Abstract
Background
Associations between psychiatric disorders and cancer incidence are inconsistent, with studies reporting cancer rates in psychiatric patients that are higher, similar, or lower than the general population. Exploration of these associations is complicated by difficulties in establishing the timing of onset of psychiatric disorders and cancer, and the associated possibility of reverse causality. Some studies have dealt with this problem by excluding patients with cancers pre-dating their psychiatric illness; others have not considered the issue.
Methods
We examined associations between psychiatric hospitalization and cancer incidence in a cohort of 1,165,039 Swedish men, and explored the impact of different analytical strategies on these associations using real and simulated data.
Results
Relative to men without psychiatric hospitalization, we observed consistent increases in smoking-related cancers in those with psychiatric hospitalizations, regardless of analytical approach (for example, hazard ratio (95% confidence interval): 1.73 (1.52, 1.96)). However, associations with nonsmoking-related cancers were highly dependent on analytical strategy. In analyses based on the full cohort, we observed no association or a modest increase in cancer incidence in those with psychiatric hospitalizations (1.14 (1.07, 1.22)). In contrast, analyses excluding men whose cancer predated their psychiatric hospitalizations, resulted in a reduction in future cancer incidence in psychiatric patients (0.72; 0.67, 0.78). Results from simulated data suggest that even modest exclusions of this type can lead to strong artefactual associations.
Conclusions
Psychiatric disorder-cancer incidence associations are complex and influenced by analytical strategy. A greater understanding of the temporal relationship between psychiatric disorder and cancer incidence is required.
Introduction
Psychiatric disorders are relatively common in the general population1-2 and an extensive literature reports excess mortality among individuals with different types of mental disorder.2-3 Psychiatric patients also have a higher risk of specific physical illness such as cardiovascular disease.4-5 These poor health outcomes may be due to worse health behaviours, e.g. individuals with mental illness are more likely to smoke,6 be overweight,7 be less physically active,8 or have a poor diet.7, 9-10 It has also been hypothesised that those with psychiatric disorders have worse access to healthcare;11 that psychiatric staff, with whom they have most contact, are less efficient at diagnosing physical problems, particularly in the context of psychiatric comorbidities;12 or that there are negative side effects to long-term medication for the treatment of certain psychiatric disorders. On this basis, individuals with psychiatric disorders might also be expected to experience an excess risk of cancer incidence. However, in spite of numerous reports, associations between mental health and cancer incidence remain unclear with individual studies reporting cancer experience in psychiatric patients that is worse,13-14 similar to,15-16 or better17-18 than the general population. Two recent reviews of schizophrenia-cancer incidence associations5,19 both highlight the high degree of heterogeneity across studies.
There are limitations to many existing studies of psychiatric disorder and cancer incidence. For example, many are based on follow-up of clinical samples, severely restricting generalizability; the majority focus on all cancers combined, limiting insights into the aetiological processes; and often distinction is not made between fatal and non-fatal cancers when risk factors may differ. Another major challenge in interpreting the relationship between psychiatric disorders and cancer incidence is establishing the temporal relationship between exposure and outcome. Both diseases have potentially long induction periods during which individuals have only minor symptoms, if any, making it difficult to establish the precise period “at risk”. In addition, evidence suggests that cancer patients have an excess of psychiatric disorders following their diagnosis,20-22 particularly adjustment disorders and depression.20 This raises the problem of reverse causality, with cancer influencing the incidence of psychiatric illness rather than the converse. This temporal relationship has not been explored in detail, and studies that do consider the issue deal with it by excluding individuals whose cancer predates their mental health diagnosis.18, 23-24 However, this approach may be overly conservative when the exact time of onset of both conditions is in doubt.
The purpose of the present analyses, based on cohort and simulated data, was to (i) explore different analytical approaches used to examine the temporal relationship between psychiatric hospitalization and cancer incidence and (ii) investigate whether apparently conflicting results in the literature are an artefact of analytical strategy.
Methods
The record linkage used to generate the cohort has been reported previously.25-26 All non-adopted men born in Sweden from 1950-1976 with both biological parents identified in the Multi-Generation Register were identified and linked to the Population and Housing Censuses records (1960/1970), and Military Service Conscription, Cause of Death, Cancer, and National Hospital Discharge Registers, resulting in 1,379,531 successful matches. Study approval was obtained from the Regional Ethics Committee, Stockholm.
Data linkage
Incident cancers for 1958-2004 were identified from the Swedish Cancer Register, a catalogue regulated by law in which clinicians and pathologists record all new cancer cases. Analyses are based on first cancer registrations identified during follow-up. Individual cancers are distinct disease entities and do not have a unifying aetiology. However, the aim of our study was to ascertain whether analytical strategy explains previously inconsistent cancer associations with psychiatric illness, and we therefore followed the approach taken in the majority of previous studies by considering all cancers combined. In the context of psychiatric illness, smoking is likely to play an important role in explaining psychiatric disorder-cancer associations, and so we also explored the link between psychiatric illness and cancers considered to be related to smoking. Smoking-related cancers were defined as: lung, oral cavity, nasopharynx, oropharynx, hypopharynx, nasal cavity and paranasal sinuses, larynx, oesophagus, stomach, pancreas, liver, kidney, ureter, urinary bladder, uterine cervix and myeloid leukaemia;27 the remaining cancers were considered unrelated to smoking.
