Abstract
Since Broca's studies on language processing, cortical functional specialization has been considered to be integral to efficient neural processing. A fundamental question in cognitive neuroscience concerns the type of learning that is required for functional specialization to develop. To address this issue with respect to the development of neural specialization for letters, we used functional magnetic resonance imaging (fMRI) to compare brain activation patterns in pre-school children before and after different letter-learning conditions: a sensori-motor group practised printing letters during the learning phase, while the control group practised visual recognition. Results demonstrated an overall left-hemisphere bias for processing letters in these pre-literate participants, but, more interestingly, showed enhanced blood oxygen-level-dependent activation in the visual association cortex during letter perception only after sensori-motor (printing) learning. It is concluded that sensori-motor experience augments processing in the visual system of pre-school children. The change of activation in these neural circuits provides important evidence that ‘learning-by-doing’ can lay the foundation for, and potentially strengthen, the neural systems used for visual letter recognition.
Introduction
A fundamental question in developmental cognitive neuroscience concerns how changes in neural activation during development can inform theories of cognitive development. If we can understand how the child's brain changes with specific experiences, we will come closer to uncovering how learning happens – a crucial component in understanding human behaviour. Demonstrating neural changes during learning and development can inform and constrain cognitive theory, leading to further experimentation using purely behavioural measures as well as methods such as functional magnetic resonance imaging (fMRI). Although research into the relationship between human behaviour and brain function continues to lead to important discoveries, very little research is devoted to documenting the emergence of a given neural response pattern during development. The study of emergence provides insights into the potential cause of particular neural responses. To study the emergence of a neural response, however, one must be able to document the absence of the response followed by its presence; that is, to document a change. One method of investigating the emergence of neural responses is through the study of the developing brain using fMRI. Many typical neural responses that are present in the adult brain are absent or different in a child's brain (Casey, Tottenham Liston & Durston, 2005). Documenting the stages that lead to adult-like neural signatures can help us to understand why the brain responds to certain stimuli the way it does, which in turn can inform theories of cognitive development.
One type of neural response that is well documented in the adult brain is functional specialization – the tendency for brain areas or networks to respond more to one category of stimulus than to others. Functional specialization is considered to be integral to efficient processing, and has been shown to occur for many categories of stimuli in the adult (Kanwisher, McDermott & Chun, 1997; Downing, Jiang, Shuman & Kanwisher, 2001; Cohen & Dehaene, 2004; Epstein, Harris, Stanley & Kanwisher, 1999). In many cases, functional specialization is thought to emerge from extensive experience (Gauthier, Skudlarski, Gore & Anderson, 2000a), but the type of experience seems to be important. That is, mere exposure to a particular stimulus category will not lead to functional specialization in the adult (Gauthier & Tarr, 2002). One well-known example of functional specialization is the neural response associated with single-letter (e.g. James, James, Jobard, Wong & Gauthier, 2005; Flowers et al., 2004) and word perception in the adult visual system (e.g. Cohen & Dehaene, 2004). That is, a region in the left fusiform gyrus has been found to respond more to individual letters than to letter strings, words, digits or Chinese characters (James et al., 2005). In contrast, a more posterior region in the left fusiform, often described as the ‘visual word form area’ (VWFA), has been found to respond more to letter strings or words than to individual letters (e.g. Cohen & Dehaene, 2004; James et al., 2005). The specialization found in the VWFA is, however, controversial, given that this same area also responds more to objects than to words in some cases (Price & Devlin, 2003; Moore & Price, 1999). Given the proximity of, and in some cases overlap between, the VWFA and the Lateral Occipital Complex, an area known to be object-selective(Grill-Spector et al., 2001), the degree to which the VWFA responds in a specific way to words will be variable. Although we assume that functional specialization for letters reflects our extensive experience with reading text, the specific type of experience that is necessary for the development of this pattern of neural response is not known. The requirements for specialization to emerge for individual letters may be different from those for words. For example, letters are learned before words during development, and children learn to write individual letters before they learn to read words (sometimes 3 or 4 years before). However, how children process letters in isolation is rarely studied. We believe that the question of how children learn to recognize letters is an important one, and one that can help us to understand how functional specialization develops. Can this specialization develop as a result of familiarity? By mere exposure? Or is a certain type of learning experience needed? Here, we make use of a learning paradigm that keeps familiarity and exposure constant while manipulating how letters are learned in order to try to gain an understanding of the experiences that may lead to the emergence of neural responses that reflect functional specialization in the developing brain.
Although functional specialization has often been characterized as a neural response pattern that is stimulus-specific, it may, in fact, reflect the recruitment of a specialized type of processing that is required for efficient recognition of a particular stimulus category (Gauthier, 2000). For instance, the visual processing of face stimuli reveals functional specialization of the right fusiform gyrus (Kanwisher et al., 1997). This specialization could reflect category specificity, but it could also reflect the recruitment of a specialized holistic or configural process that is necessary for efficient face recognition, that is also recruited for efficient recognition of stimuli from other, non-face categories (Gauthier et al., 2000a). Functional specialization for perceiving letters may reflect category specificity of this brain region (a putative ‘letter area’), but it may also reflect the recruitment of a feature-based, analytic, or ‘local’ analysis of a stimulus (Marsolek, 1999; James, Servos, Kilgour, Huh & Lederman, 2006); that is, it may reflect a processing difference.
