Abstract
Background
Amyotrophic Lateral Sclerosis (ALS) is a rapidly fatal neurodegenerative disease with few therapeutic options. Mild obesity is associated with greater survival in ALS patients and calorie-dense diets increase survival in an ALS mouse model. We therefore hypothesized that hypercaloric diets might lead to weight gain and slow ALS disease progression.
Methods
In this double-blind, placebo-controlled, multi-center clinical trial, we enrolled adults with ALS without a history of diabetes, significant liver or cardiovascular disease, who were already receiving percutaneous enteral nutrition. We randomly assigned participants to one of three dietary interventions: replacement calories using an isocaloric diet (controls) vs. a high-carbohydrate hypercaloric diet (HC/HC), vs. a high-fat hypercaloric diet (HF/HC). Participants received the intervention diets for four months and were followed for five months. The primary outcomes were safety and tolerability. Secondary outcomes included measures of disease progression, survival, and metabolism. This trial is registered with Clinicaltrials.gov, number NCT00983983.
Findings
A total of 24 participants were enrolled of whom 20 initiated study diet (six control, eight HC/HC, six HF/HC). Baseline demographics were similar among the three study arms. The HC/HC diet was better tolerated with fewer serious adverse events than the control diet (zero vs. nine, p<0·001) and fewer dose discontinuations due to adverse events (0% vs. 50%). There were no deaths in the HC/HC arm vs. three deaths (43%) in the control arm (logrank p = 0·03). The HF/HC arm was not statistically different from the controls in adverse events, tolerability, deaths or disease progression.
Interpretation
Our results suggest that hypercaloric enteral nutrition is safe and tolerable in ALS and support the study of nutritional interventions at earlier stages of the disease.
Funding
The Muscular Dystrophy Association with additional support from the National Center for Research Resources, the National Institutes of Health, and the Harvard NeuroDiscovery Center.
Background
Amyotrophic lateral sclerosis is a rapidly progressive neurodegenerative disorder of motor neurons which affects approximately 2 per 100,000 persons per year.1 Median survival is only 30 months, with mortality most often from respiratory failure.1 Weight loss of both muscle and fat is a common symptom of ALS and is hypothesized to be the result of decreased intake due to dysphagia, depression, anorexia, difficulty with the mechanics of feeding, and increased energy expenditure due to a hypermetabolic state.2–4 Enteral nutrition by percutaneous endoscopic gastrostomy (PEG) or radiologic inserted gastrostomy tube (RIG) is generally recommended once patients have lost 10% of their pre-morbid weight and before they are at increased risk of intubation from the procedure.5–7 For patients with feeding tubes, there are no nutritional guidelines nor is there consensus within the community regarding recommendations for enteral nutrition in ALS.8
Multiple groups have reported an association between body mass index (BMI) and ALS survival, with BMI<18.5 associated with shorter survival9–11 and moderate obesity (BMI 30–35) associated with slower disease progression and longer survival.11, 12 Two recent prospective studies found a reduction in ALS risk in persons who were overweight and obese.13, 14 Additionally, studies in the G86R and G93A superoxide dismutase 1 (SOD1) mouse model of ALS have shown that a calorie-dense high fat diet leads to weight gain and a delay in disease progression15, 16 while calorie restriction reduces survival.17 Given this epidemiologic and preclinical data, we hypothesized that a dietary intervention to increase body weight could improve survival in patients with ALS. We designed this phase II study to test whether hypercaloric diets with or without excess fat calories would be safe and tolerable in people with advanced ALS receiving enteral nutrition.
Methods
Study Design and Oversight
The High Fat/High Calorie Diet versus Optimal Nutrition in ALS clinical trial was an investigator-initiated, phase II, prospective, double-blind, placebo-controlled, randomized, multicenter clinical trial. The protocol and consent forms were approved by the Partners Institutional Review Board (IRB) and the IRB of 12 participating ALS centers, including the five clinics which form the Muscular Dystrophy Association (MDA) Clinical Trial Network (see complete list of participating sites, Appendix 1). The study was listed on clinicaltrials.gov (NCT00983983). The full protocol for this trial and statistical plan along with the supporting CONSORT checklist for trials of nonpharmacologic treatments are available as supplementary materials. All of the authors vouch for the fidelity of the study to the protocol. E.M. and A.M.W. had access to all the data and analyzed the data. A.M.W. was responsible for the decision to submit the manuscript.
Role of the funding source
The study was funded by the MDA with additional support from the National Center for Research Resources, the National Institutes of Health, and the Harvard NeuroDiscovery Center. Dietary supplements were purchased at cost from Abbott Pharmaceuticals who provided no financial support for the study. The sponsors had no role in the study design, data collection, analysis, interpretation, manuscript writing, or decision to submit.
Participants
From December 2009 to November 2012, adults with ALS receiving percutaneous enteral nutrition were recruited from ten of the twelve participating ALS centers. Recruitment was slower than anticipated due to the advanced nature of the disease, difficulty tolerating pre-study tube feeds, dependence on care-givers for transportation and help with feeding, fear of weight gain, limited projected survival, and eligibility for hospice services. In addition to advertising the study to providers and patients with the help of the MDA, the ALS Association, and the Northeast ALS Consortium, the home care agency Apria Healthcare sent mailings to 425 ALS patients receiving enteral nutrition, however this did not increase enrollment.
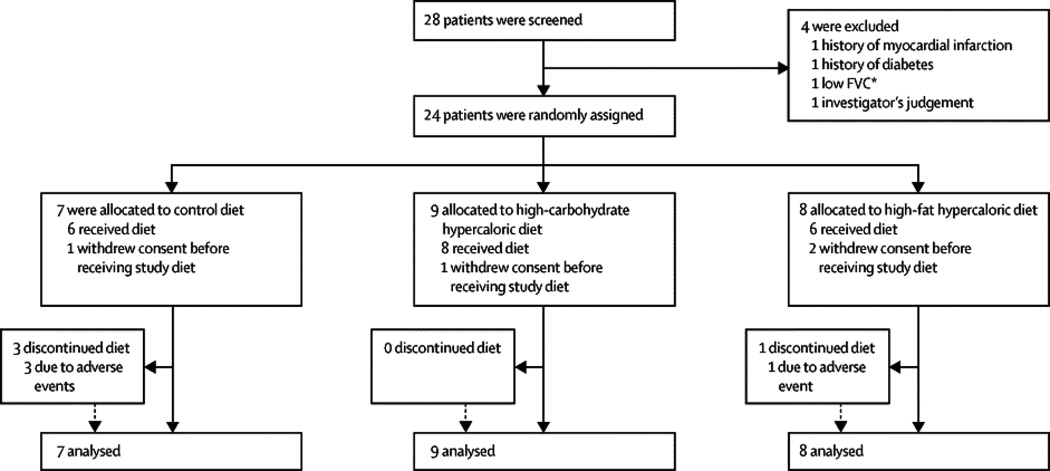
All participants provided informed consent prior to screening procedures. In the rare cases in which participants were unable to physically sign the informed consent, verbal consent was given in the presence of a witness. At screening, eligible participants had to have a diagnosis of suspected, possible, probable, or definite ALS by El Escorial criteria,18 and had to have already tolerated enteral nutrition (for full Inclusion/Exclusion criteria, see Supplementary Table 1). Four people were ineligible based upon the exclusion criteria, which included requiring non-invasive ventilatory support for more than 10 hours/day, diabetes, myocardial infarction, or stroke (Figure 1, Enrollment and Outcomes). Cholesterol-lowering medications were prohibited during the study.