Hospital admissions data from 1969-2004 were based on the Swedish Hospital Discharge Register, which covered a third of the Swedish population in 1970, rising to 71% in 1977, and 100% from 1987. The shortfall in the 1970/80s occurred in counties of varying population density and socioeconomic composition, and there were no systematic differences in psychiatric hospitalization-cancer associations in counties included and not included in the register during these early years. From these data we identified the earliest hospital admission for a psychiatric disorder during follow-up. Again our aim was to investigate the role of analytical strategy and our main analyses focused on all psychiatric hospitalizations combined. However, we also repeated analyses to investigate the individual impact of hospitalization for six specific disorders: schizophrenia, non-affective psychosis, bipolar disorders, depressive disorders, neurotic and adjustment disorders, and personality disorders.
Childhood SES was based on the highest occupation of either parent from 1960/1970 Population and Housing Censuses. Population and Housing censuses records (1990) were used to ascertain adult SES, based on the study member’s own occupation for those with an occupational code and otherwise on household SES.
Statistical methods
Although cancer registration data were available from 1958, psychiatric admissions were only available from 1969, making identification of early psychiatric admissions in men born before 1969 problematic. The main analyses are therefore based on men who were examined at military service conscription from 1969-1994 at average age 18 years (range: 16-25). During these years, the law required this conscription examination; only men of foreign citizenship or with severe disability were excused.
After ascertaining that proportional hazards assumptions were not violated, Cox proportional hazards regression was used to explore psychiatric hospitalization-cancer associations. Follow-up began from the date of conscription and ended on the earliest of: date of cancer registration, death, emigration, or 31 December 2004. Analyses were adjusted for year of birth, age at conscription, conscription centre, and separately for childhood and adult SES. Results presented here are based on all men with complete data but we also explored associations after excluding men with cancer registrations or psychiatric admissions prior to conscription.
As a result of inherent uncertainties regarding the timing of both cancer and psychiatric events, we used three different analytical approaches. Method 1 followed the approach taken in previous studies that aimed to address the issue of reverse causality. Under this method we excluded men whose first cancer registration pre-dated their first psychiatric admission and compared cancer incidence in men with at least one psychiatric admission during follow-up versus those with none. However, given the lag time involved in the identification of both cancer and psychiatric disorders, this approach may be overly conservative. Specifically, if an individual had a pre-existing (undiagnosed) psychiatric disorder when his cancer was diagnosed then his exclusion would be inappropriate. In the current analysis, psychiatric disorders were identified from hospital admissions and this may have been some time after the genuine onset of disease. We therefore carried out two further analyses with these men included. In Method 2 we again compared men with psychiatric admission during follow-up versus those with none; in this model men whose cancer pre-dated their first psychiatric admission contributed to analyses as “exposed” to psychiatric problems and having a “positive” outcome of interest (cancer) and we ignore any impact of reverse causality. In Method 3 psychiatric admissions were entered as a time-dependent variable. In this model men with psychiatric admissions were considered to have two periods of follow-up: (i) pre-admission, during which they were “unexposed” and (ii) post-admission, during which they were “exposed”. Men whose cancer registration followed their psychiatric admission contributed to analyses first as “unexposed-no cancer” and then as “exposed-cancer”; men whose cancer registration predated their psychiatric admission contributed once as “unexposed-cancer” and were censored before their first psychiatric admission.
To put our findings in the context of the published literature, we updated part of a previous meta-analysis19 of cancer incidence associations with schizophrenia, the psychiatric diagnosis most widely studied in relation to cancer. We included all published studies that reported associations between schizophrenia and all cancers combined in men. We present separately those studies that stated that pre-existing cancers or cancers predating the identification of schizophrenia were excluded from analyses versus those studies where no such statement was made. We also included schizophrenia hospitalization-cancer (any site) associations from our cohort (i) excluding men whose cancer predated their schizophrenia admission and (ii) based on all men.
The three analytical strategies all have strengths and weaknesses and the most appropriate approach is unclear. We therefore carried out two series of Monte Carlo simulations to explore these issues in more detail. In the first we simulated data with “cancer” and “psychiatric disorder” incidence rates and ages at “diagnosis” based on those in our cohort and with no association between the two conditions. We then compared results from the three analytical approaches. In the second we carried out 10,000 repeated simulations with psychiatric and cancer incidence rates based on our cohort and no association between the two. However, rather than defining ages at diagnoses, we randomly excluded increasing proportions of subjects with both cancer and psychiatric disorder. For comparison with the majority of the published literature we calculated Standardised Incidence Ratios (SIR) as:
with expected incidence = overall cancer incidence in our cohort. We recorded the number of datasets in which we observed an apparent reduction in cancer incidence associated with psychiatric disorder, determined by an upper 95% confidence limit of <100.
