Abstract
Valuable stimuli are invariably localized in space. While our knowledge regarding the neural networks supporting value assignment and comparisons is considerable, we lack a basic understanding of how the human brain integrates motivational and spatial information. The amygdala is a key structure for learning and maintaining the value of sensory stimuli and a recent non-human primate study provided initial evidence that it also acts to integrate value with spatial location, a question we address here in a human setting. We measured haemodynamic responses (fMRI) in amygdala while manipulating the value and spatial configuration of stimuli in a simple stimulus–reward task. Subjects responded significantly faster and showed greater amygdala activation when a reward was dependent on a spatial specific response, compared to when a reward required less spatial specificity. Supplemental analysis supported this spatial specificity by demonstrating that the pattern of amygdala activity varied based on whether subjects responded to a motivational target presented in the ipsilateral or contralateral visual space. Our data show that the human amygdala integrates information about space and value, an integration of likely importance for assigning cognitive resources towards highly valuable stimuli in our environment.
Keywords: Amygdala, Anterior cingulate cortex, Value, Emotion, Spatial coding, fMRI
Highlights
-
•
Amygdala responds to valuable stimuli in a spatial specific manner.
-
•
Amygdala–dACC connectivity varies according to the spatial location of value cues.
-
•
Amygdala integrates information about stimulus value and its spatial representation.
-
•
Dorsal ACC may supply information about spatial location to the amygdala.
Introduction
Fundamental for approach and avoidance behaviour is the need to localize value in space. The amygdala is a structure widely implicated in encoding the value of stimuli (Jenison et al., 2011; Morrison and Salzman, 2010; Paton et al., 2006). Electrophysiological recordings in nonhuman primates show how the amygdala contains neurons with sustained preferences for positive or negative affective value, a value signal that is also related to the animal's approach or avoidance behaviour (Paton et al., 2006). Though such influence on behaviour could be the result of a general arousal state mediated by the amygdala (Davis and Whalen, 2001), this is contradicted by demonstration of amygdala activity linking spatial and motivational representations (Peck et al., 2013).
Amygdala is not the only brain region involved in assigning and updating stimulus value. A growing literature provides a complex picture of brain regions that contribute to value encoding (Clithero and Rangel, 2013; Rangel and Hare, 2010) and several of these brain areas also serve other cognitive and emotional functions (Barrett and Satpute, 2013). However, in order to act upon valuable stimuli, it is essential to localize them in space. While regions such as orbitofrontal cortex (OFC) and ventral striatum both carry value related signals (Kable and Glimcher, 2009), it is also the case that they show low or even absent spatial selectivity (Lansink et al., 2012; Padoa-Schioppa and Assad, 2006). Interestingly, both of these regions share close anatomical connections with the amygdala (Haber and Knutson, 2010), with dynamic and complex relationships between these areas characterizing a range of value-guided decisions and behaviours (Barberini et al., 2012; Morrison et al., 2011).
The integration of stimulus value with its spatial configuration in the human amygdala remains little investigated. While functional neuroimaging studies (Basten et al., 2010; De Martino et al., 2006; Gottfried et al., 2003; Ousdal et al., 2012) and electrophysiological recordings (Belova et al., 2008; Paton et al., 2006; Schoenbaum et al., 2003) implicate the amygdala in encoding the value of stimuli, there has been little exploration of whether it is important for localizing motivational stimuli in space. It is of interest that patients with isolated amygdala lesions do support sensitivity to spatial information. For instance, patient SM who has bilateral amygdala lesions and difficulties recognizing fearful facial expressions, shows a resolution of this deficit when her attention is directed towards the informative eye region of the faces, suggesting a spatial attention aspect to her deficit (Adolphs et al., 2005). Moreover, humans detect emotional images or words faster than their neutral counterparts (Anderson, 2005; Eastwood et al., 2001; Fox, 2002), and locations previously associated with highly valuable events interfere with the search for present targets (Anderson et al., 2011). In addition, though many studies report amygdala activity in response to passive obtainment of rewards, active responding for a reward in space appears to yield significantly greater BOLD amygdala response then is the case for passive receipt (Elliott et al., 2004).
If indeed information about stimulus' value and its spatial location converge in the human amygdala, then this begs questions as to the origin of the spatial information. Both the dorsal visual stream (Ungerleider and Haxby, 1994) and the lateral (i.e. ventrolateral and dorsolateral) prefrontal cortex (Corbetta and Shulman, 2002) contain neurons sensitive to object localisation. However, both of these brain areas have few connections to the amygdala (Freese and Amaral, 2009). Another possibility is that the dorsal anterior cingulate cortex (dACC), which conjointly encodes spatial attention and reward value (Kaping et al., 2011), and shares bilateral connections with the amygdala (Beckmann et al., 2009; Ghashghaei et al., 2007), might provide the necessary spatial information.
We investigated the spatial sensitivity of a value signal in amygdala during a simple stimulus–reward task in which outcome value and the motor response requirements were kept constant, while the spatial specificity of the reward varied. We hypothesized that amygdala would encode value in space, with the greatest activity manifest when these two stimulus' attributes had to be integrated in order for optimal decisions. Furthermore, to address the question of access to spatial information by the amygdala we tested whether the functional association between amygdala and dACC varied according to the spatial representation of a rewarding event.
