Abstract
Background
Vitamin D was used to treat tuberculosis in the pre-antibiotic era, and its metabolites induce antimycobacterial immunity in vitro. Clinical trials investigating the effect of adjunctive vitamin D on sputum culture conversion are lacking.
Methods
We conducted a multi-centre randomised controlled trial of adjunctive vitamin D in adults with sputum smear-positive pulmonary tuberculosis in London, UK. 146 participants were allocated to receive 2.5 mg vitamin D3 or placebo at baseline and at 2, 4 and 6 weeks after starting standard tuberculosis treatment. The primary endpoint of the trial was time from initiation of antimicrobial therapy to sputum culture conversion. Participants were genotyped for TaqI and FokI polymorphisms of the vitamin D receptor (VDR), and interaction analyses were conducted to determine the influence of VDR genotype on response to vitamin D. This trial is registered with ClinicalTrials.gov (NCT00419068).
Findings
126 participants were included in the primary efficacy analysis (62 allocated to intervention, 64 allocated to placebo). Median time to sputum culture conversion was 36.0 days in the intervention arm and 43.5 days in the placebo arm (adjusted HR 1.39; 95% CI 0.90-2.16, P=0.14). TaqI genotype modified the effect of vitamin D supplementation on time to sputum culture conversion (Pinteraction=0.03), with enhanced response seen only in participants with the tt genotype (HR 8.09, 95% CI 1.36-48.01, P=0.02). FokI genotype did not modify the effect of vitamin D supplementation (Pinteraction=0.85). Mean serum 25-hydroxyvitamin D at 8 weeks was 101.4 nmol/L vs. 22.8 nmol/L in intervention vs. placebo arms (95% CI for difference 68.6-88.2 nmol/L, P<0.001).
Interpretation
Administration of four doses of 2.5 mg vitamin D3 elevated serum 25-hydroxyvitamin D concentrations in patients receiving intensive phase treatment for pulmonary tuberculosis and reduced time to sputum culture conversion in participants with the tt genotype of the TaqI VDR polymorphism.
Introduction
Tuberculosis (TB) is a major health problem: the World Health Organisation estimates that there were 9.4 million incident cases of TB in 2008, and 1.8 million deaths due to TB worldwide in 2008 (1). Augmentation of the immune response to Mycobacterium tuberculosis (MTB) has the potential to allow shortening of antimicrobial therapy in drug-sensitive disease, or to improve outcome in drug-resistant disease (2). Calcitriol, the active metabolite of vitamin D, induces antimycobacterial activity in vitro (3). It modulates the host response to mycobacterial infection by induction of reactive nitrogen and oxygen intermediates (4, 5), suppression of matrix metalloproteinase enzymes implicated in the pathogenesis of pulmonary cavitation (6) and induction of the antimicrobial peptide cathelicidin (7, 8) which induces autophagy (9). Calcitriol modulates immune responses by binding vitamin D receptor (VDR) expressed by antigen presenting cells and activated lymphocytes to regulate transcription of vitamin D-responsive genes. Human VDR is polymorphic: carriage of the t allele of the TaqI VDR polymorphism associates with enhanced calcitriol-induced phagocytosis of MTB in vitro (10) and more rapid sputum culture conversion in patients with pulmonary TB (PTB) (11). By contrast, carriage of the f allele of the FokI VDR polymorphism associates with reduced transcriptional activity (12), reduced calcitriol-induced phagocytosis (10) and slower sputum culture conversion in PTB (11).
Numerous case series have reported that daily doses of 625 μg to 2.5 mg vitamin D enhance response to antimicrobial therapy for PTB (13). Randomised controlled trials investigating doses of vitamin D ≤ 125 μg/day or equivalent in active TB have shown no clinical benefit (14-17), but one trial investigating a higher dose regimen (250 μg/day) did report reduced time to sputum smear conversion in the intervention arm (18). Sputum smear conversion is of limited value as a biomarker of treatment response in PTB however, because microscopy is less sensitive and less specific than culture for the detection of viable MTB bacilli in the sputum (19). Consequently multivariable analysis reveals that sputum culture conversion, but not sputum smear conversion, is an independent correlate of risk of long-term treatment failure or relapse (20). We therefore conducted a clinical trial to determine the effect of high-dose vitamin D on time to sputum culture conversion in patients receiving intensive phase antimicrobial therapy for PTB; sub-group analyses were performed to investigate whether the effect of adjunctive vitamin D on time to sputum culture conversion was modified by TaqI or FokI VDR genotype.
Results
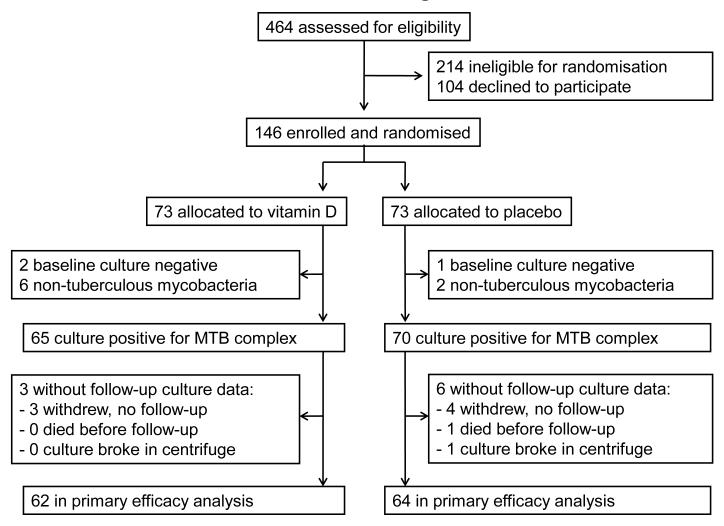
We assessed 464 patients for eligibility to participate in the trial between 25th January 2007 and 3rd July 2009: 214 were ineligible, 104 were eligible but declined randomisation and 146 were randomised. Reasons for ineligibility are presented in Table S1, Supplementary Material. There was no significant difference in median age between randomised participants vs. potentially eligible individuals who declined randomisation (31.2 years vs. 29.1 years respectively, P=0.07), but females were slightly under-represented among randomised participants vs. those declining randomisation (35/146 vs. 39/104 female respectively, P=0.02). The trial ended on the date of the final study visit of the last participant to be randomised. MTB complex was cultured from baseline sputum samples of 135 participants: follow-up sputum culture results were available for 126 participants (62 allocated to vitamin D, 64 allocated to placebo) who entered the primary efficacy analysis (Figure 1). Clinical and demographic characteristics of these participants were comparable for intervention vs. control group at baseline (Table 1). The majority of these participants were profoundly vitamin D deficient (serum 25(OH)D<20 nmol/L in 75/126) and almost all were vitamin D insufficient (serum 25(OH)D<75 nmol/L in 122/126) at baseline.
