Abstract
Objective
The findings from truly randomized community-based studies on Staphylococcus aureus nasal colonization are scarce. Therefore we have examined point prevalence and risk factors of S. aureus nasal carriage in a non-hospitalized population of Braunschweig, northern Germany.
Methods
A total of 2026 potential participants were randomly selected through the resident's registration office and invited by mail. They were requested to collect a nasal swab at home and return it by mail. S. aureus was identified by culture and PCR. Logistic regression was used to determine risk factors of S. aureus carriage.
Results
Among the invitees, 405 individuals agreed to participate and 389 provided complete data which was included in the analysis. The median age of the participants was 49 years (IQR: 39–61) and 61% were females. S. aureus was isolated in 85 (21.9%; 95% CI: 18.0–26.2%) of the samples, five of which were MRSA (1.29%; 95% CI: 0.55–2.98%). In multiple logistic regression, male sex (OR = 3.50; 95% CI: 2.01–6.11) and presence of allergies (OR = 2.43; 95% CI: 1.39–4.24) were found to be associated with S. aureus nasal carriage. Fifty five different spa types were found, that clustered into nine distinct groups. MRSA belonged to the hospital-associated spa types t032 and t025 (corresponds to MLST CC 22), whereas MSSA spa types varied and mostly belonged to spa-CC 012 (corresponds to MLST CC 30), and spa-CC 084 (corresponds to MLST CC 15).
Conclusion
This first point prevalence study of S. aureus in a non-hospitalized population of Germany revealed prevalence, consistent with other European countries and supports previous findings on male sex and allergies as risk factors of S. aureus carriage. The detection of hospital-associated MRSA spa types in the community indicates possible spread of these strains from hospitals into the community.
Introduction
The increased awareness of community-acquired methicillin-resistant Staphylococcus aureus (MRSA) requires reliable data on the prevalence of S. aureus carriage in the general (non-hospitalized) population. Population based studies from North America and Europe indicate a prevalence of S. aureus between 18% and 30% [1]–[6]. Data on prevalence in the general population of Germany - a country with a comparatively intermediate MRSA incidence in hospitals are limited. In addition, data are mostly assessed for selected population groups and epidemiological information associated with S. aureus carriage is rare [7], [8]. We therefore examined point prevalence and risk factors of S. aureus nasal carriage in a random sample of the non-hospitalized population of Braunschweig, Germany, and compared the findings with other prevalence assessments of nasal S. aureus carriage in the general population in Europe and other parts of the world. A secondary objective was to evaluate molecular epidemiological data and antimicrobial resistance patterns of S. aureus strains in the general population.
Methods
Study area, data and sample collection
This is a community based study in the general population of Braunschweig, a city with 246, 742 inhabitants in the Lower Saxony, Germany [9]. In Germany, the residents' registration office of the city is a local authority where all citizens of that particular area are obliged to register their address. Approximately 2000 men and women, 20–69 years of age were randomly selected through the resident's registration office of Braunschweig and invited to participate in the study by mail, without taking any consideration of risk factors of infection or health care contact of participants. The study was planned as a feasibility study of the German National Cohort (GNC), therefore only adult individuals 20 to 69 years of age were recruited [10]. As aimed for GNC, the proportion of younger participants (20–29 years, 30–39 years) was 10% and for older participants (40–49 years, 50–59 years and 60–69 years) the proportion was 26.7% and equal sex distribution of participants was retained for recruitment in this study [10]. Study aims and objectives were explained using information flyers to the invited participants. Information about the study was also made available through a press release in the local newspapers and on the website of the Helmholtz Centre for Infection Research, Braunschweig. A total of 405 persons agreed to participate in the study. Participants were asked to provide a self-administered nasal swab each month over a period of six months. To estimate point prevalence, this analysis is based on the data from one time point, i.e. first nasal swab received from each participant. Details of the recruitment methods, non-responder analysis, and the feasibility of obtaining serial self-collected nasal swabs, are reported separately [11]. At recruitment, the study participants were also requested to complete a questionnaire containing information on basic demographic characteristics such as age, sex, and potential risk factors for methicillin-sensitive and methicillin-resistant S. aureus (MSSA and MRSA). The questionnaire items covered skin infection, outpatient clinic visits, hospital stay of at least one night, surgery, antibiotics prescribed, or international travel occurring in the last 12 months, as well as pet animal contact, occupational animal contact, presence of allergies, and presence of diabetes mellitus. In addition, detailed and illustrated instructions on how to collect a nasal swab were provided.
Laboratory analysis
All swabs (Amies agar gel swabs 108C, Copan Diagnostics, Brescia, Italy) were inoculated on blood agar and in parallel on MRSA chrome agar plates (BD Diagnostics, Heidelberg, Germany). The plates were incubated at 37°C (Heraeus, Wehrheim, Germany). Cultures were not kept for more than 48 hours in the incubator. The appearance and growth score on blood agar were recorded. Colonies suspected to be S. aureus were characterized further with the slide coagulase test. Human blood plasma received from a local hospital and fibrinogen from human blood plasma (Sigma Aldrich, Inc. USA), were used for coagulase testing. DNA was extracted from colonies with DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany). All S. aureus were subjected to PCR targeting the spa and mecA genes, using standard primers as described elsewhere [12], [13]. Amplification conditions consisted of 3 min at 94°C, followed by 30 cycles of 30 sec at 94°C, 30 sec at 55°C, and 30 sec at 72°C, with a final step of 4 min at 72°C. The DNA fragments were separated by gel electrophoresis on a 1.5% agarose gel (Invitrogen, Merekel, Belgium) stained with ethidium bromide.