Materials and methods
Subjects
Eighteen healthy subjects (mean age ± SD = 25 ± 6 years; 9 women) were included in the study after giving written informed consent. The study was approved by the Norwegian Regional Committees for Medical and Health Research Ethics. Prior to participation, subjects were screened to exclude somatic and psychiatric illness, substance abuse and MRI-incompatibility. All subjects received 300 Norwegian Kroner (kr) (150 kr for a screening interview and 150 kr for the fMRI session) for their participation and kept additional money won in the experiment described below.
fMRI paradigm
We created a new paradigm using visual numbers presented pairwise in an event-related design. Each trial consisted of a number pair presented for 800 ms, and subjects had to make their response while the numbers were presented on the screen. Trials that did not have a response within 800 ms were coded as “missed responses”. The order of trial presentation was randomized across subjects. Trials were separated by a jittered inter-trial interval lasting 5 ± 2 s. For each trial, purple numbers were presented in pairs on a black background. The numbers occurred horizontally to each other and were either similar or different in magnitude. The number value corresponded to an amount a subject could win in Norwegian Kroner (kr; 1 kr equals approx. 0.17 USD), and was either 0, 2 or 4 kr (see Fig. 1). The task for subjects was to press a response button corresponding to the amount of kr the subject wanted to obtain, which under the assumption that they wanted to maximise their gains corresponded to the highest number. Subjects were given verbal instructions prior to scanning and also performed a practice version of the task. During the practice, subjects completed one trial of each condition, and thus familiarized themselves with the visual appearance of the stimuli and the time limits for responding. Before the practice, subjects were told that they were free to respond however they preferred, but that one response should be given for each trial. The combination of two similar numbers (i.e. 2–2 or 4–4), called the non-spatial value condition, had no preferred response. In the spatial value condition the numbers differed, and one response was more rewarding than the other (the one with the highest number). The number pairs in spatial value trials always consisted of a zero paired with a valuable stimulus (i.e. 4–0 or 2–0). The 0–0 condition provided a baseline. By creating these five conditions, we could independently manipulate reward magnitude and spatial specificity. We hypothesised that amygdala would activate more in trials requiring greater spatial specificity for reward obtainment than trials with less necessity for localisation, despite equal outcome values. The use of hands was counterbalanced. Subjects were told that they could keep all the money earned in the experiment, but they would not receive any feedback during the task indicating the outcome of the trail or their overall earnings. Twenty trials of each condition were presented, with the total scan time lasting 12.2 min. The paradigm rationale is displayed in Fig. 1.
Fig. 1.
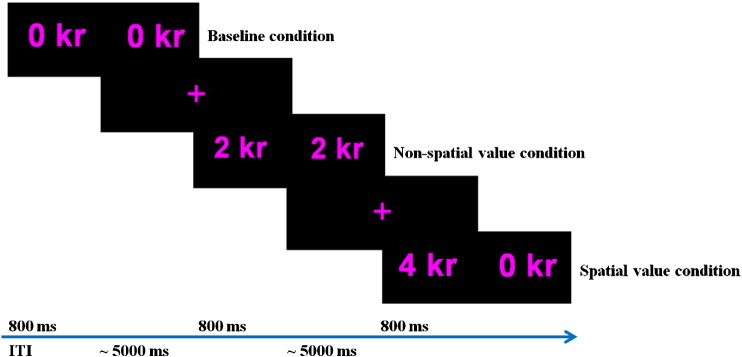
The paradigm rationale. Pairs of numbers were presented horizontal to each other. The numbers corresponded to the amount the subject could win in Norwegian kroner (kr). Each pair was made up of either two similar numbers (i.e. 2–2 or 4–4) or a zero paired with a valuable stimulus (i.e. 2–0, 0–2, 4–0, 0–4). The 0–0 condition was a baseline. The task was to press the response button corresponding to the amount of kr the subject wanted to obtain.
Apparatus
E-prime software (Psychology Software Tools, Inc.; Pittsburgh, PA, USA) was used to program and control the experiment. In the scanner, stimuli were presented through VisualSystem goggles (NordicNeuroLab, Bergen, Norway) and responses were collected by ResponseGrip (NordicNeuroLab, Bergen, Norway).
Image acquisition
Whole-brain, T2*-weighted, echo-planar images (TR = 2000 ms, TE = 25 ms, flip angle 90°, 260 mm × 260 mm field of view, 64 × 64 matrix) were acquired using a 3T scanner (General Electric Company; Milwaukee, WI, USA) supplied with a standard eight-channel head coil. A total of 272 volumes were acquired, and the first 3 volumes were discarded as dummies to allow for magnetization equilibrium. Each volume consisted of 36 slices acquired parallel to the AC–PC plane (sequential acquisition; 3.5 mm thick with a 0.5 mm gap). For anatomical comparison purposes, FSPGR T1-weighted anatomical images (TR = 7.7 ms, TE = 3.0 ms, flip angle 12°) were acquired prior to the functional imaging.
Behavioural data analysis
The behavioural data was analysed using SPSS (Statistical Package for Social Sciences 16.0. SPSS Inc., Chicago, USA). To test for differences in response time or response accuracy between the non-spatial and spatial value conditions of the experimental task, paired-sample t-tests were performed. In addition, paired-sample t-tests were used to investigate differences in response times or accuracy as a function of increasing reward magnitude (i.e. (4–0 + 0–4 + 4–4) > (2–0 + 0–2 + 2–2)) and the various response hands.
fMRI data analysis
Prior to analysis, the functional images were visually inspected for artefacts, extreme variance and signal dropout in the amygdala. None of the subjects had to be excluded due to artefacts or signal dropout. Data preprocessing and analysis were conducted using SPM8 software package (http://www.fil.ion.ucl.ac.uk/spm). All volumes were unwarped and realigned to the first volume (Friston et al., 1995) and the mean functional and anatomical images were co-registered. No participants moved more than 3 mm in any direction. The images were then spatially normalized to a standard EPI template based upon the Montreal Neurological Institute (MNI) reference brain (Evans et al., 1992), and resampled to a voxel size of 3 × 3 × 3 mm. The images were smoothed using an 8 mm full width — half maximum (FWHM) Gaussian isotropic kernel. Data were high-pass filtered using a cut-off value of 128 s and corrected for auto-correlation between scans.
