Abstract
Mercury (Hg) is neurotoxic, and children may be particularly susceptible to this effect. A current major challenge is identification of children who may be uniquely susceptible to Hg toxicity because of genetic predisposition. We examined the possibility that common genetic variants that are known to affect neurologic functions or Hg handling in adults would modify the adverse neurobehavioral effects of Hg exposure in children. Three hundred thirty subjects who participated as children in the recently completed Casa Pia Clinical Trial of Dental Amalgams in Children were genotyped for 27 variants of 13 genes that are reported to affect neurologic functions and/or Hg disposition in adults. Urinary Hg concentrations, reflecting Hg exposure from any source, served as the Hg exposure index. Regression modeling strategies were employed to evaluate potential associations between allelic status for individual genes or combinations of genes, Hg exposure, and neurobehavioral test outcomes assessed at baseline and for 7 subsequent years during the clinical trial. Among boys, significant modification of Hg effects on neurobehavioral outcomes over a broad range of neurologic domains was observed with variant genotypes for 4 of 13 genes evaluated. Modification of Hg effects on a more limited number of neurobehavioral outcomes was also observed for variants of another 8 genes. Cluster analyses suggested some genes interacting in common processes to affect Hg neurotoxicity. In contrast, significant modification of Hg effects on neurobehavioral functions among girls with the same genotypes was substantially more limited. These observations suggest increased susceptibility to the adverse neurobehavioral effects of Hg among children, particularly boys, with genetic variants that are relatively common to the general human population. These findings advance public health goals to identify factors underlying susceptibility to Hg toxicity and may contribute to strategies for preventing adverse health risks associated with Hg exposure.
Keywords: Mercury, Children, Neurobehavioral function, Neurotoxicity, Single nucleotide polymorphism, Genetic susceptibility, Allelic status
1. Introduction
Mercury (Hg), particularly in its elemental (Hg0) and methylated (CH3Hg+) forms, poses a risk of neurotoxicity to humans (Clarkson, 2002). Hg0 in the vapor form, once inhaled, readily crosses the blood brain barrier and preferentially distributes to those portions of the central nervous system (CNS) that affect personality and behavior, the toxic effects, hence, being largely neuropsychiatric in nature. Methylmercury (MeHg), in contrast, more preferentially distributes to those portions of the brain that control sensorimotor function, the principal effects being disturbances in coordination, equilibrium and motor control. Continuous exposure to MeHg, particularly in utero, may also lead to decrements in cognitive functions in offspring (Grandjean et al., 1997). Two large prospective studies initiated in the mid to late 1980s among residents of the Seychelle Islands and the Faroe Islands evaluated the potential developmental toxicity of MeHg among children exposed in utero from mothers’ consumption of fish and/or pilot whale, respectively. No adverse neurobehavioral effects were observed among children in the Seychelle Child Development study (Myers et al., 1995), whereas mild deficits in learning, memory and attention, as well as in visuospatial and motor functions, were observed among Faroe Island children evaluated 7 years after birth when stratified on umbilical cord blood Hg levels (Grandjean et al., 1997). In the assessment of the potential neurobehavioral effects of Hg0 in children, 2 randomized clinical trials (DeRouen et al., 2006; Bellinger et al, 2006) conducted between 1996-2006 compared the performance of children with and without Hg-containing dental amalgam fillings on a extensive battery of neurobehavioral tests over a period of 5 to 7 years following dental treatment beginning at a mean age of 10 years. Neither study found significant differences in neurobehavioral performance over the course of the trial when comparing children with and without dental amalgam fillings.
None of the aforementioned studies was designed to evaluate special sensitivities, such as genetic predisposition, that might affect Hg neurotoxicity in children. Such studies are relevant, however, in light of recent findings (Basu, et al., 2013; Echeverria et al., 2005, 2006, 2010; Goodrich et al., 2011; Gundacker et al., 2007, 2009; Heyer et al., 2004, 2008, 2009; Schläwicke Engstrom et al., 2008; Wang et al., 2012) identifying common variants of numerous genes that modify the effects of Hg on neurologic functions and/or Hg handling in human subjects. Identification of genetic polymorphisms that affect Hg neurotoxicity is of particular importance with regard to assessment of Hg risks in children, who may be uniquely susceptible, compared with adults, because of their smaller body mass and more rapidly developing, hence more fragile, nervous and metabolic systems (Landrigan and Goldman, 2011).
The aim of the present study was to address this concern by identifying altered risks of Hg neurotoxicity potentially associated with common variants (single nucleotide polymorphisms [SNPs], insertions/deletions) in specific candidate genes that are postulated to independently confer increased susceptibility to neurological disorders and/or to modify Hg metabolism in human subjects. Specifically, we evaluated the extent to which performance on a wide variety of neurobehavioral tests was modified by Hg exposure in relation to genotype (wildtype or variant) for 27 variants in 13 different genes among subjects who participated as children and adolescents in a recently completed prospective, randomized dental amalgam clinical trial between ages 8 and 18 and for whom longitudinal (annual) neurobehavioral assessments and quantitative measures of Hg exposure over 7 years of follow-up were available. For these studies, we employed urinary Hg concentrations, representing Hg exposure from any source whether or not related to dental amalgam, as the Hg exposure measure. We evaluated the potential modifying effects of genetic variants on the dose-response effects of Hg exposure on neurobehavioral functions within a wide range of neurologic domains including Attention, Learning & Memory, Executive Function, Visual Spatial acuity, and Motor function. Additionally, because previous studies (Woods et al., 2007) suggested likely sex-related differences in Hg handling and susceptibility to He toxicity, we made these assessments independently in boys and girls.
2. Methods
2.1. The study population
The present study included 330 subjects who participated as children in the Casa Pia Clinical Trial of Dental Amalgams in Children (DeRouen et al., 2002; 2006) conducted between 1996 and 2006. Participants in the clinical trial included 279 boys and 228 girls, aged 8–12 years at baseline, who were students of the Casa Pia school system in Lisbon, Portugal. Founded more than 200 years ago to provide an education for orphaned and homeless children, the Casa Pia system now uses a combination of public and private support to provide a quality education for children from a much wider variety of backgrounds of which approximately 20% are boarding students and 80% are from middle- to upper-class socioeconomic families who pay tuition for their children to attend. Current enrollment of the 10 schools comprising the Casa Pia system exceeds 4,700 students in grades from 1 through 12. For the clinical trial, children were initially randomized to Hg amalgam (treatment) or composite resin (control) dental treatment groups. Subjects were evaluated at baseline and at 7 subsequent annual intervals following initial dental treatment using an extensive battery of neurobehavioral assessments (Martins et al., 2005). Follow-up data were obtained on a similar number of subjects in each treatment group. Baseline urinary Hg concentrations were 1.5 ± 1.2 (0.1-7.7) and 1.4 ± 1.1 (0.0-8.6) μg/g creatinine for amalgam and composite groups, respectively. These compare with a mean urinary Hg level of 0.358 (0.313-0.408) μg/creatinine among a national representative sample of 12-19 year old U.S. children (Centers for Disease Control and Prevention, 2007). The possible sources of exposure accounting for this difference in urine Hg levels and public health implications have been discussed in detail (Woods et al., 2012, 2013). Mean urinary Hg concentrations by treatment group and by sex for each year of the clinical trial have been previously described (Woods et al., 2007). A detailed description of the study design and methods, including factors measured over the course of the study and how these factors were considered in constructing analytical models has been published (DeRouen et al., 2002).
2.2. Neurobehavioral tests
A comprehensive neurobehavioral test battery was used in these analyses, including measures from the Rey Auditory Verbal Learning Test (RAVLT), subtests from the Wide Range Assessment of Visual Motor Abilities (WRAVMA), the Wechsler Adult Intelligence Scale-III (WAIS-III), the Wechsler Memory Scale for Adults-III (WMS-III), Standard Reaction Time (SRT), Finger Tapping, Trailmaking A and B, and the Stroop word, color and word-color tests. The validity and rationale underlying the selection and use of these tests in the clinical trial as well as the baseline neuropsychological performance of all subjects have been described (Martins et al., 2005; Townes et al., 2008).
Table 1 lists the 23 neurobehavioral tests that were assessed and their test abbreviations referenced in subsequent tables. Tests are organized within the 5 behavioral domains (Attention, Learning & Memory, Executive Function, Visual Spatial acuity and Motor function) that were evaluated in the clinical trial (DeRouen et al., 2006). Arrows depict whether the test score increases or decreases in magnitude with improved performance. Diminished or adversely affected performance associated with Hg exposure or gene variant status is described as occurring in the direction of impaired performance, whereas enhanced or beneficially affected performance associated with either of these variables is described as occurring in the direction of improved performance. The Comprehensive Test Of Nonverbal Intelligence (CTONI) (Portuguese translation) was given to each child at the beginning of the clinical trial to obtain a measure of IQ at baseline. Social and cultural issues associated with measurements and interpretation of IQ test results among children in cross-cultural contexts have been previously described (Martins et al., 2005; Woods et al., 2013).
Table 1.
Neurobehavioral tests assessed with mean scores for Year 7 (final year of clinical trial)
| Test / Domain | Test Abbreviation | Measurea | Boys Mean (SD) | Girls Mean (SD) |
|---|---|---|---|---|
| Attention (6 tests) | ||||
| Stroop Test – Color | strpcol | # correct ↑ | 66.16 (11.97) | 69.25 (10.39) |
| Word | strpwd | # correct ↑ | 89.93 (15.16) | 91.54 (15.19) |
| Color-Word | Strpc/wd | # correct ↑ | 41.74 (9.76) | 43.93 (8.73) |
| WAIS-III – Digit Span | digspn | # correct ↑ | 14.30 (3.67) | 14.14 (2.76) |
| WAIS- III – Spatial Span | spatspn | # correct ↑ | 15.83 (3.03) | 15.61 (3.12) |
| Adult Trailmaking A | TrailsA | Time (sec) ↓ | 26.43 (10.63) | 30.25 (11.42) |
| Visual-Spatial (3 tests) | ||||
| Standard Reaction Time | SRT | Time (sec) ↓ | 0.74 (0.15) | 0.77 (0.13) |
| WAIS III – Digit Symbol | digitsym | # correct ↑ | 72.02 (16.48) | 76.58 (13.85) |
| Symbol Search | symsea | # correct ↑ | 32.99 (8.82) | 34.59 (8.01) |
| Executive Functioning (2 tests) | ||||
| Wisconsin Card Sort – Categories Completed | Card Sort-Cat | # categories ↑ | 3.05 (1.38) | 3.09 (1.47) |
| Adult Trailmaking B | TrailsB | Time (sec) ↓ | 65.97 (26.94) | 63.10 (23.80) |
| Learning & Memory (8 tests) | ||||
| RAVLT Trial 1 – List A | ravlt tr1 | # correct ↑ | 5.62 (1.49) | 6.12 (1.86) |
| Trial 5 – List A (fifth repetition) | ravlt tr5 | # correct ↑ | 11.23 (2.20) | 11.55 (2.23) |
| Trial 6 – List B | ravlt tr6 | # correct ↑ | 4.73 (1.38) | 5.28 (1.56) |
| Trial 7 – List A/Post B | ravlt tr7 | # correct ↑ | 9.85 (2.57) | 10.23 (2.48) |
| Trial 8 – List A after 20' | ravlt tr8 | # correct ↑ | 9.29 (2.73) | 10.08 (2.76) |
| WMS-III – Visual Reproductions – Immediate | VisRep-Imm | # correct ↑ | 34.69 (4.66) | 36.62 (2.97) |
| -Delayed | VisRep-Del | # correct ↑ | 32.01 (6.98) | 34.93 (4.02) |
| CVMT d-Prime | CVMT | Score ↑ | 1.50 (.94) | 1.61 (.87) |
| Motor (4 tests) | ||||
| WRAVMA –Pegs – Dominant Hand | pegdom | # Pegs ↑ | 47.35 (8.51) | 49.92 (6.28) |
| Non Dominant Hand | pegndom | # Pegs ↑ | 44.37 (7.59) | 45.03 (6.10) |
| Finger Tapping – Dominant Hand | ftapdom | # Taps ↑ | 52.66 (5.53) | 48.55 (5.83) |
| Non Dominant Hand | ftapndom | # Taps ↑ | 46.54 (5.79) | 42.53 (5.78) |
Arrows show direction of improved performance.