PCR products were purified with the QIAquick Nucleotide Removal Kit (Qiagen, Hilden, Germany). Molecular typing was performed by means of spa-typing which is based on polymorphisms in the X-region of the spa gene. PCR amplification and sequencing of this region was performed as described previously [12]. The software Ridom StaphType (Ridom GmbH, Münster, Germany) was used for spa sequence analysis and attribution to types [14]. Based Upon Repeat Pattern (BURP) algorithm was used to define spa clonal complexes (spa-CCs) with Ridom Staph Type software [15]. Sequences containing less than 5 repeats were excluded from the analysis and spa types were clustered if the cost was less than or equal to 4, in order to prevent the formation of too large and non-specific spa clusters.
As spa types can unambiguously be mapped to the corresponding clonal lineages defined by multi locus sequence typing (MLST) to a large extent [12], we have also enlisted MLST based clonal complexes (MLST CC) according to the Ridom database (www.ridom.com).
The antibiotic susceptibility patterns of S. aureus isolates were checked for penicillin (susceptible ≤0.03), erythromycin (susceptible ≤0.25), ciprofloxacin (susceptible ≤0.5), tetracycline (susceptible ≤1), moxifloxacin (susceptible ≤0.25), oxacilin (susceptible ≤0.25), clindamycin (susceptible ≤0.25), gentamicin (susceptible ≤0.5), linezolid (susceptible ≤2), daptomycin (susceptible ≤0.25), vancomycin (susceptible ≤0.5), tigecycline (susceptible ≤0.12), fosfomycin (susceptible ≤8), fusidic acid (susceptible ≤0.5), rifampicin (susceptible ≤0.5) and trimethoprim/sulphamethoxazole (susceptible ≤10). The antibiotic susceptibility patterns were determined with the VITEK II system (AST-P611 card, bioMérieux, SA, Craponne, France) according to the manufacturer's instructions.
Statistical analysis
In descriptive statistics, frequency and proportions were calculated for categorical variables and median and interquartile range (IQR) was reported for continuous variables. Possible determinants for S. aureus (including both MRSA and MSSA) nasal carriage were first checked through univariable logistic regression analysis. We applied multiple logistic regression by stepwise backward selection of variables with biological plausibility and a significance level of <0.10 for entry into the model. All statistical tests were considered significant with a p-value <0.05. Data were analyzed with IBM SPSS Statistics for Windows version 19.
Ethics statement
The study was approved by the Ethics Committee of the State Board of Physicians of the German Federal State of Lower Saxony and the German Federal Commissioner for Data Protection and Freedom of Information (Approval letter numbers: Bo/13/2010 and 3137/2011). Written informed consent was obtained from all study participants.
Results
Characteristics of the sample
Among the 2026 invited persons, 405 (20%) agreed to participate and 389 (96%) of those participants sent a nasal swab along with the questionnaire during the period of July – September 2012. The study population included 237 (60.9%) female participants and the median age of the study population was 49 years (IQR: 39–61 years, range: 20–70 years). In the total of 389 participants, 276 (71.0%) visited outpatient clinics, 44 (11.3%) had been in the hospital and 158 (40.6%) were prescribed antibiotics, all in the last 12 months (Table 1).
Table 1. Descriptive characteristics of the study participants of the general population of Braunschweig, Germany.
| Variables | Categories | N (%) |
| Sex | Female | 237 (60.9) |
| Male | 152 (39.1) | |
| Median age in years (inter quartile range) | 49 (39–61) | |
| Age | 20–30 years | 53 (13.6) |
| 31–40 years | 51 (13.01) | |
| 41–50 years | 98 (25.2) | |
| 51–60 years | 89 (22.9) | |
| 61–70 years | 98 (25.2) | |
| Allergies | No | 182 (46.8) |
| Yes | 176 (45.2) | |
| Diabetes mellitus | No | 365 (93.8) |
| Yes | 16 (4.1) | |
| Skin infection in the last 12 months | No | 272 (69.9) |
| Yes | 104 (26.7) | |
| Outpatient clinic visits in the last 12 months | No | 110 (28.3) |
| Yes | 276 (71.0) | |
| Hospital stay in the last 12 months | No | 341 (87.7) |
| Yes | 44 (11.3) | |
| Surgery in the last 12 months | No | 339 (87.1) |
| Yes | 49 (12.6) | |
| Prescribed antibiotics in the last 12 months | No | 225 (57.8) |
| Yes | 158 (40.6) | |
| Pet animal contact | No | 185 (47.6) |
| Yes | 201 (51.7) | |
| Occupational animals contact | No | 377 (96.9) |
| Yes | 7 (1.8) | |
| International travel in the last 12 months | No | 162 (41.6) |
| Yes | 224 (57.6) |
N = 389 (100%): Missing are included therefore percentage do not always add up to 100 percent.