The primary aim of the neuroimaging analysis was to investigate if the human amygdala activity reflected variations in outcome value per se or to the integration of value in space. Further, we wanted to investigate if the value signal was associated with spatial configuration selectivity, i.e. if the amygdala differentiated between positively valued stimuli occurring in the same or the opposite visual hemifield. We built a model by convolving stick functions for the onsets of stimuli presentation with a canonical haemodynamic response function (HRF). Trials were sorted into five types, reflecting the spatial specificity of reward presentation and the response hand usage. The five trial types were “right response in non-spatial value trials” (i.e. (2–2 + 4–4)right response), “right response in spatial value trials” (i.e. (0–2 + 0–4)), “left response in non-spatial value trials” (i.e. (2–2 + 4–4)left response), “left response in spatial value trials” (i.e. (2–0 + 4–0)) and the baseline (i.e. 0–0). In addition, head movement parameters derived from spatial realignment during the image preprocessing and wrong responses, were added as covariates of no interest. A general linear model (GLM) was used to estimate parameters of activity for each participant across the whole brain for each experimental condition compared to baseline. The individual contrast images were entered into a 2 × 2 ANOVA analysis separating the main effect of spatial specificity and response hand, in addition to their interaction. Crucially, the interaction was balanced with respect to spatial specificity and response hand usage. Consequently, activations identified in the interaction, were not attributable to the effect of spatial specificity or response hand alone.
To elaborate on the spatial selectivity of amygdala responses, we contrasted trials in which a left (or right) hand response was cued with trials in which a left (or right) hand response was not cued, i.e. (2–0 + 4–0) > (2–2 + 4–4)left response. This contrast was balanced according to response hand, but it differed with respect to the spatial configuration of the value cues. Electrophysiological recordings in primate amygdala indicate that though amygdala activity is modulated by the value of both ipsilateral and contralateral stimuli, the response to contralateral rewarding cues is stronger and with shorter latencies (Peck et al., 2013). Based on this, we expected trials with value cues presented in the left hemifield alone; to be dominated by right amygdala responses and vice versa, compared to when the value cues appeared in both hemifields. Critically, we also contrasted the spatial value trials per se according to the required response hand (i.e. (0–2 + 0–4) > (2–0 + 4–0)). The last contrast only differed with respect to the hemispheric appearance of the value cue, and thus any significant amygdala responses should reflect variations in their spatial representation.
Due to the fact that some of the subjects were extreme right- or left-lateralized (i.e. responding mainly with their right or left hand) during non-spatial value trials, we also created a model where the non-spatial value trials were pseudo-randomly distributed between two conditions, each balanced according to reward magnitude (i.e. containing equal numbers of 2–2 or 4–4 trials). We replicated the “spatial value trials > non-spatial value trials” using this model. Finally, to assess outcome value processing, we investigated the effect of reward obtainment per se (i.e. 4–4 > 0–0), and parametric effects of increasing stimulus value (i.e. 0–0/2–2/4–4) on amygdala activity. To avoid any effects related to response hand usage, only right hand response trials were used.
To correct for multiple comparisons, whole-brain family-wise error (FWE) correction was used (FWE < 0.05, k > 10 voxels). In addition, as we had an a-priori hypothesis regarding the amygdala, small volume correction based on anatomically defined bilateral amygdala region of interests (ROIs) and FWE corrected p-values were used. The anatomically defined ROIs were created using the SPM Wake Forest University (WFU) Pickatlas toolbox (http://www.fmri.wfubmc.edu/cms/software, version 2.3) (Maldjian et al., 2003).
Psychophysiological interaction (PPI) analysis
To investigate if functional connectivity of the amygdala differed based on whether the subject responded to a spatial valuable stimulus appearing in the ipsi- or the contralateral visual hemifield, we implemented a generalized psychophysiological interaction (gPPI) analysis. We created a gPPI analysis (gPPI toolbox; http://www.nitrc.org/projects/gppi) with the left amygdala as a seed region, and investigated if the connectivity of left amygdala varied when making spatial cued right as compared to left hand responses (i.e. (0–2 + 0–4) > (2–0 + 4–0)). Only left amygdala was used as right amygdala displayed no significant responses in the second level analysis. For each subject¸ mean corrected activity was extracted from volumes of interest (first eigenvariate from the activated voxels within the anatomically (WFU PickAtlas (Maldjian et al., 2003)) defined left amygdala). The individual BOLD signal of the seed region was deconvolved to obtain an estimate of the underlying neuronal activity. Subsequently, the estimated neuronal activity from the seed region was multiplied with regressors modelling all task effects and then reconvolved with the canonical HRF. Hence, the gPPI GLM includes a psychophysiological regressor for all conditions (McLaren et al., 2012). Based on the proposal by Peck et al. (2013), we were interested in the dACC, as this region might be an input for spatial information to the amygdala. Thus, the aim was to test for differences in regression slopes between right and left hemispheric cued trials as a measure of difference in regional connectivity (i.e. between seed region and other areas). To test for interaction effects, individual GLMs containing both the PPI and the task regressors, in addition to six motion regressors and the mean time course of the seed region were created. The individual t-contrast images of the interaction gained from the gPPI were then entered into a random effects one-sample t-test. As we had a-priori hypothesis for the dACC, small volume correction using anatomically defined bilateral dACC (WFU Pickatlas toolbox (Maldjian et al., 2003)) was performed.
Results
Behavioural results
In order to be included in the analyses, accuracy in all the experimental conditions had to be above 50%. None of the subjects had to be excluded due to behavioural performance not meeting this criterion (total accuracy: 94.95 ± 8.05%). However, four of the subjects were strongly biased to respond with their left or the right hand (i.e. extreme left- or right lateralized) during the 2–2 or 4–4 trials, and were therefore excluded from the 2 × 2 ANOVA analysis. The response time and accuracy for the spatial and non-spatial value conditions are presented in Table 1.