Figure 1. Trial Profile.
Table 1. Baseline characteristics by allocation.
| Vitamin D | N | Placebo | N | |
|---|---|---|---|---|
| Median age, years (IQR) | 30.7 (24.5 to 41.5) | 62 | 30.5 (24.8 to 38.4 ) | 64 |
| Female | 14 (23%) | 62 | 14 (22%) | 64 |
| Ethnic group | 62 | 64 | ||
| - Asian / Asian British | 30 (48%) | 25 (39%) | ||
| - Black / Black British | 17 (27%) | 26 (41%) | ||
| - White / Latin American | 15 (24%) | 13 (20%) | ||
| Occupation | 62 | 64 | ||
| - Student | 10 (16%) | 9 (14%) | ||
| - Employed | 46 (74%) | 49 (77%) | ||
| - Unemployed | 6 (10%) | 6 (9%) | ||
| HIV status | 62 | 64 | ||
| - Sero-positive | 3 (5%) | 2 (3%) | ||
| - Sero-negative | 43 (69%) | 39 (61%) | ||
| - Undetermined | 16 (26%) | 23 (36%) | ||
| Diabetes mellitus | 3 (5%) | 62 | 2 (3%) | 64 |
| Median duration of symptoms pre-diagnosis, months (IQR) | 2.0 (1.5 to 4.0) | 62 | 2.0 (1.0 to 3.0) | 64 |
| Median duration of treatment pre-enrolment, days (IQR) | 2.0 (0.0 to 4.0) | 62 | 2.0 (0.0 to 3.8) | 64 |
| Administration of antimicrobial treatment | 62 | 64 | ||
| - Daily, self-administered | 61 (98%) | 62 (97%) | ||
| - 3 × weekly, directly-observed | 1 (2%) | 2 (3%) | ||
| Baseline sputum smear | 62 | 64 | ||
| - ≤3 AFB per high-power field | 27 (44%) | 31 (48%) | ||
| - >3 AFB per high-power field | 35 (57%) | 33 (52%) | ||
| Rifampicin-resistant isolate | 0 (0%) | 62 | 4 (6%) | 64 |
| Serum 25(OH)D, nmol/L | 21.1 (20.0) | 62 | 21.3 (19.0) | 64 |
| Serum 25(OH)D <20 nmol/L | 36 (58%) | 62 | 39 (61%) | 64 |
| Serum 25(OH)D <75 nmol/L | 59 (95%) | 62 | 63 (98%) | 64 |
| Serum corrected calcium, mmol/L | 2.45 (0.08) | 62 | 2.45 (0.09) | 64 |
| Urinary calcium:creatinine ratio | 0.34 (0.28) | 59 | 0.37 (0.26) | 58 |
| Haemoglobin, g/dL | 12.6 (1.9) | 62 | 12.6 (1.8) | 64 |
| Mean corpuscular volume, fl | 82.7 (7.2) | 59 | 82.7 (7.1) | 61 |
| Total white blood cell count × 109/L | 9.2 (3.1) | 62 | 8.1 (2.6) | 64 |
| Neutrophil count × 109/L | 6.8 (2.8) | 62 | 5.7 (2.3) | 64 |
| Lymphocyte count × 109/L | 1.4 (0.6) | 62 | 1.4 (0.5) | 64 |
| Monocyte count × 109/L | 0.8 (0.4) | 62 | 0.8 (0.3) | 64 |
| Platelet count × 109/L | 409 (136) | 62 | 381 (149) | 64 |
| Erythrocyte sedimentation rate, mm/h | 62.1 (23.1) | 57 | 60.9 (17.4) | 60 |
| C reactive protein, mg/L | 71.4 (49.5) | 62 | 60.5 (45.0) | 62 |
| Body mass index, kg/m2 | 20.1 (3.1) | 60 | 20.2 (2.7) | 64 |
| Baseline chest radiograph | ||||
| - Cavities present | 36 (58%) | 62 | 36 (56%) | 64 |
| - Zones affected | 2.8 (1.3) | 47 | 2.8 (1.3) | 49 |
| TaqI vitamin D receptor genotype | 62 | 64 | ||
| - TT | 30 (48%) | 29 (45%) | ||
| - Tt | 27 (44%) | 28 (44%) | ||
| - tt | 5 (8%) | 7 (11%) | ||
| FokI vitamin D receptor genotype | 62 | 64 | ||
| - FF | 29 (47%) | 27 (42%) | ||
| - Ff | 25 (40%) | 24 (38%) | ||
| - ff | 8 (13%) | 13 (20%) |
Data are number (%) or mean (standard deviation) except where stated. IQR, inter-quartile range.
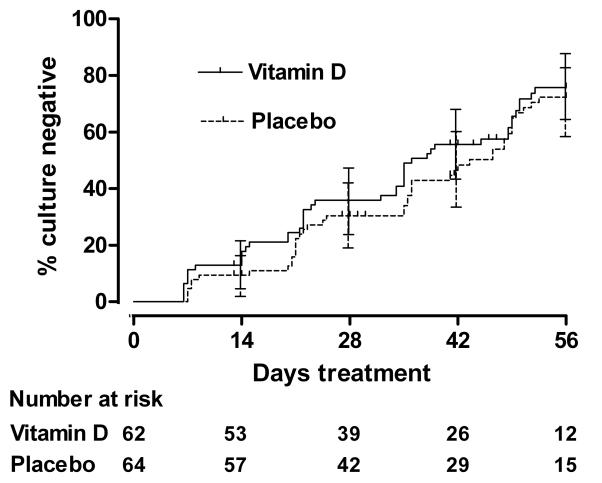
The median time to sputum culture conversion was 36 days (95% CI 31.8-40.2 days) in the intervention arm and 43.5 days (95% CI 36.5-50.5 days) in the control arm (Figure 2; unadjusted P=0.41, logrank test). White/Latin American ethnic origin, presence of >3 AFBs per high-power field on baseline sputum smear, baseline neutrophil count ≥7.5×106/ml and older age were associated with delayed sputum culture conversion on univariate analysis (table 2); the hazard ratio (HR) for effect of allocation after adjustment for these factors in the Cox regression model was 1.39 (95% CI 0.90-2.16, P=0.14). The effect of allocation on time to sputum culture conversion was significantly modified by TaqI genotype (Pinteraction=0.03): vitamin D significantly hastened sputum culture conversion in participants with the tt genotype (HR 8.09, 95% CI 1.36-48.01, P=0.02), but not in those with Tt genotype (HR 0.85, 95% CI 0.45-1.63, P=0.63) or TT genotype (HR 1.13, 95% CI 0.60-2.10, P=0.71). There was no evidence that participants’ FokI genotype modified the effect of adjunctive vitamin D on time to sputum culture conversion however (Pinteraction=0.85).