S. aureus nasal carriage prevalence and risk factors
S. aureus was detected in nasal swabs of 85 participants (21.9%; 95% confidence interval [CI]: 18.0–26.2%). MRSA was detected in five participants (1.29%; 95% CI: 0.55–2.98%), three of whom were female. The age of the five MRSA positive cases ranged from 28 to 64 years, and all five cases had a prior upper respiratory tract infection plus at least one of the following medical conditions: skin infection, gastrointestinal infection, herpes, allergies, or cardiovascular disease. Four of them had been prescribed antibiotics in the 12 months prior to the survey (Table 2).
Table 2. Profile of MRSA-positive cases identified in a random sample of 389 participants from the general population of Braunschweig, Germany.
| Individual characteristics | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
| Age | 28 | 49 | 56 | 64 | 29 |
| Sex | male | female | female | female | male |
| Comorbidities | skin diseases, URTI | URTI, GIT, herpes, bladder infection | URTI, cardiovasculardiseases, allergies | URTI, LRTI, herpes, bladder infection | URTI, GIT |
| Family member working in health care | yes | no | no | no | yes |
| Family member working in child care | no | no | yes | no | no |
| Outpatient clinic visits in the last 12 months | yes | yes | yes | yes | yes |
| Hospital stay in the last 12 months | no | no | yes, operated in gynecology | no | no |
| Prescribed antibiotics in the last 12 months | yes | yes | yes | yes | no |
| Pet animal contact | cat | no | no | no | dog |
| International travel in the last 12 months | yes | no | yes | no | yes |
| S. aureus spa type | t032 | t032 | t025 | t032 | t032, *t330 |
Abbreviations: URTI; Upper respiratory tract infections, LRTI; Lower respiratory tract infections, GIT; Gastrointestinal tract infections.
*t330 was an MSSA.
In univariable logistic regression analysis, associations were observed between nasal S. aureus carriage and male sex (odds ratio [OR] = 2.66, 95% CI: 1.63–4.34) and presence of allergies (OR = 1.85, 95% CI: 1.10–3.09); no significant association was observed between nasal S. aureus carriage and pet animal contact, diagnosis of diabetes mellitus, occupational animal contact or outpatient clinic visits, prescribed antibiotics and international travel in the last 12 months (Table 3). In multiple logistic regression analysis, nasal carriage of S. aureus was also significantly associated with male sex (OR = 3.50, 95% CI: 2.01–6.11) and presence of allergies (OR = 2.43, 95% CI: 1.39–4.24).
Table 3. Univariable and multiple logistic regression analyses of factors associated with nasal S. aureus carriage among study participants of the general population of Braunschweig, Germany.
| Variables | Categories | Univariable logistic regression OR (95% CI) | p-value | Multiple logistic regression OR (95% CI) | p-value |
| Sex | Female | 1 | 1 | ||
| Male | 2.66 (1.63–4.34) | <0.001 | 3.50 (2.01–6.11) | <0.001 | |
| Age | 61–70 years | 1 | 1 | ||
| 20–30 years | 2.14 (1.00–4.56) | 0.049 | 2.52 (1.08–5.88) | 0.085 | |
| 31–40 years | 0.77 (0.31–1.91) | 0.578 | 0.63 (0.23–1.71) | ||
| 41–50 years | 1.28 (0.64–2.53) | 0.487 | 1.24 (0.59–2.64) | ||
| 51–60 years | 0.98 (0.47–2.03) | 0.960 | 1.03 (0.46–2.29) | ||
| Allergies | No | 1 | 1 | ||
| Yes | 1.85 (1.10–3.09) | 0.002 | 2.43 (1.39–4.24) | 0.002 | |
| Diabetes mellitus | No | 1 | |||
| Yes | 1.21 (0.38–3.84) | 0.751 | |||
| Skin infection in the last 12 months | No | 1 | |||
| Yes | 1.35 (0.79–2.29) | 0.262 | |||
| Outpatient clinic visits in the last 12 months | No | 1 | |||
| Yes | 0.94 (0.56–1.60) | 0.833 | |||
| Hospital stay in the last 12 months | No | 1 | |||
| Yes | 0.52 (0.21–1.28) | 0.157 | |||
| Surgery in the last 12 months | No | 1 | |||
| Yes | 1.04 (0.51–2.13) | 0.922 | |||
| Prescribed antibiotics in the last 12 months | No | 1 | |||
| Yes | 0.85 (0.51–1.39) | 0.506 | |||
| Pet animal contact | No | 1 | 0.423 | ||
| Yes | 0.82 (0.51–1.33) | ||||
| Occupational animal contact | No | 1 | |||
| Yes | 1.44 (0.27–7.55) | 0.667 | |||
| International travel in the last 12 months | No | 1 | |||
| Yes | 1.43 (0.87–2.36) | 0.159 |
Abbreviations: OR; odds ratio, CI; confidence interval.