2.3. Genotyping assays
Genotyping was performed on DNA extracted from buccal cell samples that were obtained from study subjects following completion of the clinical trial (n=199) or from blood samples that were acquired at baseline for various clinical assessments (n=152). Genotyping was performed by the Functional Genomics and Proteomics Laboratory of the NIEHS Center for Ecogenetics and Environmental Health at the University of Washington using commercially available genotyping assays. Details of genotyping methods, including quality control procedures employed, are described in previous publications (Echeverria et al., 2010; Heyer et al., 2004; 2009; Woods et al., 2005).
2.4. Human subjects considerations
All parents or guardians of children who participated in the clinical trial gave written consent, and all children provided signed assent, for the treatments and assessments made during the course of the trial, including collection of blood samples. Written consent was also obtained from all participants who provided buccal cell samples for genotyping subsequent to completion of the clinical trial. The study protocols for both the clinical trial and the present genotyping study were approved by the institutional review boards at the University of Lisbon and the University of Washington.
2.5. Urinary Hg analysis
A urine sample was collected from each child at baseline of the clinical trial and at each subsequent annual visit to the University of Lisbon School of Dental Medicine for dental, neurologic, and neurobehavioral evaluations. Strictly maintained sterile conditions and handling procedures precluded contamination of urine samples by Hg or any other substance. Analysis of total mercury (Hg) was performed by continuous flow, cold vapor spectrofluorometry, as previously described (Pingree et al., 2001). Urinary creatinine concentrations were measured using a standard colorimetric procedure (Sigma #555-A; Sigma-Aldrich, St. Louis, MO, USA). Urinary Hg concentrations (HgU) were calculated as micrograms per gram creatinine (μg/g creatinine).
2.6. Assessment of Hg exposure
In the present genotyping study, we modified the clinical trial approach in 2 essential ways. First, rather than using the assignment to Hg amalgam or composite resin treatment groups, we employed urinary Hg concentrations (HgU) measured annually for all participants to calculate acute and chronic measures of Hg exposure. This allowed us to capture the effects of all Hg exposure, whether related to dental amalgam, diet, or other environmental sources. This decision was based on the fact that assignment to Hg amalgam or composite resin treatment group accounted for, at most, 17% of the variation in HgU among boys (r2=0.171) and 15% among girls (r2=0.154), both occurring in year 2 of the clinical trial, and indicating considerable background Hg exposure unrelated to dental amalgam (Woods et al., 2013). Moreover, recent studies (Sherman et al., 2013) demonstrate that urine may contain inorganic Hg derived from demethylation of ingested methylmercury from fish, a major constituent of the Portuguese diet. Secondly, we evaluated the potential modifying effects of specific genetic variants on the dose-response effects of Hg exposure, as represented by urinary Hg levels, on neurobehavioral performance. Because previous studies (Woods et al. 2007) suggested possible sex-related differences in Hg handling and susceptibility to Hg toxicity, we made these assessments separately in boys and girls.
2.7. Study design
We examined whether allelic status of specific genes affected the dose-response relationship between urinary Hg concentration and tests of neurobehavioral functions among children who had been evaluated annually from baseline through 7 years of follow-up of the clinical trial. The wide range in ages of subjects at the beginning of the trial (8-12 years), the duration of the study (which included passage through puberty for most subjects), and the associated change in specific tests administered to subjects based upon their age group during the course of the trial (e.g., child versus adult versions of some tests) introduced significant complexity into the interpretation of repeated measures analyses for this study. We, therefore, evaluated the acute effects of Hg exposure on performance using the concurrent urinary Hg concentrations (HgU) and neurobehavioral test performance measures at the end of the 2nd year of follow-up (Year 2), where mean HgU reached a peak among both boys and girls in the clinical trial cohort and where cumulative effects of Hg would be minimal. Similarly, we estimated the chronic effects of Hg exposure by examining the relationship between a cumulative measure of HgU over the entire study period and performance outcomes during the final study year (Year 7).
The measure of acute Hg exposure was calculated as the natural log of HgU adjusted by 1 (ln[HgU+1]). The natural log best accommodates how exposures are distributed biologically, whereas adding 1 minimizes the influence of very small changes in HgU at very low levels (which could have otherwise dominated the analyses). The measure of chronic Hg exposure was evaluated using cumulative HgU calculated as the natural log of the sum of HgU from baseline and each year of follow-up also adjusted by 1 (ln[(∑HgU)+1]). A small number of subjects (28) who were followed for the full 7 years of the clinical trial had missed one or more intermediate annual evaluations. Subjects missing 3 or more evaluations (n=2, both female) were eliminated from the chronic exposure analyses. Those missing 1 (n=22, 9 female) or 2 (n=4, 3 female) annual evaluations were included in the chronic Hg exposure analyses with their mean HgU concentrations substituted for the missing years. Chronic Hg exposure analyses conducted with and without these subjects did not significantly differ. Of note, the acute and chronic measures of Hg exposure were found to have a Pearson correlation of 0.36, indicating that they share only 13% common variance (r2) and, hence, are not highly correlated.
2.8. Statistical analyses
The effect of specific gene variants on the dose-response association between Hg exposure and neurobehavioral performance was the principal focus of this study. Thus, our analytical protocol focused on gene-Hg interactions (genexHg), independently evaluating the impact of allelic status of any gene individually and in combination on performance of each behavioral test. In all cases, boys and girls were evaluated independently, as were the effects of acute and chronic Hg exposures. Statistical analyses were performed using SPSS Version 19 (IBM®SPSS®, Chicago, IL, USA).
Initial analyses were conducted using a full interaction model consisting of Hg exposure (either acute or chronic as defined above), dichotomous allelic status for each gene (WT or not WT [HetMut or Mut]), and their interaction terms. Covariates in this model included age at assessment, race, and non-verbal IQ (determined at baseline). These covariates were selected because of their potential to bias neurobehavioral test performance in relation to Hg exposure (Echeverria et al. 1995) or other stressors (Krieg et al. 2001) and because data pertinent to these specific variables were available from the clinical trial from which subjects in the present study were acquired (Martins et al. 2005; Townes et al. 2003). Restricting our analyses to these 3 covariates, which are not highly correlated, minimized the possibility of having an over-determined model. Other factors potentially affecting behavioral test performance in relation to Hg exposure, including home environment, parent's socioeconomic status, or medical histories, were comparable among essentially all subjects (DeRouen et al., 2002) and were, therefore, not included as covariates. All behavioral tests with significant interaction terms in the above model were re-evaluated for dose-response associations between Hg exposure and test performance independently for subjects with WT or not WT allelic status for each SNP. This final model, which included Hg exposure, age at assessment, race and non-verbal IQ at baseline, provided analyses of Hg dose-response relationships for behavioral performance within each genotypic group unencumbered by interaction terms, and where the differences in dose-response by allelic status (interactions) could be directly compared. Further details pertinent to these analyses have been described (Woods et al., 2013).
Additional analyses were conducted to evaluate the possibility of interaction between combinations of genes that might potentially modify Hg neurotoxicity via shared mechanisms on the same or related neurologic processes. These analyses were performed using multi-regression techniques as described above but included dummy variables for potentially modifying gene alleles as additional covariates in the regression models. Clustering of effects was assessed by determination of significant dose-response associations between Hg exposure and test performance with or without additional alleles in the analysis.
Prior to fitting the regression models, we examined the assumptions of the model by scrutinizing the distributions and variances of all neurobehavioral tests and ln(HgU). Most distributions had no significant deviation from normality or inflated variance. After fitting each model, we examined the standardized residuals for statistical outliers. In the rare event that an outlier was found, it was removed and the model was refit.
For all variants, we evaluated the effects of genotype on test outcomes independent of Hg exposure. Few associations were observed, and most were of only borderline significance in terms of suggesting independent effects of gene status on neurobehavioral performance. Findings pertinent to variants of CPOX are published (Woods et al., 2012).
2.9. Selection of candidate genes
Using a search of the PubMed database and the current literature, we identified 13 candidate genes that, as variant forms, are reported to be associated with various neurobehavioral/neuropsychiatric disorders and/or changes in Hg handling in adults and that, therefore, might be expected to modify the effects of Hg on neurobehavioral performance among children. We limited the selection of candidate genes to those whose variants were considered a priori to modify the effects of Hg on as broad a range of neurobehavioral and Hg toxicokinetic functions as possible, with minimal overlap or redundancy. These included variants of 9 genes (CPOX, COMT, TDO2, GRIN2A, GRIN2B, SLC6A4, KIBRA, APOE) that are reported to modify performance in adults on at least 1 of the 5 neurologic domains evaluated, whereas those affecting Hg handling included variants of 4 genes (MT1M, MT2A, GSTT1, SEPP1) that are reported to modify Hg toxicokinetics and/or tissue distribution via possibly different mechanisms or processes. When minor allelic frequencies (MAF) of any gene were less than 20%, power calculations were performed to ensure that statistical significance could be attained given the numbers of boys and girls genotyped as wildtype (WT) and variant (Het, Mut, deletion, insertion/deletion), respectively. These genes and their variants, along with the distribution of their wildtype and variant forms and their minor allelic frequencies, are presented in Table 2. Conventional abbreviations for gene names are employed. A synopsis of pathways or processes affected by each gene, along with their neurologic relevance, is presented in Table 3.
Table 2.
Summary of genes and gene variants investigated
| Gene# | Gene | Chromosome | SNP | Variation | N | WT | HET | MUT | MAF% |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CPOX | 3 | rs1131857 | A>C | 328 | 224 | 91 | 13 | 24 |
| rs1729995 | G>A | 329 | 190 | 117 | 22 | 24 | |||
| 2 | MT1M | 16 | rs2270837 | G>A | 330 | 204 | 114 | 12 | 27 |
| rs2270836 | C>T | 330 | 134 | 126 | 70 | 33 | |||
| 3 | MT2A | 16 | rs10626 | G>C | 330 | 200 | 106 | 24 | 24 |
| 4 | COMT | 22 | rs4680 | G>A | 330 | 115 | 162 | 53 | 39 |
| rs4633 | C>T | 330 | 114 | 165 | 51 | 39 | |||
| rs4818 | C>G | 330 | 120 | 156 | 54 | 32 | |||
| rs6269 | A>G | 330 | 83 | 172 | 75 | 37 | |||
| 5 | TDO2 | 4 | rs13434811 | G>T | 330 | 269 | 56 | 5 | 11 |
| rs2271537 | G>T | 330 | 117 | 147 | 66 | 48 | |||
| rs2292537 | A>G | 330 | 254 | 71 | 5 | 11 | |||
| rs3755907 | G>A | 330 | 242 | 78 | 10 | 18 | |||
| rs3755908 | T>C | 330 | 242 | 78 | 10 | 18 | |||
| rs3755910 | C>A | 330 | 312 | 18 | 0 | 3 | |||
| rs3775085 | A>C | 330 | 242 | 78 | 10 | 2 | |||
| 6 | GRIN2A | 16 | rs727605 | C>T | 330 | 134 | 150 | 46 | 26 |
| rs1070503 | C>T | 330 | 232 | 89 | 9 | 25 | |||
| 7 | GRIN2B | 12 | rs1806201 | G>A | 330 | 206 | 106 | 18 | 31 |
| rs7301328 | G>C | 330 | 114 | 157 | 59 | 40 | |||
| 8 | BDNF | 11 | rs6265 | G>A | 330 | 238 | 80 | 12 | 23 |
| 9 | GSTT1 | 22 | gene deletion | 330 | 246a | 84 | 25 | ||
| 10 | SLC6A4 | 17 | 5-HTTLPR ins/del | 330 | 194 LL | 91 LS | 45 SS | 15 | |
| 11 | KIBRA | 5 | rs17070145 | C>T | 330 | 126 | 144 | 38 | 46 |
| 12 | APOE | 19 | rs429358 | T>C | 330 | 237 | 85 | 8 | 15 |
| rs7412 | C>T | 330 | 287 | 38 | 5 | 7 | |||
| 13 | SEPP1 | 5 | rs7579 | C>T | 330 | 223 | 92 | 15 | 27 |
WT:Homozygous major variant; HET:Heterozygous; MUT:Homozygous minor variant; MAF: Minor Allelic Frequency
Data for GSTT1 are no deletion (WT) vs deletion (Mut).
Table 3.
