Abstract
The interactions between cardiovascular disease (CVD) and insulin resistance syndromes suggest the possibility of joint targets for cardiometabolic research. The best drugs would go beyond minimizing adverse effects and have protective actions against both metabolic disease and CVD. In this perspective, we will outline a few examples in which a deep mechanistic understanding of the many cellular pathways that contribute to type 2 diabetes and CVD, regardless of cell type, have resulted in common upstream pathogenic pathways that can be therapeutically targeted.
The interrelationships between cardiovascular disease (CVD) and insulin resistance syndromes provide both challenges and opportunities to translational cardiometabolic research. On the one hand, the accelerating worldwide epidemics of obesity and insulin resistance are and will continue to be the major drivers of CVD (1). Conversely, one of the major hurdles for the development of new drugs for type 2 diabetes (T2D) is cardiovascular safety. Indeed, the U.S. Food and Drug Administration has stipulated that all new human-tested T2D drugs must undergo a clinical trial to show absence of adverse cardiovascular effects. In reality, the best T2D drugs would go beyond this minimum requirement and have protective actions against both metabolic disease and CVD. To meet this ideal, we must have a deep mechanistic understanding of the many cellular pathways that contribute to T2D and CVD, regardless of cell type, and then identify common upstream pathogenic pathways that can be therapeutically targeted. In this Perspective, we will outline a few examples that have emerged from the recent literature in these areas (2–5) (Fig. 1).
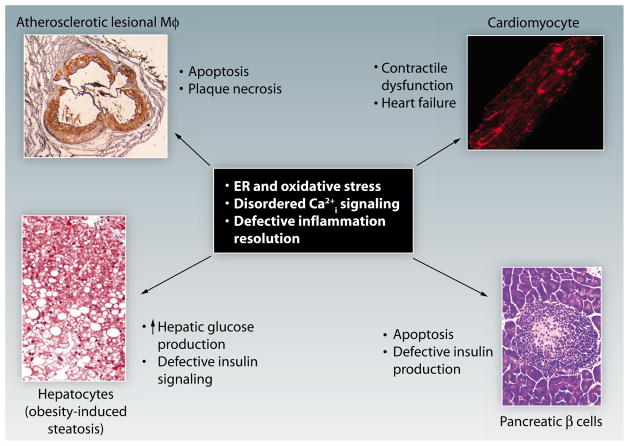
Fig. 1. Common upstream pathogenic targets in cardiometabolic disease.
Cardiometabolic disease represents the integrative pathophysiology of insulin-resistant syndromes and CVD. Understanding the mechanisms of integration is critical because of the acceleration of heart disease in subjects with insulin resistance and because all new diabetes drugs have to go through cardiac safety trials. Shown here are examples of four separate components of the cellular pathobiology of cardiometabolic disease: macrophage apoptosis in advanced atherosclerosis [panel adapted with permission from (2)], cardiomyocyte dysfunction in heart failure [panel adapted with permission from (3)], altered hepatocyte function associated with excessive HGP and defective insulin signaling [panel adapted with permission from (4)], and pancreatic β cell apoptosis in diabetes [panel adapted with permission from (5)]. Despite the different cell types and different pathobiological end points, all four processes involve the common pathogenic processes of ER and oxidative stress, disordered intracellular calcium (Ca2+i) signaling, and defective inflammation resolution. Thus, therapeutic targeting of these processes in metabolic disease will increase the probability that the drugs will not have adverse cardiac effects and, in the best case scenario, will have the added benefit of being proactively cardioprotective.
Atherothrombotic coronary artery disease (CAD) is a major cause of death among subjects with T2D (6). Acute CAD results from the progression of a relatively small percentage of atherosclerotic lesions into plaques that undergo rupture or erosion, which then leads to local exposure of thrombogenic material and acute lumenal thrombosis (7). A key process in this progression is the development of a “necrotic core” in the plaque that promotes inflammation, tissue degradation, and biomechanical stress. Macrophages, which represent the major cell type in developing atherosclerotic lesions, undergo apoptosis in the plaque and contribute to necrosis (8). These apoptotic macrophages become secondarily necrotic because of inefficient phagocytic clearance of the dead cells (efferocytosis), which is a defect known to occur in advanced human lesions.