Spa typing and BURP analysis
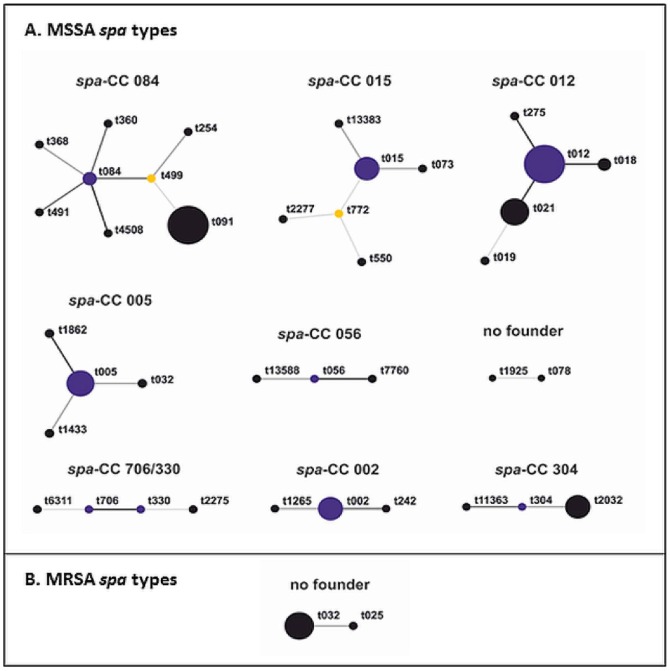
Among the 85 isolates, fifty four different MSSA spa types were found, that clustered into nine distinct groups and two different MRSA spa types were clustered into one group (figure 1, table 4). Among the MSSA isolates, one cluster had no founder and 12 spa types (12/54, 22%) could not be clustered into a spa-CC and were classified as singletons. Six isolates (6/80, 8%) were excluded from the analysis as the X-region amplimer contained less than five repeats. Most MSSA spa types belonged to spa-CC 012 (16/80, 20%) and spa-CC 084 (15/80, 19%). Four of the MRSA belonged to spa type t032 and one to spa type t025 and had no founder (corresponds to MLST CC 22). From one sample, two different spa types, t032 (MRSA, spa-CC 005) and spa type t330 (MSSA, spa-CC 330/706) were isolated. MSSA spa types varied widely, 7 (9%) belong to spa types t012 (spa-CC 012, corresponds to MLST CC 30), 7 (9%) to spa types t091 (spa-CC 084, corresponds to MLST CC 15) and 5 (6%) were spa types t021 (spa-CC 012). One MSSA also had a spa type t032. We observed three new spa type's t13383, t13449 and t13588. One isolate was not typable (NT). Relatedness of the spa types belonging to the same clonal lineages identified by BURP analysis is shown in figure 1.
Figure 1. BURP analysis of the S. aureus spa types isolated from nasal swabs of 389 participants of the general population of Braunschweig, Germany.
Each spa type identified is depicted with circles. Related spa types are connected with a black line; resultant clonal complexes are written in bold. The predicted founder of each clonal complex is indicated in blue and sub-founders are labelled in yellow. The size of the circle is proportional to the frequency of the spa type in the population. Each clonal complex (CC) is defined by the predicted founder spa type or by the spa types it contains.
Table 4. Distribution of spa-clonal complexes (spa-CCs) among isolates collected from nasal swabs of 389 participants of the general population of Braunschweig, Germany.
| MSSA spa types | ||||
| spa-CC | spa types | Number of strains (% of all strains) | Number of spa-types (% of all spa-types) | MLST CC (ST) |
| spa-CC 084 | t084, t091, t254, t360, t368, t491, t499, t4508 | 15 (19) | 8 (15) | CC 15 |
| spa-CC 015 | t015, t073, t550, t772, t2277, t13383 | 7 (9) | 6 (11) | CC 45 |
| spa-CC 005 | t005, t032, t1433, t1862 | 5 (6) | 4 (7) | CC 22 |
| spa-CC 012 | t012, t018, t019, t021, t275 | 16 (20) | 5 (9) | CC 30 |
| spa-CC 706/330 | t330, t706, t2275, t6311 | 4 (5) | 4 (7) | |
| spa-CC 002 | t002, t242, t1265 | 4 (5) | 3 (6) | CC 5 |
| spa-CC 304 | t304, t2032, t11363 | 4 (5) | 3 (6) | CC 8 |
| spa-CC 056 | t056, t7760, t13588 | 3 (4) | 3 (6) | ST 101 |
| No founder | t078, t1925 | 2 (3) | 2 (4) | CC 45 |
| Singletons | t159, t160, t537, t1057, t1541, t2313, t3741, t5337, t7088, t10983, t13449, t13587 | 14 (18) | 12 (22) | |
| Excluded | t026, t132, t1977, t6115 | 6 (8) | 4 (7) | |
Total samples n = 85 (100%), MSSA (n = 80): 9 groups, 12 singletons, 4 spa-types excluded,
MRSA (n = 5): 1 group.
