Summary
Neuregulin 1 (Nrg1) is a susceptibility gene of schizophrenia, a disabling mental illness that affects 1% of the general population. Here we show that ctoNrg1 mice, which mimic high levels of NRG1 observed in forebrain regions of schizophrenic patients, exhibit behavioral deficits and hypofunction of glutamatergic and GABAergic pathways. Intriguingly, these deficits were diminished when NRG1 expression returned to normal in adult mice that had been symptomatic, suggesting that damage which occurred during development is recoverable. Conversely, increase of NRG1 in adulthood was sufficient to cause glutamatergic impairment and behavioral deficits. We found that the glutamatergic impairment by NRG1 overexpression required LIM domain kinase 1 (LIMK1), which was activated in mutant mice, identifying a novel pathological mechanism. These observations demonstrate that synaptic dysfunction and behavioral deficits require continuous NRG1 abnormality in adulthood, suggesting that relevant schizophrenia may benefit from therapeutic intervention to restore NRG1 signaling.
Introduction
Schizophrenia is a common and disabling mental illness that affects 1% of the population worldwide and accounts for 3% of the total economic burden of human disease (Murray and Lopez, 1996). Schizophrenia is believed to be a neural developmental disorder with strong genetic factors (Lewis and Levitt, 2002; Weinberger, 1987). Neuregulin 1 (NRG1) is a large family of EGF-domain-containing trophic factors (Mei and Xiong, 2008). Its gene, Nrg1, has been identified as a schizophrenia susceptibility gene in diverse populations (Shi et al., 2009; Stefansson et al., 2009; Stefansson et al., 2003; Stefansson et al., 2002; Yang et al., 2003). Exactly how Nrg1 gene variations lead to schizophrenia remains unclear.
Most of the single nucleotide polymorphisms (SNPs) in the Nrg1 gene that are associated with schizophrenia are localized in intronic, non-coding regions (Mei and Xiong, 2008), raising a possibility that they may regulate the expression of the Nrg1 gene. Expression of isoform 1 alpha of NRG1 was lower in brains of schizophrenic patients (Bertram et al., 2007; Parlapani et al., 2010). Nrg1 hypomorphs are impaired in relevant behaviors (Bjarnadottir et al., 2007; Chen et al., 2008; Gerlai et al., 2000; O'Tuathaigh et al., 2007; Rimer et al., 2005; Stefansson et al., 2002). Recently, elevated NRG1 levels or signaling have been implicated in schizophrenia. The HapICE risk haplotype is associated with increased expression of NRG1 in the brain (Weickert et al., 2012). Moreover, mRNA and protein of NRG1 are increased in the prefrontal cortex (PFC) and hippocampus of schizophrenia patients (Chong et al., 2008; Hashimoto et al., 2004; Law et al., 2006; Petryshen et al., 2005). The increase did not correlate with antipsychotics treatment (Chong et al., 2008; Law et al., 2006), suggesting an association with the disorder instead of medication. Likewise, NRG1 signaling was increased in the forebrain of patients (Hahn et al., 2006). In agreement, transgenic mice overexpressing NRG1 exhibit relevant behavioral deficits (Deakin et al., 2009; Deakin et al., 2012; Kato et al., 2010)
Consistent with the neurodevelopmental hypothesis of schizophrenia, NRG1 has been implicated in brain development (Barros et al., 2009; Fazzari et al., 2010; Flames et al., 2004; Makinodan et al., 2012; Mei and Xiong, 2008; Ting et al., 2011). However, it remains unclear whether damage done by abnormal NRG1 signaling during development is reversible. NRG1 is known to regulate neurotransmission and synaptic plasticity (Bjarnadottir et al., 2007; Chang and Fischbach, 2006; Chen et al., 2010; Gu et al., 2005; Huang et al., 2000; Kwon et al., 2005; Li et al., 2007; Pitcher et al., 2011; Wen et al., 2010; Woo et al., 2007), raising another question whether relevant behavioral deficits require continuous abnormal NRG1 signaling in adulthood. To address these critical questions, we generated ctoNrg1 mice which overexpress type I NRG1, mimicking high levels of NRG1 in schizophrenic patients (Hashimoto et al., 2004; Law et al., 2006; Petryshen et al., 2005). Expression of NRG1 transgene in ctoNrg1 mice was restricted to forebrain regions including PFC and hippocampus, areas increasingly implicated in schizophrenia (Harrison, 2004; Weinberger et al., 1986). The ctoNrg1 mice showed relevant behavioral deficits and were impaired in glutamatergic and GABAergic transmission. Unexpectedly, both synaptic dysfunction and behavioral deficits disappeared when expression of the NRG1 transgene was switched off in adult mice. Moreover, turning-on the transgene expression in adulthood alone was sufficient to cause impaired glutamatergic transmission and behavioral deficits. We studied mechanisms underlying the synaptic dysfunction in ctoNrg1 mice. Results indicate that glutamatergic hypofunction caused by NRG1 overexpression requires LIMK1, but not ErbB4, identifying a novel pathogenic mechanism. Together, these observations demonstrate that synaptic dysfunction and behavioral deficits require continuous NRG1 abnormality in adulthood. Our results suggest that relevant schizophrenia may benefit from therapeutic intervention to restore NRG1 signaling.
Results
Spatial and temporal control of type I NRG1 expression in ctoNrg1 mice
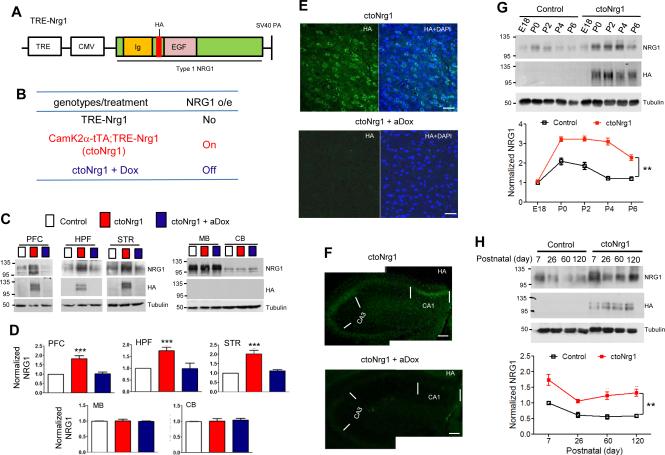
To mimic high levels of NRG1 type I in schizophrenic patients, we generated transgenic mice, TRE-Nrg1, which carry the type I NRG1β cDNA under the control of the tetracycline-responsive promoter element (TRE) tetO (Figure 1A). An HA tag was inserted between the Ig and EGF domains, which has no effect on NRG1 processing and function (Wang et al., 2001). TRE-Nrg1 mice did not express NRG1 transgene in the absence of tetracycline transactivator (tTA) (Figures 1B and 1C). Since the majority of NRG1 in the brain is produced in excitatory neurons (Brinkmann et al., 2008; Liu et al., 2011) and NRG1 increase was observed in forebrain areas of schizophrenic patients (Chong et al., 2008; Hashimoto et al., 2004; Law et al., 2006), TRE-Nrg1 mice were crossed with CamK2α-tTA mice that express tTA in excitatory neurons (Mayford et al., 1996). Resulting bitransgenic CamK2α-tTA;TRE-Nrg1 mice (ctoNrg1 for CamK2α promoter driven tet-off NRG1) produced HA-NRG1 in excitatory neurons in the absence of doxycycline (Dox) (Figures 1B and 1C). Compared with TRE-Nrg1 or control mice, NRG1 was increased by 50-100% in forebrain of ctoNrg1 mice (Figures 1C and 1D), similar to levels in forebrain of schizophrenic patients. The increase was due to expression of the transgene, which was detectable by anti-HA antibody (Figures 1C, 1E and 1F). Moreover, the increase was forebrain-specific, and not detectable in the midbrain and cerebellum (Figures 1C and 1D), in agreement with the expression of CamK2α-tTA (Mayford et al., 1996). The expression of transgene occurred as early as postnatal day 0 and remained in adulthood (Figures 1G and 1H). Finally, the overexpression of NRG1 could be switched off by Dox (Figures 1C-1F). Together, the results indicate that ctoNrg1 mice express higher levels of NRG1 in the forebrain during development, and the expression could be turned off efficiently by Dox.