Synopsis of gene functions and neurologic relevance.
| Gene(s) | Variant(s) | Pathway/Process | Neurologic Relevance |
|---|---|---|---|
| CPOX | rs1131857 | Heme biosynthesis | Neuronal receptor processing Energy (ATP) Production Modulation of neuronal gene expression |
| MTIM | rs2270837 | Hg dispersal/storage | Protection & maintenance of neurologic processes |
| MT2A | rs10636 | Hg dispersal/storage | Protection & maintenance of neurologic processes |
| COMT | rs4680 | Catecholamine deactivation | Maintenance of cognitive & executive functions |
| TDO2 | rs3755907 | Tryptophan catabolism | Serotonin (5-HT) availability & signaling |
| GRIN2A | rs727605 rs1070503 |
N-methyl-D-aspartate receptor processing | Glutamatergic (excitatory) neurotransmission |
| GRIN2B | rs1806201 rs7301328 |
N-methyl-D-aspartate receptor processing | Glutamatergic (excitatory) neurotransmission |
| BDNF | rs6265 | Neuronal growth and differentiation | Memory and motor functions |
| GSTT1 | Deletion | Hg-GSH conjugation | Facilitation of Hg excretion |
| SLC6A4 (5-HTTLPR) | Insertion/deletion | Serotonin uptake | Maintenance of mood, cognitive & motor functions |
| KIBRA | rs17070145 | Synaptic plasticity | Verbal & visual episodic memory |
| APOE | rs429358 rs7412 |
Cholesterol & fatty acid transport; neuronal repair | Maintenance of neuronal and cognitive integrity |
| SEPP1 | rs7579 | Hg binding | Protection & maintenance of neurologic processes |
3. Results
3.1. Genetic Variants that modify the effects of Hg on a Broad Range of Neurobehavioral functions in Children
Of the 13 genes evaluated, 4 (CPOX, MT1M, MT2A, COMT) were found to have variant forms that significantly modified the effects of Hg exposure on neurobehavioral performance outcomes within 3 or more neurological domains in children. Significant findings for these as well as for other genes evaluated are summarized in Table 4 (Boys) and Table 5 (Girls).
Table 4.
Individual Gene SNPxHg Results Among BOYS. Data depict significant (p<0.05) dose-response effects for Acute and Chronic Hg exposure on performance on specific neurobehavioral tests among boys genotyped as variant for the gene indicated. No significant dose-response measures among boys genotyped as WT for any gene were observed (not shown) .
| Gene | SNP (rs#) | Test | Domain | Acute Hg Exposure | Chronic Hg Exposure | ||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | p value | Beta | SE | p value | ||||
| CPOX | 1131857 | WT = 114, Het = 45, Mut = 1 | WT = 82, Het = 38, Mut = 1 | ||||||
| strpwd | Attn | −18.1 | 4.89 | 0.001 | |||||
| strpcol | Attn | −8.4 | 2.8 | 0.005 | −12.55 | 4.05 | 0.004 | ||
| strpc/wd | Attn | −3.5 | 1.68 | 0.04 | |||||
| digspn | Attn | −3.57 | 1.12 | 0.003 | |||||
| spatspn | Attn | −2.45 | 0.97 | 0.02 | |||||
| trailsA* | Attn | 11.25 | 3.83 | 0.006 | |||||
| SRT* | VisSpa | 0.15 | 0.05 | 0.007 | |||||
| digitsym | VisSpa | −20.7 | 6.08 | 0.002 | |||||
| trailsB* | ExFn | 22.26 | 7.03 | 0.003 | |||||
| ravlt tr5 | L&M | −1.78 | 0.8 | 0.03 | |||||
| ravlt tr7 | L&M | −2.24 | 0.82 | 0.01 | |||||
| ravlt tr8 | L&M | −2.14 | 0.83 | 0.01 | |||||
| pegdom | Motor | −7.16 | 2.9 | 0.02 | |||||
| pegndom | Motor | −5.34 | 2.7 | 0.06 | |||||
| ftapdom | Motor | −3.25 | 1.53 | 0.04 | −3.46 | 1.65 | 0.04 | ||
| ftapndom | Motor | −5.83 | 1.66 | 0.001 | |||||
| MT1M | 2270837 | WT = 106, Het = 49, Mut = 3 | WT = 82, Het = 35, Mut = 3 | ||||||
| ravlt tr5 | L&M | −1.22 | 0.58 | 0.04 | −1.56 | 0.53 | 0.006 | ||
| ravlt tr8 | L&M | −1.69 | 0.67 | 0.02 | −2.11 | 0.74 | 0.007 | ||
| ftapdom | Motor | −4.75 | 1.53 | 0.003 | |||||
| MT2A | 10636 | WT = 96, Het = 49, Mut = 13 | WT = 74, Het = 36, Mut = 10 | ||||||
| strpcol | Attn | −6.88 | 2.62 | 0.01 | |||||
| digitsym | VisSpa | −12.9 | 4.77 | 0.01 | |||||
| symsea | VisSpa | −8.21 | 2.62 | 0.003 | |||||
| ravlt tr5 | L&M | −1.96 | 0.86 | 0.03 | |||||
| ravlt tr8 | L&M | −6.49 | 2.72 | 0.007 | |||||
| COMT | 4680 | WT = 53, Het = 79, Mut = 27 | WT = 40, Het = 57, Mut = 23 | ||||||
| strpwd | Attn | −12.43 | 6.21 | 0.06 | |||||
| strpcol | Attn | −9.32 | 4.09 | 0.03 | |||||
| strpc/wd | Attn | −7.92 | 2.76 | 0.009 | |||||
| digitspn | Attn | −2.11 | 0.93 | 0.03 | |||||
| spatspn | Attn | −5.05 | 1.95 | 0.02 | |||||
| ravlt tr5 | L&M | −2.66 | 1.08 | 0.02 | |||||
| ravlt tr6 | L&M | −1.68 | 0.69 | 0.02 | |||||
| ravlt tr7 | L&M | −2.83 | 1.19 | 0.03 | |||||
| ravlt tr8 | L&M | −3.70 | 1.48 | 0.02 | |||||
| ravlt 1-5 | L&M | −12.21 | 4.26 | 0.01 | |||||
| digitsym | VisSpa | −32.46 | 11.28 | 0.01 | |||||
| symsea | VisSpa | −16.49 | 5.52 | 0.008 | |||||
| COMT | 4633 | WT = 50, Het = 84, Mut = 25 | WT = 38, Het = 61, Mut = 21 | ||||||
| strpcol | Attn | −7.60 | 3.70 | 0.05 | −13.79 | 6.23 | 0.04 | ||
| strpc/wd | Attn | −7.98 | 2.73 | 0.01 | |||||
| digitspn | Attn | −2.04 | 0.98 | 0.05 | |||||
| spatspn | Attn | −4.58 | 1.84 | 0.02 | |||||
| ravlt tr5 | L&M | −2.7 | 1.06 | 0.02 | |||||
| ravlt tr6 | L&M | −1.65 | 0.69 | 0.02 | |||||
| ravlt tr7 | L&M | −2.8 | 1.16 | 0.03 | |||||
| ravlt tr8 | L&M | −3.69 | 1.44 | 0.02 | |||||
| ravlt 1-5 | L&M | −12.38 | 4.2 | 0.008 | |||||
| digitsym | VisSpa | −35.71 | 10.87 | 0.005 | |||||
| symsea | VisSpa | −16.19 | 5.53 | 0.01 | |||||
| COMT | 6269 | WT = 42, Het = 83, Mut = 34 | WT = 34, Het = 60, Mut = 26 | ||||||
| strpcol | Attn | −5.31 | 2.59 | 0.05 | |||||
| digitspn | Attn | −1.72 | 0.79 | 0.04 | |||||
| digitsym | VisSpa | −17.92 | 6.8 | 0.01 | |||||
| symsea | VisSpa | −8.48 | 3.83 | 0.04 | |||||
| ravlt tr5 | L&M | −2 | 0.78 | 0.01 | |||||
| ravlt tr7 | L&M | −1.96 | 0.94 | 0.04 | |||||
| ravlt tr8 | L&M | −2.63 | 1.04 | 0.02 | |||||
| ravlt 1-5 | L&M | −8.41 | 3 | 0.008 | |||||
| TDO2 | 3755907 | WT = 119, Het = 38, Mut = 3 | WT = 88, Het = 29, Mut = 3 | ||||||
| strpwd | Attn | −14.66 | 7.12 | 0.05 | |||||
| SRTa | VisSpa | 0.16 | 0.06 | 0.005 | |||||
| pegsdom | Mot | −3.72 | 1.8 | 0.05 | −9.36 | 3.9 | 0.02 | ||
| pegndom | Mot | −3.59 | 1.97 | 0.04 | |||||
| ftapdom | Mot | −2.83 | 1.35 | 0.04 | |||||
| GRIN2A | 727605 | WT = 57, Het = 77, Mut = 24 | WT = 42, Het = 61, Mut = 17 | ||||||
| strpwd | Attn | −6.28 | 2.78 | 0.03 | |||||
| spatspn | Attn | −1.54 | 0.64 | 0.02 | |||||
| ravlt tr1 | L&M | −0.71 | 0.34 | 0.04 | |||||
| GRIN2A | 1070503 | WT = 114 , Het = 40, Mut = 4 | WT = 82, Het = 35, Mut = 3 | ||||||
| ravlt tr5 | L&M | −1.13 | 0.65 | 0.05 | |||||
| ftapndom | Mot | −4.03 | 1.38 | 0.006 | |||||
| GRIN2B | 7301328 | WT = 54, Het = 80, Mut = 24 | WT = 41, Het = 60 , Mut = 19 | ||||||
| ravlt tr8 | L&M | −1.07 | 0.58 | 0.07 | |||||
| VisRep-Del | L&M | −4.55 | 1.54 | 0.004 | |||||
| ftapndom | Motor | −3.21 | 1.06 | 0.003 | −3.65 | 1.26 | 0.005 | ||
| BDNF | 6265 | WT = 118, Het = 35, Mut = 5 | WT = 88, Het = 29, Mut = 3 | ||||||
| ravlt tr7 | L&M | −2.11 | 0.82 | 0.01 | |||||
| ravlt tr8 | L&M | −1.99 | 0.89 | 0.03 | |||||
| ravlt 1-8 | L&M | −0.71 | 0.34 | 0.04 | |||||
| GSTT1 | Deletion | No Deletion = 110, Deletion = 48 | No Deletion = 81, Deletion = 39 | ||||||
| strpwd | Attn | −9.76 | 3.29 | 0.004 | |||||
| CMVT | L&M | −0.42 | 0.22 | 0.05 | |||||
| pegsdom | Motor | −2.26 | 1.02 | 0.03 | |||||
| SLC6A4 | Ins/del | WT = 103, Het = 41, Mut = 14 | WT = 70, Het = 38, Mut = 12 | ||||||
| (5-HTTLPR) | digitspn | Attn | −2.32 | 0.86 | 0.01 | ||||
| TrailsAa | Attn | 5.72 | 2.76 | 0.04 | |||||
| ravlt 1-8 | L&M | −7.09 | 2.22 | 0.003 | |||||
| ravlt 6 | L&M | −0.68 | 0.33 | 0.05 | |||||
| KIBRA | 17070145 | WT = 65, Het = 75, Mut = 18 | WT = 48, Het = 60, Mut = 12 | ||||||
| TrailsAa | Attn | 6.42 | 2.71 | 0.02 | |||||
| symsea | VisSpa | −6.34 | 2.42 | 0.01 | |||||
| ravlt 1-5 | L&M | −7.22 | 2.68 | 0.01 | |||||
| APOE | ε4 | ε3(ε3/ε3) = 112, ε4(ε3/ε4 + ε4/ε4) = 44 | |||||||
| ravlt 1-8 | L&M | −9.15 | 3.71 | 0.02 | |||||
Trailmaking A and SRT increase in magnitude (time) with impaired performance. All other tests decrease in magnitude (score) with impaired performance. Beta is the standardized value.
Table 5.
