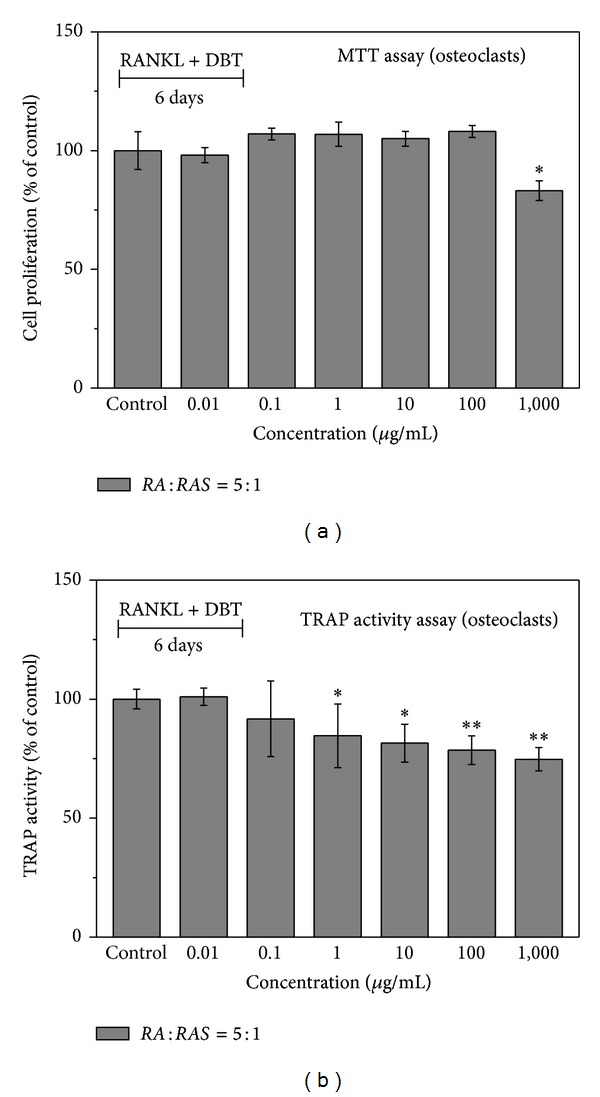
Effect of DBT extract prepared from Radix Astragali and Radix Angelicae Sinensis at a ratio of 5 : 1 on osteoclast proliferation and differentiation by (a) MTT assay and (b) TRAP activity assay, respectively, after various concentrations of DBT extract were added for 6 days (proliferative and differentiation phases). Results are expressed as percentage of control (*P < 0.05 and **P < 0.01 versus control).
Figure 8.
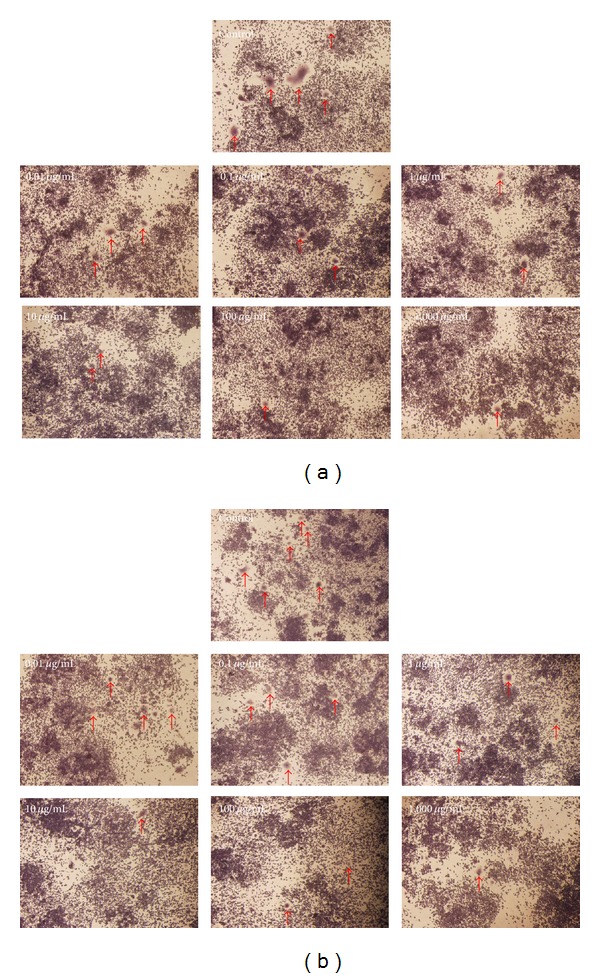
TRAP staining of osteoclasts treated with different concentrations of DBT extract (a) for 6 days and (b) for 2 days at day 7 to 8. Arrows demonstrate osteoclasts.