Table 1.
Accuracy and response time by conditions in the stimulus-reward task.
| Mean response time (ms) | Median response time (ms) | Accuracy (%) | |
|---|---|---|---|
| Spatial value condition | 439 ± 72 | 442 | 91.7 ± 12.1a |
| Non-spatial value condition | 453 ± 84 | 473 | 98.2 ± 4.0 |
Subjects chose the 0 kr in 6.9% of all spatial value trials. 1.4% of all the spatial value trials were missed responses.
Paired-sample t-tests revealed a significant shorter response time in the spatial compared to the non-spatial value condition (t = 2.48, p = 0.02). There was a trend for a significant difference in accuracy between these two conditions (t = 2.09, p = 0.05), suggesting that the improved response times in spatial trials were at the expense of reduced accuracy of responses. No difference in response time (t = 0.94, p = n.s.) or accuracy (t = 1.43, p = n.s.) was found for increasing reward magnitude or between the two response hands (accuracy; t = 0.72, p = n.s., reaction times; t = 0.07, p = n.s.) for the value trials.
Imaging results
ANOVA
Fourteen subjects were included in the final 2 × 2 ANOVA analysis. The results revealed trend significant right amygdala activity in response to the main effect of spatial specific values (right amygdala peak voxel; x = 18, y = − 4, z = − 17, F = 8.11, psvc = 0.06), attributable to significant greater amygdala responses for spatial as compared to non-spatial value trials (right amygdala peak voxel; x = 18, y = − 4, z = − 17, Z = 2.73, psvc = 0.03). In addition, there was a main effect of response hand on left amygdala activity (left amygdala peak voxel; x = − 21, y = − 4, z = − 14, F = 10.87, psvc = 0.02), attributable to significantly enhanced left amygdala activity when making a right compared to a left hand value response (left amygdala peak voxel; x = − 21, y = − 4, z = − 14, Z = 3.13, psvc = 0.01). There was no interaction between spatial coding of value and response hand usage. Additional post-hoc t-tests demonstrated a significantly greater right amygdala response when making a spatially coded left hand response as compared to when a left response was chosen without spatial cuing (right amygdala peak voxel; x = 21, y = − 4, z = − 14, Z = 2.60, psvc < 0.05). Equivalently, the same contrast for right hand responses revealed bilateral significant amygdala responses without any clear lateralization effect (right amygdala peak voxel; x = 21, y = − 4, z = − 26, Z = 2.59, psvc < 0.05, left amygdala peak voxel; x = − 30, y = − 4, z = − 23, Z= 2.72, psvc = 0.03). Crucially, the contrast (0–2 + 0–4) > (2–0 + 4–0) yielded significant responses in left amygdala alone (left amygdala peak voxel: x = − 18, y = − 4, z = − 14, Z = 3.39, psvc = 0.006) supporting a spatial specificity of amygdala responses. The opposite contrast did not yield significant amygdala responses.
To confirm the effect of spatial coding in amygdala, we created an additional model in which the left and right spatial value trials were compared to the non-spatial trials without controlling for response hand, but balancing the contrast according to reward magnitude. None of the subjects had to be excluded from this model. The model revealed bilateral amygdala responses for the contrast spatial value trials versus non-spatial value trials (right amygdala peak voxel; x = 21, y = − 4, z = − 23, Z = 3.70, psvc = 0.002, left amygdala peak voxel; x = − 18, y = − 7, z = − 20, Z = 3.13, psvc = 0.01) (Fig. 2). There was a significant response in right amygdala to reward obtainment per se (right amygdala peak voxel x = 21, y = 2, z = − 23, Z = 2.97, psvc = 0.02), but no parametric effects of increasing stimulus value, not even with a more lenient threshold (i.e. p = 0.05, uncorrected).
Fig. 2.
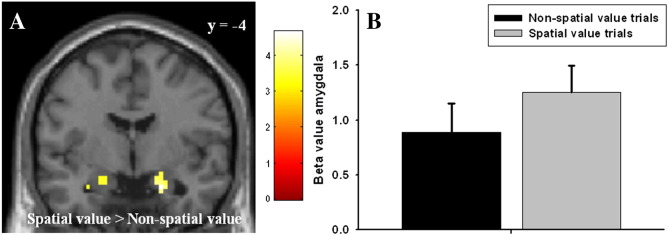
BOLD fMRI responses in the amygdala obtained for the contrast “spatial value trials” > “non-spatial value trials”. (A) Statistical parametric maps (SPM) demonstrating the responses in amygdala for the given contrast. The image is small volume corrected (PFWE). The colours refer to t-values as coded in the bar to the right of the image. (B) Beta values for the peak voxel in right amygdala (x = 21, y = − 4, z = − 23) for the conditions “spatial value trials” and “non-spatial value trials” illustrating the effect sizes.