Individual Gene SNPxHg Results Among GIRLS. Data depict significant (p≤0.05) dose-response effects for Acute and Chronic Hg exposure on performance on specific neurobehavioral tests among girls genotyped as variant for the gene indicated. No significant dose-response measures among girls genotyped as WT for any gene were observed (not shown) .
| Gene | SNP (rs#) | Test | Domain | Acute Hg Exposure | Chronic Hg Exposure | ||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | p value | Beta | SE | p value | ||||
| CPOX | 1131857 | WT = 97, Het = 42, Mut = 12 | WT = 73, Het = 34, Mut = 11 | ||||||
| ravlt tr5 | L&M | −1.34 | 0.47 | 0.006 | |||||
| ravlt tr8 | L&M | −1.69 | 0.55 | 0.003 | |||||
| ravlt 1-8 | L&M | −4.06 | 1.64 | 0.02 | |||||
| trailsB* | ExFn | 8.88 | 3.33 | 0.008 | |||||
| MT1M | 2270837 | WT = 86, Het = 57, Mut = 8 | WT = 68, Het = 44, Mut = 7 | ||||||
| ravlt tr8 | L&M | −1.74 | 0.65 | 0.01 | |||||
| COMT | 4680 | WT = 56, Het = 74, Mut = 22 | WT = 45, Het = 58, Mut = 16 | ||||||
| trailsA* | Attn | 7.49 | 1.51 | 0.0001 | |||||
| trailsB* | ExFn | 6.52 | 2.81 | 0.03 | |||||
| ravlt tr8 | L&M | −2.29 | 1.06 | 0.05 | |||||
| COMT | 4633 | WT = 58, Het = 72, Mut = 22 | WT = 46, Het = 57, Mut = 16 | ||||||
| trailsA* | Attn | 6.18 | 2.00 | 0.007 | |||||
| trailsB* | ExFn | 6.52 | 2.81 | 0.03 | |||||
| COMT | 4818 | WT = 55, Het = 70, Mut = 27 | WT = 43, Het = 54, Mut = 22 | ||||||
| strpcol | Attn | 8.39 | 3.16 | 0.01 | |||||
| strpwd | Attn | 11.90 | 4.68 | 0.02 | |||||
| strpcolwd | Attn | 4.84 | 1.85 | 0.02 | |||||
| COMT | 6269 | WT = 35, Het = 81, Mut = 36 | WT = 28, Het = 61, Mut = 30 | ||||||
| ravlt tr6 | L&M | 0.81 | 0.37 | 0.04 | |||||
| TDO2 | 3755907 | WT = 108, Het = 37, Mut = 6 | WT = 89, Het = 25, Mut = 5 | ||||||
| Card Sort-Cat | ExFn | 6.63 | 3.27 | 0.05 | |||||
| Visrep-Imm | L&M | 2.36 | 1.00 | 0.03 | |||||
| GRIN2B | 7301328 | WT = 51, Het = 68, Mut = 32 | WT = 40 Het = 55, Mut = 24 | ||||||
| ravlt tr7 | L&M | −0.70 | 0.35 | 0.05 | |||||
| ravlt tr8 | L&M | −0.92 | 0.51 | 0.08 | |||||
| pegsdom | Motor | 2.67 | 0.76 | 0.001 | |||||
| BDNF | 6265 | WT = 107, Het = 37, Mut = 7 | WT = 89, Het = 28, Mut = 5 | ||||||
| ravlt tr1 | L&M | −7.19 | 2.25 | 0.008 | |||||
| ravlt tr8 | L&M | −1.80 | 0.77 | 0.03 | |||||
| GSTT1 | deletion | No Deletion = 119, Deletion = 32 | No Deletion = 93, Deletion = 26 | ||||||
| Trails A | Attn | 6.80 | 3.20 | 0.04 | |||||
| pegsndom | Motor | −3.94 | 1.87 | 0.04 | |||||
| APOE | ε4 | ε3(ε3/ε3) = 104, ε4(ε3/ε4 + (ε4/ε4) = 41 | |||||||
| strpcol | Attn | 8.14 | 3.18 | 0.04 | |||||
| ftapdom | Motor | 7.13 | 2.46 | 0.005 | |||||
Trailmaking A and B increase in magnitude (time) with impaired performance. All other tests decrease in magnitude (score) with impaired performance. Values in italics indicate results that significantly improved with Hg exposure (in the unexpected direction). Beta is the standardized value.
3.1.1CPOX
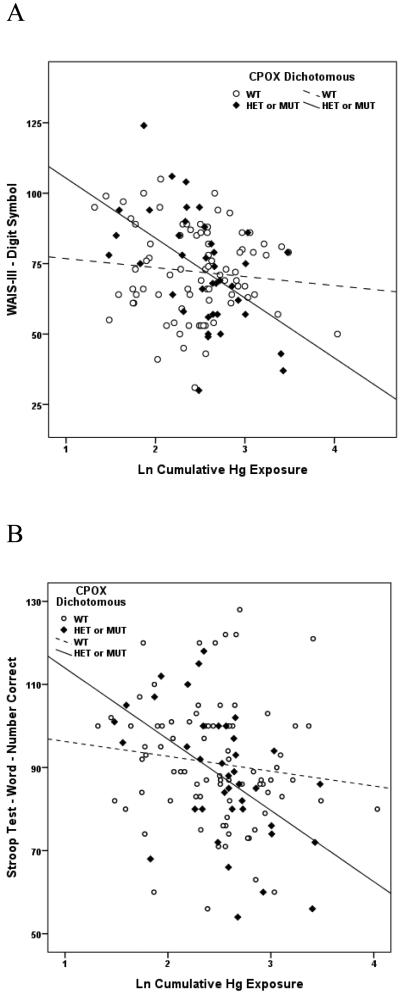
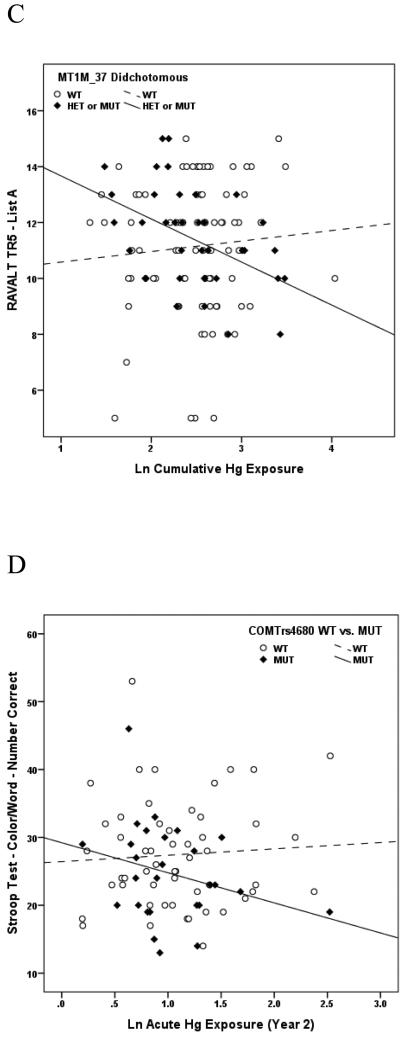
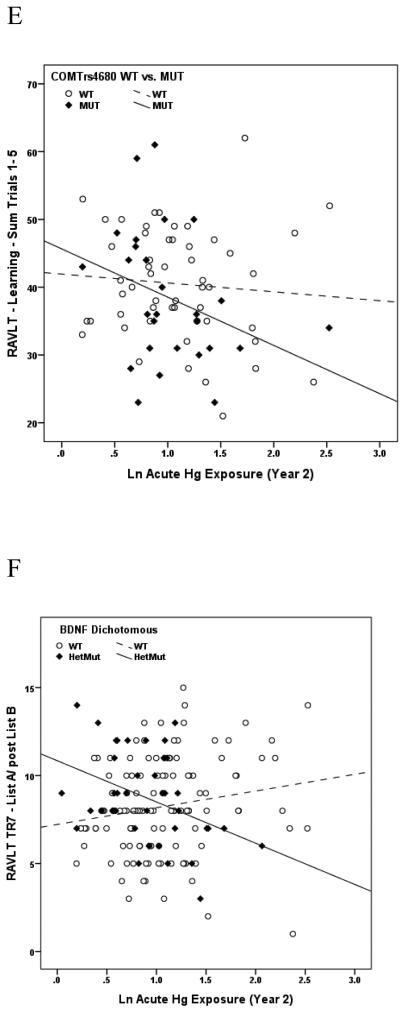
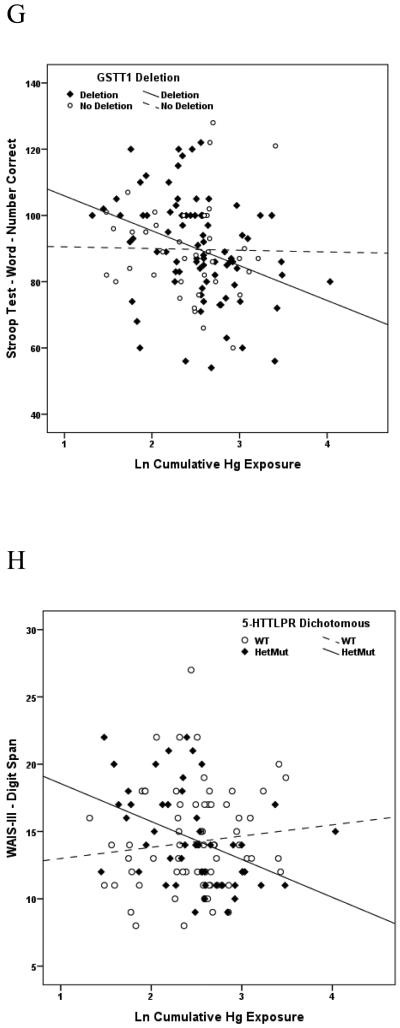
Of the 27 variants evaluated, a SNP in exon 4 of the gene encoding the heme pathway enzyme, coproporphyrinogen oxidase (CPOX4, rs1131857), conveyed the broadest range of effects in the present study, modifying performance on multiple neurobehavioral tests within all 5 neurological domains (Attention, Learning & Memory, Executive Function, Visual Spatial acuity and Motor function) in response to chronic Hg exposure among boys. Whereas no dose-response measures among boys genotyped as CPOX WT were significant, 15 of 23 dose-response associations between Hg and neurobehavioral test performance among boys genotyped as CPOX4 Het or Mut were significant, and all were in the direction of impaired performance with increasing Hg exposure (Table 4). A similar dichotomy of effects was observed between girls genotyped as CPOX WT or CPOX4 Het or Mut, although with substantially fewer significant findings, and limited largely to tests of Learning and Memory in response to acute Hg exposure (Table 5). Scatter plots depicting the associations between specific tests of neurobehavioral performance and chronic Hg exposure among boys genotyped as WT or Het/Mut for CPOX rs1131857 are shown in Figures 1A and 1B. A second CPOX variant (CPOX5, rs1729995), which encodes a synonymous mutation in the CPOX enzyme (E330E) was also evaluated in terms of mediating effect modification of Hg exposure on neurobehavioral test performance in children. None was observed for either boys or girls gentoyped as CPOX5 in response to either acute or chronic Hg exposure. These findings have been described in detail (Woods et al., 2012) and are the first to demonstrate genetic susceptibility to the adverse neurobehavioral effects of Hg exposure in children.
Figure 1.
Figures 1A-1H present scatter plots and simple linear regression fit lines of neurobehavioral test scores by acute or cumulative (chronic) Hg exposure plotted to distinguish boys genotyped as wildtype (WT) from those genotyped as variant with respect to the gene identified.
Figure 1A. Association between performance on the WAIS-III Digit Symbol test of Visual Spatial acuity and cumulative (chronic) Hg exposure among boys genotyped as WT (dashed line, open symbols) or Het/Mut (solid line, closed symbols) for coproporphyrinogen oxidase rs1131857 (CPOX4). Liner regression r2s are 0.015 and 0.259, respectively, indicating that Hg exposure accounted for 1.5% of performance variance among boys with WT status but for nearly 26% of performance variance among boys with Het or Mut gene status.
Figure 1B. Association between performance on the Stroop Word test of Attention and cumulative (chronic) Hg exposure among boys genotyped as WT (dashed line, open symbols) or Het/Mut (solid line, closed symbols) for coproporphyrinogen oxidase rs1131857 (CPOX4). Liner regression r2s are 0.011 and 0.251, respectively, indicating that Hg exposure accounted for 1.1% of performance variance among boys with WT status but for 25.1% of performance variance among boys with Het or Mut gene status.
Figure 1C. Association between performance on the Rey Auditory Verbal Learning Test (RAVLT) Trial5 List A test of Learning & Memory and cumulative (chronic) Hg exposure among boys genotyped as WT (dashed line, open symbols) or Het/Mut (solid line, closed symbols) for metallothionein MT1M rs2270837. Liner regression r2s are 0.006 and 0.210, respectively, indicating that Hg exposure accounted for 0.6% of performance variance among boys with WT status but for 21% of performance variance among boys with Het or Mut gene status.
Figure 1D. Association between performance on the Stroop Color/Word test of Attention and acute Hg exposure among boys genotyped as WT (dashed line, open symbols) or Mut (solid line, closed symbols) for catechol O-methyltransferase (COMT) rs4680. Liner regression r2s are 0.004 and 0.180, respectively, indicating that Hg exposure accounted for 0.4% of performance variance among boys with WT status but for 18% of performance variance among boys with Mut gene status.