Figure 1. Temporal control of NRG1 expression in forebrains of ctoNrg1 mice.
(A) Transgene structure. Full length NRG1 type I β1a was cloned in pMM400 between the promoter complex of TRE and CMV (cytomegalus virus minimal promoter) and SV40 polyadenylation signal.
(B) Genotypes and NRG1 expression. Unless otherwise indicated, blank, red and blue histograms/curves in the paper represent data from control, ctoNrg1, and aDox-treated ctoNrg1 mice, respectively. aDox, Dox treatment in adulthood; dDox, Dox treatment during development (see Figure 7); o/e, overexpression.
(C) Expression of NRG1 transgene in PFC, hippocampus (HPF), and striatum (STR), but not midbrain (MB) and cerebellum (CB). ctoNrg1 mice, 8 weeks of age, were treated with Dox for two weeks. Homogenates of various brain regions of indicated mice were subjected to western blot analysis with indicated antibodies.
(D) Quantification of NRG1 expression in different brain regions. Expression in ctoNrg1 samples were normalized by those from control littermates. n = 3 per genotype; *** p < 0.001 for PFC, HPF and STR, one-way ANOVA.
(E) HA staining in ctoNrg1 PFC. Sections were stained with anti-HA antibody and DAPI. Bar, 50 μm.
(F) HA staining in ctoNrg1 hippocampus. Sections were stained with anti-HA antibody. Bar, 100 μm.
(G, H) NRG1 expression of developing forebrains. Top, representative western blots; bottom, quantitative analysis. n = 3 per genotype at each time point. Data were normalized by NRG1 levels of E18 control mice (in G) or of P7 control mice (in H). In G, ** Genotype F (1, 20) = 184, p < 0.01, two-way ANOVA. In H, ** Genotype F (1, 16) = 33.03, p < 0.01, two-way ANOVA.
Behavioral deficits in ctoNrg1 mice
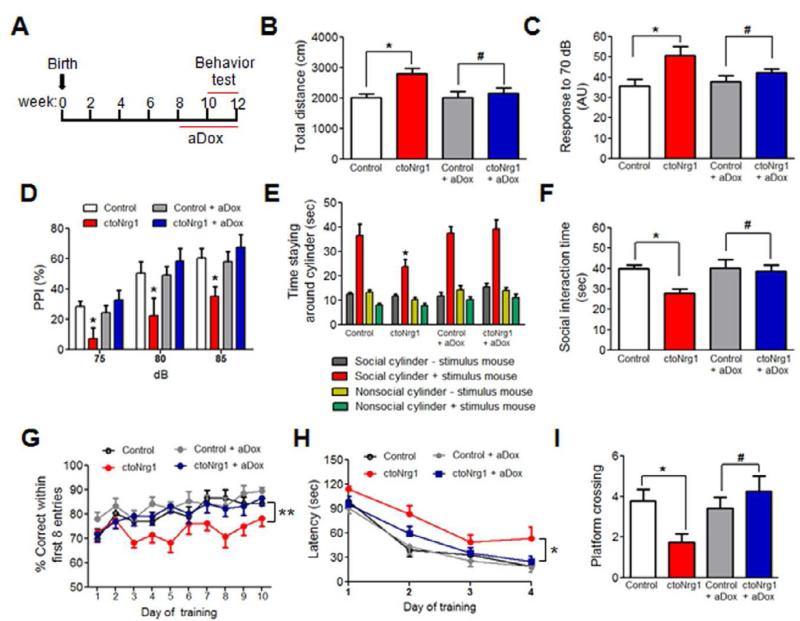
Compared to controls, ctoNrg1 mice displayed normal motor skill learning in rotarod test (Figure S1A) and did not exhibit anxiety-like phenotype in elevated plus maze (Figures S1B-S1E). However, they were hyperactive in open field test (Figure 2B), a phenotype thought to correspond to psychomotor agitation of schizophrenic patients (Snyder, 1973). Prepulse inhibition (PPI) is a common test of sensory-motor gating that is often decreased in schizophrenic patients. ctoNrg1 mice had a slightly elevated response to 70 dB background noise (Figure 2C), suggesting no deficit in hearing, and produced similar startle amplitude (Figure S1F). However, PPI was impaired in ctoNrg1 mice, compared to controls (Figure 2D).
Figure 2. Behavioral deficits in ctoNrg1 mice were attenuated after aDox treatment.
(A) Diagram of aDox treatment and behavioral test. Dox was added in the drinking water when mice were 8 weeks old and during entire behavioral tests, which started two weeks after treatment.
(B) Travel distance in open field was increased in ctoNrg1 mice, compared to controls, but became similar between the genotypes after aDox treatment. n = 12 per group; * p < 0.05, # p > 0.05, one-way ANOVA. Blank, red, grey and blue histograms/curves represent data from control, ctoNrg1, aDox-treated control and aDox-treated ctoNrg1 mice.
(C) Elevated response to 70dB background noise in ctoNrg1 mice, compared to controls, and the elevation was ameliorated by aDox treatment. AU, arbitrary units. n = 9 per group; * p < 0.05, # p > 0.05, one-way ANOVA.
(D) PPI was impaired in ctoNrg1 mice, compared to controls, and the impairment was diminished after aDox treatment. n = 9 per group; * Genotype F (3, 84) = 10.2, p < 0.05, two-way ANOVA.
(E) ctoNrg1 mice spent less time with social cylinder with stimulus mouse, compared to controls, and the interaction time was similar between two genotypes after aDox treatment. n = 12 per group; * Genotype F (3, 172) = 7.01, p < 0.05, two-way ANOVA.
(F) Social interaction time with stimulus mice without cylinder was reduced in ctoNrg1 mice, and the reduction was diminished after aDox treatment. n = 12 for each group; * p < 0.05, # p > 0.05, one-way ANOVA.
(G) Decreased correct entries in ctoNrg1 mice, compared to controls, and the difference was diminished after aDox treatment. n = 12 per group; ** Genotype F (3, 424) = 16.14, p < 0.01, two-way ANOVA.
(H) Increased latency for ctoNrg1 mice to reach the hidden platform in MWM, compared to controls, and the two genotypes showed similar latency after aDox treatment. n = 9 for control, n = 8 for ctoNrg1, n = 8 for both groups of control + aDox and ctoNrg1 + aDox; * Genotype F (3, 87) = 6.4, p <0.05, two-way ANOVA.
(I) Reduced platform crossing by ctoNrg1 mice in MWM probe test, compared to controls, and the two genotypes showed similar platform crossing after aDox treatment. n = 9 for control, n = 8 for ctoNrg1, n = 8 for both groups of control + aDox and ctoNrg1 + aDox; * p < 0.05, # p > 0.05, one-way ANOVA.