In accordance with the latter interpretation of functional specialization, we suggest that the specialized processing that occurs during letter perception may be the result of our sensori-motor experience with letters. Physical interaction with the environment through sensori-motor interactions informs visual processing, and could potentially be crucial for normal object recognition to develop. Consistent with this stance is that perceptual processes are not encapsulated modules, but instead receive feedback from sensori-motor networks, the strength of which is based on previous experience with the class of stimulus. Previous research suggests that recognition performance is particularly enhanced when learning requires integration across sensory and motor systems (Wexler & van Boxtel, 2005; Harman, Humphrey & Goodale, 1999; James, Humphrey & Goodale, 2001), suggesting that sensori-motor experience may be a crucial force in the emergence of functional specialization.
Neural activation patterns change after motor experience with objects. For example, when we visually perceive objects that we have had motor experience with, the motor system is active (Chao & Martin, 2000; Grezes & Decety, 2002; James & Atwood, 2009). This has recently been found to occur when we view letters as well (James & Gauthier, 2006), suggesting that our history of interacting with letters through writing is stored and perhaps re-activated upon visual presentation. We have proposed that the motor system is active during the visual presentation of letters because letters are learned using a combination of sensory and motor behaviours, including seeing, hearing, speaking and writing. In addition, and crucial to this hypothesis, we propose that these sensori-motor experiences are stored and may lead to functional specialization for letters. Recently we have shown that adults who learn to write pseudo-letters develop functional specialization for these stimuli (greater activation to studied pseudo-letters than to unstudied pseudo-letters) in the left fusiform gyrus – the same region that is specialized for letters (James & Atwood, 2009). In contrast, adults who learned these stimuli with only visual experience did not develop the same specialized response. We interpret this as evidence supporting our claim that sensori-motor experience with letters through writing may be a crucial component for the development of functional specialization. To truly test this hypothesis however, we needed to use real letters of the alphabet in a system that is not already efficient at letter processing – the brain of the pre-school child.
Materials and methods
Participants
Twelve healthy children (4 years 3 months to 5 years 4 months) with no known neurological diseases or psychological disorders participated in the present study. Seven of the children were female and five were male. All were native English speakers, and parents reported normal visual acuity. All children preferred to draw with their right hands spontaneously, and parents reported a right-hand preference for all children. Upon arriving at the first imaging appointment, the participants were randomly assigned to either the experimental or the control group. All research was approved by the Indiana University Protection of Human Participants Board. Informed written consent was obtained from the parents and they were compensated with gift certificates; the participants were compensated with a small toy or book.
Stimuli and procedure
Imaging sessions
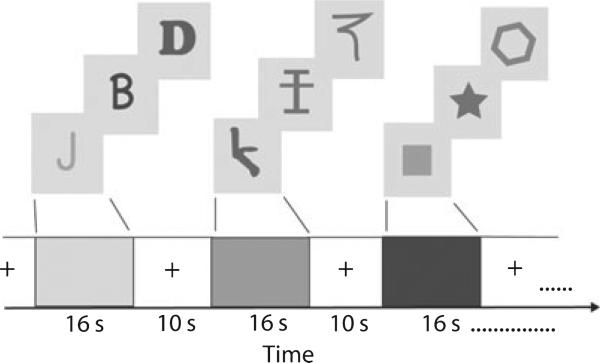
Prior to the initial imaging session, children were acclimated to an MRI environment by allowing them to watch a short cartoon in our MR simulator, an artificial MRI environment. This environment also allows simulated sound to ensure that the participants are not afraid of loud noises. We are also able to monitor head movement, and stop the cartoon when excessive head movement occurs. This technique allows the children to learn how much they can move in the environment. If they felt comfortable lying still in this simulated environment they were then acclimated to the actual MRI machine. Both imaging sessions proceeded in the same manner. All stimuli were back-displayed via a Mitsubishi XL30 projector onto a screen that was viewed through a mirror from the bore of the Siemens Trio 3T scanner. Stimuli were presented via SuperLab Pro 2.0.4 software via Dell Inspiron 6000 laptops. After initial familiarization with the MRI environment, children passively viewed blocked presentations of isolated letters, isolated false fonts (stimuli that have the same features as letters, but are not actual letters), and simple shapes (see Figure 1). Sixteen-second blocks of stimuli were interspersed with 10-s fixation blocks; a block of each stimulus type was repeated three times within a given run, resulting in approximately 4-minute runs (Figure 1). Stimuli were in 3″ × 2″ squares (see Figure 1) presented centrally, for 2 seconds each. Between each stimulus, a 500-ms fixation cross was presented. Three runs were administered per experimental session, allowing data to be collected from nine blocks of a given stimulus type. Participants were required simply to view the stimuli passively. Neural activation, measured by the blood oxygen-level-dependent (BOLD) signal in the entire brain, was then recorded during exposure to the stimuli. Imaging sessions took approximately 20 minutes in total. Beginning one week after the first scanning session, participants returned once a week for four weeks to complete training sessions.
Figure 1.
Graphical depiction of fMRI design and stimuli used in the present study (see text for design details).
fMRI acquisition
Imaging was performed using a 3-T Siemens Magnetom Trio whole-body MRI system and a phased-array eight-channel head coil, located at the Indiana University Department of Psychological and Brain Sciences. Whole Brain axial images were acquired using an echo-planar technique (TE = 30 ms, TR = 2000 ms, flip angle = 90°) for BOLD-based imaging. The field of view was 22 cm × 22 cm × 9.9 cm, with an in-plane resolution of 64 × 64pixels and 33slicespervolumethat were 4 mm thick with a 0 mm gap between them. The resulting voxel size was 3.0 mm × 3.0 mm × 4.0 mm. Functional data underwent slice-time correction, 3D motion correction, linear trend removal, and Gaussian spatial blurring (FWHM 4 mm) using the analysis tools in Brain Voyager™. Individual functional volumes were co-registered to anatomical volumes with an intensity-matching, rigid-body transformation algorithm. Voxel size of the functional volumes was standardized at 1 mm × 1 mm × 1 mm using trilinear interpolation. High-resolution T1-weighted anatomical volumes were acquired prior to functional imaging using a 3D Turbo-flash acquisition (resolution: 1.25 mm × 0.62 × 0.62, 128 volumes).