Figure 1.
Consort flow diagram of enrollment and outcomes.
Randomization and Masking
Participants were randomized 1:1:1 to one of three dietary interventions: calorie replacement using an isocaloric diet with a goal of weight stability (control or Cntl), vs. a high-carbohydrate, hypercaloric diet with a goal of modest weight gain (high-carbohydrate/hypercaloric or HC/HC), vs. a hypercaloric diet high in fat calories with a goal of modest weight gain (high-fat/hypercaloric or HF/HC). The randomization schedule was developed by the Biostatistics Center at Massachusetts General Hospital (MGH) with stratification by site in permuted blocks of three. Site investigators and coordinators were not aware of the block size. The Department of Metabolism & Nutrition Research Bionutritionist at MGH Clinical Research Center re-labeled tube feed cans prior to delivery to sites to maintain the blind. All coordination center staff, participants, investigators, site coordinators and site evaluators were blinded to treatment group assignment throughout the study.
Study Procedures
Energy requirements were estimated using indirect calorimetry adjusted for physical activity to define two levels of nutritional support: a replacement calorie (control) group, and two hypercaloric intervention groups (with or without modified fat content. At each in-person visit, the measured resting energy expenditure (MREE) was obtained using VMAX Encore metabolic carts (SensorMedics, Yorba Linda, CA) or equivalent to perform indirect calorimetry after 12 hours of fasting. Prior to randomization, normal caloric intake was calculated by nutrient analysis of four-day food records using Nutrition Data Systems for Research (NDS-R) version 2009.19 Physical activity coefficients were estimated using the Bouchard Three-Day Physical Activity Log.20, 21 Estimated energy requirements were calculated by multiplying the physical activity coefficient times the MREE, or based on the daily intake required to maintain weight between the Screening and Baseline visits (a study window of 12–21 days), whichever was greater. Participants randomized to the control diet were prescribed Jevity 1.0 (Abbott Laboratories, Abbott Park, IL) to replace 100% of their estimated energy requirements.
Interventions
Because the high-fat diet used in the pre-clinical SOD1 mouse studies was 23% more calorically dense than the control diet,16 we designed our two intervention arms to receive approximately 125% times the estimated energy requirements using either Jevity 1.5 or Oxepa (both Abbott Laboratories) with a goal of gaining approximately 0·5 kg/week. Oxepa contains 55% calories from fat compared to 29% in the Jevity products, including the omega-3 fatty acids eicosapentaenoic acid and gamma-linolenic acid. The percent of calories from protein (17%) was the same across all formula. If any participant experienced weight loss despite > 90% compliance with their tube feed formula, the Coordination Center Bionutritionist had the ability to increase their prescribed diet in a blinded manner. Because some participants were still able to consume food by mouth, the number of calories consumed could exceed the prescribed diets. The coordination center bionutritionist provided frequent phone consultations with each site to ensure protocol compliance and to encourage uniformity in nutritional management. A web-based electronic data capture system (Pharmaengine) was used to record all study data. The study intervention was designed to be four months long because the average survival time after PEG insertion is 4·5–8 months.22 Study activities were completed during five in-person visits over 4·5–5 months with a final follow-up phone call one month after discontinuing study diet. At the end of the four months on study diet, surviving participants resumed their pre-study diets or were prescribed new diets by their care providers. Participants were not informed of the total prescribed calories during or after the study in order to prevent unblinding. If participants agreed to long-term follow-up, vital status was verified at the end of the study.
Outcome Measures
Safety End Points
The primary outcomes of the study were safety and tolerability. Adverse events (AE) and serious adverse events (SAE) were coded using the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Interim safety analyses were performed every six months during the course of the study by an independent data and safety monitoring board.
Tolerability
Participants kept daily diaries of their tube feed intake as well as any additional foods that they consumed by mouth. Participants were defined as tolerating the diet if they initiated study diet and were compliant with more than 80% of the prescribed study diet during the four months of the intervention. Participants were considered intolerant if they initiated study diet but failed to complete month four of the study on the originally assigned treatment for any reason.
Secondary Metabolic Outcomes
MREE and weight were measured at every in-person visit and participants were instructed to measure their weight at home on a weekly basis. Fat mass and lean body mass (LBM) were measured using Dual-energy X-ray Absorptiometry (DXA) using a Hologic Discovery A (Hologic Inc, Bedford, MA) or equivalent at baseline and month four at sites which had access to a DXA machine. Serum was collected at in-person visits after an overnight fast, frozen at −80°C, and sent to the MGH Research Laboratory for measurement of fasting lipids, beta hydroxybutyrate, albumin, pre-albumin, insulin, leptin, interleukin-6 (IL-6), a nd high sensitivity C-reactive protein (hs-CRP).
Secondary Efficacy Outcomes
Due to the small sample size, efficacy measures were included only as exploratory end points. Forced vital capacity (FVC) was collected at every in-person visit and the ALS Functional Rating Scale-Revised (ALSFRS-R) was collected at baseline and month four in order to reduce subject burden.