Social withdrawal is a negative symptom of schizophrenia (Corcoran et al., 1995). ctoNrg1 and control mice showed no difference in the time spent around a cylinder without a stimulus mouse inside (Figure 2E). However, the time spent around the social cylinder with a stimulus mouse inside was significantly reduced in ctoNrg1 mice, compared to controls (Figure 2E). Moreover, the social interaction time of ctoNrg1 mice with stimulus mouse was reduced, compared to controls (Figure 2F). These results suggest impaired social activity in ctoNrg1 mice. To determine the effect of NRG1 overexpression on cognitive function, we tested spatial working memory and reference memory in 8-arm radial maze (ARM) and Morris water maze (MWM), respectively (Hodges, 1996; Morris, 1984). To exclude potential influence of hyperactivity in the 8 ARM test, we monitored correct entries during the first eight entries. They were reduced in ctoNrg1 mice compared to controls (Figure 2G), indicating deficient spatial working memory. In MWMs, ctoNrg1 mice had no problem in visualizing the platform (Figure S1G) and were able to learn to locate submerged platforms. However, the latency to find the platform was increased, compared to controls (Figure 2H), indicating compromised ability to learn. In the probe trial, the number of times that ctoNrg1 mice swam across the platform location decreased, compared to controls (Figure 2I). These results indicated that ctoNrg1 mice are impaired in spatial reference memory. The above results demonstrated that NRG1 overexpression in the forebrain causes behavioral deficits in locomotor activity, sensory-motor gating, social interaction, and cognitive function, in agreement with recent studies (Deakin et al., 2009; Deakin et al., 2011; Kato et al., 2010). Finally, administration of clozapine, an antipsychotic medication for schizophrenic patients (Krakowski et al., 2006), ameliorated behavioral deficits of ctoNrg1 mice in open field, PPI and social interaction tests (Figure S2).
Hypofunction of glutamatergic and GABAergic transmission in ctoNrg1 mice
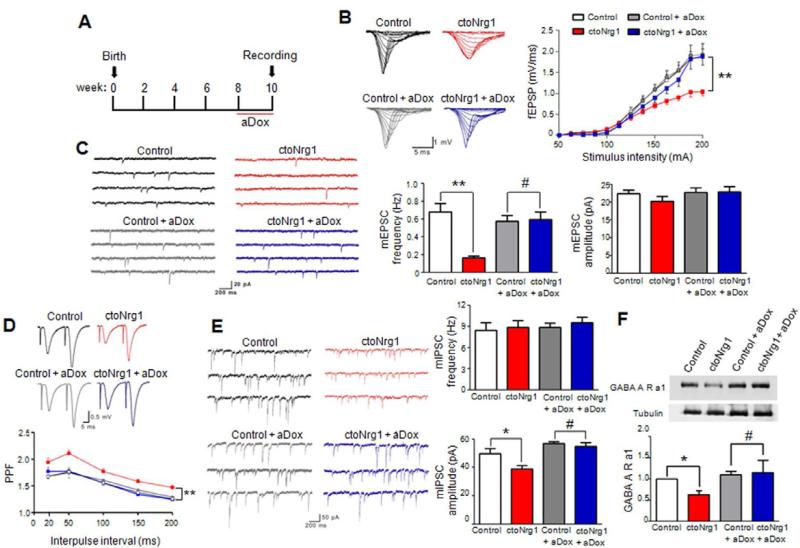
ctoNrg1 mice showed normal laminar structure and densities of excitatory and parvalbumin (PV)-positive neurons in the somatosensory cortex and hippocampus (Figure S3). Intriguingly, there was downward shift of input – output (I/O) curves of fEPSPs (field excitatory postsynaptic potentials) at Schaffer collateral (SC) – CA1 synapses in ctoNrg1 hippocampus, compared to controls (Figure 3B), suggesting hypofunction of glutamatergic transmission. To determine if the impairment is caused by reduced glutamate receptors or decreased glutamate release, we measured miniature excitatory postsynaptic currents (mEPSCs) in CA1 pyramidal neurons in the presence of TTX to block action potentials. No difference was observed in mEPSC amplitudes between ctoNrg1 and control slices (Figure 3C). Moreover, the ratio of AMPAR to NMDAR EPSCs (AMPA/NMDA ratio) (Figure S4A) and protein levels of NMDAR, AMPAR, and PSD95 were similar between two genotypes (Figure S4B). These results suggest that NRG1 overexpression did not alter glutamate receptor density or composition. However, mEPSC frequency was decreased in ctoNrg1 hippocampus (Figure 3C) and PFC (Figure S4C). Since reduction of mEPSC frequency suggests impaired glutamate release or fewer functional synapses, we measured fEPSPs evoked by two presynaptic stimulations delivered at different intervals (i.e., paired-pulses). The paired pulse facilitation (PPF) of initial slopes of fEPSPs was increased in ctoNrg1 slices, compared to controls, suggesting lowered release probability (Figure 3D). Moreover, rate of synaptic fatigue during high-frequency stimulation was slower in ctoNrg1 slices (Figure S4D). Together, these observations suggest that the glutamatergic pathway is hypofunctional in ctoNrg1 mice, probably due to impaired glutamate release.
Figure 3. Glutamatergic and GABAergic impairment in ctoNrg1 mice was reduced after aDox treatment.
(A) Diagram of aDox treatment and behavioral test. Mice were fed with Dox-containing drinking water at 8 weeks old for 2 weeks before recording. Dox was kept in the slice perfusion solution.
(B) Depressed I/O curves in ctoNrg1 mice, compared to controls, and I/O curves were similar between the genotypes after aDox treatment. Left, representative traces of fEPSPs at SC-CA1 synapses at different stimulus intensities; right, I/O curves of four groups. n = 10 slices from 6 control mice, n = 10 slices from 6 ctoNrg1 mice, n = 9 slices from 4 aDox-treated control mice, n = 8 slices from 4 aDox-treated ctoNrg1 mice; ** Genotype F (3, 429) = 19.87, p < 0.01, two-way ANOVA.
(C) Reduced mEPSC frequency in ctoNrg1 CA1 pyramidal neurons, compared to controls and mEPSC frequency was similar between the two genotypes after aDox treatment. Left, representative mEPSC traces; right, quantitative data. n = 10 cells from 4 mice per group; ** p < 0.01, # p > 0.05, one-way ANOVA.
(D) Elevated PPF in ctoNrg1 SC-CA1 synapses, compared to controls and the PPF was similar between the two genotypes after aDox treatment. Top, representative traces; bottom, quantitative data. n = 10 slices from 6 control mice, n = 10 slices from 6 ctoNrg1 mice, n = 8 slices from 4 aDox-treated control mice, n = 8 slices from 4 aDox-treated ctoNrg1 mice; *** Genotype F (3, 201) = 29.76, p < 0.001, two-way ANOVA.
(E) Attenuated mIPSC amplitudes in ctoNrg1 CA1 pyramidal neurons, compared to controls and mIPSC amplitudes were similar between the two genotypes after aDox treatment. Left, representative mIPSC traces; right, quantitative data. n = 11 cells from 5 control mice, n = 10 cells from 4 ctoNrg1 mice, n = 11 cells from 5 aDox-treated control mice, n = 10 cells from 4 aDox-treated ctoNrg1 mice; * p < 0.05, # p > 0.05, one-way ANOVA.