Training sessions
Training sessions were modelled after those used in a recent study on the effects of writing practise on letter recognition in children (Longcamp, Zerbato-Poudou & Velay, 2005). Each training session involved three types of task. First, all participants performed a four-alternative forced-choice letter identification task to assess their letter recognition ability (Figure 2a). Next, an experimenter read a short story to all participants, sitting next to the child so that s/he could view the text. The letters and words that the child was to practise were highlighted within the text and pointed to by the experimenter (Figure 2b). The children in the experimental (sensori-motor training) group then copied the letters and words that were highlighted in the story from a piece of paper (Figure 2c), and were given feedback on writing accuracy. In the control (visual training only) group, the participants simply identified the words and letters from the story instead of writing them (Figure 3c); they too were given feedback on their verbal response. The number of stimuli and time of exposure were the same in both groups. In both groups children were shown, and practised, both upper- and lower-case letters. Finally, in the first training session, all children were asked several questions from the Bader reading inventory (Bader, 2005) to assess their phonological and visual processing of letters and words. These included: an assessment of print concepts; phoneme awareness; letter knowledge phonological awareness and language comprehension. This last task was administered to ensure that all participants were at a similar level in terms of letter and word knowledge at the beginning of the study. Each training session took approximately 30 minutes. One week after the last training session, the participants returned for the post-training scanning session.
Figure 2.
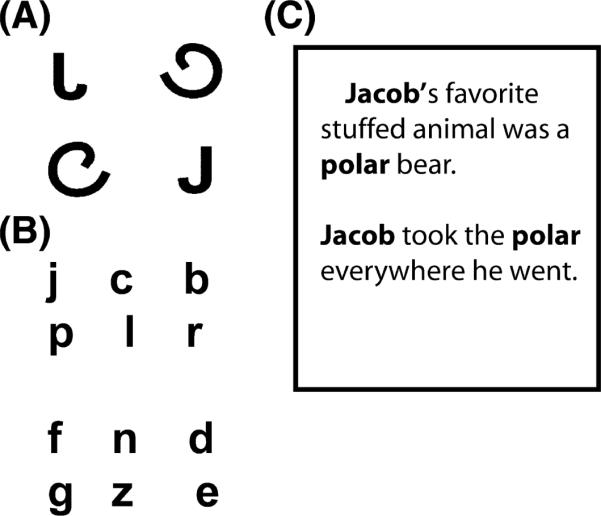
(A) An example of the four-alternative forced-choice task: participants were asked to ‘point to the “J”. (B) Words that contained the letters to be studied were highlighted in the story text. Participants were given examples in both upper and lower case. (C) The letters were presented in this format on a piece of paper: participants then either named or wrote the letters with feedback.
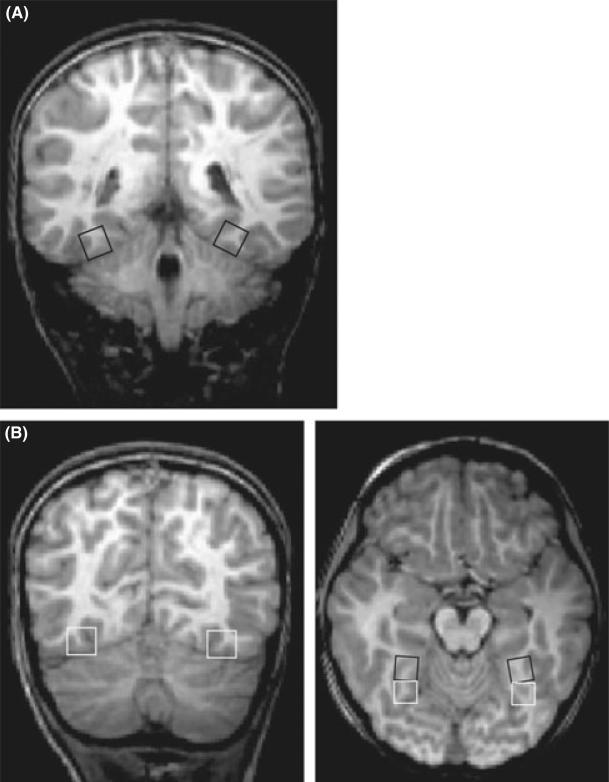
Figure 3.
(A) Anatomical locale of the anterior fusiform gyrus presented on a coronal slice. (B) anatomical locale of the posterior fusiform presented on a coronal slice, left, and both the anterior and posterior fusiform presented on the transverse slice. Because these are not transformed into Talairach space, coordinates are not provided.
Imaging-data analyses
Data analysis procedures reflected the question of primary interest: whether or not BOLD activation in the fusiform gyrus changes after sensori-motor training with letter stimuli. BOLD activation patterns were computed using the Brain VoyagerTM analysis package, and any functional data that exceeded 4 mm of motion on any axis were excluded from the analyses. This criterion resulted in the exclusion of three blocks of data from one participant and of two blocks of stimuli from another. Exclusion of these data does not significantly alter the power of the present analyses. Data were not transformed into a common stereotactic space (e.g. Talairach & Tournoux, 1988), based on the premise that there may be large individual differences in neural processing among children in this age group and because there is some disagreement regarding the validity of normalizing children's brains to an adult-defined space (Gaillard, Grandin & Xu, 2001).