Figure 2. Time to sputum culture conversion by allocation.
Error bars, 95% confidence intervals. The number of participants with positive sputum culture remaining in follow-up (number at risk) at 0, 2, 4, 6 and 8 weeks is presented (36).
Table 2. Cox regression analysis, time to sputum culture conversion.
| Univariate analysis | Multivariable analysis1 | |||||
|---|---|---|---|---|---|---|
| N | HR (95% CI) | P | HR (95% CI) | P | ||
| Allocation | Active | 62 | 1.18 (0.77 to 1.80) | 0.46 | 1.39 (0.90 to 2.16) | 0.14 |
| Placebo | 64 | ref | - | ref | - | |
| Age, yr | 126 | 0.97 (0.95 to 0.99) | 0.006 | 0.97 (0.95 to 1.00) | 0.02 | |
| Sex | Female | 28 | 1.23 (0.75 to 2.02) | 0.41 | - | - |
| Male | 98 | ref | - | - | - | |
| Ethnic group | Asian / Asian British | 55 | 2.84 (1.45 to 5.59) | 0.002 | 2.80 (1.40 to 5.62) | 0.004 |
| Black / Black British | 43 | 2.66 (1.34 to 5.26) | 0.005 | 2.65 (1.33 to 5.27) | 0.005 | |
| White / Latin American | 28 | ref | - | ref | - | |
| Baseline chest radiograph | Cavitation present | 72 | 0.97 (0.64 to 1.49) | 0.89 | 1.15 (0.73 to 1.80) | 0.54 |
| Cavitation absent | 54 | ref | - | ref | - | |
| Baseline sputum smear | ≤3 AFB per HPF | 58 | 2.03 (1.31 to 3.15) | 0.002 | 1.85 (1.16 to 2.97) | 0.01 |
| >3 AFB per HPF | 68 | ref | - | ref | - | |
| Baseline neutrophil count | <7.5 × 106/ml | 92 | 1.97 (1.18 to 3.29) | 0.01 | 1.88 (1.09 to 3.25) | 0.02 |
| ≥7.5×106/ml | 34 | ref | - | ref | - | |
| Baseline serum 25(OH)D | <20 nmol/L | 75 | 1.27 (0.82 to 1.96) | 0.28 | - | - |
| ≥20 nmol/L | 51 | ref | - | - | - | |
| TaqI genotype | TT | 59 | 1.30 (0.61 to 2.78) | 0.50 | - | - |
| Tt | 55 | 1.22 (0.57 to 2.62) | 0.62 | - | - | |
| tt | 12 | ref | - | - | - | |
| FokI genotype | FF | 56 | 1.00 (0.57 to 1.78) | 0.99 | - | - |
| Ff | 49 | 0.66 (0.36 to 1.22) | 0.19 | - | - | |
| ff | 21 | ref | - | - | - | |
Ref, reference category. AFB, acid-fast bacilli. HPF, high power field.
Adjusted for age, ethnicity, baseline sputum smear, neutrophil count and presence / absence of cavitation on baseline chest radiograph
Non-statistically significant trends towards faster rise in DTP in the intervention arm (9% faster rise; 95% CI −23%-43%, P=0.57; Fig S2) and faster sputum smear conversion in the intervention arm (adjusted HR 1.26, 95% CI 0.83-1.93, P=0.28; Fig S3) were also seen. The proportion of participants with negative sputum culture on solid medium at 8 weeks was similar in intervention and control arms of the study (41/52 vs. 45/56 respectively, P=0.85).
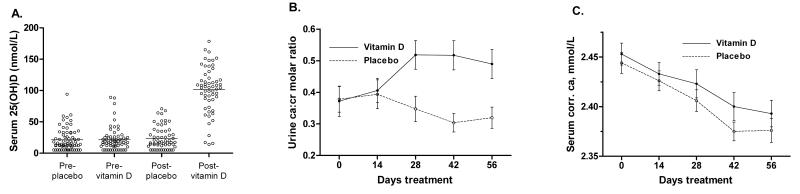
Mean serum 25(OH)D concentration at 8 weeks was 101.4 nmol/L in the intervention arm of the study vs. 22.8 nmol/L in the control arm (95% CI for difference 68.6-88.2 nmol/L, P<0.001; Fig 3A). Allocation to the intervention arm of the study also induced an increase in urinary calcium:creatinine ratio from the 4-week timepoint onwards (P<0.001, GEE; Fig 3B). Mean serum corrected calcium fell in both arms over the course of the study (P<0.001, GEE), but the rate of fall was not influenced by allocation (P=0.67, GEE; Fig 3C). Allocation to vitamin D was associated with an increase in lymphocyte count and a borderline significant decrease in monocyte count (Table 3), such that mean lymphocyte: monocyte ratio was significantly higher in the intervention vs. control arm of the study at 8 weeks (3.52 vs. 2.70, 95% CI for difference 0.16-1.11, P=0.009). No statistically significant effect of allocation on body mass index, extent of CXR involvement, CRP or ESR was observed at 8 weeks (Table 3).
Figure 3.
A. Serum 25-hydroxyvitamin D concentration by allocation at baseline and 8 weeks. B. Mean urinary calcium: creatinine molar ratio by allocation at baseline and 2, 4, 6 and 8 weeks. Error bars, standard errors of the mean. C. Mean serum corrected calcium by allocation at baseline and 2, 4, 6 and 8 weeks. Error bars, standard errors of the mean.