Results
Data analyses
Of 1,379,531 men in the original cohort, 214,492 (15.5%) had missing data for at least one variable leaving 1,165,039 (84.5%) men in our analytic sample. During an average 22.2 years of follow-up (range 0-35), 65,243 (5.6%) men had at least one psychiatric hospital admission, 2,419 (0.2%) developed a cancer related to smoking, and 13,322 (1.1%) developed a cancer unrelated to smoking. Men excluded from the analyses due to missing data were less likely to have a psychiatric admission (1.1% versus 5.6% in men excluded versus included in analyses) or to develop cancer (0.5% versus 1.4%) although unadjusted analyses including these men (not shown) were similar to those presented here, suggesting that selection bias was not an issue. Among men included in the analyses, 10,446 (0.9%) had a psychiatric admission and 1,070 (0.1%) had a cancer registration prior to conscription; results from analyses excluding these men (not shown) were almost identical to those presented here.
The mean (standard deviation) age at first psychiatric admission and cancer registration was 30.8 (8.3) and 37.1 (9.1) years respectively. In total, 1,314 (0.1%) men had both cancer registration (any site) and psychiatric admission. Of these, 410 had a cancer registration predating their first psychiatric admission. Although this is a small proportion (0.04%) of the full analytic sample, it is almost a third of those with both psychiatric admission and cancer.
Associations of psychiatric admissions with smoking-related, nonsmoking-related, and all-cause cancer registrations are shown in Table 1. Regardless of the analytical approach, results for smoking-related cancers were consistent with an increased risk in men with psychiatric admissions. Results from analyses excluding men whose cancer predated their first psychiatric admission (Method 1) suggested that psychiatric hospitalization was associated with a 73% increase in smoking-related malignancies (unadjusted Hazard ratio (95% confidence interval): 1.73 (1.52, 1.96)). In analyses including these men, associations were slightly stronger and there was little difference between standard and time-dependent Cox regression models (Method 2 versus 3: 2.03 (1.81, 2.28) versus 2.15 (1.90, 2.44)). Adjustments for childhood and adult SES partially attenuated these associations.
Table 1.
Hazard ratio (95% confidence interval) for incidence of (a) smoking-related cancer, (b) nonsmoking-related cancer, and (c) all-cause cancer in relation to psychiatric admission: based on 1,165,039 cohort members with complete data
| N (no cancer / cancer) | Adjusted for conscription age, centre, year of birth |
Additionally adjusted for SES in child and adulthood |
|
|---|---|---|---|
| Smoking related cancers1 | |||
| Method 12 | |||
| No psychiatric admission | 1,097,713 / 2,083 | 1.00 | 1.00 |
| 1+ psychiatric admission | 64,549 / 284 | 1.73 (1.52, 1.96) | 1.58 (1.39, 1.79) |
| Method 23 | |||
| No psychiatric admission | 1,097,713 / 2,083 | 1.00 | 1.00 |
| 1+ psychiatric admission | 64,907 / 336 | 2.03 (1.81, 2.28) | 1.87 (1.66, 2.10) |
| Method 34 | |||
| No psychiatric admission | 1,097,713 / 2,083 | 1.00 | 1.00 |
| 1+ psychiatric admission | 64,907 / 336 | 2.15 (1.90, 2.44) | 1.96 (1.73, 2.24) |
| Non-smoking related cancers | |||
| Method 12 | |||
| No psychiatric admission | 1,087,452 / 12,344 | 1.00 | 1.00 |
| 1+ psychiatric admission | 64,213 / 620 | 0.72 (0.67, 0.78) | 0.72 (0.67, 0.78) |
| Method 23 | |||
| No psychiatric admission | 1,087,452 / 12,344 | 1.00 | 1.00 |
| 1+ psychiatric admission | 64,265 / 978 | 1.14 (1.07, 1.22) | 1.15 (1.07, 1.22) |
| Method 34 | |||
| No psychiatric admission | 1,087,452 / 12,344 | 1.00 | 1.00 |
| 1+ psychiatric admission | 64,265 / 978 | 1.06 (0.98, 1.15) | 1.06 (0.98, 1.15) |
| All cancers | |||
| Method 12 | |||
| No psychiatric admission | 1,085,369 / 14,427 | 1.00 | 1.00 |
| 1+ psychiatric admission | 63,929 / 904 | 0.88 (0.83, 0.94) | 0.87 (0.81, 0.93) |
| Method 23 | |||
| No psychiatric admission | 1,085,369 / 14,427 | 1.00 | 1.00 |
| 1+ psychiatric admission | 63,929 / 1,314 | 1.28 (1.21, 1.36) | 1.27 (1.20, 1.34) |
| Method 34 | |||
| No psychiatric admission | 1,085,369 / 14,427 | 1.00 | 1.00 |
| 1+ psychiatric admission | 63,929 / 1,314 | 1.26 (1.18, 1.35) | 1.24 (1.15, 1.33) |
Smoking-related cancers were defined as: lung, oral cavity, nasopharynx, oropharynx, hypopharynx, nasal cavity and paranasal sinuses, larynx, oesophagus, stomach, pancreas, liver, kidney (body and pelvis), ureter, urinary bladder, uterine cervix and myeloid leukaemia;
Standard Cox regression excluding men whose first cancer registration pre-dated their first psychiatric admission;
Standard Cox regression including men whose first cancer registration pre-dated their first psychiatric admission;
Time-dependent Cox regression including men whose first cancer registration pre-dated their first psychiatric admission
Analyses of nonsmoking cancers were based on a larger number of cases, and exclusions resulted in a greater proportional loss from the group with both psychiatric admissions and cancer. In contrast to smoking-related cancers, psychiatric admission-nonsmoking-related cancer associations depended strongly on the analytical strategy. Results from Model 1 were consistent with a marked reduction in nonsmoking cancers in men with a psychiatric admission (0.72 (0.67, 0.78)). In contrast, results from analyses based on the full analytic sample were consistent with a modest increase in hazard (Model 2: 1.15 (1.07, 1.22)) or with no effect (Model 3: 1.06 (0.98, 1.15)). Adjustments for SES in childhood and adulthood had no impact on associations.