Selection of regions of interest
For this analysis, we did not perform specific contrasts to display in a functional map, but instead performed a region-of-interest (ROI) analysis based on anatomically localized ROIs (see Figure 3). The reason for using anatomical localization instead of functional localization is simply that many of our participants may not show differences among our conditions. In fact, we hypothesized that our entire control group (pre- and post-training) may not show any differences among conditions. This renders functional localization based on contrasts among conditions ineffective. Because of the evidence that the fusiform gyrus is significantly involved in letter processing (e.g. James et al., 2005) we were interested primarily in our results in this region, and therefore localized this region using anatomical markers. The entire fusiform gyrus is bounded by the lateral occipital sulcus laterally, by the collateral sulcus medially, and by the anterior and posterior collateral sulci rostrally and caudally (Duvernoy, 1999). The distance between the lateral occipital sulcus and collateral sulcus was, on average, 10 mm – this provided the extent of the ROI in the X-dimension. In the Z-, or dorsal-ventral, dimension, our ROIs began on the ventral surface of the temporal lobe and extended 10 mm dorsally. In the Y-, rostral to caudal, dimension, we took a 20-mm distance from the anterior to the posterior collateral sulcus. Because of this large area, we split this into a 10-mm posterior and a 10-mm anterior fusiform ROI. This left us with four ROIs (two in each hemisphere), an anterior fusiform ROI that was 10 × 10 × 10 mm and a posterior fusiform area also 10 × 10 × 10 mm (see Figure 3). We then extracted the percentage signal-change data in each condition from these regions and compared them in statistical analyses.
Results
Imaging results
Four 2 × 2 × 3 mixed-model ANOVAs were performed on the resultant data with Training Group (sensori-motor and control) as the between-participants variable and Imaging Day (pre-training or post-training) and Stimulus Type (letters, pseudo-letters and shapes) as within-participant variables. Separate ANOVAs were performed within each ROI for ease of interpretation. For our dependent measure, we used the peak percentage signal change that occurred after the first two time points and before the last two time points in the response function during each condition.
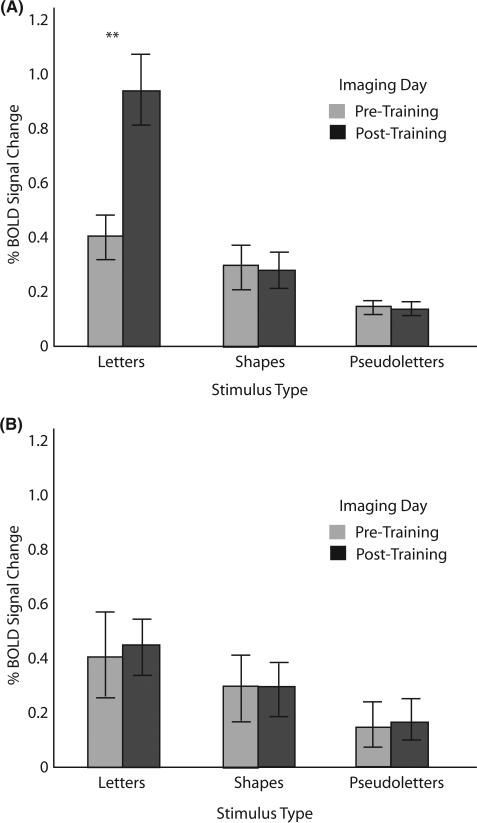
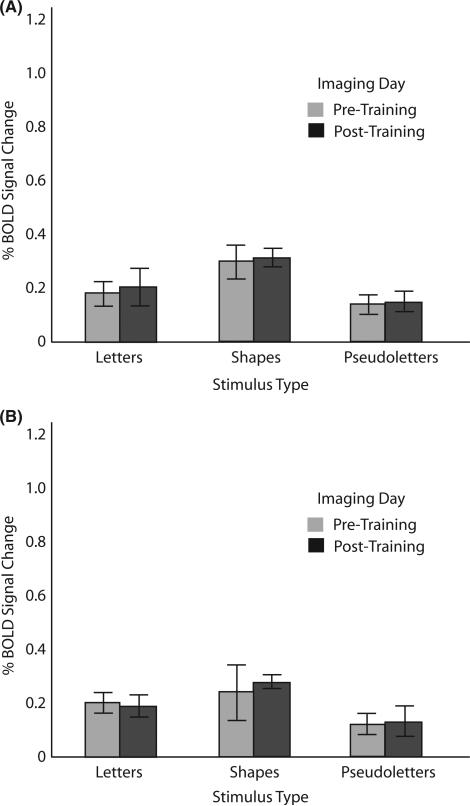
Posterior left fusiform gyrus (Figure 4)
Figure 4.
Left posterior fusiform region of interest. (A) Percentage blood oxygen-level-dependent (BOLD) signal change as a function of stimulus type during pre-training and post-training imaging sessions for the sensori-motor training group. (B) Percentage BOLD signal change as a function of stimulus type during pre-training and post-training imaging sessions for the visual-only training group. On this and all other graphs error bars represent standard error of the mean; ** depict significant differences at p < .01; and * depict differences at p < .05.
This 2 × 2 × 3 ANOVA revealed significant main effects for Imaging Day (F(1,10) = 7.6, p < . 02, MSe = .13), as well as for Stimulus Type (F(2,20) = 22.8, p < .0001, MSe = .79). Importantly, there was no main effect of Training Group (F(1,10) = .88, ns, MSe = .10). Main effects must be interpreted in light of our four significant interactions: one between Training Group and Imaging Day (F(1,10) = 5.1, p < .04, MSe = .01), one between Training Group and Stimulus (F(2,20) = 4.2, p < .05, MSe = .03), one between Imaging Day and Stimulus (F(2,20) = 11.3, p < .001, MSe = .01), and a three-way interaction among Training Group, Imaging Day and Stimulus (F(2,20), p < .001, MSe = .01). This three-way interaction supports the hypothesis that only after sensori-motor training do letters activate the left fusiform more than other, similar stimuli (see Figure 4) (t(10) = 5.77, p < .001 for letters pre-training versus post-training).