Statistical Analysis
Safety and tolerability analyses were performed on all participants who initiated study diets. Variables were summarized as frequencies and percentages, means and standard deviations, or medians and inter-quartile ranges as appropriate. Categorical and continuous variables were compared at baseline by Fisher’s exact and Kruskal-Wallis tests, respectively. Survival was analyzed using intention-to-treat analysis, including all enrolled participants. Survival curves were estimated by Kaplan–Meier product-limit methods and compared by log-rank test. Survival time was defined as time to the earlier of death or tracheotomy with censoring at the end of planned follow-up or date lost to follow-up among surviving participants regardless of compliance with assigned study diet. Given the small sample size, significance of the log-rank test was confirmed by permutation test. Change over time of continuous, longitudinal measures was analyzed using random-slopes models. This analysis is unbiased if loss to follow-up was non-informative and data missing due to mortality were predictable from observed trajectories given assumptions of the model. Given the small number of participants, no adjusted analyses were performed. As an exploratory measure, not included in our original analysis plan, we performed the recently described combined assessment of function and survival (CAFS) using methods previously described.23 Two-sided p-values less than 0·05 were considered statistically significant without correction for multiple comparisons. All analyses were performed using SAS version 9·3 (SAS Institute, Cary N.C.).
The target sample size for this study was originally 60 subjects; however due to slow enrollment, we revised our target to 30 subjects or 10 per study diet. With this revised sample size, the study had 89% power to detect adverse events expected to occur among 20% of participants on a given study diet.
Results
Study Population
A total of 28 people with ALS were screened and 24 participants were enrolled between December, 2009 and November, 2012. Seven participants were randomized to the control diet, nine to the HC/HC diet, and eight to the HF/HC diet. Four participants (one control, one HC/HC, and two HF/HC) withdrew after randomization but before starting study diet due to the burden of participation. The median number of participants per site was two (range one-six) and there was no imbalance in treatment allocation across sites (Figure 1. Enrollment and Outcomes). Baseline demographics and disease characteristics were well-balanced across the three study arms (Table 1) except that participants in the HF/HC group had a higher ALSFRS-R (p=0·04). The controls had more males, fewer patients with bulbar onset, and higher LDL/HDL cholesterol levels (reported to be associated with improved survival in ALS).24 The HC/HC group had a lower baseline BMI, were slightly younger, had a shorter time since diagnosis, and had fewer participants who had been prescribed BIPAP, however these differences were not significant.
Table 1.
Baseline characteristics of participants according to treatment group shown in frequency (N) and percents, mean and standard deviations (SD), or median and inter-quartile ranges (IQR); Bulbar onset= symptoms of ALS beginning in the cranial nerves; BIPAP=Bilevel positive airway pressure; ALSFRS-R= ALS Functional Rating Scale-Revised; Duration=time (in months) from diagnosis to screening; FVC= Forced Vital Capacity (percent of predicted); BMI= body mass index; LDL= low-density lipoprotein; HDL= high-density lipoprotein; IL-6= interleukin-6 (normal range 1·2 to 15·3 pg/mL); hs-CRP= high sensitivity C-reactive protein (normal range 0·0–3·0 mg/L). Two HC/HC participants had laboratory-supported probable ALS and one participant in the control arm had lower motor neuron features only.
| P values | ||||||
|---|---|---|---|---|---|---|
| Baseline Characteristic of Participants |
Control N=7 | HC/HC N=9 | HF/HC N=8 | Overall | HC/HC vs. Cntl |
HF/HC vs. Cntl |
| Male N(%) | 6 (86%) | 4 (44%) | 3 (38%) | 0•16 | 0•15 | 0•12 |
| Age (mean±SD yrs) | 63•2±9•4 | 57•5±15•4 | 64•0±6•9 | 0•28 | 0•19 | 0•82 |
| White Not-Hispanic N(%) | 7 (100%) | 9 (100%) | 8 (100%) | >•99 | >•99 | >•99 |
| Probable or Definite ALS N(%) | 6 (86%) | 7 (78%) | 8 (100%) | 0•61 | >•99 | 0•47 |
| Bulbar Onset N(%) | 3 (43%) | 6 (67%) | 6 (75%) | 0•50 | 0•61 | 0•31 |
| Taking Riluzole N(%) | 4 (57%) | 6 (67%) | 7 (88%) | 0•47 | >•99 | 0•28 |
| ALSFRS-R (mean±SD) | 23•0±3•5 | 25•5±9•4 | 30•7±7•0 | 0•16 | 0•85 | 0•04* |
| Duration (median (IQR) months) | 14•9 (8•8, 27•3) | 10•9 (3•3, 23•3) | 16•7 (9•5, 19•1) | 0•77 | 0•49 | 0•91 |
| FVC (mean±SD % predicted) | 54•4±14•5 | 59•3±22•4 | 54•5±22•2 | 0•82 | 0•63 | 0•38 |
| Prescribed BIPAP N(%) | 5 (71%) | 4 (44%) | 5 (63%) | 0•59 | 0•36 | >•99 |
| BMI (mean±SD kg/m2) | 25•0±4•5 | 22•4±3•0 | 24•7±4•4 | 0•34 | 0•15 | 0•91 |
| Percent Lean Body Mass (mean ±SD) | 64•8±16•0 | 66•1±11•5 | 62•1±12•5 | 0•78 | 0•77 | >•99 |
| Percent Body Fat (Mean ±SD) | 29•7±11•6 | 30•2±11•4 | 34•5±13•2 | 0•69 | 0•91 | 0•46 |
| Percent body weight loss (mean ±SD) | 19•6±6•8 | 19•2±9•1 | 19•4±11•0 | 0•96 | 0•87 | 0•82 |
| Total Cholesterol (mean ±SD mg/dL) | 217•2±32•2 | 218•7±26•6 | 212•8±33•8 | 0•96 | 0•88 | 0•86 |
| LDL Cholesterol (mean ±SD mg/dL) | 135•0±30•5 | 126•3±26•8 | 110•8±31•6 | 0•35 | 0•46 | 0•23 |
| HDL Cholesterol (mean ±SD mg/dL) | 47•2±5•5 | 59•1±13•7 | 63•2±13•3 | 0•06 | 0•05 | 0•03* |
| LDL/HDL ratio (mean±SD) | 2•89±0•69 | 2•25±0•78 | 1•83±0•69 | 0•14 | 0•19 | 0•07 |
| Uric Acid (mean ±SD mg/dL) | 5•9±0•7 | 4•4±1•7 | 4•7±0•8 | 0•07 | 0•06 | 0•05 |
| Pre-albumin (mean ±SD mg/dL) | 31•7 ±4•0 | 31•8 ±1•8 | 31•4 ±9•3 | 0•82 | 0•79 | 0•65 |
| IL-6 (mean ±SD pg/mL) | 1•9±0•6 | 2•1±1•0 | 2•8±0•6 | 0•07 | 0•88 | 0•03* |
| hs-CRP (median (IQR) mg/L) | 0•7 (0•5, 3•0) | 1•8 (1•1, 9•3) | 2•9 (1•8, 6•3) | 0•16 | 0•13 | 0•09 |
Safety and Tolerability Outcomes
Control participants were more likely to discontinue the study diet due to adverse events (three Cntl vs. zero HC/HC vs. one HF/HC) and less likely to complete the study on the intervention diet (17% vs. 88%, vs. 83%; p=0·03 and 0·08 for the difference in tolerability between Cntls and the HC/HC and HF/HC groups, respectively).