Antimicrobial susceptibility testing
Table 5 provides antibiotic resistance patterns of the 85 S. aureus strains isolated from 389 study participants. Only 35 (41.2%) were sensitive to all tested antibiotics. Among the 85 isolates, resistance to penicillin was most common 49 (57.7%), and frequent resistance to fluoroquinolones 9 (10.6%) was also observed. All MRSA were resistant to penicillin and fluoroquinolones and one MRSA was also resistant to erythromycin and clindamycin.
Table 5. Antimicrobial resistance patterns of S. aureus isolated from the nasal swabs of the 389 participants of the general population of Braunschweig, Germany.
| Antibiotic resistance | Frequency | Percent |
| Sensitive | 35 | 41.2 |
| PEN | 38 | 44.7 |
| PEN, CIP, MFL | 2 | 2.4 |
| PEN, CIP, MFL, ERY, CLI | 1 | 1.2 |
| PEN, ERY | 1 | 1.2 |
| PEN, ERY, CLI | 1 | 1.2 |
| PEN, OXA, CIP, MFL | 4 | 4.7 |
| PEN, OXA, CIP, MFL, ERY, CLI | 1 | 1.2 |
| PEN, TET | 1 | 1.2 |
| CIP, MFL | 1 | 1.2 |
| Total | 85 | 100.0 |
Abbreviations: PEN: Penicillin, ERY: Erythromycin, CIP: Ciprofloxacin, TET: Tetracycline, MFL: Moxifloxacin, OXA: Oxacilin, CLI: Clindamycin.
All isolates tested were susceptible to vancomycin, tigecycline, gentamicin, linezolid, daptomycin, fosfomycin, fusidic acid, rifampicin and trimethoprim/sulphamethoxazole.
Discussion
We determined the prevalence of MSSA and MRSA carriage in a random population-based study in one city of northern Germany. Every fifth participant in our study was colonized with S. aureus. In Europe, only two studies from the UK were based on random population-based samples, as is our study [4], [16]. Gamblin et al found 28% nasal S. aureus colonization in Southampton, which is close to a previous estimate of 23% by Abudu et al in Birmingham [4], [16]. Gamblin et al used a method similar to the one used in our study [4], [16]. The participants were requested to collect nasal swabs at home and return them by mail. However, the selection of participants from a single urban NHS general practice may have overestimated the prevalence in contrast to our study where a random sample was taken from the entire population of the city. We had a majority of female participants, which might lead to an underestimation of the prevalence detected in our study. In Italy and Norway, the prevalence of S. aureus is reported to be 25.9% and 27% respectively in the general community [3], [17]. In Italy, the study included those people who had visited university hospital for a voluntary check-up and in Norway nasal S. aureus carriage was checked in visitors of the two largest shopping centres in the region [3], [17]. The sampling approach in the Italian and Norwegian studies likely included an even stronger probability for recruiting bias, which might partly account for the higher S. aureus prevalence as compared to our study [3], [17]. In the US, only the studies by Gorwitz and Mainous were truly based on a random population based samples, as was the case in our study but resulted in higher S. aureus prevalence estimates [2], [18].
For the following reasons, we believe S. aureus prevalence assessed in this study to be an underestimate of the true population prevalence rather than an over estimate: (1) our study does not include high risk age groups such as children or people over 69 years of age (2) swabs from body sites other than anterior nares, such as pharyngeal and skin swabs, were not taken, (3) enrichment culture techniques were not used to enhance S. aureus detection.
We observed 1.3% MRSA prevalence in the community. This MRSA prevalence is lower than the previous estimate of MRSA colonization observed in one of the studies at the time of hospital admission in the state of Saarland, Germany [19] but quite similar to the estimates in the UK (1.1%), France (1.02%) and Italy (0.12%) taking the reported confidence intervals into account [3], [4], [20]. MRSA prevalence assessed in this study is not much different from other European studies and is slightly lower compared to the US (2.1%) as reported in one of the studies conducted on a large population-based random sample of 4666 adult participants [18]. MRSA prevalence appears to be significantly lower in Europe as compared to other parts of the world such as India (5.3%), China (3.6%) and Pakistan (2.8%) [21]–[23]. However, a high MRSA prevalence in these studies may be due to selection bias in the study design or less stringent antimicrobial treatment regimens and hygiene measures up to some extent.
Our finding that males were more likely to carry S. aureus is consistent with other studies [17], [18], [24], [25]. One of the recent population based studies conducted in Denmark on middle-aged and elderly twins also reported that men had a higher risk of S. aureus nasal carriage [24], indicating gender specific risk factors, not yet well understood, but also in line with observations from Norway, Denmark, Australia, New Zealand and the USA [17], [18], [24]–[28]. In contrast, more female carriers were observed in the National Health and Nutrition Examination Survey (2001) in the USA [2], [29]. Interestingly, a recent study in the South West of Germany found a significantly higher proportion of S. aureus colonization among females taking hormonal contraceptives [8]; this finding revives the discussion on hormonal disposition to S. aureus carriage. The literature is inconclusive with respect to differences in age [18], [24], [26]. Some studies found a higher prevalence in elderly people while others found a higher prevalence in younger adults.