Figure 1E. Association between performance on the Rey Auditory Verbal Learning Test (RAVLT) sum of Trials 1-5 test of Learning & Memory and acute Hg exposure among boys genotyped as WT (dashed line, open symbols) or Mut (solid line, closed symbols) for catechol O-methyltransferase (COMT) rs4680. Liner regression r2s are 0.007 and 0.150, respectively, indicating that Hg exposure accounted for 0.7% of performance variance among boys with WT status but for 15% of performance variance among boys with Mut gene status.
Figure 1F. Association between performance on the Rey Auditory Verbal Learning Test (RAVLT) Trial7 List A/Post B test of Learning & Memory and acute Hg exposure among boys genotyped as WT (dashed line, open symbols) or Het/Mut (solid line, closed symbols) for brain-derived neurotropic factor (BDNF) rs6265. Liner regression r2s are 0.031 and 0.156, respectively, indicating that Hg exposure accounted for 3.1% of performance variance among boys with WT status but for 15.6% of performance variance among boys with Het or Mut gene status.
Figure 1G. Association between performance on the Stroop Word test of Learning & Memory and cumulative (chronic) Hg exposure among boys genotyped as no deletion (dashed line, open symbols) or deletion mutation (solid line, closed symbols) for glutathione-S-transferase theta-1 (GSTT1). Liner regression r2s are 0.0003 and 0.114, respectively, indicating that Hg exposure accounted for essentially 0% of performance variance among boys without the deletion mutation but for 11.4% of performance variance among boys with the deletion polymorphism.
Figure 1H. Association between performance on the WAIS III Digit Span test of Visual Spatial acuity and cumulative (chronic) Hg exposure among boys genotyped as WT (LL) (dashed line, open symbols) or deletion (LS or SS) (solid line, closed symbols) for solute carrier 6A4 (SLC6A4), also known as 5-HTTLPR. Liner regression r2s are 0.011 and 0.175, respectively, indicating that Hg exposure accounted for 1.1% of performance variance among boys without the deletion mutation but for 17.5% of performance variance among boys with one (LS) or two (SS) alleles for the deletion polymorphism.
3.1.2 MT1M and MT2A
The Metallothioneins (MT) are a multigene family of proteins that are involved in Hg distribution and excretion (Aschner et al., 2006). Two MT isoforms, MT1 and MT2, in particular, are implicated in the dispersal and storage of Hg in the CNS (West et al., 2008). Studies in humans have suggested that SNPs of these MT isoforms, MT1M (rs2270837) and MT2A (rs10636), affect Hg body burden, and therefore potentially modify the adverse effects of Hg on neurobehavioral functions (Gundacker et al., 2007; Wang et al., 2012). Testing this hypothesis in children, we observed numerous significant adverse effects, particularly in relation to chronic Hg exposure, among boys genotyped as HetMut for either MT1M or MT2A, occurring principally within the domains of Visual Spatial acuity and Learning & Memory, but also on tests of Attention and Motor function (Table 4). A scatter plot depicting the association between performance on the ravlt tr1-5 test of Learning & Memory and chronic Hg exposure among boys genotyped as WT or Het/Mut for MT1M rs2270837 is shown in Figure 1C. Additionally, we found significant potentiation of the adverse effects of chronic Hg exposure on tests of Attention, Visual Spatial acuity, Learning & Memory, and Motor Function among boys carrying HetMut variants of both MT1M and MT2A genes (Woods et al., 2013). In contrast, we found no significant modification of Hg effects on any tests of neurobehavioral function among boys genotyped as WT for either gene. Among girls, we found significant modification of Hg effects on neurobehavioral function only for the ravlt tr8 test of Learning and Memory (p<0.01)in relation to chronic Hg exposure among those genotyped as HetMut for MT1M rs70837 (Table 5). These results support a role for metallothioneins in protection from the adverse neurobehavioral effects of Hg in children and demonstrate increased susceptibility to those adverse effects among boys carrying Het or Mut variants of these genes. Detailed findings are published (Woods et al., 2013).
3.1.3 COMT
Catechol-O-methyltransferase (COMT) is a ubiquitously expressed enzyme that maintains neurologic functions by regulating the availability of key neurotransmitters such as dopamine (Weinshilboum et al., 1999). Polymorphisms in the COMT gene that modify the activity of the COMT enzyme are known to underlie a number of neuropsychiatric disorders as well as process-specific functions that accompany neurobehavioral tasks (Barnett et al., 2009; Nackley et al., 2009). We examined the hypothesis that 4 genetic variants of COMT (rs4680, rs4633, rs4818 and rs6269), individually or in combination, would modify the adverse neurobehavioral effects of Hg exposure in children. Analyses demonstrated that 3 of these 4 COMT SNPs (rs4680, rs4633 and rs6269) modified the effects of Hg exposure on a wide range of neurobehavioral functions within the domains of Attention and Learning & Memory, among boys. We found comparable effects among boys having a COMT haplotype that included the Mut form of rs4680 but not among those whose haplotype did not include rs4680 Mut. Therefore, we attribute the adverse Hg effects observed to rs4680, a variant form of COMT common to approximately 40% of subjects in the general population. Scatter plots depicting the associations between specific tests of neurobehavioral performance and acute Hg exposure among boys genotyped as WT or Mut for COMT rs4680 are shown in Figures 1D and 1E.
In additional studies, we evaluated the potential contribution of CPOX4, MT1M and MT2A to Hg effects on neurobehavioral functions among subjects genotyped as COMT WT or Mut, to test the possibility that clusters of SNPs of different genes affecting the same test outcomes would explain more of the effects associated with COMT variants alone. Significantly less impaired performance was observed in each case among both boys and girls when CPOX or MT variants were included in the models, suggesting that these variants do not act in concert to modify the effects of Hg on neurobehavioral functions among subjects genotyped as COMT Mut. Details of these findings are published (Woods et al., 2014).
3.2. Genetic Variants that modify the effects of Hg on a Limited Range of Neurobehavioral functions in Children
Among the 13 genes evaluated (Table 2), 8 were found to have variant forms that significantly modified the effects of Hg exposure on neurobehavioral test performance within a limited number of neurologic domains in children. We note that, for all genetic variants evaluated, the numbers of significant findings may reflect the limited population size when stratified by gender and may be more fully substantiated when assessed in a larger population of Hg-exposed subjects.
3.2.1 TDO2
Tryptophan 2,3-dioxygense (TDO2) is the first and rate-limiting enzyme in the catabolism of tryptophan, the precursor of serotonin (5-HT). TDO2 is a hemoprotein whose synthesis and activity are dependent on heme availability (Ren and Correia, 2000). Thus, heme deficiency impairs TDO2 activity and tryptophan degradation, leading to increased tryptophan availability and, consequently, increased brain 5-HT levels. Elevated 5-HT in the CNS may underlie neurobehavioral disorders associated with porphyria (Litman and Correia, 1983) and autism (Nabi et al., 2004) as well as with increased anxiety-related behaviors (Kanai et al., 2009). We evaluated the possibility that genetic variants of TDO2 that presumably compromise TDO2 activity and increase 5-HT levels would exacerbate the adverse effects of Hg on neurobehavioral functions in children. Among the 7 TDO2 SNPs we evaluated (Table 2), only rs3755907 (TDO2-907) substantially modified the adverse effects of Hg exposure among children in this study. Significant effects among boys were seen principally on tests of Motor function including pegs dominant (p<0.05), pegs non-dominant (p<0.04) and finger tapping dominant (p<0.04) relative to acute Hg exposure. Significant effects among boys were also observed on the Stroop word test of Attention (p<0.05) and the SRT test of Visual-Spatial acuity (p<0.005) in relation to chronic Hg exposure (Table 4). In contrast, among girls, tests of Executive Function and Learning & Memory were modified in relation to chronic Hg exposure, however, in the direction of improved performance (Table 5). SNP rs3755907 (TDO2-907) is in linkage disequilibrium with rs3755908, rs3755910 and rs3775085 (Comings et al., 1996), but we found no modification of Hg effects among subjects who were HetMut for these SNPs. It is possible, therefore, that effects observed in relation to TDO2-907 are the result of chance observation. Additionally, we evaluated the effects of Hg on neurobehavioral functions among boys genotyped as HetMut for both TDO2-907 + CPOX4 (rs1131857), hypothesizing that the activity of the enzyme encoded by TDO2-907 would be further compromised by heme deficiency associated with CPOX4 + Hg (Li and Woods, 2009). We found no substantial differences in test performance among such subjects than observed among boys genotyped as CPOX4 and Hg alone.
3.2.2 GRIN2A and GRIN2B
GRIN2A and GRIN2B (glutamate receptor, ionotropic, N-methyl D-aspartate 2A and 2B) encode subunits of N-methyl-D-aspartate (NMDA) glutamatergic receptors, which mediate excitatory neurotransmission in the CNS. The NMDA channel has been shown to be involved in long-term potentiation, an activity-dependent increase in the efficiency of synaptic transmission thought to underlie Attention as well as Learning & Memory domains that are detrimentally affected by Hg (Adams et al., 2004; Baraldi et al., 2002; Endele et al., 2010). We assessed the possibility that variants of these genes would increase susceptibility to the adverse effects of Hg exposure in children, particularly on tests within the domains of Attention and Learning & Memory.
For GRIN2A, we found 4 significant genexHg interactions among boys genotyped as HetMut for GRIN2A rs727605, including 2 tests of Attention (Stroop Word [p<0.03] and Spatial Span [p<0.05]) as well as one test of Learning & Memory (ravlt tr1 [p<0.04]) (Table 4). Among boys genotyped as HetMut for GRIN2A rs1070503, we found 2 significant interactions including ravlt tr5 (Learning & Memory)(p<0.05) and Finger Tapping-non dominant (Motor function) (p<0.006). We also found 4 significant genexHg interactions among boys who were HetMut for both GRIN2A SNPs. These include ravlt Sum 1-8 (Learning& Memory) (p<0.04), WCS #categories correct (Executive Function) (p<0.009), Finger Tapping-non-dominant (Motor function) (p<0.004), and WMS-III Spatial span (Attention) (p<0.02) (not shown). Additionally, since heme is postulated to regulate the expression of N-methyl-D-aspartate receptors in neurons (Chernova et al., 2006; Smith et al, 2012), we evaluated the effects of Hg on neurobehavioral functions among boys genotyped as HetMut for [GRIN2A rs727605 + CPOX rs1131857] or [GRIN2A rs100503 + CPOX rs1131857], hypothesizing that GRIN2A-associated glutamatergic processes that regulate cognition via NMDA receptors (Adams et al., 2004) would be further compromised by heme deficiency associated with CPOX rs1131857 (CPOX4) +Hg (Li and Woods, 2009). Consistent with this hypothesis, both [GRIN2A rs727605 + CPOX4] and [GRIN2A rs1070503 + CPOX4] were associated with impaired the Executive Function test WCS #categories correct, (p<0.04 and p<0.05, respectively) among boys genotyped as HetMut for both variants in relation to acute Hg exposure. These findings support the view that both GRIN2A and CPOX participate in the processing of glutamatergic receptors effecting development of executive function and that compromised processing associated with SNPs of either or both genes is further impaired by Hg exposure. No comparable interactive effects were observed among girls.
For GRIN2B, we found no significant genexHg interactions among boys genotyped as HetMut for rs1806201 for any tests of neurobehavioral function. However, among boys genotyped as HetMut for GRIN2B rs7301328, we observed significant genexHg interactions for ravlt tr8 (Learning & Memory)(p<0.07), WSM-III visual reproductions delayed (Learning & Memory)(p<0.004), and Finger Tapping-non dominant (Motor)(p<0.003) (Table 4). These findings suggest modification of Hg effects by SNPs of GRIN2A and/or GRIN2B on tests of Attention and Learning & Memory as well as for Motor function among boys. Among girls (Table 5), we found significant effect modification only by GRIN2B rs7301328 on ravlt tr7 (p<0.05) in relation to acute Hg exposure, and ravlt tr8 (p<0.03) in relation to chronic Hg exposure, both tests of Learning & Memory. These findings are consistent with the postulated role of GRIN2A and GRIN2B in neuronal mechanisms underlying Attention and Learning & Memory and an adverse effect of Hg on these processes among subjects carrying variant forms of these genes.