The majority of previous studies have focused on all cancers combined and, for comparison, we also present these results. Given the numbers of smoking- and nonsmoking-related cancers, it is unsurprising that results for all cancers combined were similar to those for nonsmoking-related cancers. Analyses excluding men with cancers predating their psychiatric hospitalization (Method 1) were consistent with a decrease in risk (0.88 (0.83, 0.94)), while analyses based on all men suggested an increase in risk (Method 2: 1.28 (1.21, 1.36); Method 3: 1.26 (1.18, 1.35)).
Separate analyses of hospitalizations for schizophrenia, non-affective psychosis, bipolar disorders, depressive disorders, neurotic and adjustment disorders, and personality disorders (not shown) were very similar to those presented here. Associations with smoking-related cancers were consistent with an increase in hazard in those hospitalized for any of these mental disorders regardless of analytical strategy. Results for nonsmoking cancers were dependent on analytical strategy: analyses that excluded cancers predating psychiatric hospitalization were consistent with a decrease in hazard in men with an admission for any of the six specific conditions, while analyses that included all men suggested that nonsmoking cancer incidence was similar or greater in those with condition-specific hospitalizations. The differences due to analytical strategy were most marked for depression and neurotic and adjustment hospitalizations.
Meta-analysis of schizophrenia-cancer associations
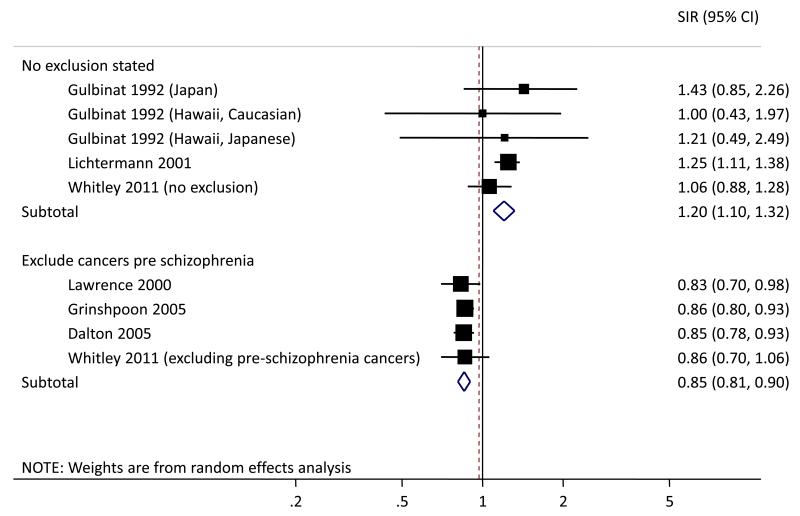
Altogether 6,669 (0.6%) men in our analytic sample had at least one hospital admission for schizophrenia during follow-up and 22 had a cancer predating this admission. For consistency with published studies, we present results for all cancers combined. Schizophrenia hospitalization-cancer associations including and excluding men with a cancer preceding their schizophrenia hospitalization are shown in Figure 1 along with corresponding SIRs from published studies. There is considerable heterogeneity between published studies in terms of populations, exposures and outcomes, and our classification is based only on reading the relevant paper so exclusions may have been made but not stated. However, it is notable that results from the current analysis based on the full cohort and those studies in which no exclusions were mentioned14, 28 were all consistent with no association or a small increase in incidence. Conversely, associations from the current cohort with pre-schizophrenia hospitalization cancers excluded and those studies in which exclusions were made18, 23-24 were strikingly consistent and all indicate a reduction in cancer incidence in schizophrenia patients of about 15%.
Figure 1.
Standardised incidence ratio (SIR) (95% confidence interval (CI)) for overall cancer risk in men with versus without schizophrenia based on random effects meta-analysis.1 Published studies are subdivided into those in which a specific statement was made that men with a cancer predating their first diagnosis of schizophrenia were excluded from analyses and those in which no such statement was made. Analyses from the present paper (Whitley 2011) are based on the full analytical sample (no exclusions) or excluding men whose first cancer predated their first schizophrenia admission (excluding pre-schizophrenia cancers).