Our findings confirm previously reported associations between allergies and S. aureus carriage [24], [25]. S. aureus toxins have been shown to accelerate allergic reactions in atopic diseases, asthma and allergic rhinitis, but a satisfactory mechanistic explanation for this observation has, so far, not been provided [24], [28], [30]–[31]. The other known risk factors could not be confirmed with significant associations in our study. Although the respective trends were visible, our study population most likely had insufficient power regarding less prevalent risk factors in the general population.
MRSA spa types t032 and t025 (MLST CC 22) represent the most frequent epidemic MRSA in German hospitals and are disseminated worldwide [32], [33], [34]. Spread of MRSA CC22 to the community was recently reported from countries with high prevalence of MRSA in hospitals [35], [36]. The high prevalence of particular clonal complexes observed in this study such as CC 15, CC30, and CC45 was also reported for isolates from nasal carriage by the study of Holtfreter et al., performed in Germany in 2006 and also for isolates from the Netherlands, the USA and Portugal [7], [37], [38], [39]. These clonal complexes obviously have evolved as very successful colonizers of humans. The reasons for this success remain unknown so far. As nasal colonization is a main reservoir from which infections can start, it is no surprise to see isolates attributed to CC15 and to CC30 among the most frequent ones in a European study on S. aureus from invasive infections [40]. The multiple strain carriage was rare as only person was carrying MRSA spa type t032 and MSSA spa type t330.
More than half (60%) of the strains were resistant to one or more antibiotics and resistance to penicillin was most common. The MRSA isolates were also resistant to multiple antibiotics. Among MSSA isolates, the prevalence of resistance to fluoroquinolones, macrolides, aminoglycosides and other antibiotic classes (except penicillin) was still very low.
One of the limitations of this study is the possibility of selection bias. Although selection of the potential participant through the resident's registration office was random, response and participation in the study can be affected by age, sex and health related factors within the individuals. We had significantly more female participants (61%) as compared to the general population in Braunschweig (51%) [41]. Our response proportion of 20% is comparable with other studies based on invitation to participants through mail [4], [42]. However, the response proportion of the invitation accepting participants to send a nasal swab and the questionnaire was over 90%, which is also comparable with other studies [4], [42]. Use of self-swabbing may affect the quality of swab sampling. Sampling suitability was checked by the presence of microorganisms in general and only 3 (0.8%) had no bacterial growth [11]. Perfect agreement in terms of viral pathogen detection by PCR was observed in a previous study comparing staff-collected and participant-collected nasal swabs [43].
Conclusion
This is the first study of S. aureus and MRSA point prevalence based on a random sample in a non-hospitalized population in Germany. It shows that S. aureus colonization in the Braunschweig population is similar to the reports available from other European countries. MRSA carriage is relatively uncommon in this community. The detection of hospital-associated MRSA spa types in the community indicate possible spread of these strains from hospitals into the community. Given the positive experience with self-sampling in a general population based design, a larger point prevalence study, including children and elderly people might help to establish risk factors for MRSA in the general population, which would in turn contribute relevant evidence for screening algorithms and other prevention methods.
Acknowledgments
We are grateful to Iris Plumeier and Manuela Höer for their assistance in laboratory analysis and Johannes Horn for his guidance on statistical analysis. We are also thankful to Martin Andrzejak, Anja Schultze, Sabrina Wieghold, and the entire staff of the Department of Epidemiology for organizational support. We thank Klaus Schughart for his support in the early phase of the study. We are also thankful to Kishanda Vyboh (The Montreal Chest Institute, McGill University Health Centre, Montreal, Canada) for a critical reading of the manuscript. JM is supported by a joint doctoral scholarship from the German Academic Exchange Service (Deutscher Akademischer Austausch Dienst: DAAD) and the Higher Education Commission (HEC) of Pakistan.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This project was financed through internal funds of the Helmholtz Centre for Infection Research, Braunschweig, Germany. JM is supported by a joint doctoral scholarship from the German Academic Exchange Service (Deutscher Akademischer Austausch Dienst: DAAD) and the Higher Education Commission (HEC) of Pakistan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wardyn SE, Forshey BM, Smith TC (2012) High prevalence of Panton-Valentine leukocidin among methicillin-sensitive Staphylococcus aureus colonization isolates in rural lowa. Microb Drug Resist 18: 427–433 10.1089/mdr.2011.0239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mainous AG, Hueston WJ, Everett CJ, Diaz VA (2006) Nasal carriage of Staphylococcus aureus and methicillin-resistant S. aureus in the United States, 2001–2002. Ann Fam Med 4: 132–137 10.1370/afm.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zanelli G, Sansoni A, Zanchi A, Cresti S, Pollini S, et al. (2002) Staphylococcus aureus nasal carriage in the community: a survey from central Italy. Epidemiol Infect 129: 417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gamblin J, Jefferies JM, Harris S, Ahmad N, March P, et al. (2013) Nasal self-swabbing for estimating the prevalence of Staphylococcus aureus in the community. J Med Microbiol 62: 437–440 10.1099/jmm.0.051854-0 [DOI] [PubMed] [Google Scholar]
- 5. Schweickert B, Noll I, Feig M, Claus H, Krause G, et al. (2012) MRSA-surveillance in Germany: data from the Antibiotic Resistance Surveillance System (ARS) and the mandatory surveillance of MRSA in blood. Eur J Clin Microbiol Infect Dis 31: 1855–1865 10.1007/s10096-011-1511-8 [DOI] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control (2012) Antimicrobial resistance surveillance in Europe 2011: Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) Stockholm ECDC. Available: http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-surveillance-europe-2011.pdf. Accessed 20113 Nov 14.