Psychophysiological interaction (PPI) analysis
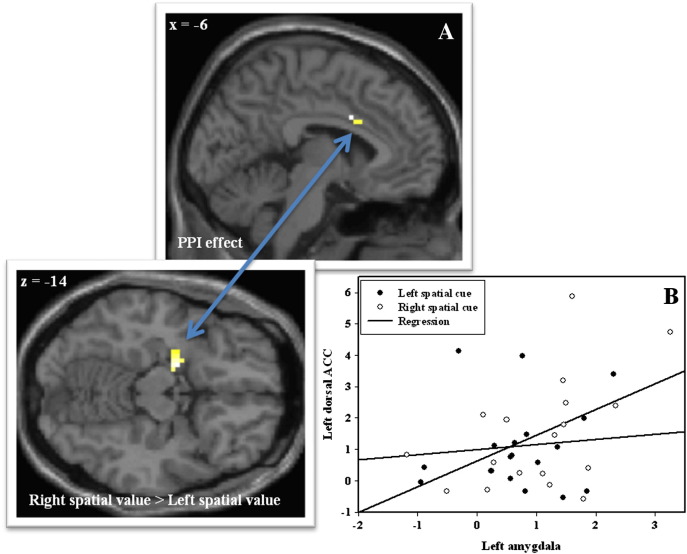
A PPI-analysis with left amygdala as seed region revealed, as predicted, a significantly increased connectivity with left dACC (left dACC peak voxel; x = − 6, y = 8, z = 31, Z = 3.20, psvc = 0.02) and a trend significant connectivity with right dACC (right dACC peak voxel; x = 6, y = 8, z = 31, Z = 2.68, psvc = 0.06) when responding to right as compared to left cued value stimuli (see Fig. 3).
Fig. 3.
The results of the generalized psychophysiological interaction analysis. (A) Statistical parametric map (SPM) showing the cluster in left dorsal anterior cingulate cortex that showed condition-specific BOLD signal changes with left amygdala activity. The SPM below illustrates the significant cluster in left amygdala that showed greater activity towards spatial value cues requiring right as compared to left responses (i.e. (0–2 + 0–4) > (2–0 + 4–0)). Both images are small volume corrected (PFWE). (B) Scatter plot with regression lines demonstrating the pattern of functional connectivity. The x-axis represents activity in the left amygdala (beta values) cluster and the y-axis represents activity in the left dorsal anterior cingulate cluster (beta values). In the right spatial value condition there was a positive correlation between left amygdala and left dorsal anterior cingulate cortex activity (r = 0.48, p = 0.04). However, in the left spatial value condition, no correlation appeared (r = 0.10, p = n.s.).
Discussion
The main finding of this study was that amygdala activity did not only reflect variations in reward magnitude, but also reflected an association of stimulus value and its spatial configuration. The idea that amygdala encodes or calculates value is not new (Paton et al., 2006). Further, there is a growing literature in animals and humans indicating that the amygdala generates a value signal during decision making, and that this value signal may subsequently inform decision processes in downstream brain structures (Hampton et al., 2007; Park et al., 2011; Schoenbaum et al., 2003; Zeeb and Winstanley, 2013). However, the possibility that the amygdala combines information about stimulus value with its spatial properties has only been shown in a single non-human primate study (Peck et al., 2013). Our demonstration here of such an effect in humans extends the role of amygdala in reward to include an integration of spatial location with reward value. Furthermore, variation in functional connectivity between amygdala and dACC dependent on the spatial representation of valuable cues provides a possible source by which the amygdala might access the position of highly valuable events.
Spatial value trials required subjects to localize the more valuable of two simultaneously presented stimuli, and respond accordingly. Our finding that amygdala activity differentiated trials of equal outcome value based on the spatial configuration of the reward-predictive stimuli, supports the notion that amygdala integrates value and space. Critically, the comparison of spatial value trials that only differed with respect to the hemispheric presentation of the value cue, confirmed this finding. To the best of our knowledge, this is the first study demonstrating that amygdala encodes spatial attributes of motivational stimuli in humans, though a number of behavioural studies in humans indirectly support this notion. For example, subjects detect emotional faces more readily (Frischen et al., 2008) and recognize emotional objects (Ohman et al., 2001) faster than neutral targets in visual search tasks, and this emotional advantage is reduced in subjects with amygdala lesions (Anderson and Phelps, 2001). In addition, the presence of task-irrelevant positive or negative valued distracter stimuli (Anderson et al., 2011; Vuilleumier et al., 2001) slows the target detection, perhaps reflecting the value-driven capture of attention. Indeed, attentional capture by high-value distracters occurs in a spatially specific manner, so that response times are slower if the target appears in a location formerly occupied by the high-value distracter compared to when the distracter appeared in another location (Anderson et al., 2011). This hints that amygdala acts to associate the emotional or rewarding value of stimuli with their spatial location, allowing them to be acted upon more easily than if spatial information was not encoded.
The possibility of spatial coding in the amygdala was directly tested in a study where amygdala neural activity and behaviour of primates were predicted by the association of stimulus value and its spatial configuration (Peck et al., 2013). Importantly, there was a systematic relationship between the valence and the location of the stimuli, so that neurons responding with a signal increase to the presence of a reward, demonstrated the largest increase when the reward occurred in the contralateral visual hemifield. By contrast, neurons preferentially responding to the absence of reward showed a response decrease, and this was more pronounced in trials where the reward occurred in the contralateral hemifield. In keeping with Peck et al. (2013), we observed a main effect of response hand in left amygdala in the present ANOVA analysis attributable to increased left amygdala activity when responding to valuable stimuli occurring in the right, as compared to the left, visual field. Further, the comparison of trials in which the value cue was presented in the right hemifield with trials in which it occurred on the left side, yielded significant responses in the left amygdala alone. To the best of our knowledge, this is the first human study investigating whether value responsive neurons in the human amygdala show spatial selectivity. However, we note prior indirect support for this notion comes from a study where targets appearing at a previously emotional cued left visual hemifield location resulted in enhanced right amygdala responses, as well as faster response times, compared to when neutral cues preceded the targets (Noesselt et al., 2005). Furthermore, Palminteri and colleagues observed that activity in ventromedial prefrontal cortex tracked the value of contralateral options in an instrumental learning task (Palminteri et al., 2009), with the amygdala potentially serving as an input region through intrahemispheric connections (Carmichael and Price, 1995).