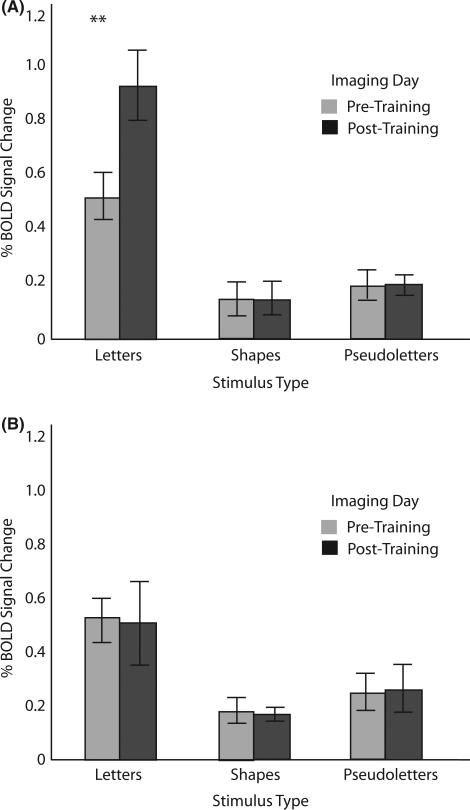
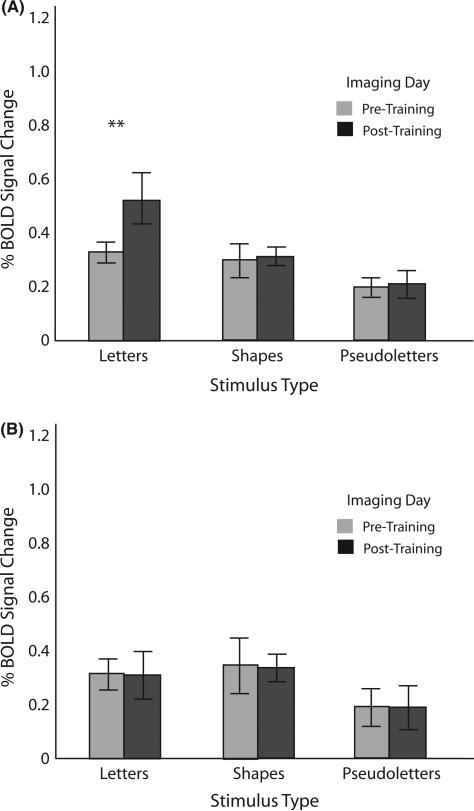
Anterior left fusiform gyrus (Figure 5)
Figure 5.
Left anterior fusiform gyrus. (A) Percentage blood oxygen-level-dependent (BOLD) signal change as a function of stimulus type during pre-training and post-training imaging sessions for the sensori-motor training group. (B) Percentage BOLD signal change as a function of stimulus type during pre-training and post-training imaging sessions for the visual-only training group.
The results of the 2 × 2 × 3 ANOVA in this region also revealed significant main effects of Imaging Day (F(1,10) = 7.4, p < .05, MSe = .01) and Stimulus (F(2,20) = 51.1, p < .0001, MSe = 03), with no main effect of Training Group (F(1,10) = 2.6, ns). Significant interactions also surfaced, one between Training Group and Imaging Day (F(1,10) = 8.2, p < .01, MSe = .09), one between Imaging Day and Stimulus (F(2,20), p < .005, MSe = .01), and a three-way interaction among Training Group, Imaging Day and Stimulus (F(2,20) = 10.5, p < .001, MSe = .01). As can be seen in Figure 5, this interaction is driven by the greater percentage signal change in the sensori-motor training group for letters pre-training versus post-training (t(10) = 4.48, p < .01). It should be noted here that no other simple effects in this interaction were significant.
Posterior right fusiform gyrus (Figure 6)
Figure 6.
Right posterior fusiform gyrus. (A) Percentage blood oxygen-level-dependent (BOLD) signal change as a function of stimulus type during pre-training and post-training imaging sessions for the sensori-motor training group. (B) Percentage BOLD signal change as a function of stimulus type during pre-training and post-training imaging sessions for the visual-only training group.
In this region, the 2 × 2 × 3 ANOVA resulted in a significant difference among the test stimuli (F(2,20) = 7.0, p < .005, MSe = .02), with a greater percentage signal change to shapes than to letters (t(10) = 6.23, p < .0001) and a greater activation to letters than to pseudo-letters (t(10) = 6.01, p < .004). No interactions were significant in this region.
Anterior right fusiform gyrus (Figure 7)
Figure 7.
Right anterior fusiform gyrus. (A) Percentage blood oxygen-level-dependent (BOLD) signal change as a function of stimulus type during pre-training and post-training imaging sessions for the sensori-motor training group. (B) Percentage BOLD signal change as a function of stimulus type during pre-training and post-training imaging sessions for the visual-only training group.
There was a significant effect of Stimulus Type in this region, with letter perception producing a greater percentage signal change overall (F(2,20) = 6.6, p < .005, MSe = .02). There were also two significant interactions: one between Imaging Day and Stimulus (F(2,20) = 4.3, p < .05, MSe = .006), and a three-way interaction among Training Group, Imaging Day and Stimulus (F(2,20) = 3.9, p < .05, MSe = .006). As depicted in Figure 7, this interaction is a result of the greater response of the sensori-motor training group to letter stimuli only after the training session (t(10) = 5.48, p < .005 letters pre-training versus post-training).