ENDOPLASMIC RETICULUM STRESS
The causes of advanced lesional macrophage apoptosis are multifactorial. However, according to mechanistic studies in cultured macrophages, molecular-genetic causation studies in mice, and observational studies that use human atherosclerotic plaques, an apoptotic signaling pathway triggered by prolonged endoplasmic reticulum (ER) stress signaling appears to be particularly important (8). ER stress signaling manifested by the unfolded protein response (UPR) is normally transiently activated in response to short-term ER functional disequilibrium and serves to restore homeostasis (9). Although physiological ER stress signaling is involved to ensure proper function and survival in many cell types, severe and/or non-resolving ER stress in certain disease processes leads to prolonged activation of the UPR, which can trigger cell death pathways (10). In advanced lesional macrophages, various cytotoxic lipids can cause this scenario, leading to prolonged expression of a proapoptotic UPR effector called CHOP, or GADD153, which is amplified in the face of insulin resistance. Indeed, there is a strong correlation among necrotic plaques, CHOP expression, and cell death in human coronary artery lesions, and genetic disruption of CHOP in mice suppresses plaque necrosis (11, 12). Moreover, the UPR is activated in other types of cells in advanced lesions, namely endothelial cells and smooth-muscle cells, which further implicates the involvement of ER stress in atherosclerosis.
These cell-intrinsic atherothrombotic processes in arterial wall lesions can be exacerbated by systemic risk factors. Among the most important of these are dyslipidemia and hyperinsulinemia driven by obesity and insulin resistance (6). Just as chronic ER stress in arterial wall cells drives advanced plaque progression, there is increasing evidence that chronic ER stress in hepatocytes drives insulin resistance and dyslipidemia in obesity (13). Although most of the data exploring hepatic ER stress in obesity comes from animal studies, there is an association between relief of ER stress and improvement in metabolic parameters after bariatric surgery in humans (14). Among the likely causes of hepatocyte ER stress in obesity are intracellular accumulation of saturated fatty acids and excessive glucagon signaling. Saturated fatty acids probably disturb ER membrane structure, and excessive glucagon action suppresses a protective arm of the UPR involving a transcription factor called activating transcription factor 6 (ATF6) and a chaperone called P58IPK (6, 15). When intracellular levels of P58IPK are decreased, there is activation of a potentially pathogenic arm of the UPR controlled by the ER kinase PERK.
One consequence of chronic UPR activation in hepatocytes is activation of the mitogen-activated protein kinase c-Jun N-terminal kinase (JNK), which disturbs insulin signaling through Ser-phosphorylation of insulin receptor substrate–1 (IRS1) (16). Moreover, PERK activation leads to the induction of a transcription factor called ATF4, which induces an inhibitor of insulin-induced AKT activation called Tribbles 3 (15). The PERK pathway also induces another transcriptional regulator, ATF3, which contributes to dyslipidemia by promoting the secretion of apolipoprotein B-containing lipoprotein (17).
The detrimental metabolic consequences of chronic UPR activation in hepatocytes contributes over time to activation of the UPR in another key metabolic cell type, the pancreatic β cell (18). In response to hepatic insulin resistance, as well as excessive hepatic glucose production, β cells compensate by secreting high levels of insulin. The protein translational stress imposed by this scenario triggers a chronic, pathogenic ER stress response in β cells. Thus, similar to the effect of chronic UPR activation in macrophages in advanced atherosclerotic lesions, chronic ER stress in β cells can trigger apoptosis, leading to end-stage diabetes.