In line with the study by Peck and colleagues (Peck et al., 2013), we investigated how the spatial configuration of two valuable cues affected the allocation of cognitive resources and subsequent behaviours. Despite differences in task design, both studies show that the paired presentation of a high and low value cue biases attention towards the high valuable item, resulting in shorter response latencies compared to when two equally valuable cues were presented simultaneously. Furthermore, both tasks also assess spatial selectivity at the level of visual hemifield, demonstrating comparable spatial sensitivity in the human and primate amygdala. However, while the valuable cues were separated from the decision phase in the Peck et al. study, the high and low valuable cues served as decision variables in the present task. This was motivated by evidence that the amygdala may be important not only for the valuation phase of economic decisions, but also for choice per se (Grabenhorst et al., 2012). Hence, the present findings of a spatial reward representation in the amygdala at choice points support a role for the amygdala in behavioural guidance beyond the evaluation of choice options.
If the amygdala encodes value in space, one outstanding question is how this representation of space and value is created. One possibility is that the amygdala receives spatial information from the brain areas known to contain neurons with high spatial specificity. Neurons in the dACC in monkeys are sensitive to the spatial location of attentional targets (Kaping et al., 2011), and may be essential for linking reward-related information to action (Hayden and Platt, 2010; Williams et al., 2004). Further, the amygdala and dACC share reciprocal connections (Beckmann et al., 2009; Ghashghaei et al., 2007), and their interaction is essential for adaptive aversive learning (Klavir et al., 2013). Thus, the dACC was suggested as a candidate region by which amygdala inherits spatial information (Peck et al., 2013). We show that left amygdala activity covaried more strongly with left dACC for right as compared to left cued value trials. There are at least two possible interpretations of this finding. One interpretation is that amygdala receives spatial information from the dACC, in line with the spatial modulation present in this region (Kaping et al., 2011). Alternatively, another independent set of brain areas code locations, and further modulates the amygdala–dACC projections accordingly. Spatial selective areas like the frontal eye field, parietal cortex or the dorsal striatum share few or none connections with the amygdala (Freese and Amaral, 2009), which would require indirect transitions across several synapses. Consequently, such a transition would be slow, and not benefit an amygdala based orienting of attention. Although the current data doesn't allow us to exclude this last interpretation, reciprocal amygdala–dACC connections (Beckmann et al., 2009; Ghashghaei et al., 2007) together with the observed spatial coding in dACC (Kaping et al., 2011), favour the first interpretation.
Our decisions may reflect a choice between stimuli predicting various rewards or between actions necessary to obtain a rewarded outcome (O'Doherty, 2011; Rangel and Hare, 2010). In order to choose between actions, the associated reward value yielded by an action (i.e. action value) has to be computed. It is possible that the spatial value trials were more cognitive demanding than the non-spatial value trials, as the former entailed a greater response specificity to obtain the reward. This might result in greater action costs for the spatial value trials, with a concomitant decrease in action values compared to non-spatial value trials. Though we cannot exclude that the present results reflect differences in action values or cognitive demands between the two conditions, two of our findings argue against such an interpretation. Increased cognitive effort is associated with slower response times (Lavie et al., 2004), but we found that subjects responded faster to the more challenging spatial value as compared to non-spatial value trials. Furthermore, we also contrasted spatial value trials that only differed in the spatial configuration of the value cues, and this contrast was balanced according to cognitive effort or action values. Finally, we note that previous studies have failed to demonstrate an action value signal in the amygdala, at least with the spatial selectivity presented here (Croxson et al., 2009; FitzGerald et al., 2012; Wunderlich et al., 2009).
While primate electrophysiology studies consistently report that amygdala encodes a value signal related to the amount of positive and negative reinforcements (Morrison and Salzman, 2010), findings in humans are inconsistent (Clithero and Rangel, 2013). While we could show amygdala activity in response to reward obtainment per se, we failed to find linear effects of increasing reward magnitude. This contrasts with other studies that report amygdala activity as reflecting reward magnitude at the time of choice (Bermudez and Schultz, 2010; Smith et al., 2009). There are several possible reasons for this apparent discrepancy between our study and the primate electrophysiology literature. First, the spatial resolution of fMRI does not allow separation of positive and negative value neurons, and thus, represent a sum across various neuronal subtypes. Secondly, amygdala activity is also sensitive to stimulus' salience or ambiguity (Pessoa, 2010), which can explain contradictory high responses to low value events. Finally, it is possible that the increase from 2 to 4 kr was not experienced as a significant increase in reward magnitude or subjective utility by the participant. Both represent relatively modest rewards, and thus a greater difference in reward magnitude might be necessary to detect such effects. Based on the notion that reward obtainment was deterministic (and not probabilistic) during the task, and that spatial and non-spatial value trials led to equivalent outcomes, we do not think that the results represent variations in arousal. Especially, choosing the zero in spatial value trials did not lead to punishment, but instead hindered the subject from obtaining the maximum reward.
The finding that amygdala responds in a manner compatible with spatial encoding of value provides a new avenue for studies of amygdala in attentional processes. The amygdala is postulated to play a crucial role in the enhanced perception of, and attention to, highly valuable stimuli due to its intimate connections with the ventral visual stream (Vuilleumier, 2005; Vuilleumier et al., 2004). However, recent studies point to a role for amygdala in guiding frontoparietal attention network as well, potentially mediated through an amygdala–cingulate cortex pathway (Mohanty and Sussman, 2013). Indirect support comes from studies demonstrating that this spatial attention network interacts with amygdala during a search for motivational stimuli (Mohanty et al., 2008, 2009), and that dACC–amygdala interactions are necessary for successful avoidance of punishments (Klavir et al., 2013). Though it is possible that the amygdala enhances processing of highly valuable events in general, directing attention towards the location of emotional relevant events would be more efficient in promoting fast behavioural responses. Moreover, the number of brain areas linked to computation of stimulus value at the time of choice is vast (Kable and Glimcher, 2009; Rangel and Hare, 2010; Rushworth et al., 2011), and unsurprisingly several of these areas are also implicated in emotion and reward (Barrett and Satpute, 2013). Of particular interest is the OFC, a core region for computation of stimulus value tied to the guidance of decisions (Rushworth et al., 2011). The OFC shares close anatomical connections with the amygdala, and interactions between these regions are essential for learning and its behavioural expression in both aversive and appetitive settings (Morrison et al., 2011). Interestingly, though OFC neurons compute value, they do not encode the spatial location of highly valuable events (Padoa-Schioppa and Assad, 2006). Thus, the observation of a unique integration of value and space in the amygdala may help delineate the various contributions of these brain areas, ultimately improving our understanding of how value guided decisions and behaviour are coded in the brain.