(F) Reduced GABAA receptor α1 subunit (GABAARα1) in ctoNrg1 forebrain and the reduction was diminished after aDox treatment. Top, representative blots; bottom, quantitative data. Data from other three groups were normalized by control. n = 3 per group; * p < 0.05, # p > 0.05, one-way ANOVA.
To determine if NRG1 overexpression alters GABAergic transmission, miniature inhibitory postsynaptic currents (mIPSCs) were first recorded at a holding potential of + 10 mV in a Cs-methanesulfonate-based internal solution (Zhou et al., 2009). mIPSC amplitudes were reduced in ctoNrg1 mice, compared to controls (Figure S5A). To enhance the driving force of Cl- currents, we also recorded mIPSCs at a holding potential of -70 mV with high concentration of CsCl in internal solution (Gonzalez-Islas et al., 2009). mIPSC amplitudes under this condition were similarly reduced in ctoNrg1 slices (Figure 3E). These results suggest reduced GABAA receptor density in ctoNrg1 mice. This notion was supported by biochemical data that GABAA receptor α1 subunit (GABAAR α1) was decreased in ctoNrg1 forebrain (Figure 3F). Notably, mIPSC frequency was similar between ctoNrg1 and control mice by the two methods (Figures S5A and 3E), suggesting no change in GABA spontaneous release. The paired-pulse ratios (PPRs) of evoked inhibitory postsynaptic currents (eIPSCs) in response to two stimulations were comparable between two genotypes (Figure S5B). These observations indicate that GABAergic transmission was impaired in ctoNrg1 mice, mainly due to a postsynaptic mechanism.
Dependence of behavioral and synaptic deficits on continuous NRG1 overexpression
To determine whether behavioral deficits in ctoNrg1 mice require continuous NRG1 overexpression, mice were subjected to aDox treatment (adult Dox treatment) (Figure 2A). To eliminate possible compounding effects of Dox, control mice were subjected to identical aDox treatment. aDox treatment effectively shut off the NRG1 overexpression (Figure 1C, 1D). Notably, aDox-treated ctoNrg1 and control mice traveled similar distance in the open field (Figure 2B), suggesting normal locomotive activity of aDox-treated ctoNrg1 mice. Moreover, no difference was observed in PPI between aDox-treated control and ctoNrg1 mice (Figure 2D). In addition, social interaction deficits in ctoNrg1 mice were ameliorated by aDox treatment (Figures 2E and 2F). Finally, aDox-treated ctoNrg1 mice performed normally in 8-ARM and MWM test (Figures 2G-2I), indicating normal cognitive function. These results show that behavioral deficits in ctoNrg1 mice require continuous NRG1 overexpression in adulthood and are reversible by aDox treatment.
If synaptic dysfunction is a contributing mechanism, it should be diminished when NRG1 expression returned to normal. To test this, aDox-treated mice were subjected to electrophysiological studies (Figure 3A). I/O curves at SC-CA1 synapses were similar between aDox-treated control and ctoNrg1 mice (Figure 4B), indicative of normal AMPAR-mediated glutamatergic transmission. mEPSC frequencies of aDox-treated ctoNrg1 and controls were also similar (Figure 3C). The PPF, which was increased in ctoNrg1 mice, showed no difference between the two genotypes after aDox treatment (Figure 3D). In addition, mIPSC amplitude (Figure 3E) and GABAAR α1 level (Figure 3F) were also similar between aDox-treated control and ctoNrg1 mice. These observations demonstrate the dependence of synaptic dysfunction in ctoNrg1 mice on continuous NRG1 overexpression and underscore the importance of synaptic dysfunction in behavioral deficits.
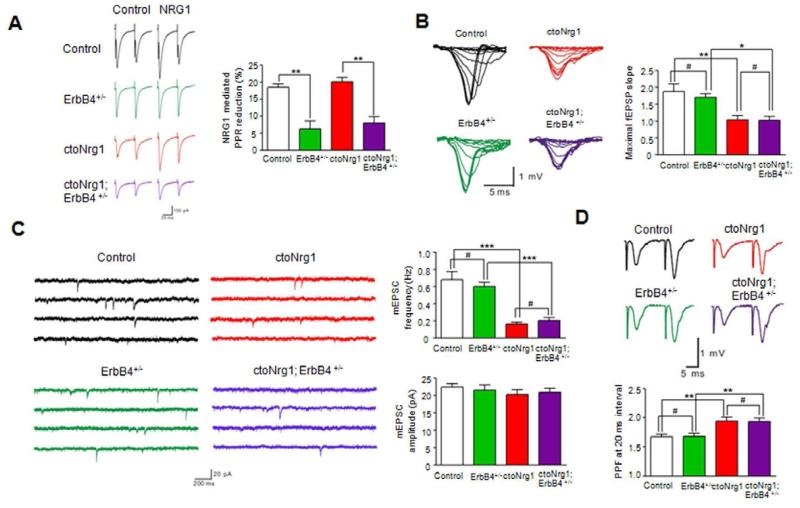
Figure 4. No effect of ErbB4 mutation on glutamatergic impairment in ctoNrg1 mice.
(A) ErbB4 heterozygous mutation attenuated NRG1-mediated PPR reduction in both control and ctoNrg1 hippocampal slices. Slices were subjected to a pair of stimulation in the absence or presence of NRG1. Left, representative eIPSC traces in ctoNrg1 and ctoNrg1;ErbB4 +/- slices; right, quantitative data. n = 10 cells from 3 mice for each group. ** p < 0.01, one-way ANOVA.
(B) No effect of ErbB4 heterozygous mutation on maximal fEPSP slope in ctoNrg1 hippocampal slices. Left, representative I/O curves in control, ErbB4 +/-, ctoNrg1 and ctoNrg1;ErbB4 +/- slices; right, quantitative data. n = 12 slices from 3 mice for each group. ** p < 0.01, * p < 0.05, # p > 0.05, one-way ANOVA.
(C) Reduced mEPSC frequency in ctoNrg1 CA1 pyramidal neurons was not altered by ErbB4 heterozygous mutation. Left, representative mEPSC traces in control, ErbB4 +/-, ctoNrg1 and ctoNrg1; ErbB4 +/- slices; right, quantitative data. n = 10 cells from 3 mice for each group. *** p < 0.001, # p > 0.05, one-way ANOVA.
(D) ErbB4 heterozygous mutation had no effect on elevated PPF at ctoNrg1 SC-CA1 synapses. Top, representative traces in control, ErbB4 +/-, ctoNrg1 and ctoNrg1; ErbB4 +/- slices; right, quantitative data at 20 ms interval, n = 12 slices from 3 mice for each group. ** p < 0.01, # p > 0.05, one-way ANOVA.
ErbB4 is dispensable for impaired glutamate release in ctoNrg1 mice
ErbB4, a critical receptor for NRG1, has been implicated in regulation of GABAergic transmission and synaptic plasticity (Chen et al., 2010; Pitcher et al., 2008; Wen et al., 2010; Woo et al., 2007). To investigate if ErbB4 is involved in synaptic dysfunction in ctoNrg1 mice, we determined whether ErbB4 expression was altered. ErbB4, enriched in cortical P2 fractions (Huang et al., 2000), was at similar level between ctoNrg1 and control mice, regardless of Dox treatment (Figure S6A). ErbB4 activation in forebrain, revealed by anti-phospho-ErbB4 antibody, showed no difference between the two genotypes, regardless of dDox (for developmental Dox, see below) treatment (Figures S6B and S6C). Finally, ErbB4 tyrosine phosphorylation, in response to NRG1 stimulation, in acutely isolated forebrain slices was similar between the genotypes (Figure S6B). Together, the results suggest that basal and NRG1-induced ErbB4 activity is similar between control and ctoNrg1 mice.