Monte Carlo simulations
Results from simulated data with incidence and ages at diagnosis based on our cohort but with no association between psychiatric disorder and cancer are shown in Table 2. Of 1,000,000 individuals in the simulated dataset, 56,203 had a psychiatric disorder, 13,970 had cancer, 789 had both, and 229 (0.02% of full sample; 29.0% of those with both diagnoses) had cancer predating psychiatric disorder. In analyses based on the full dataset (Methods 2 and 3) hazard ratios were, as expected, consistent with no association (1.01 (0.94, 1.08) and 1.02 (0.94, 1.11) respectively). However, analysis excluding cancers predating psychiatric disorder (Method 1) demonstrated a strong artefactual reduction in risk (0.71 (0.66, 0.78)).
Table 2.
Hazard ratio (95% confidence interval) for incidence of cancer in relation to psychiatric admission: based on simulated data1
| N (no cancer / cancer) | Hazard ratio (95% confidence interval) |
|
|---|---|---|
| Method 12 | ||
| No psychiatric disorder | 930,616 / 13,181 | 1.00 |
| Psychiatric disorder | 55,414 / 560 | 0.71 (0.66, 0.78) |
| Method 23 | ||
| No psychiatric disorder | 930,616 / 13,181 | 1.00 |
| Psychiatric disorder | 55,414 / 789 | 1.01 (0.94, 1.08) |
| Method 34 | ||
| No psychiatric disorder | 930,616 / 13,181 | 1.00 |
| Psychiatric disorder | 55,414 / 789 | 1.02 (0.94, 1.11) |
Data were simulated to represent 1,000,000 individuals, of whom 5.6% developed a “psychiatric disorder” at mean age 30.8, 1.4% developed “cancer” at mean age 37.1, and with no association between the two diagnoses;
Standard Cox regression excluding men whose cancer pre-dated their psychiatric disorder;
Standard Cox regression including men whose cancer pre-dated their psychiatric disorder;
Time-dependent Cox regression including men whose cancer pre-dated their psychiatric disorder
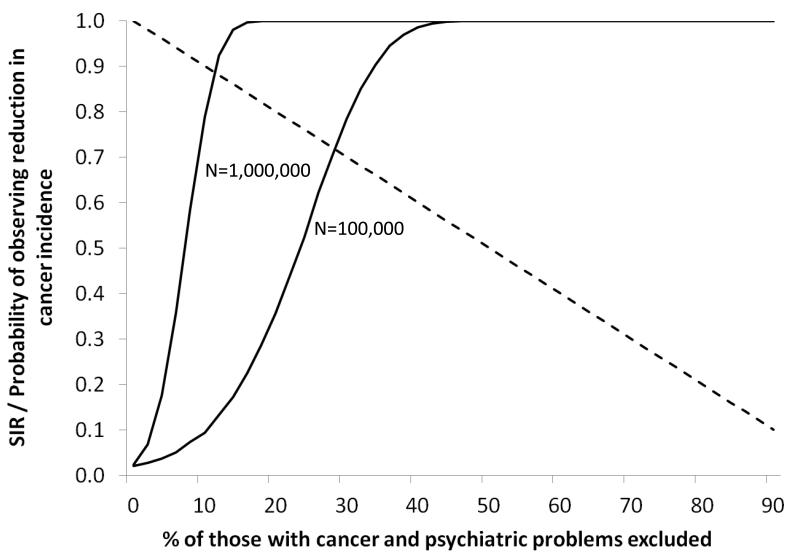
Results from simulations in which proportions of those with both cancer and psychiatric disorders were randomly excluded are presented in Figure 2. The mean SIR calculated over repeated simulations is shown as a dashed line and, as expected, declines approximately in line with the percentage reduction. The proportions of simulations with an apparently protective effect are shown by solid lines separately for total population sizes of 1,000,000 and 100,000. Based on a psychiatric disorder rate of 5.6%, these population sizes correspond to between 5,600 and 56,000 psychiatric patients and are broadly concordant with much of the published literature. Under the null hypothesis of no association we would expect 2.5% of simulations to show a reduction in risk and this is the case when no exclusions are made. However, as more individuals are excluded, this proportion increases rapidly. Based on these simulations, we estimate that randomly excluding 5% of those with cancer and psychiatric disorder results in over a quarter of analyses with N=1,000,000 and 4% of those with N=100,000 demonstrating an artefactual protective effect. With 10% exclusions these proportions rise to 79% and 9% and the corresponding figures for 20% exclusions are 100% and 36%. When 30% are excluded, 100% of analyses with N=1,000,000 and 78% of analyses with N=100,000 show an artefactual protective effect.
Figure 2.
Results of repeated Monte Carlo simulations of N individuals with “psychiatric disorder” and “cancer” incidence of 5.6% and 1.4% respectively and no association between the two diagnoses. Dashed line shows mean SIR by percentage of individuals with both “cancer” and “psychiatric disorders” excluded; solid lines show proportion of simulations in which an apparent reduction in cancer incidence was observed.