Participants on the HC/HC diet experienced fewer adverse events (AEs) and serious adverse events (SAEs) compared to control and HF/HC participants (24 AEs and 0 SAEs in HC/HC vs. 42 AEs and 9 SAEs in controls, vs. 49 AEs and 3 SAEs in the HF/HC arm, Table 2). The most common AEs were gastrointestinal (50% of HC/HC vs. 100% of Cntl vs. 100% of HF/HC). None of the participants in the two hypercaloric arms experienced elevated serum bicarbonate (compared to three participants in the control arm), as we had postulated might occur due to respiratory weakness.25 In addition, there were no cardiovascular AEs or SAEs in the hypercaloric arms and the HF/HC diet was not associated with increased cholesterol or hs-CRP levels (Table 3). Finally, there was no evidence that the hypercaloric diets led to diabetes based on fasting blood glucose levels and serum insulin levels (Table 3).
Table 2.
Adverse Events coded by Common Terminology Criteria for Adverse Events version 3.0 body system. Frequency of adverse events shown in N=absolute numbers of adverse events; Pts(%)= number and percent of participants who experienced at least one adverse event; AE= Adverse Events; SAE= Serious Adverse Events; Cntl=Control diet; HC/HC=high-carbohydrate/hypercaloric diet; HF/HC= high-fat/hypercaloric diet. Specific AEs listed parenthetically under the CTCAE body systems are a selection from all CTCAE terms reported.
| Adverse Events by Body Sytem |
Cntl | HC/HC | HF/HC | p values (events) | p values (% Pts) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | N | Pts (%) |
N | Pts (%) |
N | Pts (%) |
Overall | HC/HC vs. Cntl |
HF/HC vs. Cntl |
Overall | HC/HC vs. Cntl |
HF/HC vs. Cntl |
|
| Allergy/Immunology (Allergic rhinitis) | AE | 0 | 0 (0) | 1 | 1 (13) | 0 | 0 (0) | 0•81 | 0•69 | >0•99 | >0•99 | >0•99 | >0•99 |
| SAE | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | |
| Cardiac (Atrial fibrillation, elevated troponin) | AE | 2 | 1 (17) | 0 | 0 (0) | 0 | 0 (0) | >0•99 | 0•97 | 0•97 | 0•6 | 0•43 | >0•99 |
| SAE | 0 | 0(0) | 0 | 0 (0) | 0 | 0 (0) | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | |
| Constitutional Symptoms (Fatigue, weight change) | AE | 7 | 2 (33) | 2 | 2 (25) | 5 | 2 (33) | 0•41 | 0•2 | 0•78 | >0•99 | >0•99 | >0•99 |
| SAE | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | |
| Dermatology (Abration) | AE | 0 | 0 (0) | 0 | 0 (0) | 1 | 1 (17) | 0•71 | >0•99 | 0•51 | 0•6 | >0•99 | >0•99 |
| SAE | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | |
| Gastrointestinal (detached feeding tube, bloating, dyspepsia, diarrhea) | AE | 13 | 6 (100) | 8 | 4 (50) | 21 | 6 (100) | 0•04 | 0•11 | 0•21 | 0•02 | 0•09 | >0•99 |
| SAE | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | |
| Hematology/Coagulation (Thrombocytopenia) | AE | 1 | 1 (17) | 0 | 0 (0) | 0 | 0 (0) | >0•99 | 0•97 | 0•64 | 0•6 | 0•43 | >0•99 |
| SAE | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | |
| Infection (Urinary tract, pneumonia) | AE | 5 | 2 (33) | 3 | 3 (38) | 6 | 4 (67) | 0•46 | 0•41 | 0•82 | 0•62 | >0•99 | 0•57 |
| SAE | 1 | 1 (17) | 0 | 0 (0) | 0 | 0 (0) | 0•6 | 0•43 | >0•99 | 0•6 | 0•43 | >0•99 | |
| Metabolic/Laboratory (Elevated alkaline phosphatase, hypokalemia) | AE | 2 | 1 (17) | 0 | 0 (0) | 0 | 0 (0) | >0•99 | 0•97 | 0•97 | 0•6 | 0•43 | >0•99 |
| SAE | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | |
| Musculoskeletal (Falls, weakness) | AE | 0 | 0 (0) | 5 | 1 (13) | 4 | 2 (33) | >0•99 | 0•97 | 0•97 | 0•45 | >0•99 | 0•45 |
| SAE | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | >0•99 | |
| Neurology/Psychiatry (Dizziness, depression) | AE | 1 | 1 (17) | 1 | 1 (13) | 1 | 1 (17) | 0•97 | 0•9 | 0•99 | >0•99 | >0•99 | >0•99 |
| SAE | 0 | 0 (0) | 0 | 0 (0) | 1 | 1 (17) | 0•6 | >0•99 | >0•99 | 0•6 | >0•99 | >0•99 | |
| Pain (Abdomin, neck, limb) | AE | 3 | 2 (33) | 2 | 2 (25) | 4 | 3 (50) | 0•58 | 0•46 | 0•72 | 0•84 | >0•99 | >0•99 |
| SAE | 2 | 1 (17) | 0 | 0 (0) | 0 | 0 (0) | 0•18 | 0•18 | 0•5 | 0•6 | 0•43 | >0•99 | |
| Pulmonary/Upper Respiratory (Dyspnea, hypoxia, aspiration, cough) | AE | 8 | 3 (50) | 1 | 1 (13) | 6 | 3 (50) | 0•16 | 0•075 | 0•72 | 0•28 | 0•24 | >0•99 |
| SAE | 6 | 3 (50) | 0 | 0 (0) | 2 | 2 (33) | 0•007 | 0•006 | 0•29 | 0•07 | 0•06 | >0•99 | |
| Total | AE | 42 | 6 (100) | 23 | 8 (100) | 48 | 6 (100) | 0•06 | 0•06 | 0•73 | >0•99 | >0•99 | >0•99 |
| SAE | 9 | 4 (67) | 0 | 0 (0) | 3 | 3 (50) | <0•001 | <0•001 | 0•15 | 0•03 | 0•015 | >0•99 | |
Table 3. Change over time in secondary and metabolic outcome measures, by treatment group.