In sum, the imaging data suggest that prior to any training, the left fusiform gyrus was engaged more during letter perception than during the perception of other stimuli, but that after sensori-motor training the amplitude of the percentage signal change significantly increased during letter perception. This is not as a result of familiarity with the stimuli, as the increase occurred only after sensori-motor training and not after visual training. The increase in neural response to letters after sensori-motor training also occurred in the right anterior fusiform gyrus, but no changes were observed in the right posterior fusiform.
Behavioural results
Behavioural results measured before and after training revealed a trend towards an interaction among training group and pre-and post-training letter recognition (F(1,5) = 5.0, p < .07, partial eta squared = .50). Because of the non-significance of this effect, we did not pursue further analyses, but observed that, in the experimental group, performance increased with training (from M = 7.5, SD = .56 to M = 8.0, SD = .57), whereas performance stayed the same in the control group (M = 7.8, SD = .60). Thus, the sensori-motor experience with the letters in this experiment did improve performance more than visual-only training, although not significantly (see below for further discussion).
Discussion
The current study is the first to investigate the effects of sensori-motor experience on the neural activation patterns of young children. It is also the first study to document neural responses in pre-school children to letters, shapes and unfamiliar 2D objects (pseudo-letters). As a result, we can report several novel and interesting findings. First, we have found that, even for pre-literate children, there appear to be some hemispheric differences in how the brain responds during letter and shape perception. The left fusiform gyrus, especially in the anterior portion, responded more to letters than to shapes and pseudo-letters, even prior to our training manipulation (Figures 4 and 5). In contrast, however, the right fusiform responded similarly to letters, shapes and pseudo-letters (Figures 6 and 7). This finding suggests that early on, before children learn to read, the brain is organizing to achieve a left-hemisphere dominance for perceiving print. It seems that this hemispheric dominance does not simply result from the complexity of the letter stimuli, because the pseudo-letters contained exactly the same features as the letters, only in a different organization – arguably, these stimuli are just as complex as letters. We cannot say, of course, when this hemispheric dominance for letters begins to develop; however, it should be noted that these children did have significant experience with letters despite not being able to read. That is, all children could sing the alphabet song, and all could name a minimum of 80% of the alphabet. In addition, all children could print their name. This suggests that perhaps familiarity with these stimuli has led to some hemispheric specialization. However, the children were also very familiar with the simple shapes, and could draw them as well. Even at this early age, letters are being processed differently from other, similar objects. This may be as a result of the emphasis that parents and other caregivers assign to these stimuli even at this age. It may also be as a result of perceiving letters in groups (words), even though the groups are ‘meaningless’ to a certain extent. We contend that part of the difference among letters and other shapes may lie in the manual construction that is happening when children learn to write letters.
Moreover, our second result of interest was the dramatic increase in activation that occurred in a putative visual area only after sensori-motor, that is, printing, experience. This increase supports the idea that printing practice, resulting in interactions among sensori-motor systems, may lead to the functional specialization that develops in three of our ROIs (Figures 4, 5 and 7). The increase in percentage signal change after printing training cannot be caused by familiarity, because both groups were familiar with the stimuli and had very similar exposures. The only difference in the experiences of the two groups was in the type of interactions that the participants had with the stimuli – one visual-motor and one visual only. It is acknowledged, however, that the visual-motor association may not be the only type of association that changes the response properties in this area. We did not exhaustively test all possible associations, but were interested primarily in sensory-motor associations. That being said, the ‘visual-only’ practice involved saying the letters aloud (as did the printing practice), which is also a motor response. Thus, another way of stating our results is that the manual motor associations resulted in a different response in visual association areas than did an oral motor association.
The visual training did not result in increased activation in the VWFA, unlike the case for sensori-motor training. This brings into question the role of the VWFA as a visual perceptual area of the ventral processing stream: although it may be involved in word processing it does not change its response based on visual-only experience. The response does change here, though, with sensori-motor training, supporting the idea that some visual association areas accumulate input from other sensori-modalities as well as from the motor system (James et al., 2005). The VWFA then may be important in reading, but this may be a reflection of the integration of information among neural systems rather than of visual processing per se.
An alternative explanation for our results is that the sensori-motor training condition resulted in greater attention to the stimuli than did the visual training condition, leading to differences in brain activation. Although this possibility will be addressed with further studies, we do not believe that attentional differences are the only reason for these results. First, if attention were significantly different in the two conditions then one would certainly expect to see a behavioural advantage for the more attentionally demanding condition. However, our two training conditions, although producing different neural activation patterns, did not result in significant behavioural differences. Second, we have evidence from adult participants who learned either to write, type or visually recognize pseudo-letters that attention differences are probably not causing these results (James & Atwood, 2009). In that study, the training included a typing condition, and, arguably, typing and writing require the same attentional demands. In fact, because the pseudo-letters in that study were mapped onto the familiar keyboard, the typing condition may have required even more attention than the printing condition. But results demonstrated that writing training recruited different neural substrates than did the typing training and the visual training, suggesting that the difference is in the type of sensori-motor training required and not in the attentional demands. Because the present work does not directly address this possibility however, we are currently investigating this interpretation.
Nonetheless, the importance of sensori-motor interactions with the environment is sometimes overlooked by cognitive psychologists and neuroscientists alike. Recently, though, there has been renewed support from a variety of disciplines for the idea that sensori-motor interactions influence many cognitive operations (e.g. Thelan & Smith, 1994; Sporns, 2002, Wexler & van Boxtel, 2005; Schutz-Bosbach & Prinz, 2007; Knoblich & Prinz, 2001). Here, we have addressed one aspect of this framework, which is that perceptual learning is most efficient when observers are allowed to explore the environment through the combination of sensory and motor systems. Although neural responses will change as a result of any learning (it would not be a very efficient system if it did not), here we show that in a putative visual area, learning through visual–motor interactions results in a different activation pattern than does visual learning alone. This is intuitive – we do not learn about, and adapt to, our environment by passively sampling and encoding sensory information. Rather, we move through our environment voluntarily, and attend, explore, manipulate and handle the pieces of our environment that we choose. We actively gather information that allows us to adapt to our surroundings. In short, we are active perceivers.