For a closer examination (group 2, mature phase), after RAW 264.7 cells were treated with 50 ng/mL RANKL for 6 days, DBT was then added to the mature osteoclasts from day 7 to day 8 (for 2 days). Figure 7(a) clarifies that DBT did not affect the proliferation of mature osteoclasts. TRAP activity assay revealed that DBT at concentrations of 0.01–1,000 μg/mL produced significant decreases in TRAP activity (Figure 7(b)). When DBT inhibited TRAP activity, the number of osteoclasts was lower than the control group (Figure 8(b)). These results suggest that DBT can inhibit the RANKL-induced osteoclast differentiation of RAW 264.7 cells.
Figure 7.
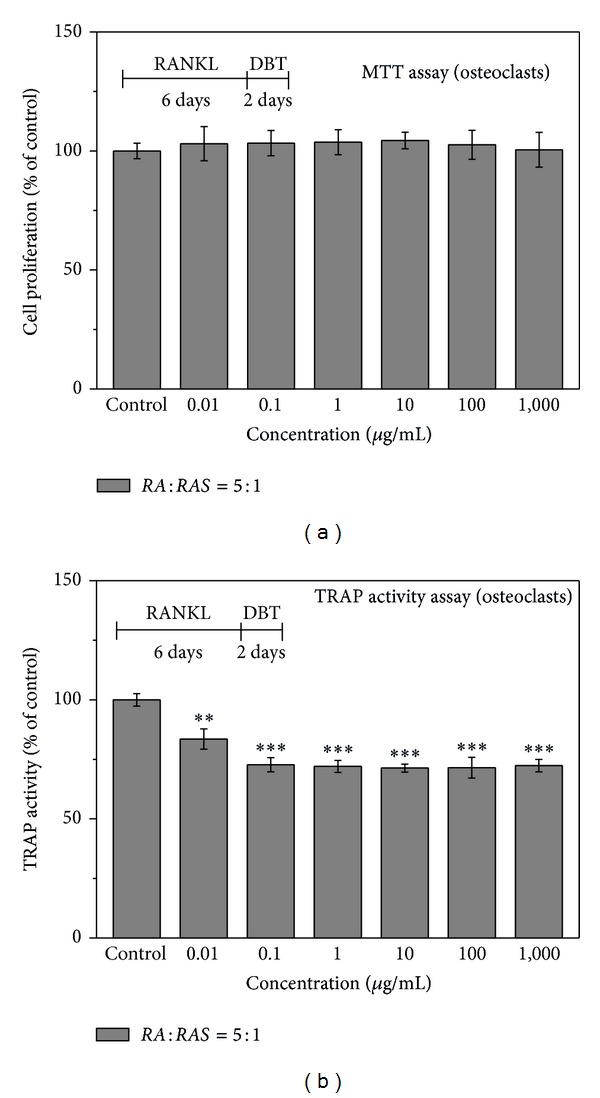
Effect of DBT extract prepared from Radix Astragali and Radix Angelicae Sinensis at a ratio of 5 : 1 on osteoclast proliferation and differentiation by (a) MTT assay and (b) TRAP activity assay, respectively, after various concentrations of DBT extract were added for 2 days at day 7 to 8 (mature phase). Results are expressed as percentage of control (**P < 0.01 and ***P < 0.001 versus control).