Parameter estimates for the interaction between treatment and time are shown (95% confidence intervals in parentheses) for BMI= body mass index; FVC: functional vital capacity percent of predicted; LDL= low-density lipoprotein; HDL= high-density lipoprotein; hs-CRP= high sensitivity C-reactive protein. Individual pair-wise p-values were calculated from contrasts using fixed effect solutions from models that included all three treatment groups.
| Change over time in Secondary and Metabolic Outcome Measures |
Parameter Estimates (95% CI) | Comparison p-values | ||||
|---|---|---|---|---|---|---|
| Control | HC/HC | HF/HC | Ove rall |
HC/HC vs Cntl |
HF/HC vs Cntl |
|
| Weight (kg/month) | 0•11 (−0•64, 0•86) | 0•39 (−0•16, 0•94) | −0•46 (−1•11, 0•18) | 0•12 | 0•54 | 0•24 |
| BMI (units/month) | −0•04 (−0•34, 0•26) | 0•22 (0•00, 0•44) | −0•17 (0•43, 0•08) | 0•06 | 0•17 | 0•51 |
| ALSFRS-R (units/month) | −2•17 (−3•24, −1•10) | −1•07 (−1•71, −0•42) | −1•54 (−2•36, −0•73) | 0•17 | 0•07 | 0•32 |
| FVC (% predicted/ month) | −3•06 (−6•33, 0•21) | −3•39 (−5•47, −1•31) | −3•84 (−6•40, −1•29) | 0•92 | 0•86 | 0•70 |
| Total Cholesterol (mg/dL/month) | 3•01 (−7•80, 13•81) | 0•45 (−6•65, 7•54) | −4•00 (−12•93, 4•94) | 0•56 | 0•69 | 0•31 |
| LDL Cholesterol (mg/dL/month) | 0•80 (−6•97, 8•57) | −1•90 (−6•81, 3•02) | −8•19 (−14•46, −1•93) | 0•16 | 0•55 | 0•08 |
| HDL Cholesterol (mg/dL/month) | 0•63 (−3•66, 4•92) | −0•58 (−3•65, 2•48) | −1•65 (−5•37, 2•07) | 0•71 | 0•64 | 0•41 |
| Insulin (uU/mL) | 1•50 (0•23, 2•77) | −0•14 (−0•98, 0•71) | −0•13 (−1•16, 0•90) | 0•06 | 0•025 | 0•04 |
| Pre-albumin (mg/dL/month) | −0•22 (−2•72, 2•28) | 0•07 (−1•28, 1•42) | −0•03 (−1•58, 1•51) | 0•98 | 0•83 | 0•89 |
| hs-CRP (mg/L/month) | 0•51 (−0•93, 1•96) | 0•20 (−0•71, 1•10) | 0•33 (−0•79, 1•45) | 0•92 | 0•69 | 0•83 |
Secondary Metabolic Outcomes
Participants randomized to the control arm were essentially weight-stable gaining on average 0·11 kg/month (95% CI −0·64, 0·86), although there was substantial variation in weight in the control arm (see Supplementary Figure 2A). On average, control participants consumed 1·21±0·26 times their estimated energy requirements, including both prescribed enteral nutrition and oral intake. Participants in the HC/HC arm gained on average 0·39 kg/month (95% CI −0·16, 0·95), consuming 1·54±0·33 times their estimated energy requirements. Participants in the HF/HC arm lost 0·46 kg/month (95% CI −1·11, 0·18) despite consuming on average 1·51±0·33 times their estimated energy requirements.
Based on the eleven participants who underwent repeated DXA measurements after four months, participants who gained weight overall during the study gained primarily fat mass compared to LBM (2·23 ±2·25 kg vs. 0·17 ±1·29 kg) while those with net weight loss lost 1·61 ±0·99 kg LBM while still gaining a small amount of fat mass (0·39 ±0·35 kg).
Secondary Efficacy Outcomes
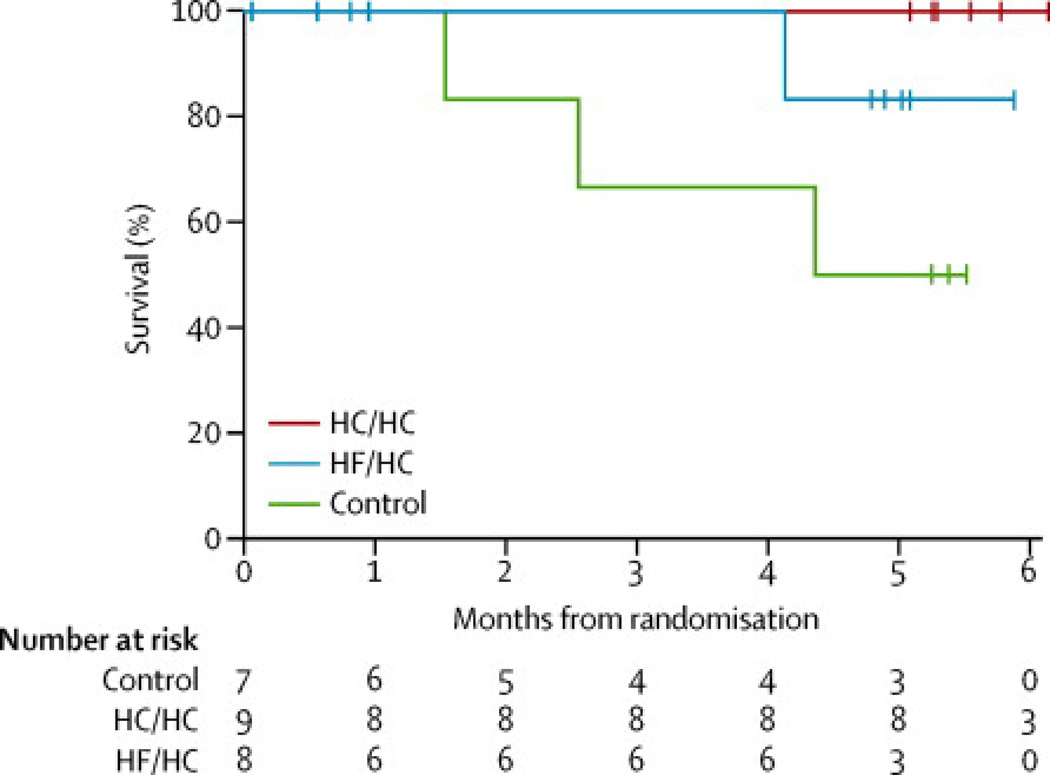
There were no deaths in the HC/HC arm compared to three deaths in the control arm during the five-month follow-up schedule (log-rank test for survival p=0·03; Figure 2). There was one death in the HF/HC arm (log-rank p=0·23 compared to controls). While all of the deaths occurred within 30 days of stopping study diet, all deaths were due to respiratory failure and no deaths were considered related to study diet. Long term survival, recorded at the end of the study (up to 19 months after completing scheduled follow-up) is shown in Supplementary Figure 1. ALSFRS-R scores declined more slowly in the HC/HC arm −1·06 (95% CI −1·71, −0·41) points/month vs. −2·17 (95% CI −3·25, −1·1) in the surviving controls vs. −1·59 (95% CI −2·44, −0·74) in the HF/HC group, although this did not reach significance (p=0·07 for the comparison between the HC/HC and control groups, Supplementary Figure 2C). As the ALSFRS-R analysis excluded participants who died during the study because only a single follow-up assessment was completed, we also performed an exploratory post-hoc CAFS analysis. The mean (SD) joint rank scores were 14·8 (12·9) in the HC/HC arm vs. 6·0 (12·1) in the control arm vs. 9·3 (12·1) in the HF/HC arm (higher is better). The difference between the HC/HC and control arm was significant (Kruskal-Wallis p=0·01) while the difference between the HF/HC and control arm was not (p=0·26). There was no difference in the rate of decline in FVC (Table 3, Supplementary Figure 2D).