To determine if ErbB4 is involved in glutamatergic hypofunction in ctoNrg1 mice, we determined if the deficits could be attenuated by ErbB4 heterozygous mutation, to avoid compounding effect of null mutation on the assembly and function of GABAergic circuitry (Fazzari et al., 2010; Li et al., 2012; Tan et al., 2012). Heterozygous mutation does not alter the number of GABAergic interneurons in cortex and hippocampus (Flames et al., 2004) and did not alter fEPSP slopes, mEPSC frequency and amplitude, and PPF of fEPSPs in control mice (Figures 4B-4D), in agreement with previous studies (Fazzari et al., 2010; Huang et al., 2000; Pitcher et al., 2011). However, it ameliorated NRG1 reduction of eIPSC PPR (Figure 4A), a phenomenon known to depend on ErbB4 (Woo et al., 2007). Intriguingly, the maximal fEPSP slope and mEPSC frequency in ctoNrg1 slices remained depressed after ErbB4+/- mutation (Figures 4B and 4C). Moreover, ErbB4+/- mutation did not attenuate the elevated PPF of fEPSPs in ctoNrg1 slices (Figure 4D). Finally, to circumvent possible compensatory or secondary effects of ErbB4+/- mutation during development, we acutely inhibited ErbB4 in ctoNrg1 slices with PD158780, an inhibitor of ErbB4 (Pitcher et al., 2008; Tan et al., 2012). Glutamatergic transmission remained deficient in treated slices (Figure S7). Together, these results demonstrate that glutamatergic hypofunction in ctoNrg1 mice may be independent of ErbB4.
Regulation of synaptic LIMK1 signaling by overexpressed NRG1
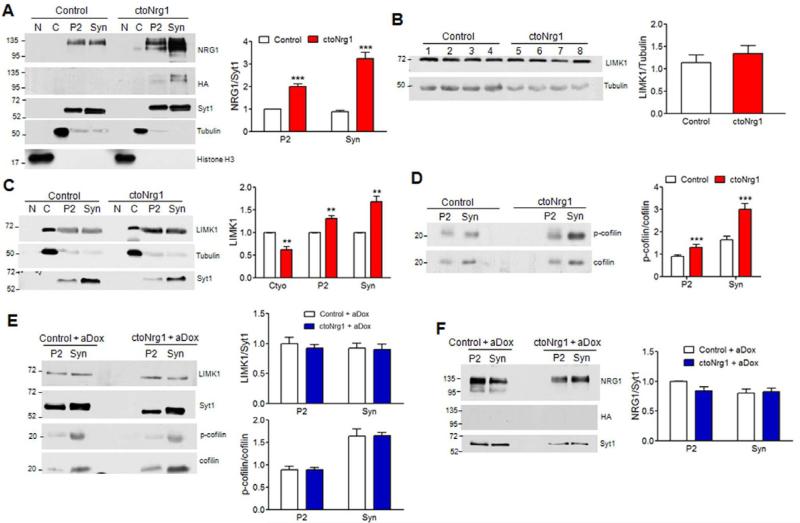
LIMK1 interacts with the intracellular domain of NRG1 (Wang et al., 1998). It phosphorylates and thus inactivates cofilin, an actin depolymerization factor that binds to F-actin and severs the filament (Arber et al., 1998; Yang et al., 1998). F-actin polymerization could inhibit glutamate release by preventing vesicle fusion at active zone (Cingolani and Goda, 2008). Accordingly, LIMK1 has been implicated in regulation of glutamatergic transmission and plasticity (Meng et al., 2004; Meng et al., 2002). We wondered if LIMK1 contributes to glutamatergic hypofunction in ctoNrg1 mice. Both endogenous and transgenic NRG1 are enriched in P2 fraction and synaptosomes (Figure 5A). NRG1 overexpression had no effect on overall level of LIMK1 (Figure 5B), but increased its amount in P2 fraction and synaptosomes (Figure 5C), suggesting that overexpressed NRG1 may recruit LIMK1 to synaptosomes (Figure 5C). Accordingly, phosphorylated cofilin (p-cofilin) increased in ctoNrg1 synaptosomes, compared to controls (Figure 5D). Notably, the increase of both LIMK1 and p-cofilin in synaptosomes was diminished after aDox treatment, which turned off the expression of the NRG1 transgene (Figures 5E, 5F) and attenuated glutamatergic hypofunction (Figures 3B-3D). These observations suggested that increase of LIMK1 and p-cofilin in synaptosomes correlated with impaired glutamatergic transmission.
Figure 5. Synaptic recruitment and activation of LIMK1 by overexpressed NRG1.
(A) NRG enrichment in P2 and synaptosomes in ctoNrg1 forebrains. Subcellular fractions were subjected to western blotting with indicated antibodies. Left, representative blots; right, quantitative data where NRG1 levels were normalized by synaptagmin1 (Syt1), and those in controls were taken as 1. n = 3 per genotype; *** Genotype F (1, 8) = 124.48, p < 0.001, two-way ANOVA. N, nucleus; C, cytoplasm; Syn, synaptosomes.
(B) Similar levels of LIMK1 in control and ctoNrg1 forebrains. Homogenates of individual control (lanes 1-4) and ctoNrg1 (5-8) mice were subjected to western blotting with antibodies against LIMK1 and α-tubulin. Left, representative blots; right, quantitative data where LIMK1 was normalized by α-tubulin. n = 4 per genotype; p > 0.05, t-test.
(C) Increased LIMK1 in P2 and synaptosomes of ctoNrg1 forebrains. Left, representative blots; right, quantitative data where LIMK1 in cytoplasm and P2 or synaptosomes were normalized by tubulin and Syt1, respectively. Levels in control mice were taken as 1. n = 3 per genotype; ** Genotype F (1, 12) = 17.73, p < 0.01, two-way ANOVA.
(D) Increased p-cofilin in P2 and synaptosomes of ctoNrg1 mice. Left, representative blots; right, quantitative data where p-cofilin was normalized by total cofilin. Data from controls were taken as 1. n = 3 per genotype; *** Genotype F (1, 8) = 26.68, p < 0.001, two-way ANOVA.
(E) Similar levels of LIMK1 and p-cofilin in P2 and Syn in aDox-treated control and ctoNrg1 forebrains. Left, representative blots; right, quantitative data where LIMK1 in cytoplasm was normalized by tubulin and that in P2 and synaptosomes by Syt1, p-cofilin was normalized by total cofilin; and those in controls were taken as 1; n = 3 per genotype; For LIMK1, Genotype F (1, 8) = 0.26, p > 0.05, two-way ANOVA; for p-cofilin, Genotype F (1, 8) = 0.00, p > 0.05, two-way ANOVA.
(F) HA-NRG1 was not detectable in forebrains of aDox-treated mice. Left, representative blots; right, quantitative data where NRG1 levels were normalized by Syt1, and those in controls were taken as 1; n = 3 per genotype; Genotype F (1, 8) = 1.57, p > 0.05, two-way ANOVA.