Table 3. Secondary outcome measures at 8 weeks, by allocation.
| Vitamin D | Placebo | 95% CI for difference | P | |
|---|---|---|---|---|
| Haemoglobin, g/dL | 13.27 (1.64) | 13.35 (1.54) | −0.49 to 0.31 | 0.67 |
| Mean corpuscular volume, fl | 85.45 (7.25) | 83.78 (6.99) | 0.06 to 2.59 | 0.04 |
| Total white blood cell count × 109/L | 7.32 (2.71) | 6.82 (2.11) | −0.70 to 0.84 | 0.85 |
| Lymphocyte count × 109/L | 1.80 (0.71) | 1.60 (0.60) | 0.03 to 0.40 | 0.02 |
| Monocyte count × 109/L | 0.57 (0.26) | 0.66 (0.25) | −0.17 to 0.01 | 0.09 |
| Neutrophil count × 109/L | 4.61 (2.34) | 4.24 (1.94) | −0.78 to 0.65 | 0.86 |
| Platelet count × 109/L | 304 (104) | 294 (105) | −30 to 37 | 0.83 |
| Erythrocyte sedimentation rate, mm/hr | 29.90 (25.91) | 30.11 (25.22) | −9.27 to 9.76 | 0.96 |
| C reactive protein, mg/L | 19.59 (25.23) | 17.82 (22.73) | −7.14 to 9.71 | 0.76 |
| Body mass index | 21.29 (2.72) | 21.18 (2.75) | −0.28 to 0.46 | 0.63 |
| Chest radiograph: zones affected | 2.30 (1.29) | 2.28 (1.18) | −0.45 to 0.27 | 0.62 |
Data are mean (standard deviation)
Two patients died during the study, one in each arm; neither death was attributed to study medication (Table 4). Eight serious adverse events (SAE) were observed in 7 out of the 71 participants who received at least one dose of vitamin D; two SAE were observed in 2 out of the 70 participants who received at least one dose of placebo. Study medication was discontinued in three participants in the intervention arm of the study who experienced adverse events (AE) that were judged to be potentially related to vitamin D by physicians blinded to allocation. One of these participants experienced a paradoxical upgrading reaction (enlarging paraspinal abscess) and mild hypercalcaemia (corrected calcium 2.68 mmol/L) after receiving two doses of 2.5 mg vitamin D; one experienced a paradoxical upgrading reaction (enlarging psoas abscess) without hypercalcaemia after receiving two doses of 2.5 mg vitamin D; and one experienced asymptomatic mild hypercalcaemia (corrected calcium 2.72 mmol/L) after receiving 2 doses of 2.5 mg vitamin D. Participants with paradoxical upgrading reaction underwent drainage of collections and made a good recovery; corrected serum calcium normalised within 2 weeks of discontinuing vitamin D in both participants who experienced hypercalcaemia. The frequency of all other AEs was similar between arms with the exception of symptoms of upper respiratory tract infection, which were reported by 1/71 patients receiving ≥ 1 dose of vitamin D vs. 6/70 patients receiving ≥1 dose of placebo.
Table 4. Adverse events.
| Vitamin D (n=71) | Placebo (n=70) | |
|---|---|---|
| Serious adverse events 1 | ||
| Multi-organ failure secondary to alcoholic liver disease2 | 0 | 1 |
| Lobar pneumonia2 | 1 | 0 |
| Paradoxical upgrading reaction | 2 | 0 |
| Nausea / vomiting | 1 | 0 |
| Epistaxis | 1 | 0 |
| Rifampicin-induced haemolytic anaemia3 | 1 | 0 |
| Bowel perforation3 | 1 | 0 |
| COPD exacerbation | 1 | 0 |
| Non-compliance with medication | 0 | 1 |
| Number experiencing any serious adverse event | 7 | 2 |
| Non-serious adverse events | ||
| Nausea / vomiting | 9 | 7 |
| Pruritis | 5 | 5 |
| Arthralgia | 4 | 7 |
| Hepatitis | 4 | 1 |
| Upper respiratory tract infection | 1 | 6 |
| Hypercalcaemia | 2 | 0 |
| Hypocalcaemia | 5 | 2 |
| Urinary calcium:creatinine molar ratio ≥ 1 | 16 | 9 |
| Other non-serious adverse event | 21 | 21 |
| Number experiencing any non-serious adverse event | 37 | 32 |
Adverse events were classified as serious if they caused death or were life-threatening, or if they required hospitalisation or prolongation of existing hospitalisation
These adverse events were fatal
Both adverse events were experienced by the same participant
Discussion
We report that administration of four doses of 2.5 mg vitamin D3 to patients receiving intensive phase treatment for smear-positive PTB significantly hastened sputum culture conversion in a sub-group of participants with the tt genotype of the TaqI vitamin D receptor polymorphism. A large increase in mean serum 25(OH)D concentration was observed among participants in the intervention arm of the trial.
Our study has several strengths. Participants in the intervention arm of the trial received a preparation with verified vitamin D3 content at a dose sufficient to increase mean serum 25(OH)D concentration >100 nmol/L, while those in the placebo arm of the trial were almost universally vitamin D insufficient throughout the study; the very high prevalence of vitamin D deficiency among TB patients in London has previously been reported, and has been attributed to lack of sunshine at latitude 51°N(21). We determined the effect of adjunctive vitamin D on sputum culture conversion - the only phase II TB trial outcome measure shown to correlate with long-term risk of relapse (27). We also demonstrated an inverse relationship between MTB CFU/ml and DTP in liquid medium, and investigated the effect of vitamin D supplementation on the speed of elimination of bacilli from sputum, as estimated by rate of rise of DTP in liquid culture of serial sputum specimens. This represents an important methodological advance, and builds on the work of the Oflotub Consortium who have utilised non-linear mixed effects modelling of serial sputum colony counts to assess sterilising activity of fluoroquinolones (28). All sputum samples in our study were analysed in a single laboratory that was close to study sites, ensuring standardisation of culture and microscopy methods and minimising loss of bacillary viability in transit. Additionally, we investigated the effect of adjunctive vitamin D on numbers of different circulating leucocyte populations, and observed an increase in lymphocyte: monocyte ratio in participants in the intervention arm of the study - a biomarker of disease resolution in animal models of TB (29). Finally, we explored the possibility that the effect of vitamin D supplementation might be modified by VDR genotype, and found that individuals with tt genotype of the TaqI polymorphism have enhanced responsiveness to vitamin D supplementation. The hypothesis that carriage of the t allele for this synonymous single nucleotide polymorphism might associate with increased VDR mRNA stability (30) has been refuted by two subsequent studies demonstrating no influence of the TaqI polymorphism on mRNA stability (31, 32); the mechanism by which this gene: environment interaction is mediated remains to be determined.
Our study has some limitations. Serum 25(OH)D concentration > 75 nmol/L was not attained in all participants in the intervention arm at 8 weeks, suggesting that some participants may not have taken all doses of study medication. Moreover, the 8-week duration of the study did not allow follow-up after completion of intensive phase therapy; this precluded analysis of the effect of adjunctive vitamin D on risk of relapse post-treatment.