- 7. Holtfreter S, Grumann D, Schmudde M, Nguyen HT, Eichler P, et al. (2007) Clonal distribution of superantigen genes in clinical Staphylococcus aureus isolates. J Clin Microbiol 45: 2669–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zanger P, Nurjadi D, Gaile M, Gabrysch S, Kremsner PG (2012) Hormonal contraceptive use and persistent Staphylococcus aureus nasal carraige. Clin Infect Dis 55: 1625–1632. [DOI] [PubMed] [Google Scholar]
- 9.Stadt Braunschweig Referat Stadtentwicklung und Statistik (2013) Entwicklung der Einwohnerzahl seit 1988. Available: http://www.braunschweig.de/politik_verwaltung/statistik/jahrbuch/jahrbuch/G02_01j.pdf. Accessed 2014 Mar 26.
- 10. Wichmann HE, Kaaks R, Hoffmann W, Jöckel KH, Greiser KH, et al. (2012) The German National Cohort. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 55: 781–787 10.1007/s00103-012-1499-y [DOI] [PubMed] [Google Scholar]
- 11. Akmatov MK, Mehraj J, Gatzemeier A, Strömpl J, Witte W, et al. (2014) Serial home-based self-collection of anterior nasal swabs to detect Staphylococcus aureus carriage in a randomized population-based study in Germany. Int J Infect Dis 25: 4–10 10.1016/j.ijid.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 12. Strommenger B, Kettlitz C, Weniger T, Harmsen D, Friedrich AW, et al. (2006) Assignment of Staphylococcus isolates to groups by spa typing, SmaI macrorestriction analysis, and multilocus sequence typing. J Clin Microbiol 44: 2533–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murakami K, Minamide W, Wada K, Nakamura E, Teraoka H, et al. (1991) Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol 29: 2240–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harmsen D, Claus H, Witte W, Rothganger J, Turnwald D, et al. (2003) Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol 41: 5442–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mellmann A, Weniger T, Berssenbrugge C, Rothganger J, Sammeth M, et al. (2007) Based Upon Repeat Pattern (BURP): an algorithm to characterize the long-term evolution of Staphylococcus aureus populations based on spa polymorphisms. BMC Microbiol 7: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abudu L, Blair I, Fraise A, Cheng KK (2001) Methicillin-resistant Staphylococcus aureus (MRSA): a community-based prevalence survey. Epidemiol Infect 126: 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skramm I, Moen AEF, Bukholm G (2011) Nasal carriage of Staphylococcus aureus: frequency and molecular diversity in a randomly sampled Norwegian community population. APMIS 119: 522–528. [DOI] [PubMed] [Google Scholar]
- 18. Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, et al. (2008) Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis 197: 1226–1234 10.1086/533494 [DOI] [PubMed] [Google Scholar]
- 19. Herrmann M, Petit C, Dawson A, Biechele J, Halfmann A, et al. (2013) Methicillin-resistant Staphylococcus aureus in Saarland, Germany: a statewide admission prevalence screening study. PLoS One 8: e73876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ficca G, Chauvel M, de Moüy D (2006) biologist members of the l'AFORCOPI-BIO, directors of private medical laboratory (2006) Prevalence of community-acquired methicillin-résistant Staphylococcus aureus. Méd Mal Infect 36: 207–212. [DOI] [PubMed] [Google Scholar]
- 21. Saxena S, Singh K, Talwar V (2003) Methicillin-resistant Staphylococcus aureus prevalence in community in the east Dehli area. Jpn J Infect Dis 56: 54–56. [PubMed] [Google Scholar]
- 22. Anwer MS, Jaffery G, Rehman Bhatti KU, Tayyib M, Bokhari SR (2004) Staphylococcus aureus and MRSA nasal carriage in general population. J Coll Physicians Surg Pak 14: 661–664. [PubMed] [Google Scholar]
- 23. Lu PL, Chin LC, Peng CF, Chiang YH, Chen TP, et al. (2005) Risk Factors and Molecular Analysis of Community Methicillin-Resistant Staphylococcus aureus Carriage. J Clin Microbiol 43: 132–139 10.1128/JCM.43.1.132-139.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andersen PS, Larsen AL, Fowler VG Jr, Stegger M, SkovRL, et al (2013) Risk factors for Staphylococcus aureus nasal colonization in Danish middle-aged and elderly twins. Eur J Clin Microbiol Infect Dis 32: 1321–1326 10.1007/s10096-013-1882-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Graham PL 3rd, Lin SX, Larson EL (2006) A US population-based survey of Staphylococcus aureus colonization. Ann Intern Med 144: 318–325. [DOI] [PubMed] [Google Scholar]