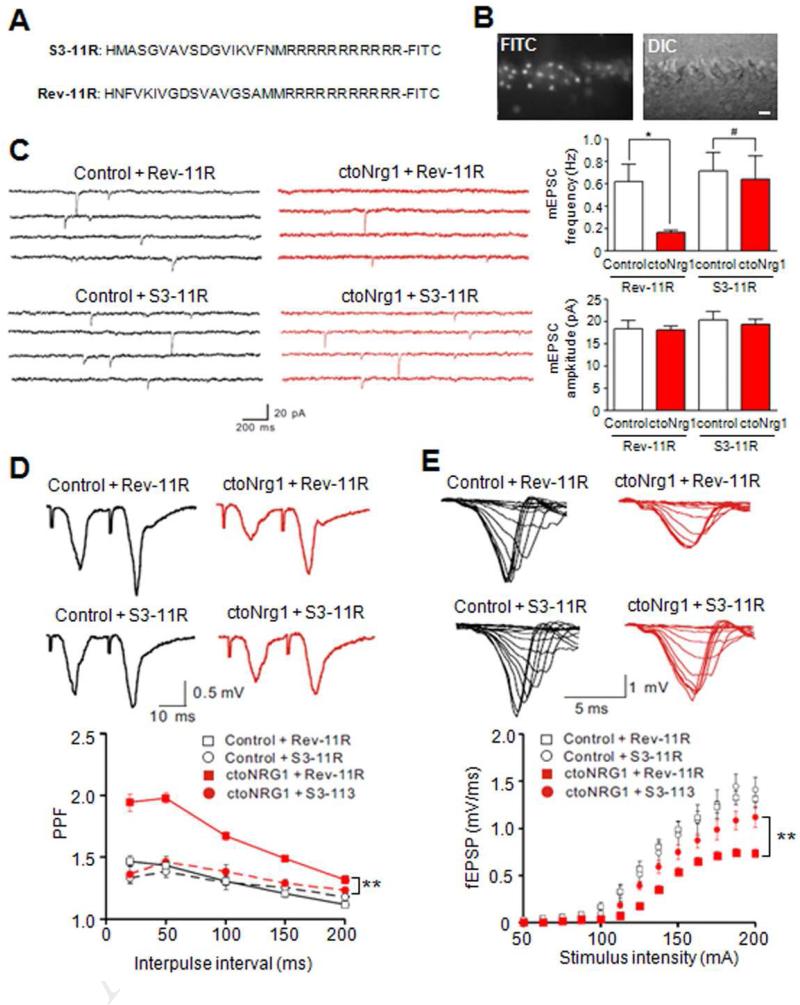
To test if LIMK1 was necessary for glutamatergic hypofunction, hippocampal slices were treated with S3-11R, a LIMK1 inhibitory peptide consisting of cofilin phosphorylation consensus site (Aizawa et al., 2001) or control peptide, Rev-11R, which contains the reversed cofilin sequence (Figure 6A). Both peptides had a stretch of eleven arginines (11R) to facilitate delivery into hippocampal neurons (Fukazawa et al., 2003) (Figure 6B). mEPSC frequency remained lower in ctoNrg1 slices in the presence of control peptide Rev-11R, compared to controls (Figure 6C). In contrast, S3-11R ameliorated the reduction of mEPSC frequency (Figure 6C). It also reduced elevated PPR (Figure 6D) and alleviated I/O depression in ctoNrg1 slices (Figure 6E). These results indicate that impaired glutamate release in ctoNrg1 slices requires LIMK1 activity. Together with correlative results above (Figure 5), these observations provide evidence that LIMK1 may be necessary for glutamatergic dysfunction due to NRG1 overexpression, identifying a novel pathophysiological mechanism.
Figure 6. Inhibition of LIMK1 ameliorated glutamatergic impairment in ctoNrg1 slices.
(A) Amino acid sequences of LIMK1 control (Rev-11R) and inhibitory peptide (S3-11R).
(B) Penetration of FITC-labeled S3-11R into neurons of hippocampal slices. Bar, 20 μm.
(C) Treatment with S3-11R, but not Rev-11R, attenuated mEPSC frequency reduction in ctoNrg1 CA1 pyramidal neurons. Hippocampal slices were treated with 1 μM S3-11R and Rev-11R, respectively. Left, representative traces; right, quantitative data. Rev-11R: n = 8 cells from 3 mice per genotype, S3-11R: n = 8 cells from 3 control mice, n = 9 cells from 3 ctoNrg1 mice; * p < 0.05, # p > 0.05, one-way ANOVA.
(D) S3-11R, but not Rev-11R, reduced PPF at SC-CA1 synapses in ctoNrg1 mice. Slices were treated as in C. Top, representative traces; bottom, quantitative data. Rev-11R: n = 9 slices from 3 control mice, n = 10 slices from 3 ctoNrg1 mice, S3-11R: n = 8 slices from 3 mice per genotype; ** Genotype F (3, 155) = 88.07, p < 0.01, two-way ANOVA.
(E) Partial recovery of I/O curves at ctoNrg1 SC-CA1 synapses by S3-11R. Top, representative traces; bottom, quantitative data. Rev-11R: n = 9 slices from 3 control mice, n = 10 slices from 3 ctoNrg1 mice, S3-11R: n = 8 slices from 3 control mice, n = 9 slices from 3 ctoNrg1 mice; ** Genotype F (3, 416) = 31.32, p < 0.01, two-way ANOVA.
Induction of synaptic and behavioral deficits by adulthood NRG1 overexpression
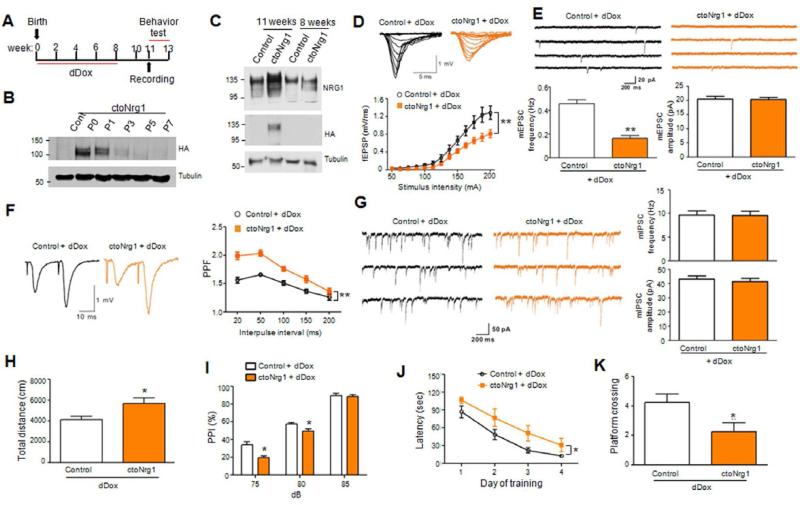
To determine whether NRG1 overexpression in adulthood is sufficient to cause synaptic dysfunction and behavioral deficits, ctoNrg1 mice were treated with dDox immediately after birth for 8 weeks (Figure 7A). HA-NRG1 expression was suppressed by dDox completely after P3 and remained undetectable until 11 weeks of age (i.e., three weeks after dDox removal) (Figures 7B and 7C). Control mice were subjected to simultaneous, identical dDox treatment to minimize possible side-effects of dDox.
Figure 7. Adult NRG1 overexpression is sufficient to cause glutamatergic impairment and behavioral deficits.
(A) Diagram of dDox treatment and tests. Mice were fed with Dox-containing drinking water from P0 till 8 weeks of age, when Dox was omitted. Three weeks later, mice were subjected to analysis. Such treatment was designated as dDox for Dox treatment during development.