In summary, our results show that amygdala integrates information about space and value, with each amygdala contributing to the efficient location of relevant events. In this frame of reference impaired amygdala function could contribute to deficits in spatial specific cognitive processes, like directing attention towards the location of emotional cues, or acting in relation to the location of valuable events.
Acknowledgments
This work was supported by grants from the KG Jebsen Foundation, the Research Council of Norway (#167153/V50, #163070/V50), South East Norway Health Authority (#39386/6051) and Wellcome Trust (098362/Z/12/Z). The authors would like to thank the NORMENT, KG Jebsen Centre for Psychosis Research study group and Anne Hilde Farstad for assisting with data collection.
References
- Adolphs R., Gosselin F., Buchanan T.W., Tranel D., Schyns P., Damasio A.R. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Anderson A.K. Affective influences on the attentional dynamics supporting awareness. J. Exp. Psychol. Gen. 2005;134:258–281. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- Anderson A.K., Phelps E.A. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Anderson B.A., Laurent P.A., Yantis S. Value-driven attentional capture. Proc. Natl. Acad. Sci. U. S. A. 2011;108:10367–10371. doi: 10.1073/pnas.1104047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberini C.L., Morrison S.E., Saez A., Lau B., Salzman C.D. Complexity and competition in appetitive and aversive neural circuits. Front. Neurosci. 2012;6:170. doi: 10.3389/fnins.2012.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett L.F., Satpute A.B. Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr. Opin. Neurobiol. 2013;23:361–372. doi: 10.1016/j.conb.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten U., Biele G., Heekeren H.R., Fiebach C.J. How the brain integrates costs and benefits during decision making. Proc. Natl. Acad. Sci. U. S. A. 2010;107:21767–21772. doi: 10.1073/pnas.0908104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M., Johansen-Berg H., Rushworth M.F. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J. Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belova M.A., Paton J.J., Salzman C.D. Moment-to-moment tracking of state value in the amygdala. J. Neurosci. 2008;28:10023–10030. doi: 10.1523/JNEUROSCI.1400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez M.A., Schultz W. Reward magnitude coding in primate amygdala neurons. J. Neurophysiol. 2010;104:3424–3432. doi: 10.1152/jn.00540.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael S.T., Price J.L. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Clithero J.A., Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Soc. Cogn. Affect. Neurosci. 2013 doi: 10.1093/scan/nst106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Croxson P.L., Walton M.E., O'Reilly J.X., Behrens T.E., Rushworth M.F. Effort-based cost-benefit valuation and the human brain. J. Neurosci. 2009;29:4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- De Martino B., Kumaran D., Seymour B., Dolan R.J. Frames, biases, and rational decision-making in the human brain. Science. 2006;313:684–687. doi: 10.1126/science.1128356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood J.D., Smilek D., Merikle P.M. Differential attentional guidance by unattended faces expressing positive and negative emotion. Percept. Psychophys. 2001;63:1004–1013. doi: 10.3758/bf03194519. [DOI] [PubMed] [Google Scholar]
- Elliott R., Newman J.L., Longe O.A., William Deakin J.F. Instrumental responding for rewards is associated with enhanced neuronal response in subcortical reward systems. Neuroimage. 2004;21:984–990. doi: 10.1016/j.neuroimage.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Evans A.C., Marrett S., Neelin P., Collins L., Worsley K., Dai W., Milot S., Meyer E., Bub D. Anatomical mapping of functional activation in stereotactic coordinate space. Neuroimage. 1992;1:43–53. doi: 10.1016/1053-8119(92)90006-9. [DOI] [PubMed] [Google Scholar]
- FitzGerald T.H., Friston K.J., Dolan R.J. Action-specific value signals in reward-related regions of the human brain. J. Neurosci. 2012;32:16417–16423a. doi: 10.1523/JNEUROSCI.3254-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E. Processing emotional facial expressions: the role of anxiety and awareness. Cogn Affect Behav Neurosci. 2002;2:52–63. doi: 10.3758/cabn.2.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese J.L., Amaral D.G. In: The Human Amygdala. Whalen P.J., Phelps E.A., editors. Guilford Press; 2009. pp. 3–42. (Ch. 1) [Google Scholar]
- Frischen A., Eastwood J.D., Smilek D. Visual search for faces with emotional expressions. Psychol. Bull. 2008;134:662–676. doi: 10.1037/0033-2909.134.5.662. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Poline J.B., Grasby P.J., Williams S.C., Frackowiak R.S., Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H.T., Hilgetag C.C., Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried J.A., O'Doherty J., Dolan R.J. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F., Hernadi I., Schultz W. Prediction of economic choice by primate amygdala neurons. Proc. Natl. Acad. Sci. U. S. A. 2012;109:18950–18955. doi: 10.1073/pnas.1212706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton A.N., Adolphs R., Tyszka M.J., O'Doherty J.P. Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron. 2007;55:545–555. doi: 10.1016/j.neuron.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Hayden B.Y., Platt M.L. Neurons in anterior cingulate cortex multiplex information about reward and action. J. Neurosci. 2010;30:3339–3346. doi: 10.1523/JNEUROSCI.4874-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenison R.L., Rangel A., Oya H., Kawasaki H., Howard M.A. Value encoding in single neurons in the human amygdala during decision making. J. Neurosci. 2011;31:331–338. doi: 10.1523/JNEUROSCI.4461-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable J.W., Glimcher P.W. The neurobiology of decision: consensus and controversy. Neuron. 2009;63:733–745. doi: 10.1016/j.neuron.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaping D., Vinck M., Hutchison R.M., Everling S., Womelsdorf T. Specific contributions of ventromedial, anterior cingulate, and lateral prefrontal cortex for attentional selection and stimulus valuation. PLoS Biol. 2011;9:e1001224. doi: 10.1371/journal.pbio.1001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavir O., Genud-Gabai R., Paz R. Functional connectivity between amygdala and cingulate cortex for adaptive aversive learning. Neuron. 2013;80:1290–1300. doi: 10.1016/j.neuron.2013.09.035. [DOI] [PubMed] [Google Scholar]