4. Discussion
Several studies have documented the feasibility of alleviating bone disorders and liver diseases following treatment with Chinese herbal decoction DBT [8–11, 13]. Specific biological advantages, which can be achieved from Chinese medicine, must include faster and more uniform bone ingrowth [3]. As is well known, osteoblasts and osteoclasts in the fracture site are actively engaged in the synthesis and secretion of collagen [22]. To repair skeletal defects, osteoblasts should populate the defects by proliferation of the transplanted cells and migration of cells into the defect from the surrounding tissue; the construct is ultimately filled by the osteoblasts and healing of large osseous defects [23]. Our previous study developed and evaluated tricalcium phosphate, gelatin, and Chinese medicine as a new bone substitute [19]. During bone repair, bone remodeling involves bone resorption by osteoclasts, which is followed by bone formation by osteoblasts. This study investigates how DBT affects bone cell activity.
The results of the biological evaluation indicate that DBT prepared from RA and RAS at a ratio of 5 : 1 had a significant osteotropic effect. Moreover, the optimal concentration of DBT prepared from RA and RAS at a ratio of 5 : 1 was 1,000 μg/mL, which obviously raised the number of osteoblasts, intracellular ALP levels, and nodule numbers, while suppressing osteoclast activity. Additionally, applying DBT to osteoblasts triggered the downstream signaling cascades including p-ERK and p-JNK signal pathways. Doing so facilitated the proliferation and differentiation of human osteosarcoma cell line MG-63, thus demonstrating excellent osteoinductive activity. Moreover, DBT could inhibit the RANKL-induced osteoclast formation in vitro.
Traditional Chinese medicine has been developed empirically based on clinical experience. Importantly, traditional Chinese medicine can be used systemically to accelerate bone formation or diminish bone resorption in order to treat bone diseases. For early stage of healing and resorption remodeling process, individual Chinese medicines (e.g., Loranthus parasiticus, Achyranthes bidentata, and Drynaria fortunei) can enhance osteoclast formation by stimulating the proliferation in bone resorption. In the middle and late phases of healing, Chinese medicines such as Cuscuta chinensis, Eucommia ulmoides, and Dipsacus asper can potentially inhibit osteoclast proliferation and promote osteoblastic proliferation and differentiation [6, 19].
5. Conclusion
This work demonstrates the biological functions of this decoction in promoting the proliferation, differentiation, and mineralization of osteoblasts in vitro as well as inhibiting osteoclast activity. Importantly, DBT is highly promising for use in accelerating fracture healing in the middle or late healing periods and treating osteoporosis.
Acknowledgments
The authors would like to thank the National Science Council of the Republic of China, Taiwan (Contract no. NSC98-2221-E-039-005-MY3), and the China Medical University (Contract nos. CMU 101-AWARD-05 and CMU101-S-01) for financially supporting this research.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Wong RWK, Rabie ABM. Traditional Chinese medicines and bone formation—a review. Journal of Oral and Maxillofacial Surgery. 2006;64(5):828–837. doi: 10.1016/j.joms.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Yan SQ, Wang G-J, Shen TY. Effects of pollen from Typha angustata on the osteoinductive potential of demineralized bone matrix in rat calvarial defects. Clinical Orthopaedics and Related Research. 1994;(306):239–246. [PubMed] [Google Scholar]
- 3.Huang H-F, You J-S. The use of chinese herbal medicine on experimental fracture healing. The American Journal of Chinese Medicine. 1997;25(3-4):351–356. doi: 10.1142/S0192415X97000391. [DOI] [PubMed] [Google Scholar]
- 4.Peng LH, Ko CH, Siu SW, et al. In vitro & in vivo assessment of a herbal formula used topically for bone fracture treatment. Journal of Ethnopharmacology. 2010;131(2):282–289. doi: 10.1016/j.jep.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 5.Lin C-Y, Sun J-S, Sheu S-Y, Lin F-H, Wang Y-J, Chen L-T. The effect of Chinese medicine on bone cell activities. The American Journal of Chinese Medicine. 2002;30(2-3):271–285. doi: 10.1142/S0192415X02000351. [DOI] [PubMed] [Google Scholar]