(B) Expression of the HA-NRG1 transgene in forebrains was not detectable after P3 in mice being treated by dDox.
(C) HA-NRG1 was not detectable in forebrains of dDox-treated mice at 8 weeks of age, but at 11 weeks of age (i.e., 3 weeks after dDox withdrawal).
(D) Depressed I/O curves in dDox-treated ctoNrg1 mice, compared to dDox-treated controls. Top, representative traces; bottom, quantitative data; n = 12 slices from 4 dDox-treated control mice, n = 11 slices from 5 dDox-treated ctoNrg1 mice; ** Genotype F (1, 273) = 40.77, p < 0.01, two-way ANOVA. Blank and yellow histograms/curves represent data from dDox-treated control and ctoNrg1 mice, respectively.
(E) Decreased mEPSC frequency, but not amplitude, in CA1 pyramidal neurons of dDox-treated ctoNrg1 mice, compared to controls. Top, representative traces; bottom, quantitative data; n = 9 cells from 3 dDox-treated control mice; and n = 10 cells from 4 dDox-treated ctoNrg1 mice; ** p < 0.01, t-test.
(F) Elevated PPF at SC-CA1 synapses in dDox-treated ctoNrg1 mice, compared to controls. Left, representative traces; right, quantitative data; n = 10 slices from 4 dDox-treated control mice; n = 10 slices from 5 dDox-treated ctoNrg1 mice; ** Genotype F (1, 90) = 70.21, p < 0.01, two-way ANOVA.
(G) Normal mIPSC in dDox-treated ctoNrg1 mice, compared to controls. Left, representative traces; right, quantitative data; n = 10 cells from 4 dDox-treated control mice; n = 12 cells from 4 dDox-treated ctoNrg1 mice; p > 0.05, t-test.
(H) Increased travel distance by dDox-treated ctoNrg1 mice in open field test for 30 min, compared to controls. n = 10 per genotype; * p < 0.05, t-test.
(I) Reduced PPI in dDox-treated ctoNrg1 mice, compared to controls. n = 10 per genotype; * Genotype F (1, 51) = 16.06, p < 0.05, two-way ANOVA.
(J) Increased latency of dDox-treated ctoNrg1 mice to reach the hidden platform in MWM, compared to controls. n = 10 per genotype; * Genotype F (1, 68) = 13, p < 0.05, two-way ANOVA.
(K) Reduced platform crossing by dDox-treated ctoNrg1 mice in MWM probe test, compared to controls. n = 10 per genotype; * p < 0.05, t-test.
The I/O curve was shifted downward in dDox-treated ctoNrg1 slices, compared to treated controls (Figure 7D), indicating impaired glutamatergic transmission. mEPSC frequency, but not amplitude, was decreased in slices from dDox-treated ctoNrg1 mice, compared to dDox-treated controls (Figure 7E). This reduction was associated with increased PPF, suggesting lowered release probability (Figure 7F). The glutamatergic deficits were similar to those observed in non-treated ctoNrg1 mice where NRG1 overexpression was higher during development and in adulthood (Figure 3). These results indicate that NRG1 overexpression only in adulthood is sufficient to cause glutamatergic hypofunction. dDox-treated ctoNrg1 mice were hyperactive and impaired in PPI, spatial learning and memory (Figures 7H-7K), demonstrating that NRG1 increase in adulthood was able to cause behavioral deficits. Notably, however, mIPSC amplitudes in dDox-treated ctoNrg1 slices were similar to those in dDox-treated controls (Figure 7G), suggesting that GABAergic deficits observed in ctoNrg1 mice (Figures 3E and S4E) cannot be induced by NRG1 overexpression in adulthood alone.
Discussion
In this study, we modeled in mice NRG1 overexpression that is observed in schizophrenic patients. The major findings of our study are as follows. First, we show that ctoNRG1 mice exhibited schizophrenia-related behavioral deficits including hyperactivity, impaired PPI, reduced social interaction and cognitive deficits (Figure 2). Intriguingly, after NRG1 returned to normal levels in adulthood, behavioral deficits were ameliorated in ctoNrg1 mice (Figure 2). The reversibility of the deficits by turning off NRG1 overexpression suggests that the phenotypes in ctoNrg1 mice were due to the transgene expression, not insertion of the transgene into the chromosome. Conversely, increase of NRG1 levels only in adulthood was sufficient to cause behavioral deficits (Figure 7). These observations demonstrate that behavioral deficits in ctoNrg1 mice not only require continuous NRG1 abnormality, but also can be sufficiently induced in adult mice by abnormal NRG1 expression. These results suggest that relevant schizophrenia may benefit from therapeutic intervention to restore normal NRG1 signaling in adulthood. Second, our studies indicate that NRG1 overexpression impaired neurotransmission at glutamatergic as well as GABAergic synapses. The glutamatergic hypofunction is likely due to impaired glutamate release whereas GABAergic dysfunction is caused by reduction of GABAA receptor (Figure 3). Notably, like behavioral deficits, synaptic dysfunction also requires continuous NRG1 overexpression in adulthood (Figure 3) and glutamatergic hypofunction can be sufficiently induced by adult NRG1 overexpression (Figure 7). These results underscore synaptic dysfunction as a cellular mechanism for behavioral deficits. Third, we show that LIMK1 level and activity in ctoNrg1 synaptosomes correlate with glutamatergic hypofunction (Figure 5). Importantly, impaired glutamate release in ctoNrg1 brain slices requires LIMK1 activity (Figure 6), identifying a novel pathophysiological mechanism and a potential target of intervention.
Both glutamatergic and GABAergic hypofunction has been implicated in schizophrenia (Lewis and Moghaddam, 2006). NRG1 and ErbB4 “APPEAR TO BE CRITICAL FOR” development and maturation of GABAergic circuitry (Abe et al., 2011; Cahill et al., 2012; Fazzari et al., 2010; Flames et al., 2004; Ting et al., 2011). Acutely, NRG1 promotes GABA release by activating ErbB4 in GABAergic neurons (Woo et al., 2007) and thus regulates the firing of excitatory neurons and LTP (Chen et al., 2010; Li et al., 2012; Tan et al., 2012; Wen et al., 2010). However, in ctoNrg1 mice, GABA release was not altered and glutamatergic dysfunction does not appear to require ErbB4 activity. First, ErbB4 level and activity is not altered in ctoNrg1 mice (Figure S6). However, ErbB4 can be activated by acute treatment with exogenous NRG1 (Figure S6B). Second, pharmacological inhibition or genetic mutation of ErbB4, which attenuates NRG1 promotion of GABA release (Figure 4A), had little effect on glutamatergic hypofunction in ctoNrg1 mice (Figures 4B-4D and S7). These observations suggest that NRG1 overexpression may cause glutamatergic dysfunction by mechanisms independent of ErbB4 activation.