3.2.3 BDNF
Brain-derived neurotrophic factor (BDNF) is a protein that regulates neuronal growth and differentiation in the peripheral and central nervous systems. A SNP (rs6265) at nucleotide 196 (G>A) in the gene encoding BDNF, resulting in a V66M substitution in the gene product, is associated with deficits in memory and motor skills and adverse mood as well as impairment of neurodevelopmental function in humans (Egan et al., 2003). Previous studies identified adults who were HetMut for rs6265 as having increased risk of neurobehavioral deficits associated with low-level occupational Hg exposure (Echeverria et al., 2005; Heyer et al., 2004). In the present study, we observed significant genexHg interactions among boys genotyped as HetMut for rs6265 for several tests of Learning & Memory, including ravlt tr7 (p<0.01), ravlt tr8 (p<0.04) and ravlt sum tr1-8 (p<0.04) in relation to acute Hg exposure (Table 4) . We also observed a significant genexHg interaction among girls for ravlt tr1 (p<0.008) in relation to acute Hg exposure and for ravlt tr8 (p<0.03) in relation to chronic Hg exposure (Table 5). These findings suggest potential modification of Hg effects by BDNF rs6265 on tests of Learning and Memory among both boys and girls. A scatter plot depicting the association between performance on the ravlt tr7 test of Learning & Memory and chronic Hg exposure among boys genotyped as WT or Het/Mut for BDNF rs6265 is shown in Figure 1F.
3.2.4 GSTT1
GSTT1 encodes glutathione S-transferase theta-1, a member of a superfamily of enzymes that catalyze the conjugation of reduced glutathione (GSH) to a variety of electrophilic and hydrophilic ligands including Hg (Goodrich et al., 2011). GSTT1 has a deletion polymorphism that results in impaired (or null) catalytic activity that may be associated with increased Hg body burden (Gundacker et al., 2007, 2009; Schläwiche Engström et al., 2008). We evaluated the effects of Hg exposure on neurobehavioral functions among children genotyped for the presence or absence of this deletion mutation. As shown in Table 4, we found significant interactions with chronic Hg exposure on tests of Attention (Stroop word [p<0.004]) and Learning & Memory (CMVT d-Prime [p<0.05]) and with acute Hg exposure for Pegs dominant (Motor) (p<0.03) among boys genotyped as having the GSTT1 deletion mutation. In addition, we observed a significant interaction with chronic Hg exposure for ravlt sum tr1-5 (Learning & Memory)(p<0.02) among girls with the deletion mutant (Table 5). These findings suggest potential modification of Hg effects among both boys and girls with deletion mutations of GSTT1, consistent with increased Hg body burden observed among subjects with this variant (Gundacker et al., 2007). A scatter plot depicting the association between performance on the Stroop Word test of Attention and chronic Hg exposure among boys genotyped as WT (no deletion) or Het/Mut (deletion mutation) for GSTT1 is shown in Figure 1G.
3.2.5 SLC6A4
Solute Carrier 6A4, also known as 5-hydroxytryptamine (5-HT, serotonin) transporter (5-HTT), transports the neurotransmitter, serotonin, into presynaptic neurons and, hence, determines the magnitude and duration of serotonergic responses. A functional polymorphism in the promoter region of the 5-HTT gene known as the short (S) allele (5-HTTLPR-S) that decreases 5-HTT function has been reported to be associated with behavioral deficits, depressive symptoms, diagnosable depression, suicidal behavior and excessive shyness in children (Arbelle et al., 2003; Caspi et al., 2003; Lesch et al., 1996). Affective spectrum disorders including anxiety, depression and aggression-related personality traits associated with this polymorphism are also reported (Collier et al., 1996). Since serotonergic neurotransmission influences many brain functions that may also be altered by Hg exposure, including cognition, attention, mood, social interaction and motor function, we hypothesized that a polymorphism that alters the functional expression of the 5-HTT gene might exacerbate Hg-induced neurobehavioral deficits in children. We evaluated the effects of Hg exposure among children genotyped as 5-HTTLPR-L (WT) versus those genotyped as LS or SS (variant). We observed significant variantxHg interactions for 2 tests of Attention, i.e.,WAIS-III- digit span (p<0.01) and Trails A (p<0.04) and 2 tests of Learning & Memory (ravlt tr6 List B (p<0.05) and ravlt sum 1-8 (p<0.003) among boys with chronic Hg exposure (Table 4). No effect modification, however, was observed for either acute or chronic Hg exposure among girls genotyped with the variant form. These findings suggest the S allele of 5-HTTLPR may predispose boys to increased risk of adverse neurologic effects of Hg exposure. A scatter plot depicting the association between performance on the Digit Span test of Visual Spatial acuity and chronic Hg exposure among boys genotyped as WT or Het/Mut for the 5-HTTLPR deletion mutation is shown in Figure 1H.
3.2.6 KIBRA
The protein KIBRA (kidney and brain expressed protein), also known as WW domain-containing protein 1 (WWC1), is implicated in human cognition via an association of the intronic SNP rs17070145 with episodic memory performance in humans (Papassotiropoulos et al., 2006). This SNP is a common T>C exchange within the 9th intron of the KIBRA gene. CC carriers perform poorer on verbal and visual episodic memory tasks than CT and TT carriers (Schaper et al 2008). Neither the effect of this SNP on memory of healthy children nor its potential interaction with Hg exposure on memory or other neurobehavioral functions is known. We evaluated the effects of Hg exposure among children genotyped as CC (homozygous mutant) for KIBRA. Among boys, we observed significant genexHg interactions for ravlt tr7 List A (p<0.03) and ravlt sum tr1-5 (p<0.01) in the Learning & Memory domain, for Trails A (Attention) (p<0.02), and for WAIS-III symbol search (Visual Spatial) (p<0.01) for chronic Hg exposure (Table 4). No significant genexHg interactions were observed among girls. These findings suggest potential modification of Hg effects on tests of Learning & Memory as well as other domain functions among boys carrying the Mut variant of KIBRA rs17070145.
3.2.7 APOE
Apolipoprotein E (APOE) is an intracellular cholesterol and fatty acid transport protein that plays an important function in neuronal metabolism as well as in synaptogenesis during neurodevelopment (Buttini et al., 1999). Three common alleles of the APOE gene (ε2, ε3 and ε4) differ only on the basis of 1 or 2 amino acids on positions 112 and 158 (Wright et al., 2003). The APOE ε4 allele is associated in some studies with poor neural repair function and has been found to modify the adverse effects of prenatal Hg exposure on tests of neurobehavioral function among 2 year-old children (Ng et al., 2013). In the present study, we genotyped children for the principal alleles of the SNPs (rs429358, rs7412) encoding ε2, ε3 and ε4 and evaluated the effects of Hg exposure on neurobehavioral functions among groups genotyped as ε3 (ε3/ε3) versus ε4 (ε3/ε4 + ε4/ε4). We found only 1 significant genexHg interaction among boys genotyped as ε4, specifically, ravlt sum tr1-8 (Learning & Memory)(p<0.02) (Table 4). Among girls, we observed 2 significant genexHg interactions, Stroop color word (Attention)(p<0.04) and Finger tapping dom (Motor) (p<0.005), both in the direction of improved performance (Table 5). While these findings may be random in terms of suggesting an effect of APOE ε4 on the neurobehavioral effects of Hg in children, they are nonetheless consistent with those previously described by others (Ng et al., 2013) and may be more clearly substantiated if assessed in a larger population of Hg-exposed subjects.
3.2.8 SEPP1
SEPP1 encodes a selenoprotein (Selenoprotein P) containing multiple selenocysteine residues that confer antioxidant and metal binding functions (Goodrich et al., 2011). Several variants encoding different isoforms have been found for this gene, although no studies describing their effects on human behavior or performance have been reported. However, SEPP1 knock-out mice have low brain Se concentrations and develop progressive neurological dysfunction characterized by impaired movement and coordination (Nakayama et al., 2006). We evaluated the effects of Hg exposure on neurobehavioral functions among children genotyped as HetMut for the relatively common 3'UTR rs7579 SEPP1 variant. We found no significant interactions in relation to either acute or chronic Hg exposure.
4. Discussion
4.1. Genetic variation in the assessment of Hg risk
This study identifies significant adverse effects of low-level Hg exposure (such as that acquired from diet or dental amalgams) on a wide range of neurobehavioral functions among children who carry genetic variants that are relatively common within the general human population. These findings have potentially important implications for assessment, prediction and management of risks of neurobehavioral dysfunction associated with Hg exposure in children and adults as well. Foremost, the minor allelic frequencies (MAFs) for most of the individual SNPs evaluated in the present study cohort ranged from between 20% and 40%, accounting for the relatively high prevalence of adverse effects among subjects in response to Hg exposure observed. Inasmuch as the present study population was largely of European (Caucasian) ancestry, the findings of susceptibility to Hg toxicity observed here should be predictably similar among all populations of comparable descent. Moreover, although substantial differences in allelic frequencies of individual SNPs are known to occur across populations of different lineage, e.g., European, African, Asian, it is notable that common combinations of alleles (haplotypes) are largely shared across different populations (Gabriel et al., 2002), suggesting that the likelihood of genetic susceptibility to Hg toxicity observed here will be broadly relevant across other population groups. We note in this context that the present findings demonstrate genetic modification of neurobehavioral test outcomes in relation to Hg exposure but not specifically by Hg alone. Such findings point to the importance of genetic variation both as an etiologic factor underlying susceptibility to Hg toxicity as well as for assessment, prediction and management of Hg risk.
4.2. Children are more susceptible than adults to genetic modification of Hg neurotoxicity
The findings of the present study support and substantiate the well-established view that children have heightened susceptibility to the adverse effects of environmental chemical exposures compared with adults, particularly, with regard to Hg exposure. Here, children who were genotyped with common variants of genes that have been previously shown to elicit no or only additive effects with Hg on neurobehavioral functions in adults, e.g., BDNF, CPOX, COMT, 5-HTTLPR (Echeverria et al., 2005, 2006, 2010; Heyer et al., 2004, 2008, 2009), displayed numerous significant interactive effects on comparable tests of neurobehavioral function at similar levels of Hg exposure. Since adverse neurobehavioral effects were found only among children having gene modifications, genexHg interactions are assumed to underlie these effects. In this regard, Hg directly affects dopaminergic (Eddins et al., 2008), serotonergic (Tsai et al., 1995) and other neurologic processes (Castoldi et al., 2008; Pieper et al., 2014), and these effects are likely to be exaggerated against enzyme or protein products of genetic variants that mediate or facilitate developing neurologic pathways. Of note, Hg2+ directly impairs the enzymatic activity of CPOX4 (Li and Woods, 2009) and also likely inhibits preferentially the S-containing met forms of the COMT val158met enzyme and the BDNF val66met protein product, both of which play critical roles in neurologic development (Barnett et al., 2009; Burkhalter et al., 2003). Moreover, genetic variants affecting Hg toxicokinetics, e.g., MT1M, MT2A, GSTT1 (Wang et al., 2012), might predispose children to higher concentrations of Hg in critical brain regions than would occur in adults with comparable exposures. Such effects directed against components of the developing central nervous system could underlie the preferential susceptibility of children and adolescents with these genotypes to the adverse neurologic effects of Hg exposure. Additionally, from a genomic perspective, pre-pubertal Hg exposure might act epigenetically to alter the timing of normal developmental regulation of gene expression and/or gene products in such as way as to maximize the impact of Hg interactions in younger children. Of note, epigenetic mechanisms have been reported to underlie the timing of toxic effects of a number of environmental chemicals, including Hg (Baccarelli and Bollati, 2009), and these alterations are particularly manifest in early development (Fleming et al., 2008). Epigenetic factors affecting the timing of gene expression might also provide a plausible mechanistic explanation for genexHg interactions whose effects were expressed differentially as a consequence of acute, e.g., COMT, BDNF, GRIN2A, GRIN2B, versus chronic, e.g., CPOX4, MT2A, GSTT1, 5-HTTLPR, KIBRA, Hg exposure among children in this investigation. Further studies are required to identify and elaborate the mechanisms by which the toxic effects of Hg are preferentially expressed in relation to genotype and timing of Hg exposure in children versus adults.