Figure 2.
Kaplan-Meier curves for overall survival. HC/HC= high-carbohydrate/hypercaloric diet; HF/HC= high-fat/hypercaloric diet; Cntl= control diet. The log-rank test for the difference across all treatments was p=0·06. The log-rank test for the difference in survival between the HC/HC and control arms was p=0·03.
Discussion
ALS patients randomized to a high-carbohydrate hypercaloric formula with a goal of weight gain were less likely to experience serious adverse events including death during the study, compared to participants on a calorie replacement diet with a goal of weight stability. The study results support hypercaloric enteral nutrition as a novel and potentially robust non-pharmacologic intervention for this fatal disease. These results should be interpreted with caution given the small sample size. Another imitation which prevents the results from being generalizable is the fact that study participants had advanced disease and were malnourished, having lost almost 20% of their body weight on average by the time of enrollment. While this was a small pilot study, the results are consistent with epidemiologic and preclinical data showing that obesity in humans with ALS and hypercaloric diets in a mouse model of ALS are associated with improved survival. In addition to the safety data, the tolerability and secondary efficacy outcomes all favored the high carbohydrate hypercaloric arm. Possible risks of weight gain (hypercarbia, diabetes, or increased vascular events) were not observed during the study.
Our DXA results support the hypothesis that weight loss due to starvation may exacerbate muscle loss due to denervation by causing muscle catabolism.2 Also consistent with prior reports, participants experienced an increase in body fat even while losing LBM.8 Unfortunately, we did not have complete DXA data to compare the rates of LBM loss among the intervention groups.
We had initially hypothesized that the enteral formula containing 55% calories from fat (HF/HC) would be most effective at causing weight gain, as it most closely mimicked the diets used in animal studies. A recent study found that oral supplements containing a modest 35% calories from fat were slightly more effective at causing weight gain than supplements containing 0% calories from fat.26 In our study, the HF/HC diet resulted in weight loss, despite participants consuming up to 174% of their estimated energy requirements. This may have been due to the lower tolerability and higher rate of gastrointestinal side effects compared to the HC/HC diet. Prior studies using Oxepa have not reported weight loss as a side effect.27
We were surprised by the number of calories required by participants to achieve weight gain. One possible explanation is that the observed MREE were inappropriately low; however, the MREE correlated well with the Harris-Benedict calculated REE and were measured by multiple machines at multiple sites. An alternative explanation is that self-reported activity levels were low; however, the estimated physical activity coefficients (mean 1·1, range 1·0–1·48) were consistent with prior doubly-labeled water experiments showing that advanced ALS patients’ total daily energy expenditure is only 1·0–1·.2 times their MREE.28, 29 Another explanation, supported by elevated hs-CRP levels in the majority of participants, could be that some participants were resistant to weight gain due to circulating cytokines as in cancer cachexia.30 Finally, the high frequency of gastrointestinal side effects in the control and HF/HC arms suggests that continued weight loss may have been due to gastrointestinal malabsorption. Our study had several strengths: first, by using enteral nutritional supplements, we were able to carefully control the number of calories administered to each participant. Second, our randomization scheme resulted in well-balanced groups, with little risk of confounding by site due our use of stratified randomization and the low number of participants enrolled at each site.
In summary, we believe that our study results provide preliminary evidence for a novel, simple, low-cost, low-risk treatment for this devastating disease. The results of this study also support growing interest in the use of dietary interventions to treat neurological diseases. Our results also support the concept that ALS is a multi-organ systemic disease, characterized by metabolic dysfunction.3 We believe that given the promising results of this pilot study and lack of treatment options for ALS, nutritional interventions should be studied in larger randomized controlled trials at earlier stages of the disease.
Supplementary Material
Research in Context.
Systematic Review
We performed a systematic search of PubMed and Ovid for all clinical trials, observational studies, and reviews published between Jan 1, 1963 and February 7, 2014 in English, Spanish, Italian, German, and French. Search terms were nutrition supplements, hypercaloric, hyperalimentation, enteral nutrition, weight gain, oral supplements dietary supplements, and amyotrophic lateral sclerosis or ALS,
Interpretation
Currently the only FDA-approved therapy for ALS is riluzole, which provides only a modest survival benefit. Two small prior studies of oral supplements have shown some efficacy in causing weight gain in ALS, although these studies were underpowered to detect differences in disease outcomes in participants randomized to oral supplements. Our pilot study provides important preliminary evidence to support the use of hypercaloric nutritional interventions as a novel, low-risk therapy for the treatment of ALS.