Discussion
We have explored psychiatric hospitalization-cancer associations in a large cohort of over one million Swedish men with almost complete population coverage. Many previously published studies have focused on all cancers combined, and results have been inconsistent. It has been estimated that between a quarter to a third of cancers in men may be attributable to smoking,30 and this may be even lower in younger populations such as those with psychiatric disorders. Results based on all cancers combined will therefore be dominated by nonsmoking cancers and this may be misleading in practise. For example, in our data, smoking-related cancers were consistently more common in men with psychiatric admissions independent of analytical strategy while, in contrast, associations with nonsmoking-related cancers were contradictory and highly dependent on the choice of model. However, for the purposes of discussion in the context of existing literature we focus here on results for all cancers combined.
Our results demonstrate that psychiatric admission-cancer associations are very sensitive to the analytical approach. Standard Cox regression analyses based on the full cohort (Method 2) were generally consistent with an increase in risk in those with psychiatric admissions. Results for schizophrenia were also consistent with published studies in which no statement regarding exclusions was made. However, given potentially lengthy lags in the diagnosis of both psychiatric disorders and cancers, and the potential for psychiatric disturbance following a cancer diagnosis, analyses that ignore the possibility of reverse causality are likely to overestimate associations. Time-dependent Cox regression (Method 3) is a potentially useful approach in this situation. However, it is only appropriate if the order of events is known explicitly and the gap between occurrence and identification of both psychiatric disorders and cancers raises doubts about this assumption.
A more common approach is the exclusion of men whose cancer predates their psychiatric disorder (Method 1). Analyses of our cohort which followed this approach were consistent with a marked reduction of risk in those with psychiatric admissions, and schizophrenia associations were highly consistent with those from published studies that made these exclusions. A number of plausible mechanisms explaining this apparent reduction in risk have been proposed and discussed in the literature. These include anti-cancer effects of antipsychotic/neuroleptic medications,31 genetic factors,19 later/poorer cancer detection in psychiatric patients,11-12 lower uptake in screening programs,32 or competing mortality.2-3
However, it is important to note that results from our meta-analysis indicate that the apparent reduction in cancer risk in psychiatric patients is specifically restricted to those studies in which exclusions were made. It is plausible that this analytical approach, designed to reduce the impact of reverse causality, is over-compensating and leads to an artefactual reduction in risk. Results from our simulation study suggest that even very modest exclusions of this type dramatically increase the probability of observing this artefactual risk reduction. This is particularly problematic when sample sizes are large, as is often the case in this context. A priori, we would expect analyses of the psychiatric disorders most commonly reported in cancer patients to be most affected by reverse causality. Depression and neurotic and adjustment disorders are the two disorders most commonly reported in patients with advanced cancers20 and these were the two specific admissions for which analytical strategy had the greatest impact in our results.
Strengths and limitations
The large size of our cohort offers superior statistical power and has allowed separate investigation of smoking and nonsmoking-related cancers; this has not been widely done in previous studies and may be an important distinction. The longitudinal design has allowed direct comparison of psychiatric patients with members of the population from which they were drawn, in contrast to many previous studies based on follow-up of clinical samples, and has allowed the potential exclusion of psychiatric hospitalization arising as a result of a cancer diagnosis.
However, there are also a number of limitations. The cohort consisted of young men at conscription, a strength in terms of psychiatric hospitalizations, which are more common in younger individuals, but, in spite of lengthy follow-up, cohort members were aged ≤55 years at the end, which is relatively young in terms of cancer development. Moreover, data are restricted to men born in Sweden from 1950-1976 which limits generalizability and we are unable to comment on cancer risk in women which is known to be distinct from that in men.
We adjusted analyses for both childhood and adult SES. Although childhood SES clearly predates psychiatric and cancer events, adult SES may be influenced by psychiatric disorder and/or cancer and its inclusion may have led to over-adjustment although, in practice, it had only limited impact on smoking-related cancer associations and no impact on nonsmoking-related cancer associations. We did not have extensive information on other factors, e.g. smoking or psychiatric medications, which might contribute to associations. The lack of complete smoking data is a shortcoming given the established associations with both psychiatric disorders and some cancers. Data on smoking status at conscription were available for a small subset (3%) of our study population and adjustment attenuated psychiatric hospitalization-smoking-related cancer associations. However, a single estimate of smoking status in early adulthood may not be an accurate representation of an individual’s life-long smoking experience. We may therefore have overestimated the link between psychiatric hospitalization and smoking-related cancers.
Finally, the use of hospital discharge data to identify psychiatric disorders, while guaranteeing clinically identified problems, also limits results to problems severe enough to warrant hospital admission, e.g. recent estimates suggest that 25% of non-affective psychosis patients are treated as out-patients only.29 In the present study we captured individuals with mental illness severe enough to pose a danger to themselves, others, or both. More moderate mental health problems are likely to elicit associations with cancer that are lower in magnitude. Hospitalization rates also vary between psychiatric diagnoses, e.g. individuals with schizophrenia are more likely to be admitted to hospital than those with depression. However, it is reassuring that results for individual psychiatric diagnoses were similar to those for all diagnoses combined. The use of hospital admissions data also means that the age of onset of psychiatric disease may have been overestimated. This restriction will have no impact on analyses based on Method 2, which simply compared men with and without diagnosis, but may have led to additional exclusions in Method 1 and a longer “unexposed” period in Method 3, both of which may have exaggerated the impact of analytical strategy. However, it is clear from simulated data that even very small exclusions have a marked impact on the results.