Philosophers (e.g. Merleau-Ponty, 1962), and early developmental psychologists (e.g. Piaget, 1953), perceptual psychologists (Gibson, 1979), educators (Montessori, 1912), and, most recently, researchers in artificial intelligence (Brooks, 1991a) have all stressed the importance of considering a perceiver as an active participant in the environment, a view exemplified by the theory of ‘embodied cognition’ (Clark, 1997). Similarly, we have shown previously that, in adults, active manipulation of objects during initial encounters can facilitate learning of object shape (Harman et al., 1999; James et al., 2001). Cognitive operations such as ‘mental rotation’ are affected by our motor experiences (Wohlschläger & Wohlschläger, 1998), as is the perception of depth and 3-D shape perception (Wexler & van Boxtel, 2005). In addition, there is a long literature indicating a deep and fundamental coordination between vision and action in perceptual development (e.g. Held & Hein, 1963). These previous studies converge with our study in suggesting a strong coupling between perceptual and motor systems. More specifically, though, ours is the first evidence that functional specialization emerges from this coupling in the developing brain.
The data from the behavioural portion of the present study exhibit what is becoming a common finding – that neural changes observed during fMRI are not always associated with behavioural changes. This is a reflection of the phenomenon that neural activation can be a more sensitive measure of cognitive operations than behavioural indices (Wilkinson & Halligan, 2004). Our lack of a significant change in behavioural performance is a good demonstration of this: our results fall just short of significance, and other studies using a similar training paradigm do show behavioural changes with larger sample sizes (Longcamp et al., 2005). Therefore, although the changes in our neural responses as a result of our manipulation are obvious, behavioural measures do not seem sensitive enough to reflect this change. Given increased sample sizes, and/or increased exposure (training), it is quite likely that we would also observe a behavioural change as a result of training. Arguably, both differences in accuracies and changes in neural activation are markers of underlying cognitive processes, and neither should take precedence over the other (see Wilkinson & Halligan, 2004 for further discussion of the issue).
The results of our study support behavioural work that has shown learning benefits from sensori-motor experiences. Educators have implemented sensori-motor learning strategies when teaching children to recognize shapes, including letters (Montessori, 1912). Indeed, some dyslexic individuals are delayed in motor tasks, implying that the motor difficulties they experience may have affected their letter-learning ability (Stoodley, Harrison & Stein, 2005). Similarly, children exhibiting developmental dyspraxia, a disorder manifesting in reduced motor skills, often have difficulty with letter identification and with learning to read (e.g. Portwood, 2000). Recent empirical work has shown that children recognize letters more efficiently after printing practice than when printing is not involved in learning (Longcamp et al., 2005). Thus, the accumulated applied evidence also converges with our results, which suggest that printing, which involves the coupling of visual and motor systems, is an important contributor to letter recognition.
The demonstration of changes in brain activation as a result of controlled experience is important for understanding why neural activation patterns emerge in given situations. The results shown here suggest that one cause of neural specialization in the left fusiform gyrus may be our sensori-motor experience with letters. In general, these results have significant implications for our understanding of how the child's brain changes with experience, and they also suggest that the type of experience may be important in causing neural changes associated with learning.
Acknowledgements
I gratefully acknowledge the parents and children who participated in this study. I also thank our MR technicians, Rebecca Ward and Thea Atwood, and MR physicist Dr Hu Cheng. In addition, for testing participants: Elaine Augustine, Laura Engelhardt, Robert Kime and Lisa Huang. For helpful comments on earlier versions of this manuscript, I gratefully acknowledge Linda B. Smith, Isabel Gauthier and Thomas James. This research was supported in part by the Indiana METACyt Initiative of Indiana University, and funded in part through a major grant from the Lilly Endowment, Inc. and by the Faculty Research Support Program, Indiana University.