Acknowledgements
We thank Drs. Jeremy Shefner, Wendy Galpern and Michael McDermott for serving on the DSMB. We thank Drs. Valerie Cwik and Sanjay Bidichandani, Karen L. Smith, Jodi Wolff and Jane Larkindale at the MDA for their unwavering support for this project. We thank the NorthEast ALS Consortium, Dr. Lucie Bruijn and the ALS Association, and Apria Healthcare for helping to publicize the study. We would also like to thank Roy Cutler, Drs. Hideo Makimura and Robert H. Brown Jr. for their useful discussions. Finally we wish to thank all of the patients and their family members and caregivers who volunteered their time and effort to participate in this study.
Sources of Funding: This study was supported by the Muscular Dystrophy Association with additional support from the Massachusetts General Hospital Clinical Research Center, grant number 1 UL1 RR025758-03, Harvard Clinical and Translational Science Center, from the National Center for Research Resources, National Institutes of Health grant number RR-00109, and the Harvard NeuroDiscovery Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors and Contributors:
Anne-Marie Wills M.D., M.P.H.: performed literature searches, initiated and designed the study, participated in data collection, data analysis, data interpretation, and wrote the first draft of the manuscript
Jane Hubbard M.S., R.D. performed literature searches, assisted in the design of the study, participated in data collection, data analysis, data interpretation, and reviewed and critiqued the manuscript
Eric A. Macklin Ph.D. performed data analysis, data interpretation, and reviewed and critiqued the manuscript
Jonathan Glass, M.D. participated in data collection, data interpretation, and reviewed and critiqued the manuscript
Rup Tandan, M.D participated in data collection, data interpretation, and reviewed and critiqued the manuscript
Ericka P Simpson, M.D. participated in data collection, data interpretation, and reviewed and critiqued the manuscript
Benjamin Brooks, M.D. participated in data collection, data interpretation, and reviewed and critiqued the manuscript
Deborah Gelinas, M.D. participated in data collection, data interpretation, and reviewed and critiqued the manuscript
Hiroshi Mitsumoto, M.D. participated in data collection, data interpretation, and reviewed and critiqued the manuscript
Tahseen Mozaffar, M.D. participated in data collection, data interpretation, and reviewed and critiqued the manuscript
Gregory P. Hanes M.D. participated in data collection, data interpretation, and reviewed and critiqued the manuscript
Shafeeq S. Ladha M.D. participated in data collection, data interpretation, and reviewed and critiqued the manuscript
Terry Heiman-Patterson, M.D. participated in data collection, data interpretation, and reviewed and critiqued the manuscript
Jonathan Katz, M.D. participated in data collection, data interpretation, and reviewed and critiqued the manuscript
Jau-Shin Lou, M.D. Ph.D participated in data collection, data interpretation, and reviewed and critiqued the manuscript
Katy Mahoney B.A. participated in data collection, and reviewed and critiqued the manuscript
Daniela Grasso B.A. participated in data collection, and reviewed and critiqued the manuscript
Robert Lawson B.S. participated in data collection and reviewed and critiqued the manuscript
Hong Yu M.S. participated in data collection, data interpretation, and reviewed and critiqued the manuscript
Merit Cudkowicz, M.D. M.Sc. assisted in the design of the study, participated in data interpretation, and reviewed and critiqued the manuscript
Conflicts of Interest: (full financial disclosures within the past 3 years)
Anne-Marie Wills M.D., M.P.H. has received research funding from the Muscular Dystrophy Association, NIH/NINDS, Schering-Plough and consultant payments from Asubio pharmaceuticals and Accordant, a CVS/Caremark disease management company.
Jane Hubbard M.S., R.D. reports no conflicts of interest/financial disclosures.
Eric A. Macklin Ph.D. serves on DSMB's for Lantheus Medical Imaging and Shire Human Genetic Therapies and was an unpaid consultant to Knopp Biosciences.
Jonathan Glass, M.D. receives research funding from MDA, ALSA, NIH, and Neuralstem, Inc.
Rup Tandan, M.D. F.R.C.P. has received research funding from the Muscular Dystrophy Association, NIH/NINDS, National ALS Association, Novartis Pharmaceuticals and Alexion Pharmaceuticals; speakers bureau payments from Athena Diagnostics; and consultant payments from Rx Solutions and Walgreens.
Ericka P Simpson, M.D. reports no conflicts of interest/financial disclosures.
Benjamin Brooks, M.D. reports grants from Muscular Dystrophy Association; grants from Cytokinetics Pharmaceuticals, Biogen-Idec Pharmaceuticals, Avanir Pharmaceuticals, and from Carolinas ALS Research Fund Carolinas Healthcare Foundation, He has served as a consultant for Cytokinetics Pharmaceuticals, Knopp Biosciences, the American Academy of Neurology, Asubio Pharmaceuticals, Bristol-Myers- Squibb Pharmaceuticals, Countervail Corporation, Biogen-Idec Pharmaceuticals, and is an unpaid member of the Board of Directors of the ALS Research Group.
Deborah Gelinas, M.D. serves on the Speaker Bureau for Avanir Pharmaceuticals.
Hiroshi Mitsumoto, M.D. reports no conflicts of interest/financial disclosures.
Tahseen Mozaffar, M.D. reports no conflicts of interest/financial disclosures.
Gregory P. Hanes M.D. reports no conflicts of interest/financial disclosures.
Shafeeq S. Ladha M.D. reports no conflicts of interest/financial disclosures.
Terry Heiman-Patterson, M.D. reports no conflicts of interest/financial disclosures.
Jonathan Katz, M.D. reports no conflicts of interest/financial disclosures.
Jau-Shin Lou, M.D. Ph.D reports no conflicts of interest/financial disclosures.
Katy Mahoney B.A. reports no conflicts of interest/financial disclosures.
Daniela Grasso B.A. reports no conflicts of interest/financial disclosures.
Robert Lawson B.S. reports no conflicts of interest/financial disclosures.
Hong Yu M.S. reports no conflicts of interest/financial disclosures.
Merit Cudkowicz, M.D. M.Sc. reports no conflicts of interest/financial disclosures.