Conclusion
The choice of analytical strategy has a strong impact on psychiatric disorder-cancer associations, which are complex and time-dependent. The exclusion of cancers predating psychiatric problems is common in this context, and may be reasonable if the timing of both events is clearly established; however, if psychiatric problems already exist but are not identified until after, or as a result of, cancer diagnosis, then these exclusions are not appropriate and the approach may be overly conservative. Moreover, this issue is not restricted to psychiatric disorder-cancer associations and similar problems may arise whenever an association is based on exposures and outcomes that both have potentially long induction periods. Recommendations and priorities for future research include: (a) separate analyses of cause-specific cancers to reflect distinct aetiology; (b) greater understanding of temporal relationships between psychiatric and cancer diagnoses; and (c) development of appropriate methods for dealing with issues of reverse causality when the exact timing of events is unclear. It is important that the true nature of the association between psychiatric disorders and cancer be established before further discussion of mechanisms takes place.
Acknowledgments
source of funding: David Batty has a Wellcome Trust Fellowship which also supports Elise Whitley. The Centre for Cognitive Ageing and Cognitive Epidemiology is supported by the Biotechnology and Biological Sciences Research Council, the Engineering and Physical Sciences Research Council, the Economic and Social Research Council, the MRC, and the University of Edinburgh as part of the cross-council Lifelong Health and Wellbeing initiative. Finn Rasmussen is funded by the Swedish Council for Working Life and Social Research.
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
SIRs estimated across different populations are not necessarily comparable and may potentially be affected by residual confounding.33 However, this problem is much reduced if the compared populations are similar and if stratum-specific SIRs from individual studies are approximately constant. The studies included in this meta-analysis are of men only. In addition, schizophrenia cases in these studies have a number of similarities, being: (i) from developed countries, (ii) identified from hospitals and specialist centres, (iii) identified from 1960s/early 1970s up to 1990/2000s, and (iv) where ages are specified, largely aged 15-45 years at identification. Very few stratum-specific SIRs are presented in these published papers but, where they are, they are broadly similar.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Eaton WW, Martins SS, Nestadt G, Bienvenu OJ, Clarke D, Alexandre P. The Burden of Mental Disorders. Epidemiol Rev. 2008;30:1–14. doi: 10.1093/epirev/mxn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris E, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11–53. doi: 10.1192/bjp.173.1.11. [DOI] [PubMed] [Google Scholar]
- 4.Mäkikyrö T, Karvonen JT, Hakko H, et al. Comorbidity of hospital-treated psychiatric and physical disorders with special reference to schizophrenia: a 28 year follow-up of the 1966 Northern Finland general population birth cohort. Public Health. 1998;112:221–228. doi: 10.1038/sj.ph.1900455. [DOI] [PubMed] [Google Scholar]
- 5.Leucht S, Burkard T, Henderson J, Maj M, Sartorius N. Physical illness and schizophrenia: a review of the literature. Acta Psychiatr Scand. 2007;116:317–333. doi: 10.1111/j.1600-0447.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 6.de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Davidson S, Judd F, Jolley D, Hocking B, Thompson S, Hyland B. Cardiovascular risk factors for people with mental illness. Aust N Z J Psychiatry. 2001;35:196–202. doi: 10.1046/j.1440-1614.2001.00877.x. [DOI] [PubMed] [Google Scholar]
- 8.Daumit GL, Goldberg RW, Anthony C, et al. Physical activity patterns in adults with severe mental illness. J Nerv Ment Dis. 2005;193:641–646. doi: 10.1097/01.nmd.0000180737.85895.60. [DOI] [PubMed] [Google Scholar]
- 9.Osborn DPJ, Nazareth I, King MB. Physical activity, dietary habits and Coronary Heart Disease risk factor knowledge amongst people with severe mental illness - A cross sectional comparative study in primary care. Soc Psychiatry Psychiatr Epidemiol. 2007;42:787–793. doi: 10.1007/s00127-007-0247-3. [DOI] [PubMed] [Google Scholar]
- 10.McCreadie RG. Scottish Schizophrenia Lifestyle Group. Diet, smoking and cardiovascular risk in people with schizophrenia - Descriptive study. Br J Psychiatry. 2003;183:534–539. doi: 10.1192/bjp.183.6.534. [DOI] [PubMed] [Google Scholar]
- 11.Cradock-O’Leary J, Young AS, Yano EM, Wang MM, Lee ML. Use of general medical services by VA patients with psychiatric disorders. Psychiatr Serv. 2002;53:874–878. doi: 10.1176/appi.ps.53.7.874. [DOI] [PubMed] [Google Scholar]