References
- Bader LA. Bader Reading and Language inventory. Merrill Prentice Hall; New York: 2005. [Google Scholar]
- Brooks R. Intelligence without representation. Artificial Intelligence Journal. 1991a;47:139–160. [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Science. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of man-made objects in the dorsal stream. Neuroimage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Clark A. Being there: Putting brain body and world together again. MIT Press; Cambridge, MA: 1997. [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. NeuroImage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain surface, blood supply, and three–dimensional sectional anatomy. Springer-Verlag; New York: 1999. [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Flowers DL, Jones K, Noble K, VanMeter J, Zeffiro TA, Wood FB, Eden GF. Attention to single letters activates left extrastriate cortex. NeuroImage. 2004;21:829–839. doi: 10.1016/j.neuroimage.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Grandin CB, Xu B. Developmental aspects of pediatric fMRI: considerations for image acquisition, analysis, and interpretation. NeuroImage. 2001;13:239–249. doi: 10.1006/nimg.2000.0681. [DOI] [PubMed] [Google Scholar]
- Garrett AS, Flowers DL, Absher JR, Fahey FH, Gage HD, Keyes JW, Porrino LJ, Wood FB. Cortical activity related to accuracy of letter recognition. NeuroImage. 2000;11:111–123. doi: 10.1006/nimg.1999.0528. [DOI] [PubMed] [Google Scholar]
- Gauthier I. What constrains the organization of the ventral temporal cortex? Trends in Cognitive Science. 2000;4:1–2. doi: 10.1016/s1364-6613(99)01416-3. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ. Unraveling mechanisms for expert object recognition: bridging brain activity and behavior. Journal of Experimental Psychology: Human Perception and Performance. 2002;28(2):431–446. doi: 10.1037//0096-1523.28.2.431. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nature Neuroscience. 2000a;3:191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- Gibson JJ. The ecological approach to visual perception. Erlbaum; Hillsdale, NJ: 1979. [Google Scholar]
- Grezes J, Decety J. Does visual perception of object afford action? Evidence from a neuroimaging study Neuropsychologia. 2002;40:212–222. doi: 10.1016/s0028-3932(01)00089-6. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Research. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Harman KL, Humphrey GK, Goodale MA. Active manual control of object views facilitates recognition. Current Biology. 1999;9:1315–1318. doi: 10.1016/s0960-9822(00)80053-6. [DOI] [PubMed] [Google Scholar]
- Held R, Hein A. Movement produced stimulation in the development of visually guided behavior. Journal of Computational Physiology and Psychology. 1963;56:872–876. doi: 10.1037/h0040546. [DOI] [PubMed] [Google Scholar]
- James KH, Atwood TP. Active motor experience changes neural activation patterns to letter-like symbols. Cognitive Neuropsychology. 2009;26:91–110. doi: 10.1080/02643290802425914. [DOI] [PubMed] [Google Scholar]
- James KH, Gauthier I. Letter processing automatically recruits a sensori-motor network. Neuropsychologia. 2006;44:2937–2949. doi: 10.1016/j.neuropsychologia.2006.06.026. [DOI] [PubMed] [Google Scholar]
- James KH, Humphrey GK, Goodale MA. Manipulating and recognizing visual objects: where the action is. Canadian Journal of Experimental Psychology. 2001;55(2):111–120. doi: 10.1037/h0087358. [DOI] [PubMed] [Google Scholar]
- James KH, James TW, Jobard G, Wong CN, Gauthier I. Letter processing in the visual system: different activation patterns for single letters and strings. Cognitive, Affective and Behavioral Neuroscience. 2005a;5:452–466. doi: 10.3758/cabn.5.4.452. [DOI] [PubMed] [Google Scholar]
- James TW, Humphrey GK, James KH, Goodale MA. Do visual and tactile object representations share the same neural substrate? In: Heller MA, Ballesteros S, editors. Touch and blindness: Psychology and neuroscience. Lawrence Erlbaum; Mahwah, NJ: 2005b. pp. 65–72. [Google Scholar]
- James TW, Servos P, Kilgour AR, Huh E, Lederman S. The influence of familiarity on brain activation during haptic exploration of 3-D facemasks. Neuroscience Letters. 2006;397:269–273. doi: 10.1016/j.neulet.2005.12.052. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich G, Prinz W. Predicting the effects of actions: interactions of perception and action. Psychological Science. 2001;12:467–472. doi: 10.1111/1467-9280.00387. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Anton JL, Roth M, Velay JL. Visual presentation of single letters activates a premotor area involved in writing. NeuroImage. 2003;19:1492–1500. doi: 10.1016/s1053-8119(03)00088-0. [DOI] [PubMed] [Google Scholar]
- Longcamp M, Zerbato-Poudou M, Velay JL. The influence of writing practice on letter recognition in pre-school children: a comparison between handwriting and typing. Acta Psychologica. 2005;119(1):67–79. doi: 10.1016/j.actpsy.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Marsolek CJ. Dissociable neural subsystems underlie abstract and specific object recognition. Psychological Science. 1999;10:111–118. [Google Scholar]
- Merleau-Ponty M. Phenomenology of perception. Routledge & Kegan Paul; London: 1962. [Google Scholar]
- Montessori M. The Montessori Method. New York: Frederick Stokes Company. 1912 [Google Scholar]
- Moore CJ, Price CJ. Three distinct ventral occipitotemporal regions for reading and object naming. NeuroImage. 1999;10(2):181–192. doi: 10.1006/nimg.1999.0450. [DOI] [PubMed] [Google Scholar]
- Piaget J. The origins of intellegence in the child. Routledge and Kegan Paul; London: 1953. [Google Scholar]
- Portwood M. Developmental dyspraxia. David Fulton Publishing; London: 2000. [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. NeuroImage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Schutz-Bosbach S, Prinz W. Perceptual resonance: action-induced modulation of perception. Trends in Cognitive Science. 2007;11(8):349–355. doi: 10.1016/j.tics.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Sporns O. Embodied cognition. In: Arbib M, editor. MIT handbook of brain theory and neural networks. MIT Press; Cambridge, MA: 2002. pp. 46–67. [Google Scholar]
- Stoodley CJ, Harrison EPD, Stein JF. Implicit motor learning deficits in dyslexic adults. Neuropsychologia. 2005;44(5):795–798. doi: 10.1016/j.neuropsychologia.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Thelen E, Smith L. A dynamic systems approach to the development of cognition and action. MIT Press; Cambridge, MA: 1994. [Google Scholar]
- Wexler M, van Boxtel JJA. Depth perception by the active observer. Trends in Cognitive Science. 2005;9:431–438. doi: 10.1016/j.tics.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Halligan P. The relevance of behavioural measures for functional-imaging studies of recognition. Nature reviews, neuroscience. 2004;5:67–73. doi: 10.1038/nrn1302. [DOI] [PubMed] [Google Scholar]
- Wohlschläger A, Wohlschläger A. Mental and manual rotation. Journal of Experimental Psychology: Human Perception and Performance. 1998;24(2):397–412. doi: 10.1037//0096-1523.24.2.397. [DOI] [PubMed] [Google Scholar]