References
- 1.Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 2.Kasarskis EJ, Neville HE. Management of ALS: nutritional care. Neurology. 1996;47(4) Suppl 2:S118–S120. doi: 10.1212/wnl.47.4_suppl_2.118s. [DOI] [PubMed] [Google Scholar]
- 3.Dupuis L, Pradat PF, Ludolph AC, Loeffler JP. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. 2011;10(1):75–82. doi: 10.1016/S1474-4422(10)70224-6. [DOI] [PubMed] [Google Scholar]
- 4.Genton L, Viatte V, Janssens JP, Heritier AC, Pichard C. Nutritional state, energy intakes and energy expenditure of amyotrophic lateral sclerosis (ALS) patients. Clin Nutr. 2011;30(5):553–559. doi: 10.1016/j.clnu.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Miller RG, Jackson CE, Kasarskis EJ, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence–based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73(15):1218–1226. doi: 10.1212/WNL.0b013e3181bc0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersen PM, Abrahams S, Borasio GD, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)–revised report of an EFNS task force. Eur J Neurol. 2012;19(3):360–375. doi: 10.1111/j.1468-1331.2011.03501.x. [DOI] [PubMed] [Google Scholar]
- 7.Greenwood DI. Nutrition management of amyotrophic lateral sclerosis. Nutr Clin Pract. 2013;28(3):392–399. doi: 10.1177/0884533613476554. [DOI] [PubMed] [Google Scholar]
- 8.Nau KL, Bromberg MB, Forshew DA, Katch VL. Individuals with amyotrophic lateral sclerosis are in caloric balance despite losses in mass. J Neurol Sci. 1995;129(Suppl):47–49. doi: 10.1016/0022-510x(95)00061-6. [DOI] [PubMed] [Google Scholar]
- 9.Kasarskis EJ, Berryman S, Vanderleest JG, Schneider AR, McClain CJ. Nutritional status of patients with amyotrophic lateral sclerosis: relation to the proximity of death. Am J Clin Nutr. 1996;63(1):130–137. doi: 10.1093/ajcn/63.1.130. [DOI] [PubMed] [Google Scholar]
- 10.Desport JC, Preux PM, Truong TC, Vallat JM, Sautereau D, Couratier P. Nutritional status is a prognostic factor for survival in ALS patients. Neurology. 1999;53(5):1059–1063. doi: 10.1212/wnl.53.5.1059. [DOI] [PubMed] [Google Scholar]
- 11.Paganoni S, Deng J, Jaffa M, Cudkowicz ME, Wills AM. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve. 2011;44(1):20–24. doi: 10.1002/mus.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reich–Slotky R, Andrews J, Cheng B, et al. Body mass index (BMI) as predictor of ALSFRS–R score decline in ALS patients. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(3):212–216. doi: 10.3109/21678421.2013.770028. [DOI] [PubMed] [Google Scholar]
- 13.Gallo V, Wark PA, Jenab M, et al. Prediagnostic body fat and risk of death from amyotrophic lateral sclerosis: the EPIC cohort. Neurology. 2013;80(9):829–838. doi: 10.1212/WNL.0b013e3182840689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Reilly EJ, Wang H, Weisskopf MG, et al. Premorbid body mass index and risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(3):205–211. doi: 10.3109/21678421.2012.735240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupuis L, Oudart H, Rene F, Gonzalez de Aguilar JL, Loeffler JP. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high–energy diet in a transgenic mouse model. Proc Natl Acad Sci U S A. 2004;101(30):11159–11164. doi: 10.1073/pnas.0402026101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattson MP, Cutler RG, Camandola S. Energy intake and amyotrophic lateral sclerosis. Neuromolecular Med. 2007;9(1):17–20. doi: 10.1385/nmm:9:1:17. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen WA, Mattson MP. No benefit of dietary restriction on disease onset or progression in amyotrophic lateral sclerosis Cu/Zn–superoxide dismutase mutant mice. Brain Res. 1999;833(1):117–120. doi: 10.1016/s0006-8993(99)01471-7. [DOI] [PubMed] [Google Scholar]
- 18.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial "Clinical limits of amyotrophic lateral sclerosis" workshop contributors. J Neurol Sci. 1994;124(Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 19.Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102(11):1621–1630. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 20.Bouchard C, Tremblay A, Leblanc C, Lortie G, Savard R, Theriault G. A method to assess energy expenditure in children and adults. Am J Clin Nutr. 1983;37(3):461–467. doi: 10.1093/ajcn/37.3.461. [DOI] [PubMed] [Google Scholar]
- 21.Otten J, Pitzi-Hellwig J, Meyers L. Dietary Reference Intakes: The essential guide to nutrient requirements. Washington DC: National Academies Press; 2006. [Google Scholar]
- 22.Shaw AS, Ampong MA, Rio A, et al. Survival of patients with ALS following institution of enteral feeding is related to pre–procedure oximetry: a retrospective review of 98 patients in a single centre. Amyotroph Lateral Scler. 2006;7(1):16–21. doi: 10.1080/14660820510012013. [DOI] [PubMed] [Google Scholar]
- 23.Berry JD, Miller R, Moore DH, et al. The Combined Assessment of Function and Survival (CAFS): a new endpoint for ALS clinical trials. Amyotroph Lateral Scler Frontotemporal Degener. 14(3):162–168. doi: 10.3109/21678421.2012.762930. [DOI] [PubMed] [Google Scholar]
- 24.Dupuis L, Corcia P, Fergani A, et al. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology. 2008;70(13):1004–1009. doi: 10.1212/01.wnl.0000285080.70324.27. [DOI] [PubMed] [Google Scholar]
- 25.Aldrich TK. Nutritional factors in the pathogenesis and therapy of respiratory insufficiency in neuromuscular diseases. Monaldi Arch Chest Dis. 1993;48(4):327–330. [PubMed] [Google Scholar]
- 26.Dorst J, Cypionka J, Ludolph AC. High–caloric food supplements in the treatment of amyotrophic lateral sclerosis: A prospective interventional study. Amyotroph Lateral Scler Frontotemporal Degener. 2013 doi: 10.3109/21678421.2013.823999. [DOI] [PubMed] [Google Scholar]
- 27.Pontes-Arruda A, Aragao AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med. 2006;34(9):2325–2333. doi: 10.1097/01.CCM.0000234033.65657.B6. [DOI] [PubMed] [Google Scholar]
- 28.Ichihara N, Namba K, Ishikawa-Takata K, et al. Energy requirement assessed by doubly–labeled water method in patients with advanced amyotrophic lateral sclerosis managed by tracheotomy positive pressure ventilation. Amyotroph Lateral Scler. 2012;13(6):544–549. doi: 10.3109/17482968.2012.699968. [DOI] [PubMed] [Google Scholar]
- 29.Kasarskis EJ, Mendiondo MS, Wells S, et al. The ALS Nutrition/NIPPV Study: design, feasibility, and initial results. Amyotroph Lateral Scler. 12(1):17–25. doi: 10.3109/17482968.2010.515225. [DOI] [PubMed] [Google Scholar]
- 30.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.