4.3. Boys are more susceptible to genetic modification of Hg neurotoxicity than girls
In all cases in which we observed significant genetic modification of Hg effects on neurobehavioral functions, such effects were found to be substantially more prominent among boys than girls in terms of both numbers of neurobehavioral domains as well as tests within such domains affected. Moreover, few adverse effects of Hg by sex were observed in the absence of genetic variation. These findings support the view that genetic predisposition is a significant factor in defining sex differences in susceptibility to the adverse neurobehavioral effects of Hg, particularly in children. Factors underlying these sex-specific effects may include differences in Hg toxicokinetics that predispose boys to greater Hg retention and tissue accumulation than girls. Notably, significant differences in Hg excretion between boys and girls were observed in the clinical trial from which subjects in the present study were drawn (Woods et al, 2007), suggesting that boys may retain and accumulate significantly higher levels of Hg than girls over the course of comparable Hg exposure. Findings from animal studies (Hultman and Nielsen, 2001) demonstrating significantly higher renal Hg concentrations and whole body Hg retention in male compared with female mice following prolonged HgCl2 exposure in the drinking water support this view. Genetic and hormonal differences affecting brain development, morphology and function between boys and girls might also underlie the sex-differences observed with respect to effects of genotype on Hg-related neurobehavioral functions. In this respect, estrogen regulation of COMT transcription (Xie et al., 1999) corresponds to significant sex differences in dopamine levels in the prefrontal cortex and other tissues (Harrison and Tunbridge, 2008), potentially contributing to sex differences in response to Hg exposure. Similarly, sex hormone regulation of BDNF signaling mediates sexually dimorphic axonal growth, resulting in generation of sex-specific neural circuits (Liu et al., 2012), also potentially underlying differences in response of boys and girls to Hg exposure (Spulber et al., 2010). Moreover, as noted above, epigenetic factors that differentially affect the timing, duration and magnitude of gene expression are also likely to contribute to the sex differences in susceptibility to Hg neurotoxicity observed (Jirtle and Skinner, 2007; Basu et al., 2013; Goodrich et al., 2013). In this regard, significant differences in DNA methylation patterns, methyl transferases, methyl binding proteins, and co-repressor proteins are currently recognized as contributing to the establishment and maintenance of sex differences in the brain and behavior (McCarthy et al., 2009), providing a plausible mechanistic basis for sex differences in response to Hg observed, both with respect to the numbers of genes and related neurologic processes affected as well as in the overall scope and direction of responses between boys and girls. Consistent with this view, the present findings are not unique to Hg exposure. Many classes of drugs and chemicals including other metals, pesticides, halogenated hydrocarbons and constituents of cigarette smoke are reported to differentially affect neurologic functions in males and females, particularly children (Woods et al., 2012). The finding of sex-specific responses to Hg exposure observed here in relation to common genetic variants highlights the importance of considering such differences in the development of strategies aimed as risk reduction from adverse Hg effects, particularly among children.
4.4. Implications for future research
The present studies focused exclusively on a candidate gene approach toward defining susceptibility to Hg neurotoxicity in children, based on a priori knowledge of genes whose variants are known or suspected to modify Hg handling and/or to increase the risk of neurologic disorders in humans. Although most of the variants investigated adversely modified the effects of Hg exposure on neurologic functions in children, the relatively small population size may have precluded detection of other significant genexHg effects associated with some variants as well as identification of potential effects associated with combinations of the variants evaluated. Replication of the present study in other, larger, cohorts is therefore essential both to confirm the present findings as well as to identify additional variants contributing to Hg toxicity. Beyond the present study design, investigations aimed at defining common pathways through which these and other variants might act to modulate the adverse effects of Hg could substantially advance our understanding of Hg toxicity. In particular, studies utilizing genome-wide genotyping and network modeling techniques (e.g., Schadt et al., 2009), could allow more comprehensive genetic characterization of variants that potentially influence Hg toxicity and elucidate a broader set of pathways and/or biological subnetworks that underlie health risk susceptibilities from Hg exposure. A pathway-based approach to Hg risk assessment could also enhance abilities to make connections between genes and pathways underlying Hg neurotoxicity with those associated with other neurologic health risks, such as attention deficit or autism spectrum disorders. Additionally, investigation of epigenetic factors underlying global expression of genes through which Hg neurotoxicity is predominantly mediated may allow better characterization of timing, magnitude, direction and gender-specificity of these effects. It is already clear from these and other studies cited herein that the genes being identified expose relevant biology. Ultimately, elucidation of genome-wide pathways underlying Hg neurotoxicity will improve the power for prediction both of Hg-mediated adverse health risks as well as better inform the extent to which Hg toxicity can be mitigated by prevention of Hg exposure.
4.5. Conclusions
Numerous common genetic polymorphisms affect susceptibility to the adverse neurobehavioral effects of Hg in children. The genexHg interactions identified in this study contribute to public health goals to define factors underlying susceptibility to Hg toxicity and suggest risk assessment and intervention strategies for preventing adverse health effects associated with Hg exposure, irrespective of source, within the general population. The present findings also establish a scientific framework for additional candidate gene studies in other Hg-exposed populations as well as more comprehensive genome-wide, network-based studies to define multiple genetic variants and associated biochemical pathways underlying susceptibility to Hg neurotoxicity, particularly in children.
Highlights.
Genotype determines the effects of Hg on neurobehavioral functions in children
Boys are more susceptible to genetic modification of Hg neurotoxicity than girls
Multiple common variants underlie the wide prevalence of Hg neurotoxicity
Genes identified expose relevant biology underlying susceptibility to Hg toxicity
Acknowledgements
We thank Jasmine Wilkerson, M.S. and Jesse M. Tsai, B.S., Functional Genomics and Proteomics Laboratory, Center for Ecogenetics and Environmental Health, University of Washington, for excellent technical assistance in the conduct of this study. We also thank Mário F. Bernardo, D.M.D., Ph.D. and Lurdes Vaz, D.D.S., Faculdade de Medicina Dentaria, Universidade de Lisboa, Lisbon, Portugal, for extraordinary effort in subject recruitment and acquisition of biological samples for this study. Finally, we acknowledge Henry B. Wallace, humanitarian, altruist and friend, for his enduring support of these research activities.
Funding
This work was supported by grants R21ES019632, P42ES04696, P30ES07033 to the University of Washington from the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health. Additional funding was provided by the Wallace Research Foundation. Funding source organizations had no direct involvement in any aspect of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Adams J, Crosbie J, Wigg K, Ickowicz A, Pathare T, Roberts W, et al. Glutamate receptor, ionotropic, N-methyl D-aspartate 2A (GRIN2A) gene as a positional candidate for attention-deficit/hyperactivity disorder in the 16p13 region. Molec. Psychiat. 2004;9:494–499. doi: 10.1038/sj.mp.4001455. [DOI] [PubMed] [Google Scholar]
- Arbelle S, Benjamin J, Golin M, Kremer I, Belmaker RH, Ebstein RP. Relation of shyness in grade school children for the long form of the serotonin transporter promoter region polymorphism. Amer. J. Psychiatr. 2003;160:671–676. doi: 10.1176/appi.ajp.160.4.671. [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JBT. Metallothioneins: mercury species-specific induction and their potential role in attenuating neurotoxicity. Exp. Biol. Med. 2006;231:1468–1473. doi: 10.1177/153537020623100904. [DOI] [PubMed] [Google Scholar]
- Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr. Opin. Pediatr. 2009;21:243–251. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraldi M, Zanoli P, Tascedda F, Blom JMC, Brunello N. Cognitive deficits and changes in gene expression of NMDA receptors after prenatal methylmercury exposure. Environ. Health Perspect. 2002;110:855–858. doi: 10.1289/ehp.02110s5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, Heron J, Goldman D, Jones PB, Xu K. Effects of catechol-O-methyltransferase on normal variation in the cognitive function of children. Am. J. Psychiatry. 2009;16:909–916. doi: 10.1176/appi.ajp.2009.08081251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu N, Goodrich JM, Head J. Ecogenetics of mercury: From genetic polymorphisms and epigenetics to risk assessment and decision making. Environ. Toxicol. Chem. 2013 doi: 10.1002/etc.2375. DOI: 10.1002/etc.2375. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Trachtenberg F, Barregard L, Tavares M, Chernichiari E, Daniel D, et al. Neuropsychological and renal effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006;295:1775–1783. doi: 10.1001/jama.295.15.1775. [DOI] [PubMed] [Google Scholar]
- Burkhalter J, Fiumelli H, Allaman I, Chatton J-Y, Martin JL. Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J. Neurosci. 2003;23:8212–8220. doi: 10.1523/JNEUROSCI.23-23-08212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M, Orth M, Bessosta S, Akeefe H, Pitas RE, Wyss-Coray T. Expression of human apolipoprotein E3 or E4 in the brains of Apoe−/− mice: isoform-specific effects on neurodegeneration. J. Neurosci. 1999;19:4867–4880. doi: 10.1523/JNEUROSCI.19-12-04867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capsi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braitwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Castoldi AF, Onishchenko N, Johansson C, Coccini T, Roda E, Vahter M, Ceccatelli S, Manzo L. Neurodevelopmental toxicity of methylmercury: Laboratory animal data and their contribution to human risk assessment. Reg. Toxicol. Pharmacol. 2008;51:215–229. doi: 10.1016/j.yrtph.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention National Health and Nutrition Examination Survey. 2007 Available: http://www.cdc.gov/nchs/nhanes.htm [24 July2014]
- Chernova T, Nicotera P, Smith AG. Heme deficiency is associated with senescence and causes suppression of N-methyl-D-aspartate receptor subunits expression in primary cortical neurons. Molec. Pharmacol. 2006;69:697–705. doi: 10.1124/mol.105.016675. [DOI] [PubMed] [Google Scholar]
- Clarkson TW. The three faces of mercury. Environ. Health Perspect. 2002;110(Suppl 1):11–23. doi: 10.1289/ehp.02110s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Gade R, Muhleman D, Chiu C, Wu S, et al. Exon and intron variants in the human tryptophan 2,3-dioxygenase gene: potential association with Tourette syndrome, substance abuse, and other disorders. Pharmacogenet. 1996;6:407–418. doi: 10.1097/00008571-199608000-00004. [DOI] [PubMed] [Google Scholar]
- Collier DA, Stober G, Li T, Heils A, Catalano M, Di Bella D. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Molec. Psychiatr. 1996;1:434–436. et l. [PubMed] [Google Scholar]
- DeRouen TA, Leroux BG, Martin MD, Townes BD, Woods JS, Leitão J, Castro-Caldas A, Braveman N. Issues in the design and analysis of a randomized clinical trial to assess the safety of dental amalgam restorations in children. Contr. Clin. Trials. 2002;23:301–320. doi: 10.1016/s0197-2456(01)00206-9. [DOI] [PubMed] [Google Scholar]
- DeRouen TA, Martin MD, Leroux BG, Townes BD, Woods JS, Leitão J, Castro-Caldas A, Luis H, Bernardo M, Rosenbaum G, Martins IP. Neurobehavioral effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006;295:1784–1792. doi: 10.1001/jama.295.15.1784. [DOI] [PubMed] [Google Scholar]
- Echeverria D, Heyer NJ, Martin MD, Naleway CA, Woods JS, Bittner AC. Behavioral effects of low-level exposure to Hg0 among dentists. Neurotoxicol. Teratol. 1995;17:161–168. doi: 10.1016/0892-0362(94)00049-j. [DOI] [PubMed] [Google Scholar]