Randomised trials have not previously investigated the effects of adjunctive vitamin D on sputum culture conversion in TB patients, to our knowledge, but trials using other primary outcome measures have been performed. The largest of these (17) reported that administration of three doses of 2.5 mg vitamin D3 at baseline, 5 and 8 months, did not influence clinical severity score or time to sputum smear conversion in 365 patients in Guinea Bissau; however in that trial, participants’ mean baseline 25(OH)D was >70 nmol/L, and the dosing regimen utilised was too low to influence serum 25(OH)D concentrations at follow-up. A smaller trial conducted in 67 patients in Indonesia (18) reported enhanced sputum smear conversion at 6 weeks, but not at 8 weeks, after initiation of antimicrobial treatment in patients receiving 250 μg vitamin D daily. The clinical significance of this finding is hard to interpret as sputum smear conversion, unlike sputum culture conversion, is not an independent correlate with long-term risk of treatment failure or relapse (20). Moreover, vitamin D content of study medication used in this study was not verified, and serum 25(OH)D concentrations in participants in this study were not reported before or after supplementation.
The incidence of hypercalcaemia in our study is much lower than that seen by Narang et al, who reported hypercalcaemia in 19/30 patients with smear positive pulmonary TB taking a daily dose of 10-95 μg vitamin D (15). The actual dose of vitamin D administered in the Narang study has been called into question, however (33); if it was significantly higher than reported, this would explain the discordance in reported incidence of hypercalcaemia between that study and our own. We also found that serum corrected calcium declined in both intervention and control groups following initiation of antimicrobial therapy. This may have occurred due to a reduction in granulomatous burden in patients responding to therapy, resulting in a decrease in extra-renal 1-alpha hydroxylation of 25(OH)D and a fall in serum 1,25-dihydroxyvitamin D concentrations. Paradoxical upgrading reactions were seen in two participants in the intervention arm vs. none in the control arm of our study. We discontinued study medication in these patients as a precaution, as some case series have reported an association between paradoxical upgrading reaction and administration of vitamin D (13). The incidence of paradoxical upgrading reaction in the intervention arm of our study (3%) was considerably lower than the 10% previously reported in HIV uninfected patients (34), and a causal relationship between vitamin D supplementation and paradoxical upgrading reaction is not demonstrated. The observation that symptoms of upper respiratory tract infection (URTI) were markedly less frequent in the intervention arm of our study is in keeping with that reported by Aloia et al who observed a reduction in URTI symptoms among vitamin D-supplemented participants in an osteoporosis trial (35).
In conclusion, we report that administration of four oral doses of 2.5 mg vitamin D corrected deficiency safely and quickly in patients receiving intensive phase therapy for pulmonary TB, but did not significantly enhance response to therapy in the study population as a whole. However, vitamin D supplementation did significantly reduce time to sputum culture in conversion among participants with the tt genotype of the TaqI VDR polymorphism, suggesting that this sub-group of TB patients may derive clinical benefit from vitamin D supplementation. Investigation of the mechanism by which this gene: environment interaction is mediated is warranted.
Methods
Participants
Study participants were recruited at 10 National Health Service Trusts in London, UK. Patients with newly-diagnosed PTB and acid-fast bacilli (AFB) visible on sputum smear microscopy were assessed for eligibility to participate. Individuals were excluded if they were aged less than 18 years; if they were known to be intolerant of vitamin D or first-line antituberculous therapy; if they were known to have a diagnosis of sarcoidosis, hyperparathyroidism, nephrolithiasis, HIV infection, pulmonary silicosis, malignancy or renal or hepatic failure at screening; if they had taken oral corticosteroid therapy, cytotoxic drugs or other immunosuppressant therapy in the month preceding enrolment; if they had taken antituberculous therapy for more than 7 days in the 6 months preceding enrolment; if they were taking antituberculous therapy other than rifampicin, isoniazid, pyrazinamide or ethambutol at enrolment; if molecular testing of MTB in their sputum indicated presence of a rpoB mutation conferring rifampicin resistance; if serum corrected calcium >2.65 mmol/L, aspartate transaminase or alanine transaminase >120 IU/L, total serum bilirubin > 40 micromol/L or serum creatinine > 250 micromol/L; or if they were pregnant or breastfeeding. The study was approved by East London and The City Research Ethics Committee 3 (REC ref 06/Q0605/83), and written informed consent was obtained from all participants before enrolment.
Procedures
Participants were randomly assigned to receive either four oral doses of 2.5 mg vitamin D (Vigantol® oil, Merck Serono, Darmstadt, Germany) or organoleptically identical placebo (Miglyol® oil, Caesar and Loretz, Hilden, Germany) with allocation ratio 1:1. We have previously demonstrated that an oral dose of 2.5 mg vitamin D elevates serum 25-hydroxyvitamin D (25[OH]D) > 75 nmol/L in TB patients at one week post-administration [1]. Randomisation was stratified according to presence / absence of cavitation on baseline chest radiograph, and assigned by permuted block randomisation with blocks of ten. The first dose of study medication was administered within 7 days of starting antimicrobial treatment, and subsequent doses were administered at 2, 4 and 6 weeks after the start of antimicrobial treatment. Study medication was discontinued if a participant’s sputum culture grew a non-tuberculous mycobacterium after randomisation. Nova Laboratories Ltd, Wigston, UK, generated the randomisation sequence and bottled study medication according to Good Manufacturing Practice; they had no other involvement in the study. Vitamin D3 content of a random sample of active and placebo medication was determined by high performance liquid chromatography at the end of the study; active medication was found to contain 97.7% to 99.8% of its original vitamin D3 content, and placebo medication was confirmed to contain no detectable vitamin D3. Treatment allocation was concealed from participants and study staff. All participants received intensive phase antimicrobial therapy comprising isoniazid, rifampicin, pyrazinamide and ethambutol according to UK national guidelines [2] in addition to study medication; after completion of 8 weeks of treatment, participants were discharged from the study and continuation-phase antimicrobial therapy was initiated.
Participants completed a baseline clinical assessment including chest radiography, measurement of height and weight and collection of a sputum sample for microscopy and culture, a urine sample for determination of urinary calcium:creatinine ratio and a blood sample for determination of full blood count (FBC), erythrocyte sedimentation rate (ESR) and serum concentrations of calcium, albumin, C reactive protein (CRP) and 25-hydroxyvitamin D (25[OH]D). Participants were reviewed at 2, 4, 6 and 8 weeks after starting antituberculous therapy to assess clinical status and to monitor for adverse events. At each timepoint, an overnight sputum specimen was collected from all patients who were able to expectorate spontaneously, weight was measured and urine and blood samples were collected as above. Sputum samples were transported to the microbiology laboratory by research staff at ambient temperature immediately after collection, and processed within 24 hours of arrival in the laboratory. Sputum samples awaiting processing in the laboratory were stored at 4°C. All units were within 16 miles (25 kilometres) of the laboratory. Chest radiography was repeated at 8 weeks.