- 6.Sun J-S, Lin C-Y, Dong G-C, et al. The effect of Gu-Sui-Bu (Drynaria fortunei J. Sm) on bone cell activities. Biomaterials. 2002;23(16):3377–3385. doi: 10.1016/s0142-9612(02)00038-8. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Zhen L, Zhang G, Wong M-S, Qin L, Yao X. Osteogenic effects of flavonoid aglycones from an osteoprotective fraction of Drynaria fortunei—an in vitro efficacy study. Phytomedicine. 2011;18(10):868–872. doi: 10.1016/j.phymed.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Tsim KWK. Danggui Buxue tang (Chinese angelica decoction): a sample trial in TCM standardization. Asia-Pacific Biotechnology News. 2004;8(23):1316–1321. [Google Scholar]
- 9.Gao Q, Li J, Cheung JKH, et al. Verification of the formulation and efficacy of Danggui Buxue Tang (a decoction of Radix Astragali and Radix Angelicae Sinensis): an exemplifying systematic approach to revealing the complexity of Chinese herbal medicine formulae. Chinese Medicine. 2007;2, article 12 doi: 10.1186/1749-8546-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Q, Populo SM, Zhang J, Yang G, Kodama H. Effect of Angelica sinensis on the proliferation of human bone cells. Clinica Chimica Acta. 2002;324(1-2):89–97. doi: 10.1016/s0009-8981(02)00210-3. [DOI] [PubMed] [Google Scholar]
- 11.Choi RCY, Gao QT, Cheung AWH, et al. A Chinese herbal decoction, Danggui buxue tang, stimulates proliferation, differentiation and gene expression of cultured osteosarcoma cells: genomic approach to reveal specific gene activation. Evidence-based Complementary and Alternative Medicine. 2011;2011:13 pages. doi: 10.1093/ecam/nen085.307548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong TTX, Zhao KJ, Gao QT, et al. Chemical and biological assessment of a Chinese herbal decoction containing Radix Astragali and Radix Angelicae Sinensis: determination of drug ratio in having optimized properties. Journal of Agricultural and Food Chemistry. 2006;54(7):2767–2774. doi: 10.1021/jf053163l. [DOI] [PubMed] [Google Scholar]
- 13.Lv J, Zhao Z, Chen Y, et al. The Chinese herbal decoction Danggui Buxue Tang inhibits angiogenesis in a rat model of liver fibrosis. Evidence-Based Complementary and Alternative Medicine. 2012;2012:11 pages. doi: 10.1155/2012/284963.284963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 15.Liu B-S, Yao C-H, Chen Y-S, Hsu S-H. In vitro evaluation of degradation and cytotoxicity of a novel composite as a bone substitute. Journal of Biomedical Materials Research A. 2003;67(4):1163–1169. doi: 10.1002/jbm.a.20017. [DOI] [PubMed] [Google Scholar]
- 16.Hoemann CD, El-Gabalawy H, McKee MD. In vitro osteogenesis assays: influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathologie Biologie. 2009;57(4):318–323. doi: 10.1016/j.patbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Chang Y-M, Shih Y-T, Chen Y-S. Schwann cell migration induced by earthworm extract via activation of PAs and MMP2/9 mediated through ERK1/2 and p38. Evidence-Based Complementary and Alternative Medicine. 2011;2011:12 pages. doi: 10.1093/ecam/nep131.395458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi N, Akatsu T, Udagawa N, et al. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123(5):2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- 19.Yao C-H, Tsai H-M, Chen Y-S, Liu B-S. Fabrication and evaluation of a new composite composed of tricalcium phosphate, gelatin, and Chinese medicine as a bone substitute. Journal of Biomedical Materials Research B: Applied Biomaterials. 2005;75(2):277–288. doi: 10.1002/jbm.b.30294. [DOI] [PubMed] [Google Scholar]
- 20.Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcified Tissue International. 1982;34(1):285–290. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- 21.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiology and Molecular Biology Reviews. 2004;68(2):320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith R. Collagen and disorders of bone. Clinical Science. 1980;59(4):215–223. doi: 10.1042/cs0590215. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Ahmad M, Gronowicz G. Effects of transforming growth factor-beta 1 (TGF-β1) on in vitro mineralization of human osteoblasts on implant materials. Biomaterials. 2003;24(12):2013–2020. doi: 10.1016/s0142-9612(02)00616-6. [DOI] [PubMed] [Google Scholar]