- Echeverria D, Woods JS, Heyer NJ, Martin MD, Rohlman DS, Farin FM, Li T. The association between serotonin transporter gene promotor polymorphism (5-HTTLPR) and elemental mercury exposure on mood and behavior in humans. J. Toxicol. Environ. Health. Pt. A. 2010;73:1003–1020. doi: 10.1080/15287390903566591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria D, Woods JS, Heyer NJ, Rohlman DS, Farin FM, Bittner AC, Jr., et al. Chronic low-level mercury exposure, BDNF polymorphism, and associations with cognitive and motor functions. Neruotoxicol. Teratol. 2005;27:781–796. doi: 10.1016/j.ntt.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Echeverria D, Woods JS, Heyer NJ, Martin MD, Rohlman DS, Farin FM, Li T, Garabedian CE. The association between a genetic polymorphism of coproporphyrinogen oxidase, dental mercury exposure and neurobehavioral response in humans. Neurotoxicol. Teratol. 2006;28:39–48. doi: 10.1016/j.ntt.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Eddins D, Petro A, Pollard N, Freedman JH, Levin ED. Mercury-induced cognitive impairment in metallithionein-1/2 null mice. Neurotoxicol. Teratol. 2008;30:88–95. doi: 10.1016/j.ntt.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of DBDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Endele S, Rosenberger G, Geider K, Popp B, Tamer C, Stefanova I, et al. Mutations in GRIN2A and GRIN2B encoding regulatory subunits of NMDA receptors cause variable neurodevelopmental phenotypes. Nature Genet. 2010;42:1021–1026. doi: 10.1038/ng.677. [DOI] [PubMed] [Google Scholar]
- Fleming JL, Huang TH, Toland AE. The role of parental and grandparental epigenetic alterations in familial cancer risk. Cancer Res. 2008;68:9116–9121. doi: 10.1158/0008-5472.CAN-08-2184. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Goodrich JM, Basu N, Franzblau A, Dolinoy DC. Mercury biomarkers and DNA methylation among Michigan dental professionals. Environ. Mol. Mutagen. 2013;54:195–203. doi: 10.1002/em.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich JM, Wang Y, Gillespie B, Werner R, Franzblau A, Basu N. Glutathione enzyme and selenoprotein polymorphisms associate with mercury biomarker levels in Michigan dental professionals. Toxicol Appl. Pharmacol. 2011;257:301–308. doi: 10.1016/j.taap.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol. Teratol. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Gundacker C, Komarnicki G, Jagiello P, Gencikova A, Dahman N, Wittmann KJ, et al. Glutathione-S-transferase polymorphism, metallothionein expression, and mercury levels among students in Austria. Sci. Total Environ. 2007;385:37–47. doi: 10.1016/j.scitotenv.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Gundacker C, Wittmann KJ, Kukuckova G, Hikkel I, Gencik M. Genetic background of lead and mercury metabolism in a group of medical studies in Austria. Environ. Res. 2009;109:786–796. doi: 10.1016/j.envres.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): A gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacol. 2008;33:3037–3045. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- Heyer NJ, Echeverria D, Bittner AC, Jr., Farin FM, Garabedian CC, Woods JS. Chronic low-level mercury exposure, BDNF polymorphism, and associations with self-reported symptoms and mood. Toxicol. Sci. 2004;81:354–363. doi: 10.1093/toxsci/kfh220. [DOI] [PubMed] [Google Scholar]
- Heyer NJ, Echeverria D, Farin FM, Woods JS. The association between serotonin transporter gene promotor polymorphism (5-HTTLPR), self-reported symptoms, and dental mercury exposure. J. Toxicol. Environ. Health, Part A. 2008;71:1381–1386. doi: 10.1080/15287390802240850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer NJ, Echeverria D, Martin MD, Farin FM, Woods JS. Catechol O-Methyltransferase (COMT) VAL158MET functional polymorphism, dental mercury exposure and self-reported symptoms and mood. J. Toxicol. Environ. Health, Part A. 2009;72:599–609. doi: 10.1080/15287390802706405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman P, Nielsen JB. The effect of dose, gender, and non-H-2 genes in murine mercury-induced autoimmunity. J. Autoimmunol. 2001;17:27–37. doi: 10.1006/jaut.2001.0521. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai M, Funakoshi H, Takahashi H, et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Molec. Brain. 2009;2:8. doi: 10.1186/1756-6606-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg EF, Jr., Chrislip DW, Letz RE, Otto DA, Crespo CJ, Brightwell WS, et al. Neurobehavioral test performance in the third National Health and Nutrition Examination Survey. Neurotoxicol. Teratol. 2001;23:569–589. doi: 10.1016/s0892-0362(01)00177-5. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Goldman LR. Children's vulnerability to toxic chemicals: a challenge and opportunity to strengthen health and environmental policy. Health Aff. 2011;30:842–850. doi: 10.1377/hlthaff.2011.0151. [DOI] [PubMed] [Google Scholar]
- Lesch K-P, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related behavior traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Li T, Woods JS. Cloning, expression, and biochemical properties of CPOX4, a genetic variant of coproporphyrinogen oxidase that affects susceptibility to mercury toxicity in humans. Toxicol Sci. 2009;109:228–236. doi: 10.1093/toxsci/kfp066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman DA, Correia MA. L-Tryptophan: A common denominator of biochemical and neurological events of acute hepatic porphyria? Science. 1983;222:1031–1033. doi: 10.1126/science.6648517. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rutlin M, Huang S, Barrick CA, Wang F, Jones KR, Tessarollo L, Ginty DD. Sexually dimorphic BDNF signaling directs sensory innervation of the mammary gland. Science. 2012;338:1357–1360. doi: 10.1126/science.1228258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins IP, Castro-Caldas A, Townes BD, Ferreira G, Rodrigues P, Marques S, Rosenbaum G. Age and sex differences in neurobehavioral performance: A study of Portuguese elementary school children. Internat. J. Neurosci. 2005;115:1687–1709. doi: 10.1080/00207450590958556. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GT, Dunn GA, Forger NG, Murray EK, Nugent BN, Schwartz JM, Wilson ME. The epigenetics of sex differences in the brain. J. Neurosci. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers GJ, Marsh DO, Davidson PW, Cox C, Shamlaye CF, Tanner M, et al. Main neurodevelopmental study of Seychellois children following in utero exposure to methylmercury from a maternal fish diet: outcome at 6 months. Neurotoxicol. 1995;16:677–688. [PubMed] [Google Scholar]
- Nabi R, Serajee FJ, Chugani DC, et al. Association of tryptophan 2,3-dioxygenase gene polymorphism with autism. Amer. J. Med. Genet. 2004;125B:63–68. doi: 10.1002/ajmg.b.20147. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Shabalina SA, Lambert JE, Conrad MS, Gibson DG, Spiridonov AN, Satterfield SK, Diatchenko L. Low enzymatic activity haplotypes of the human catechol-O-methyltransferase gene: Evidence for marker SNPs. PLOS ONE. 2009;4:e5237. doi: 10.1371/journal.pone.0005237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama A, Hill KE, Motley AK, Burk RF. All regions of mouse brain are dependent on selenoprotein P for maintenance of selenium. J. Nutri. 2007;137:690–693. doi: 10.1093/jn/137.3.690. [DOI] [PubMed] [Google Scholar]
- Ng S, Lin C-C, Hwang Y-H, Hsieh W-S, Liao H-Fang., Chen P-C. Mercury, APOE, and children's neurodevelopment. Nerutoxicol. 2013;37:85–92. doi: 10.1016/j.neuro.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Stephan DA, Huentelman MJ, Hoerndli FJ, Craig DW, Pearson JV, et al. Common KIBRA alleles are associated with human memory performance. Science. 2006;314:475–478. doi: 10.1126/science.1129837. [DOI] [PubMed] [Google Scholar]
- Pieper I, Wehe CA, Bornhorst J, Ebert F, Leffers L, Holtkamp M, Höseler P, Weber T, Mangerish A, Bürke A, Karst U, Schwerdtle T. Mechanisms of Hg species induced toxicity in cultured human astrocytes: genotoxicity and DNA-damage response. Metallomics. 2014;6:662–671. doi: 10.1039/c3mt00337j. [DOI] [PubMed] [Google Scholar]
- Pingree SD, Simmonds PL, Woods JS. Effects of 2,3-dimercapto-1-propanesulfonic acid (DMPS) on tissue and urine mercury levels following prolonged methylmercury exposure in rats. Toxicol. Sci. 2001;61:224–233. doi: 10.1093/toxsci/61.2.224. [DOI] [PubMed] [Google Scholar]
- Ren S, Correia MA. Heme: A regulator of rat hepatic tryptophan 2,3-dioxygenase? Arch. Biochem. Biophys. 2000;377:195–203. doi: 10.1006/abbi.2000.1755. [DOI] [PubMed] [Google Scholar]
- Schadt EE, Zhang B, Zhu J. Advances in systems biology and enhancing our understanding of disease and moving us closer to novel disease treatments. Genetica. 2009;136:2259–269. doi: 10.1007/s10709-009-9359-x. [DOI] [PubMed] [Google Scholar]
- Schaper K, Kolsch H, Popp J, Wagner M, Jennen F. KIBRA gene variants are associated with episodic memory in healthy elderly. Neurobiol. Aging. 2008;29:1123–1125. doi: 10.1016/j.neurobiolaging.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Schläwiche Engström KS, Stromberg U, Lundh T, Jonhansson I, Vessby B, Hallmans G, et al. Genetic variation in glutathione-related genes and body burden of methylmercury. Environ. Health Perspect. 2008;116:734–739. doi: 10.1289/ehp.10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LS, Blum JD, Franzblau A, Basu N. New insights into biomarkers of human mercury exposure using naturally occurring mercury isotopes, Environ. Sci Technol. 2013;47:3403–3409. doi: 10.1021/es305250z. [DOI] [PubMed] [Google Scholar]
- Smith AG, Raven EL, Chernova T. The regulatory role of heme in neurons. Metallomics. 2012;3:955–962. doi: 10.1039/c1mt00085c. [DOI] [PubMed] [Google Scholar]
- Spulber S, Rantamäki T, Nikkilä O, Castrén E, Weihe P, Grandjean P, Caccatelli S. Effects of maternal smoking and exposure to methylmercury on brain-derived neurotrophic factor concentrations in umbilical cord serum. Toxicol. Sci. 2010;117:263–269. doi: 10.1093/toxsci/kfq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townes BD, Martins IP, Castro-Caldas A, Rosenbaum G, DeRouen T. Repeat test scores on neurobehavioral measures over an eight-year period in a sample of Portuguese children. Int. J. Neurosci. 2008;118:79–93. doi: 10.1080/00207450601042102. [DOI] [PubMed] [Google Scholar]
- Townes BD, Rosenbaum JG, Martins IP, Castro-Caldas A. Neurobehavioral assessment in children: a cross-cultural perspective. Psychologica. 2003;34:817–820. [Google Scholar]
- Tsai CL, Jang TH, Wang LH. Effects of mercury on serotonin concentrations in the brain of tilapia, Orreochromis mossambicus. Neurosci. Lett. 1995;184:208–211. doi: 10.1016/0304-3940(94)11208-z. [DOI] [PubMed] [Google Scholar]
- Wang Y, Goodrich JM, Gillespie B, Basu N, Franzblau A. An investigation of modifying effects of metallothionein single-nucleotide polymorphisms on the association between mercury exposure and biomarker levels. Environ. Health Perspect. 2012;120:530–534. doi: 10.1289/ehp.1104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: Catechol O-methyl ransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu. Rv. Pharmacol. Toxicol. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]
- West AK, Hidalgo J, Eddins D, Levin LD, Aschner M. Metallothionein in the central nervous system: roles in protection, regeneration and cognition. Neruotoxicol. 2008;29:489–503. doi: 10.1016/j.neuro.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JS, Echeverria D, Heyer NJ, Simmonds PL, Wilkerson J, Farin FM. The association between genetic polymorphisms of coproporphyrinogen oxidase and an atypical porphyrinogenic response to mercury exposure in humans. Toxicol. Appl. Pharmacol. 2005;206:113–120. doi: 10.1016/j.taap.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Woods JS, Heyer NJ, Echeverria D, Russo JE, Martin MD, Bernardo MF, Luis HS, Vaz L, Farin FM. Modification of neurobehavioral effects of mercury by a genetic polymorphism of coproporphyrinogen oxidase in children. Neurotoxicol. Teratol. 2012;34:513–521. doi: 10.1016/j.ntt.2012.06.004. PMID:22765978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JS, Heyer NJ, Russo JE, Martin MD, Pillai PB, Farin FM. Modification of neurobehavioral effects of mercury by genetic polymorphisms of metallothionein in children. Neurotoxicol. Teratol. 2013;39:36–44. doi: 10.1016/j.ntt.2013.06.004. PMID:23827881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JS, Heyer NJ, Russo JE, Martin MD, Pillai PB, Bammler TK, Farin FM. Genetic polymorphisms of catechol-O-methyltransferase modify the neurobehavioral effects of mercury in children. J. Toxicol. Environ. Health. Pt. A. 2014;77:293–312. doi: 10.1080/15287394.2014.867210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JS, Martin MD, Leroux BG, DeRouen TA, Leitão JG, Bernardo M, Luis HS, Simmonds PL, Kushleika JV, Huang Y. The contribution of dental amalgam to urinary mercury excretion in children. Environ. Health Perspect. 2007;115:527–1531. doi: 10.1289/ehp.10249. PMID:17938746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RO, Hu H, Silverman EK, Tsaih SW, Schwartz J, Dellinger D, et al. Apolipoprotein E genotype predicts 24-month Bayley Scales Infant Development score. Pediatr. Res. 2003;54:819–825. doi: 10.1203/01.PDR.0000090927.53818.DE. [DOI] [PubMed] [Google Scholar]
- Xie T, Ho SL, Ramsden DB. Characterization and implications of estrogenic down-regulation of human catechol-O-methyl transferase gene transcription. Molec. Pharmacol. 1999;56:3–38. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]