Sputum specimens were digested and decontaminated with the N-acetyl-L-cysteine-NaOH procedure [3] using BBL MycoPrep kits (Becton Dickinson Microbiology Systems, Cockeysville, Md. USA). A drop of the concentrated decontaminated sputum sample was stained with auramine-phenol and examined for the presence of acid-fast bacilli by fluorescence microscopy. [4] The remainder of the sample was resuspended in a final volume of 1.25 ml of water; 1 ml of this suspension was inoculated into a MB/BacT bottle (Organon Teknika, Boxtel, The Netherlands) which was placed in a MB/BacT incubator for continuous monitoring until it signalled positive, when Gram and Ziehl-Nielsen (ZN) stains were performed. Where microscopy indicated contamination with another organism, bottles were re-treated with MycoPrep and culture was extended for a further 42 days; if these bottles subsequently grew another contaminant the culture was discarded. The number of days from inoculation to positive signal for mycobacteria was recorded for each uncontaminated sample; samples that did not signal positive within 42 days of inoculation were deemed culture negative, unless AFB were visible on microscopy of the original inoculum, in which case culture was extended to 60 days post-inoculation. Additionally, at baseline and 8 weeks, 0.25 ml of the resuspended sample was inoculated onto a Löwenstein-Jensen slope (Media for Mycobacteria, Penarth, UK). Slopes were incubated at 37°C and examined visually for growth; those that were not positive within 56 days of inoculation were deemed culture negative. The presence of mycobacteria on positive slopes was confirmed by ZN staining and microscopy. Isolates were speciated using the Genotype Mycobacterium CM kit (Hain Lifesciences, Nehren, Germany), and drug susceptibility testing of all baseline cultures growing MTB was performed by the resistance ratio or proportion methods [4]. An inverse relationship between colony forming units / ml of inoculum vs. days from inoculation to positive signal (DTP) was demonstrated by inoculating MB/BacT bottles with 10-fold serial dilutions of a suspension of MTB H37Rv (Supplementary Figure S1).
Concentrations of 25(OH)D2 and 25(OH)D3 were determined by isotope-dilution liquid chromatography–tandem mass spectrometry [5] and summed to give values for total 25(OH)D. Sensitivity for this assay was 10 nmol/l. CRP, albumin and total serum calcium concentrations were determined using an Architect ci8200 analyser (Abbott Diagnostics, Chicago, IL, USA). Calcium concentration was corrected for serum albumin concentration using the formula: corrected calcium (mmol/l) = total calcium (mmol/l) + 0.02 × (40 − albumin [g/l]). Full blood counts were performed using a LH750 haematology analyser (Beckman Coulter, Brea, CA, USA) and ESR was measured by the Wintrobe method using a s2000 analyser (Desaga, Wiseloch, Germany). DNA was extracted from whole blood using the Promega Wizard® SV 96 Genomic DNA Purification System on the Biomek FX robot (Beckman Coulter), quantified using the Nanodrop spectrophotometer and normalised to 5ng/ml. 10ng DNA was used as template for 5 ml TaqMan assays (Applied Biosystems, Foster City, CA, USA) performed on the ABI 7900HT platform in 384-well format and analysed with Autocaller software. A pre-developed assay was used to type the TaqI polymorphism of the vitamin D receptor; a customised assay was used for FokI typing (forward primer sequence TGGCCTGCTTGCTGTTCTTA, reverse primer sequence GGGTCAGGCAGGGAAGTG, reporter sequences ATTGCCTCCGTCCCTG and TTGCCTCCATCCCTG). Alleles at all loci conformed to the Hardy-Weinberg equilibrium. Chest radiographs were read by a consultant radiologist blinded to participant allocation. Body mass index (BMI) was calculated using the formula: BMI = weight (kg) / [height (m)].2
Statistical analysis
The primary endpoint of the study was time from initiation of antituberculous therapy to sputum culture conversion, estimated as the mid-point between the last positive sputum culture in broth and the first negative sputum culture in broth thereafter. Participants who were unable to expectorate spontaneously were deemed to be culture negative. Assuming a median time to sputum culture conversion of 5 weeks in the control group [6], follow-up of 8-weeks and accrual time of 2.5 years we calculated that a total of 122 patients (61 patients in each group) would need to be recruited in order to detect a 2-week difference in median time to culture conversion (equivalent to a hazard ratio of 1.67) between intervention and control groups with 80% power using a 2-sided test at the 5% significance level [7]. This number was increased by 20% to compensate for loss to follow-up, giving a total sample size of 146. Time to sputum smear conversion was a secondary endpoint, as were rate of rise in days-to-positivity in broth culture, proportion of participants with negative sputum culture on solid medium at 8 weeks, change in serum corrected calcium concentration and urinary calcium: creatinine ratio during the study and 8-week 25(OH)D concentration, full blood count parameters, radiographic response to treatment and BMI. The primary safety endpoint was incidence of hypercalcaemia (defined as serum corrected calcium > 2.65 mmol/L). Efficacy analysis was by modified intention-to-treat (ITT), and excluded participants whose baseline sputum culture was negative or grew non-tuberculous mycobacteria. All participants who took at least one dose of study medication were included in the safety analysis.
Analyses were performed using SPSS (version 16.0, 2007), STATA (version 10.1, 2008) and R (version 2.8.1, 2009) software packages. Significance was tested at the 5% level. The primary analysis for effect of allocation on time to sputum culture conversion was conducted using a logrank test stratified according to presence / absence of cavitation on baseline chest radiograph. A multivariable Cox proportional hazards regression model stratified according to presence / absence of cavitation on baseline chest radiograph was obtained by fitting additional potential predictors of time to sputum culture conversion in turn; those whose addition resulted in a significant reduction in the −2logL statistic for the model were retained [8]. Time to sputum smear conversion was similarly analysed. Proportional hazards assumptions for Cox regression models were checked using Grambsch and Therneau’s methods [9]. A single pre-specified interim efficacy analysis of time to sputum culture conversion was performed after enrolment of 73 participants. The influence of allocation on categorical and continuous outcome measures compared at 8 weeks was evaluated using χ2 tests and analysis of covariance respectively. Analysis of the effect of allocation on rate of rise of DTP was conducted using a linear mixed effects model; longitudinal changes in urinary calcium:creatinine ratio and serum corrected calcium were analysed using generalised estimating equations (GEE) with the assumption of an unstructured correlation matrix [10]. Median age among randomised participants vs. potentially eligible individuals who declined randomisation was compared using a Mann Whitney test.