- 12.Rigby J, Oswald A. An evaluation of the performing and recording of physical examinations by psychiatric trainees. Br J Psychiatry. 1987;150:533–535. doi: 10.1192/bjp.150.4.533. [DOI] [PubMed] [Google Scholar]
- 13.BarChana M, Levav I, Lipshitz I, et al. Enhanced cancer risk among patients with bipolar disorder. J Affect Disord. 2008;108:43–48. doi: 10.1016/j.jad.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Lichtermann D, Ekelund J, Pukkala E, Tanskanen A, Lonnqvist J. Incidence of Cancer Among Persons With Schizophrenia and Their Relatives. Arch Gen Psychiatry. 2001;58:573–578. doi: 10.1001/archpsyc.58.6.573. [DOI] [PubMed] [Google Scholar]
- 15.Dalton SO, Mellemkjær L, Olsen JH, Mortensen PB, Johansen C. Depression and Cancer Risk: A Register-based Study of Patients Hospitalized with Affective Disorders, Denmark, 1969-1993. Am J Epidemiol. 2002;155:1088–1095. doi: 10.1093/aje/155.12.1088. [DOI] [PubMed] [Google Scholar]
- 16.Carney CP, Woolson RF, Jones L, Noyes R, Jr., Doebbeling BN. Occurrence of Cancer Among People With Mental Health Claims in an Insured Population. Psychosom Med. 2004;66:735–743. doi: 10.1097/01.psy.0000133281.10749.64. [DOI] [PubMed] [Google Scholar]
- 17.Barak Y, Achiron A, Mandel M, Mirecki I, Aizenberg D. Reduced cancer incidence among patients with schizophrenia. Cancer. 2005;104:2817–2821. doi: 10.1002/cncr.21574. [DOI] [PubMed] [Google Scholar]
- 18.Grinshpoon A, Barchana M, Ponizovsky A, et al. Cancer in schizophrenia: is the risk higher or lower? Schizophr Res. 2005;73:333–341. doi: 10.1016/j.schres.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Catts VS, Catts SV, O’Toole BI, Frost ADJ. Cancer incidence in patients with schizophrenia and their first-degree relatives – a meta-analysis. Acta Psychiatr Scand. 2008;117:323–336. doi: 10.1111/j.1600-0447.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 20.Miovic M, Block S. Psychiatric disorders in advanced cancer. Cancer. 2007;110:1665–1676. doi: 10.1002/cncr.22980. [DOI] [PubMed] [Google Scholar]
- 21.Kissane DW, Grabsch B, Love A, Clarke DM, Bloch S, Smith GC. Psychiatric disorder in women with early stage and advanced breast cancer: a comparative analysis. Aust N Z J Psychiatry. 2004;38:320–326. doi: 10.1080/j.1440-1614.2004.01358.x. [DOI] [PubMed] [Google Scholar]
- 22.Couper JW, Love AW, Duchesne GM, et al. Predictors of psychosocial distress 12 months after diagnosis with early and advanced prostate cancer. Med J Aust. 2010;193:S58–S61. doi: 10.5694/j.1326-5377.2010.tb03930.x. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence D, D’Arcy C, Holman J, Jablensky AV, Threfall TJ, Fuller SA. Excess cancer mortality in Western Australian psychiatric patients due to higher case fatality rates. Acta Psychiatr Scand. 2000;101:382–388. doi: 10.1034/j.1600-0447.2000.101005382.x. [DOI] [PubMed] [Google Scholar]
- 24.Dalton SO, Mellemkjær L, Thomassen L, Mortensen PB, Johansen C. Risk for cancer in a cohort of patients hospitalized for schizophrenia in Denmark, 1969-1993. Schizophr Res. 2005;75:315–324. doi: 10.1016/j.schres.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Batty GD, Wennerstad KM, Smith GD, et al. IQ in early adulthood and later cancer risk: cohort study of one million Swedish men. Ann Oncol. 2007;18:21–28. doi: 10.1093/annonc/mdl473. [DOI] [PubMed] [Google Scholar]
- 26.Gunnell D, Magnusson PKE, Rasmussen F. Low intelligence test scores in 18 year old men and risk of suicide: cohort study. Br Med J. 2004;330:167–170. doi: 10.1136/bmj.38310.473565.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 28.Gulbinat W, Dupont A, Jablensky A, et al. Cancer incidence of schizophrenic patients - results of record linkage studies in 3 countries. Br J Psychiatry. 1992;161:75–85. [PubMed] [Google Scholar]
- 29.Jorgensen L, Ahlbom A, Allebeck P, Dalman C. The Stockholm non-affective psychoses study (snaps): the importance of including out-patient data in incidence studies. Acta Psychiatr Scand. 2010;121:389, 392. doi: 10.1111/j.1600-0447.2009.01500.x. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Moreno JM, Soerjomataram I, Magnusson G. Cancer causes and prevention: A condensed appraisal in Europe in 2008. Eur J Cancer. 2008;44:1390–1403. doi: 10.1016/j.ejca.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Dalton SO, Johansen C, Poulsen AH, et al. Cancer risk among users of neuroleptic medication: a population-based cohort study. Br J Cancer. 2006;95:934–939. doi: 10.1038/sj.bjc.6603259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howard LM, Barley EA, Davies E, et al. Cancer diagnosis in people with severe mental illness: practical and ethical issues. Lancet Oncol. 2010;11:797–804. doi: 10.1016/S1470-2045(10)70085-1. [DOI] [PubMed] [Google Scholar]
- 33.Rothman K, Greenland S, Lash T. Modern Epidemiology. 3rd Edition Lippincott Williams & Wilkins; Philadelphia: 2008. [Google Scholar]