- 26. Munckhof WJ, Nimmo GR, Schooneveldt JM, Schlebusch S, Stephens AJ, et al. (2009) Nasal carriage of Staphylococcus aureus, including community-associated methicillin-resistant strains, in Queensland adults. Clin Microbiol Infect 15: 149–155 10.1111/j.1469-0691.2008.02652.x [DOI] [PubMed] [Google Scholar]
- 27. Best N, Fraser JD, Rainey PB, Roberts SA, Thomas MG, et al. (2011) Nasal carriage of Staphylococcus aureus in healthy Aucklanders. NZ Med J 124: 31–39. [PubMed] [Google Scholar]
- 28. Halablab MA, Hijazi SM, Fawzi MA, Araj GF (2010) Staphylococcus aureus nasal carriage rate and associated risk factors in individuals in the community. Epidemiol Infect 138: 702–706 10.1017/S0950268809991233 [DOI] [PubMed] [Google Scholar]
- 29. Kuehnert MJ, Kruszon-Moran D, Hill HA, McQuillan G, McAllister SK, et al. (2006) Prevalence of Staphylococcus aureus Nasal Colonization in the United States, 2001–2002. J Infect Dis 193: 172–179. [DOI] [PubMed] [Google Scholar]
- 30. Pastacaldi C, Lewis P, Howarth P (2011) Staphylococci and staphylococcal superantigens in asthma and rhinitis: a systematic review and meta-analysis. Allergy 66: 549–555 10.1111/j.1398-9995.2010.02502.x [DOI] [PubMed] [Google Scholar]
- 31. Jappe U, Heuck D, Witte W, Gollnick H (1998) Superantigen production by Staphylococcus aureus in atopic dermatitis: no more than a coincidence? J Invest Dermatol 110: 844–846. [DOI] [PubMed] [Google Scholar]
- 32. Layer F, Cuny C, Strommenger B, Werner G, Witte W (2012) Current data and trends on methicillin-resistant Staphylococcus aureus (MRSA). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 55: 1377–1386 10.1007/s00103-012-1560 [DOI] [PubMed] [Google Scholar]
- 33. Holden MT, Hsu LY, Kurt K, Weinert LA, Mather AE, et al. (2013) A genomic portrait of the emergence, evolution, and global spread of a methicillin-resistant Staphylococcus aureus pandemic. Genome Res 23: 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chaberny IF, Bindseil A, Sohr D, Gastmeier P (2008) A Point-prevalence Study for MRSA in a German University Hospital to Identify Patients at Risk and to Evaluate an Established Admission Screening Procedure. Infection 36: 526–532 10.1007/s15010-008-7436-1 [DOI] [PubMed] [Google Scholar]
- 35. Espadinha D, Faria NA, Miragaia M, Lito LM, Melo-Cristino J, et al. (2013) Extensive dissemination of methicillin-resistant Staphylococcus aureus (MRSA) between the hospital and the community in a country with a high prevalence of nosocomial MRSA. PLoS One 8: e59960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Biber A, Abuelaish I, Rahav G, Raz M, Cohen L, et al. (2012) A typical hospital- acquired methicillin-resistant Staphylococcus aureus clone is widespread in the community in the Gaza strip. PLoS One 7: e42864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tavares A, Faria NA, de Lencastre H, Miragaia M (2014) Population structure of methicillin-susceptible Staphylococcus aureus in Portugal over a 19-year period (1992–2011). Eur J Clin Microbiol Infect Dis 33: 423–432. [DOI] [PubMed] [Google Scholar]
- 38. Melles DC, Tenover FC, Kuehnert MJ, Witsenboer H, Peeters JK, et al. (2008) Overlapping population structures of nasal isolates of Staphylococcus aureus from healthy Dutch and American individuals. J Clin Microbiol 46: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van der Donk CFM, Rijnders MIA, Donker GA, de Neeling AJ, Nys S, et al. (2013) Is living in a border region a risk for a high prevalence of resistance? Eur J Clin Microbiol Infect Dis 32: 989–995 10.1007/s10096-013-1835-7 [DOI] [PubMed] [Google Scholar]
- 40. Grundmann H, Aanensen DM, van den Wijngaard CC, Spratt BG, Harmsen D, et al. (2010) Geographic Distribution of Staphylococcus aureus Causing Invasive Infections in Europe: A Molecular-Epidemiological Analysis. PLoS Med 7: e1000215 10.1371/journal.pmed.1000215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Statistisches Bundesamt, destatis, (2013) Population based on the 2011 Census. Available: https://www.destatis.de/EN/FactsFigures/SocietyState/Population/CurrentPopulation/Tables/Census_SexAndCitizenship.html. Accessed 2014 Jul 21.
- 42. van Cleef BA, Verkade EJM, Wulf MW, Buiting AG, Voss A, et al. (2010) Prevalence of Livestock-Associated MRSA in Communities with High Pig-Densities in The Netherlands. PLoS One 5: e9385 10.1371/journal.pone.0009385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Akmatov MK, Gatzemeier A, Schughart K, Pessler F (2012) Equivalence of self- and staff-collected nasal swabs for the detection of viral respiratory pathogens. PLoS One 7: e48508 10.1371/journal.pone.0048508 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.