Supplementary Material
Acknowledgments
The study was funded by the British Lung Foundation (ref TB05/11). Merck Serono donated Vigantol® and Miglyol® oil, and assayed vitamin D3 concentrations in vials of placebo and active study medication; they had no role in the design, conduct or analysis of the study. We thank the members of our Data Monitoring Committee, Dr Guy E. Thwaites (Chair), Dr Brenda E. Jones and Dr Tuan Q. Phung. We also thank Professor Denis A. Mitchison for advice regarding trial design; Dr Thomas C. Stokes, Queen Elizabeth Hospital, London, and Dr Stefan Lozewicz, North Middlesex Hospital, London, for acting as Principal Investigators; Ms. June Parris, Ms. Olubisi Tunde-Adebayo and Ms. Wai-Yee James for assistance with patient recruitment and follow-up; Ms. Leena Bhaw-Rosun and Ms. Rosamond Nuamah, Genome Centre, Barts and The London School of Medicine, for assistance with genotyping assays; pharmacy staff at Homerton University NHS Trust and Northwick Park Hospital for overseeing dispensing of study medication; and all TB nurses and administrative staff who referred patients to the study. Finally, we thank all patients who participated in the trial.
Role of the funding source: The British Lung Foundation was not involved in the design, conduct or analysis of the study, and did not review or approve the manuscript. Merck Serono donated Vigantol® and Miglyol® oil for the trial but did not influence study design, execution or analysis. The decision to publish was made solely by the authors, and the corresponding author had full access to all data in the study.
Footnotes
Conflict of interest statement: Merck Serono donated €7,000 to Queen Mary, University of London, to support an academic meeting entitled ‘Vitamin D: mechanisms of action in health and disease’; this meeting was convened by ARM and CJG. All other authors declare that they have no conflict of interest.
References
- 1.WHO . Global tuberculosis control: a short update to the 2009 report. Geneva: 2009. [Google Scholar]
- 2.Maartens G, Wilkinson RJ. Tuberculosis. Lancet. 2007 Dec 15;370(9604):2030–43. doi: 10.1016/S0140-6736(07)61262-8. [DOI] [PubMed] [Google Scholar]
- 3.Rook GA, Steele J, Fraher L, Barker S, Karmali R, O’Riordan J, et al. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57(1):159–63. [PMC free article] [PubMed] [Google Scholar]
- 4.Rockett KA, Brookes R, Udalova I, Vidal V, Hill AV, Kwiatkowski D. 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun. 1998 Nov;66(11):5314–21. doi: 10.1128/iai.66.11.5314-5321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sly LM, Lopez M, Nauseef WM, Reiner NE. 1alpha,25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem. 2001 Sep 21;276(38):35482–93. doi: 10.1074/jbc.M102876200. [DOI] [PubMed] [Google Scholar]
- 6.Coussens A, Timms PM, Boucher BJ, Venton TR, Ashcroft AT, Skolimowska KH, et al. 1alpha,25-dihydroxyvitamin D inhibits matrix metalloproteinases induced by Mycobacterium tuberculosis infection. Immunology. 2008 Dec 18; doi: 10.1111/j.1365-2567.2008.03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006 Mar 24;311(5768):1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 8.Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, et al. IFN-{gamma}- and TNF-Independent Vitamin D-Inducible Human Suppression of Mycobacteria: The Role of Cathelicidin LL-37. J Immunol. 2007 Jun 1;178(11):7190–8. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 9.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell host and microbe. 2009 Sep 17;6(3):231–43. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Selvaraj P, Chandra G, Jawahar MS, Rani MV, Rajeshwari DN, Narayanan PR. Regulatory role of vitamin D receptor gene variants of Bsm I, Apa I, Taq I, and Fok I polymorphisms on macrophage phagocytosis and lymphoproliferative response to mycobacterium tuberculosis antigen in pulmonary tuberculosis. J Clin Immunol. 2004 Sep;24(5):523–32. doi: 10.1023/B:JOCI.0000040923.07879.31. [DOI] [PubMed] [Google Scholar]
- 11.Roth DE, Soto G, Arenas F, Bautista CT, Ortiz J, Rodriguez R, et al. Association between vitamin D receptor gene polymorphisms and response to treatment of pulmonary tuberculosis. J Infect Dis. 2004 Sep 1;190(5):920–7. doi: 10.1086/423212. [DOI] [PubMed] [Google Scholar]
- 12.Arai H, Miyamoto K, Taketani Y, Yamamoto H, Iemori Y, Morita K, et al. A vitamin D receptor gene polymorphism in the translation initiation codon: effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res. 1997 Jun;12(6):915–21. doi: 10.1359/jbmr.1997.12.6.915. [DOI] [PubMed] [Google Scholar]
- 13.Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol. 2007 Jan 11;103:793–8. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 14.Gwinup G, Randazzo G, Elias A. The influence of vitamin D intake on serum calcium in tuberculosis. Acta Endocrinol (Copenh) 1981;97(1):114–7. doi: 10.1530/acta.0.0970114. [DOI] [PubMed] [Google Scholar]
- 15.Narang NK, Gupta RC, Jain MK. Role of vitamin D in pulmonary tuberculosis. J Assoc Physicians India. 1984;32(2):185–8. [PubMed] [Google Scholar]
- 16.Morcos MM, Gabr AA, Samuel S, Kamel M, el Baz M, el Beshry M, et al. Vitamin D administration to tuberculous children and its value. Boll Chim Farm. 1998 May;137(5):157–64. [PubMed] [Google Scholar]
- 17.Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009 May 1;179(9):843–50. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 18.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006 Jan-Mar;38(1):3–5. [PubMed] [Google Scholar]
- 19.Su WJ, Feng JY, Chiu YC, Huang SF, Lee YC. Role of two-month sputum smears in predicting culture conversion in pulmonary tuberculosis. Eur Respir J. 2010 Jun 1; doi: 10.1183/09031936.00007410. [DOI] [PubMed] [Google Scholar]