- Lansink C.S., Jackson J.C., Lankelma J.V., Ito R., Robbins T.W., Everitt B.J., Pennartz C.M. Reward cues in space: commonalities and differences in neural coding by hippocampal and ventral striatal ensembles. J. Neurosci. 2012;32:12444–12459. doi: 10.1523/JNEUROSCI.0593-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie N., Hirst A., de Fockert J.W., Viding E. Load theory of selective attention and cognitive control. J. Exp. Psychol. Gen. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A., Sussman T.J. Top-down modulation of attention by emotion. Front. Hum. Neurosci. 2013;7:102. doi: 10.3389/fnhum.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A., Gitelman D.R., Small D.M., Mesulam M.M. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cereb. Cortex. 2008;18:2604–2613. doi: 10.1093/cercor/bhn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A., Egner T., Monti J.M., Mesulam M.M. Search for a threatening target triggers limbic guidance of spatial attention. J. Neurosci. 2009;29:10563–10572. doi: 10.1523/JNEUROSCI.1170-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S.E., Salzman C.D. Re-valuing the amygdala. Curr. Opin. Neurobiol. 2010;20:221–230. doi: 10.1016/j.conb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S.E., Saez A., Lau B., Salzman C.D. Different time courses for learning-related changes in amygdala and orbitofrontal cortex. Neuron. 2011;71:1127–1140. doi: 10.1016/j.neuron.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noesselt T., Driver J., Heinze H.J., Dolan R. Asymmetrical activation in the human brain during processing of fearful faces. Curr. Biol. 2005;15:424–429. doi: 10.1016/j.cub.2004.12.075. [DOI] [PubMed] [Google Scholar]
- O'Doherty J.P. Contributions of the ventromedial prefrontal cortex to goal-directed action selection. Ann. N. Y. Acad. Sci. 2011;1239:118–129. doi: 10.1111/j.1749-6632.2011.06290.x. [DOI] [PubMed] [Google Scholar]
- Ohman A., Flykt A., Esteves F. Emotion drives attention: detecting the snake in the grass. J. Exp. Psychol. Gen. 2001;130:466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Ousdal O.T., Reckless G.E., Server A., Andreassen O.A., Jensen J. Effect of relevance on amygdala activation and association with the ventral striatum. Neuroimage. 2012;62:95–101. doi: 10.1016/j.neuroimage.2012.04.035. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C., Assad J.A. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palminteri S., Boraud T., Lafargue G., Dubois B., Pessiglione M. Brain hemispheres selectively track the expected value of contralateral options. J. Neurosci. 2009;29:13465–13472. doi: 10.1523/JNEUROSCI.1500-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Q., Kahnt T., Rieskamp J., Heekeren H.R. Neurobiology of value integration: when value impacts valuation. J. Neurosci. 2011;31:9307–9314. doi: 10.1523/JNEUROSCI.4973-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J.J., Belova M.A., Morrison S.E., Salzman C.D. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck C.J., Lau B., Salzman C.D. The primate amygdala combines information about space and value. Nat. Neurosci. 2013;16:340–348. doi: 10.1038/nn.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. Emotion and cognition and the amygdala: from “what is it?” to “what's to be done?”. Neuropsychologia. 2010;48:3416–3429. doi: 10.1016/j.neuropsychologia.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A., Hare T. Neural computations associated with goal-directed choice. Curr. Opin. Neurobiol. 2010;20:262–270. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Rushworth M.F., Noonan M.P., Boorman E.D., Walton M.E., Behrens T.E. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G., Setlow B., Saddoris M.P., Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Smith B.W., Mitchell D.G., Hardin M.G., Jazbec S., Fridberg D., Blair R.J., Ernst M. Neural substrates of reward magnitude, probability, and risk during a wheel of fortune decision-making task. Neuroimage. 2009;44:600–609. doi: 10.1016/j.neuroimage.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider L.G., Haxby J.V. ‘What’ and ‘where’ in the human brain. Curr. Opin. Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn. Sci. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Armony J.L., Driver J., Dolan R.J. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Richardson M.P., Armony J.L., Driver J., Dolan R.J. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat. Neurosci. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Williams Z.M., Bush G., Rauch S.L., Cosgrove G.R., Eskandar E.N. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat. Neurosci. 2004;7:1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- Wunderlich K., Rangel A., O'Doherty J.P. Neural computations underlying action-based decision making in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2009;106:17199–17204. doi: 10.1073/pnas.0901077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb F.D., Winstanley C.A. Functional disconnection of the orbitofrontal cortex and basolateral amygdala impairs acquisition of a rat gambling task and disrupts animals' ability to alter decision-making behavior after reinforcer devaluation. J. Neurosci. 2013;33:6434–6443. doi: 10.1523/JNEUROSCI.3971-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]