Because ER stress in different cell types contributes to both advanced atherosclerosis and T2D, drugs that are able to suppress excessive and chronic ER stress, without compromising the physiologic UPR response, may have beneficial effects on both disease processes. Indeed, administration of a drug called 4-phenylbutyric acid (PBA) relieves ER stress in the liver of obese mice, where it improves markers of insulin resistance, and decreases lesional macrophage death and atherosclerosis in hypercholesterolemic mice (16, 19). Similarly, treatment of obese, insulin-resistant patients with tauroursodeoxycholic acid (TUDCA), a bile-acid derivative known to relieve ER stress, increases insulin sensitivity in liver and skeletal muscle and suppresses the development of atherosclerotic lesions in hypercholesterolemic mice (20, 21). More advanced and mechanistically defined UPR-suppressing drugs are in development and may be useful in protecting obese subjects from both T2D and CVD.
INTRACELLULAR CALCIUM SIGNALING
Another common pathway with therapeutic promise involves pathologic intracellular calcium signaling. A downstream proapoptotic mechanism of excessive CHOP expression in macrophages is prolonged increase in cytosolic calcium (22). This abnormality is triggered by a combination of oxidative stress-induced inositol 1,4,5-trisphosphate receptor (IP3R) calcium channel activation and lipid-mediated sarco(endo)plasmic reticulum Ca2+–adenosine triphosphatase (SERCA) inactivation (22, 23). Elevated cytosolic Ca2+ leads to excessive activation of a calcium-sensitive kinase called calcium/calmodulin–dependent protein kinase II (CaMKII), which in turn triggers a number of downstream apoptotic pathways in macrophages (24). Inhibition of CaMKII of ER-stressed macrophages protects them from cell death and eliminates signs of cytotoxicity, which suggests a therapeutic target to suppress macrophage death in advanced atherosclerosis.
Notably, the same combination of IP3R activation, SERCA inactivation, elevated cytosolic Ca2+, and CaMKII hyperactivation occurs in hepatocytes in the setting of obesity and contributes to both increased hepatic glucose production (HGP) and defective insulin signaling (15, 25–27). In fact, the same isoform of CaMKII, CaMKIIγ, is the predominant CaMKII isoform in both macrophages and hepatocytes. In the case of hepatocytes, IP3R activation is caused by excessive glucagon signaling, and SERCA inactivation is caused by alterations in ER phospholipids. The downstream metabolic effects of CaMKII hyperactivation in hepatocytes involve two separate pathways. One CaMKII pathway leads to elevated expression of FoxO1-induced genes that mediate HGP, and the other pathway blocks insulin signaling through the ATF6 suppressive mechanism described in the previous section. As such, genetic inhibition of CaMKII in hepatocytes in obese mice lowers plasma glucose and insulin and improves glucose and insulin tolerance.
Thus, as was the case with ER stress signaling, partial suppression of CaMKIIγ to prevent hyperactivation could be a common therapeutic target to improve metabolism in T2D and, by suppressing macrophage apoptosis and plaque necrosis in advanced atherosclerotic lesions, protect against acute cardiac events. It should also be mentioned that a closely related isoform of CaMKII, CaMKIIδ, is activated in heart failure, and it gets further activated by oxidation and O-GlcNAc modification in the setting of diabetes. Moreover, activated CaMKII amplifies the detrimental intracellular calcium signaling through a feed-forward cycle involving ryanodine receptor phosphorylation (28). Because heart failure can be induced or worsened by both T2D and some of the drugs used to treat T2D, an inhibitor that prevents the hyperactivation of both CaMKIIγ and CaMKIIδ may find a niche in this area. Last, targeting MAP-KAPK2, which mediates the metabolic actions of CaMKII in liver, may also benefit both atherosclerosis and heart failure (15, 29, 30).
INFLAMMATION RESOLUTION
A third example of a common pathologic pathway in heart disease and T2D is defective inflammation resolution. The normal inflammatory response mounts an acute, robust attack to kill pathogenic organisms and then triggers a resolution phase to prevent excessive oxidative and proteolytic collateral damage and restore tissue homeostasis (27). The resolution phase is mediated by multiple lipid-derived and protein mediators that block inflammatory cell influx and promote their egress; clear pathogens, cellular debris, inflammatory cytokines, and dead cells (efferocytosis); and carry out the necessary processes to repair tissue damage. Among the molecules that mediate resolution processes are specialized pro-resolving mediators (SPMs), which are endogenous lipids that are actively generated during inflammation (31). SPM families include lipoxins, resolvins, protectins, and maresins (31). Lipoxins are derived from omega-6 arachidonic acid, whereas resolvins, protectins, and maresins are derived from omega-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (31).