- 20.Benator D, Bhattacharya M, Bozeman L, Burman W, Cantazaro A, Chaisson R, et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet. 2002 Aug 17;360(9332):528–34. doi: 10.1016/s0140-6736(02)09742-8. [DOI] [PubMed] [Google Scholar]
- 21.Martineau AR, Nanzer AM, Satkunam KR, Packe GE, Rainbow SJ, Maunsell ZJ, et al. Influence of a single oral dose of vitamin D(2) on serum 25-hydroxyvitamin D concentrations in tuberculosis patients. Int J Tuberc Lung Dis. 2009 Jan;13(1):119–25. [PubMed] [Google Scholar]
- 22.Maunsell Z, Wright DJ, Rainbow SJ. Routine Isotope-Dilution Liquid Chromatography–Tandem Mass Spectrometry Assay for Simultaneous Measurement of the 25-Hydroxy Metabolites of Vitamins D2 and D3. Clin Chem. 2005;51(9):1683–90. doi: 10.1373/clinchem.2005.052936. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Montaner LJ, Natal S, Yongchaiyud P, Olliaro P, Rifabutin Study Group Rifabutin for the treatment of newly-diagnosed pulmonary tuberculosis: a multinational, randomized, comparative study versus Rifampicin. Tuber Lung Dis. 1994 Oct;75(5):341–7. doi: 10.1016/0962-8479(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 24.Schoenfeld DA, Richter JR. Nomograms for calculating the number of patients needed for a clinical trial with survival as an endpoint. Biometrics. 1982 Mar;38(1):163–70. [PubMed] [Google Scholar]
- 25.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–26. [Google Scholar]
- 26.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986 Mar;42(1):121–30. [PubMed] [Google Scholar]
- 27.Phillips PJ, Fielding K. Surrogate markers for poor outcome to treatment for tuberculosis: results from extensive multi-trial analysis. Int J Tuberc Lung Dis. 2008;12:S146–S7. [Google Scholar]
- 28.Rustomjee R, Lienhardt C, Kanyok T, Davies GR, Levin J, Mthiyane T, et al. A Phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008 Feb;12(2):128–38. [PubMed] [Google Scholar]
- 29.Sabin FR, Doan CA, Cunningham RS. Studies of the Blood in Experimental Tuberculosis: The Monocyte-Lymphocyte Ratio; The Anemia-Leucopenia Phase. Transactions of the Annual Meeting of the National Tuberculosis Association. 1926;22:252–6. [Google Scholar]
- 30.Morrison NA, Qi JC, Tokita A, Kelly PJ, Crofts L, Nguyen TV, et al. Prediction of bone density from vitamin D receptor alleles. Nature. 1994 Jan 20;367(6460):284–7. doi: 10.1038/367284a0. [DOI] [PubMed] [Google Scholar]
- 31.Verbeek W, Gombart AF, Shiohara M, Campbell M, Koeffler HP. Vitamin D receptor: no evidence for allele-specific mRNA stability in cells which are heterozygous for the Taq I restriction enzyme polymorphism. Biochem Biophys Res Commun. 1997 Sep 8;238(1):77–80. doi: 10.1006/bbrc.1997.7239. [DOI] [PubMed] [Google Scholar]
- 32.Durrin LK, Haile RW, Ingles SA, Coetzee GA. Vitamin D receptor 3′-untranslated region polymorphisms: lack of effect on mRNA stability. Biochim Biophys Acta. 1999 Mar 30;1453(3):311–20. doi: 10.1016/s0925-4439(99)00007-1. [DOI] [PubMed] [Google Scholar]
- 33.Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001 Feb;73(2):288–94. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- 34.Breen RAM, Smith CJ, Bettinson H, Dart S, Bannister B, Johnson MA, et al. Paradoxical reactions during tuberculosis treatment in patients with and without HIV co-infection. Thorax. 2004;59:704–7. doi: 10.1136/thx.2003.019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aloia JF, Li-Ng M. Epidemic influenza and vitamin D. Epidemiol Infect. 2007 Mar 12;:1–4. doi: 10.1017/S0950268807008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002 May 11;359(9318):1686–9. doi: 10.1016/S0140-6736(02)08594-X. [DOI] [PubMed] [Google Scholar]
Methods References
- [1].Martineau AR, Nanzer AM, Satkunam KR, Packe GE, Rainbow SJ, Maunsell ZJ, et al. Influence of a single oral dose of vitamin D(2) on serum 25-hydroxyvitamin D concentrations in tuberculosis patients. Int J Tuberc Lung Dis. 2009 Jan;13(1):119–25. [PubMed] [Google Scholar]
- [2].National Collaborating Centre for Chronic Conditions . Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. Royal College of Physicians; London: 2006. [PubMed] [Google Scholar]
- [3].Kubica GP, Dye WE, Cohn ML, Middlebrook G. Sputum digestion and decontamination with N-acetyl-L-cysteine-sodium hydroxide for culture of mycobacteria. Am Rev Respir Dis. 1963 May;87:775–9. doi: 10.1164/arrd.1963.87.5.775. [DOI] [PubMed] [Google Scholar]
- [4].Collins CH, Grange JM, Yates MD. Tuberculosis bacteriology. 2nd ed. Butterworth-Heinemann; Oxford: 1997. [Google Scholar]
- [5].Maunsell Z, Wright DJ, Rainbow SJ. Routine Isotope-Dilution Liquid Chromatography–Tandem Mass Spectrometry Assay for Simultaneous Measurement of the 25-Hydroxy Metabolites of Vitamins D2 and D3. Clin Chem. 2005;51(9):1683–90. doi: 10.1373/clinchem.2005.052936. [DOI] [PubMed] [Google Scholar]
- [6].Gonzalez-Montaner LJ, Natal S, Yongchaiyud P, Olliaro P, Rifabutin Study Group Rifabutin for the treatment of newly-diagnosed pulmonary tuberculosis: a multinational, randomized, comparative study versus Rifampicin. Tuber Lung Dis. 1994 Oct;75(5):341–7. doi: 10.1016/0962-8479(94)90079-5. [DOI] [PubMed] [Google Scholar]
- [7].Schoenfeld DA, Richter JR. Nomograms for calculating the number of patients needed for a clinical trial with survival as an endpoint. Biometrics. 1982 Mar;38(1):163–70. [PubMed] [Google Scholar]
- [8].Collett D. Modelling Survival Data in Medical Research. 2nd ed. Chapman and Hall; Bristol: 2003. [Google Scholar]
- [9].Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–26. [Google Scholar]
- [10].Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986 Mar;42(1):121–30. [PubMed] [Google Scholar]
- [11].Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002 May 11;359(9318):1686–9. doi: 10.1016/S0140-6736(02)08594-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.