In chronic disease processes in which the inciting pathologic process is not readily neutralized, the resolution phase is defective, leading to a cycle of persistent tissue injury. In both atherosclerosis and T2D, there are signs of defective inflammation resolution (32–34). For example, advanced atherosclerotic lesional macrophages are chronically activated and display defective efferocytosis, and both adipose tissue and liver in obesity have continual influx of inflammatory cells and excessive oxidative stress. In atherosclerosis, the persistent stimulus is subendothelial-retained apolipoprotein B–containing lipoproteins (35), whereas in T2D, factors in the diet or responses to the diet may promote SPM catabolism (34). Moreover, the host’s ability to activate resolution programs may be weakened by excessive nutrient sensing through pattern recognition receptors, alterations in membrane fluidity, nuclear receptor activation, and/or lipid raft formation (31, 32).
With the knowledge that failed inflammation resolution contributes to the pathology of cardiometabolic diseases, understanding new approaches to stimulate resolution are of immense interest (35). One characteristic of SPMs is that they stimulate the termination of inflammation without being immunosuppressive (36). This feature, which is critical when contemplating therapies for chronic diseases, distinguishes SPMs from classic anti-inflammatory drugs, which often compromise host defense. There are as yet no reports on the application of SPM therapy in atherosclerosis, but a recent proof-of-concept study showed that a resolution-mediator peptide called Ac2-26 packaged in a type of nanoparticle designed to target vascular lesions could suppress ischemia-reperfusion injury in mice by promoting resolution (37). In terms of metabolic disease, there are several recent examples of exogenous SPM treatment in obese T2D models (34). For example, resolvin D1 (RvD1) has been shown to promote insulin sensitivity in obese diabetic mice, in part by enhancing insulin signaling in adipose tissue (38). Moreover, defective efferocytosis, which is involved in the progression of atherosclerosis (above), is an important cause of impaired wound healing in T2D, and RvD1 rescues defective macrophage efferocytosis and enhances wound healing in diabetic subjects (39).
In conclusion, knowledge of upstream pathogenic pathways that are common features of different cells types in cardiometabolic disease—such as chronic ER stress, disturbed intracellular calcium signaling, and defective inflammation resolution—offers a potentially improved approach to new therapeutic strategies. The standard approach of targeting specific cell types and end points can lead to fragmented therapy. Moreover, because these upstream pathways have also important physiological functions in diverse biological processes, an in-depth understanding of the associated outcomes is required in order to prevent possible side effects. In the case of designing new diabetes drugs, rather than “hoping” that a drug designed to affect liver metabolism or β cell function is heart-safe, the integrated approach suggested here increases the probability of cardiac safety and even raises the possibility of a T2D drug that also suppresses one or more pathophysiological pathways in heart disease per se. However, for this type of approach to be successful more work is needed to understand the mechanisms and molecular targets of the three pathways discussed here and to identify additional common pathways. Biological systems do not exist in isolation, and only by studying the intersections of disease can we come up with integrated therapies for the expanding epidemic of cardiometabolic disease.
References
- 1.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Kotla S, Singh NK, Heckle MR, Tigyi GJ, Rao GN. The transcription factor CREB enhances interleukin-17A production and inflammation in a mouse model of atherosclerosis. Sci Signal. 2013;6:ra83. doi: 10.1126/scisignal.2004214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: Old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon JW, Yoon CS, Lim HW, Huang QQ, Kang Y, Pyun KH, Hirasawa K, Sherwin RS, Jun HS. Control of autoimmune diabetes in NOD mice by GAD expression or suppression in β cells. Science. 1999;284:1183–1187. doi: 10.1126/science.284.5417.1183. [DOI] [PubMed] [Google Scholar]
- 6.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–585. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 8.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 10.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 12.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotamisligil GS. Endoplasmic reticulum stress and atherosclerosis. Nat Med. 2010;16:396–399. doi: 10.1038/nm0410-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozcan L, Cristina de Souza J, Harari AA, Backs J, Olson EN, Tabas I. Activation of calcium/calmodulin-dependent protein kinase II in obesity mediates suppression of hepatic insulin signaling. Cell Metab. 2013;18:803–815. doi: 10.1016/j.cmet.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ai D, Baez JM, Jiang H, Conlon DM, Hernandez-Ono A, Frank-Kamenetsky M, Milstein S, Fitzgerald K, Murphy AJ, Woo CW, Strong A, Ginsberg HN, Tabas I, Rader DJ, Tall AR. Activation of ER stress and mTORC1 suppresses hepatic sortilin-1 levels in obese mice. J Clin Invest. 2012;122:1677–1687. doi: 10.1172/JCI61248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papa FR. Endoplasmic reticulum stress, pancreatic β-cell degeneration, and diabetes. Cold Spring Harb Perspect Med. 2012;2:a007666. doi: 10.1101/cshperspect.a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong Y, Zhang M, Liang B, Xie Z, Zhao Z, Asfa S, Choi HC, Zou MH. Reduction of AMP-activated protein kinase α2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 2010;121:792–803. doi: 10.1161/CIRCULATIONAHA.109.900928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, Klein S. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59:1899–1905. doi: 10.2337/db10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Ge M, Ciani L, Kuriakose G, Westover EJ, Dura M, Covey DF, Freed JH, Maxfield FR, Lytton J, Tabas I. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J Biol Chem. 2004;279:37030–37039. doi: 10.1074/jbc.M405195200. [DOI] [PubMed] [Google Scholar]
- 24.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, Tabas I. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozcan L, Wong CC, Li G, Xu T, Pajvani U, Park SK, Wronska A, Chen BX, Marks AR, Fukamizu A, Backs J, Singer HA, Yates JR, 3rd, Accili D, Tabas I. Calcium signaling through CaMKII regulates hepatic glucose production in fasting and obesity. Cell Metab. 2012;15:739–751. doi: 10.1016/j.cmet.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Li G, Goode J, Paz JC, Ouyang K, Screaton R, Fischer WH, Chen J, Tabas I, Montminy M. Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature. 2012;485:128–132. doi: 10.1038/nature10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luczak ED, Anderson ME. CaMKII oxidative activation and the pathogenesis of cardiac disease. J Mol Cell Cardiol. 2014 doi: 10.1016/j.yjmcc.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jagavelu K, Tietge UJ, Gaestel M, Drexler H, Schieffer B, Bavendiek U. Systemic deficiency of the MAP kinase-activated protein kinase 2 reduces atherosclerosis in hypercholesterolemic mice. Circ Res. 2007;101:1104–1112. doi: 10.1161/CIRCRESAHA.107.156075. [DOI] [PubMed] [Google Scholar]
- 30.Streicher JM, Ren S, Herschman H, Wang Y. MAPK-activated protein kinase-2 in cardiac hypertrophy and cyclooxygenase-2 regulation in heart. Circ Res. 2010;106:1434–1443. doi: 10.1161/CIRCRESAHA.109.213199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: State of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fredman G, Spite M. Recent advances in the role of immunity in atherosclerosis. Circ Res. 2013;113:e111–e114. doi: 10.1161/CIRCRESAHA.113.302986. [DOI] [PubMed] [Google Scholar]
- 34.Spite M, Clària J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014;19:21–36. doi: 10.1016/j.cmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamaly N, Fredman G, Subramanian M, Gadde S, Pesic A, Cheung L, Fayad ZA, Langer R, Tabas I, Farokhzad OC. Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc Natl Acad Sci USA. 2013;110:6506–6511. doi: 10.1073/pnas.1303377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 2011;25:2399–2407. doi: 10.1096/fj.10-178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang Y, Zhang MJ, Hellmann J, Kosuri M, Bhatnagar A, Spite M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes. 2013;62:618–627. doi: 10.2337/db12-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]