The intracellular domain of NRG1 interacts with LIMK1 (Wang et al., 1998), a threonine/serine protein kinase implicated in actin cytoskeletal reorganization. The kinase is localized in both presynaptic terminals and postsynaptic densities (Wang et al., 2000; Yildirim et al., 2008) and has been implicated in the regulation of glutamatergic transmission (Meng et al., 2004; Meng et al., 2002). Intriguingly, LIMK1 and p-cofilin were increased in synaptosomes of ctoNrg1 mice (Figures 5C and 5D) and reduced upon aDox treatment (Figure 5E). LIMK1-cofilin signaling causes F-actin polymerization (Arber et al., 1998; Yang et al., 1998) which has been shown to inhibit glutamate release by preventing vesicle fusion at active zone (Cingolani and Goda, 2008; Morales et al., 2000). Accordingly, some synaptic deficits in ctoNrg1 mice can be attributed to impaired glutamate release: impaired I/O curve, reduced mEPSC frequency, elevated PPF and slower synaptic fatigue (Figures 3B-3D and S4C-S4D); and these phenotypes were attenuated upon NRG1 reduction in aDox-treated ctoNrg1 mice (Figures 3B-3D). Remarkably, acute inhibition of LIMK1 attenuated glutamatergic hypofunction in ctoNrg1 slices, suggesting that LIMK1 activity is necessary (Figure 6). These observations suggest that overexpressed NRG1 may recruit LIMK1 to synapses to alter glutamate release. These observations identify a novel, cell-autonomous mechanism for NRG1 overexpression to cause synaptic dysfunction.
mRNA levels of GABAAR α1 subunit in DLPFC were found to be higher in schizophrenic patients than in control subjects (Impagnatiello et al., 1998; Ohnuma et al., 1999). Recent studies suggested a reduction in mRNA levels of GABAAR α1 subunit in the DLPFC of subjects with schizophrenia (Beneyto et al., 2011; Glausier and Lewis, 2011; Hashimoto et al., 2008). Reasons for this discrepancy are unknown, but have been attributed to difference in subject and control population (age and sex) or post-mortem intervals (Glausier and Lewis, 2011). Nevertheless, we found that GABAAR α1 subunit and mIPSC amplitude were reduced in ctoNrg1 mice (Figures 3E, 3F and S5A). Since mature type I NRG1 is diffusible, the reduction of GABAAR α1 subunit in ctoNrg1 mice might be through activation of ErbB2 or other potential co-receptors in pyramidal neurons. This hypothesis was supported by a previous study that long-term treatment of hippocampal slices with NRG1 decreases GABAAR α1 subunit levels (Okada and Corfas, 2004). Alternatively, the reduction could be due to a homeostatic mechanism in pyramidal neurons, which might decrease inhibition response to compensate for glutamatergic hypofunction (Figures 3B-3D and S4D). The reduction of mIPSC amplitudes in ctoNrg1 mice was diminished after aDox treatment (Figure 3E) and notably, mIPSC amplitudes were similar between dDox-treated control and ctoNrg1 mice (Figure 7G). These results suggest that adult overexpression of NRG1 is required to maintain, but not sufficient to cause the reduction of mIPSC amplitudes.
Our work corroborates recent findings that transgenic mice expressing NRG1 under the control of Thy-1 and EF-1α promoters exhibit similar, but not identical, behavioral deficits (Deakin et al., 2009; Deakin et al., 2012; Kato et al., 2010). For example, tremor and impaired motor skill were observed in Thy1-NRG1 transgenic mice (Deakin et al., 2009) but not in ctoNrg1 mice (Figure S1A). EF-1α-NRG1 mice, where NRG1 was expressed in all cells in the body, albeit hyperactive, were normal in PPI test (Kato et al., 2010). The differences of these two strains and between them and ctoNrg1 mice may be due to variation in expression patterns and levels of NRG1 transgene (for example hundreds folds above normal in Thy-1-NRG1 mice) (Deakin et al., 2009). Nevertheless, these observations revealed pathogenic effects of NRG1 overexpression in mice during development. Our study further demonstrates that these pathogenic effects can be repaired in adult mice and raise a possibility that relevant schizophrenia may benefit from therapeutic approaches to restore NRG1 signaling.
Experimental procedures
Generation of ctoNrg1 mice
NRG1Iβ1a (kindly provided by Dr. Douglas Falls, Emory University) was cloned into the EcoR V site of pMM400 (kindly provided by Dr. Joe Tsien, Georgia Regents University). An HA tag was inserted between Ig and EGF domains. A Not I fragment containing the transgene was used for transgenic mouse production. C57BL/6-CBA(J) F2 founders (i.e., TRE-Nrg1) were identified by PCR with primers (forward atctggtcagaaaagacaat, in pMM400; reverse tgagtgaggagtactcgg in NRG1Iβ1a). TRE-Nrg1 mice were backcrossed with C57BL/6N mice for >5 generations before crossing with CamK2α-tTA mice (Jackson Lab, stock number 007004). Animals were housed in rooms at 23 °C in a 12 h light/dark cycle and with food and water available ad libitum. In some experiments, Dox (Sigma-Aldrich, catalog number D9891) was added to drinking water at 1 mg/ml in 2.5% sucrose. Animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Georgia Regents University.
Immunohistochemical and stereological analysis
Anesthetized mice were perfused transcardially with 4% paraformaldehyde (PFA) in PBS and tissues were fixed in 4% PFA at 4 °C for 5 h. Frozen brain blocks were cut into 40-μm-thick sections on a vibrating microtome (VT1000S; Leica Microsystem). Sections were permeabilized with 0.3% Triton-X 100 and 5% BSA in PBS and incubated with primary antibodies at 4 °C overnight. After washing with PBS for 3 times, samples were incubated with Alexa Fluor-conjugated secondary antibodies (1:1000, Invitrogen) for 1 h at room temperature. Samples were mounted with Vectashield mounting medium (Vector) and images were taken by Zeiss LSM510 confocal microscope. Primary antibodies were prepared in PBS containing 0.3% Triton X-100 by the following dilutions: mouse anti-NeuN (1:1000, Millipore), rabbit anti-PV (1:1000, Swant), mouse anti-HA (1:200, Covance). For stereological analysis, brain blocks were cut transversely into 50-μm-thick serial sections. Slices for entire dorsal hippocampus were collected. Every 3rd section, total 8, was subjected to immunofluorescent staining of NeuN and PV. Unbiased stereological analysis of total neurons, PV-positive GABAergic interneurons was carried out with the optical fractionator technique by using StereoInvestigator software, as previously described (Stranahan et al., 2012).
Behavior analysis
To eliminate possible effects that may be associated with the insertion of transgenes, TRE-Nrg1 and Camk2α-tTA mice were used as controls in behavioral analysis as described previously (Kellendonk et al., 2006). Behavioral tests were performed in 3 ~ 4-months old, male mice, except social interaction where females were used. The investigators for behavioral tests were blind to genotypes and/or Dox administration. Both control and ctoNrg1 mice were treated with Dox to avoid possible compounding effects of Dox on behaviors (Kellendonk et al., 2006; Mayford et al., 1996). Mice were not tested for same behavioral paradigms more than once, to avoid effects of learning and memory (Kellendonk et al., 2006).
Statistic analysis
Two-way ANOVA was used in behavioral analysis including PPI, rotarod, 8-ARM, social interaction, training in MWM, and effects of clozapine and electrophysiological studies including I/O curve, PPR and synaptic fatigue. One-way ANOVA was used for analysis of data from three or more groups. Student's t-test was used to compare data from two groups. Data were expressed as mean ± SEM unless otherwise indicated.
Supplementary Material
Article highlights.
- Mice modeling abnormal NRG1 level in patients show schizophrenia-like deficits.
- NRG1 overexpression causes synaptic dysfunction possibly by activating LIMK1.
- Deficits are alleviated when NRG1 expression is returned to normal in adult animals.
- Schizophrenia may benefit from therapeutic intervention to restore NRG1 signaling.
ETOC/In Brief paragraph.
Yin et al. demonstrate that NRG1 overexpression in adulthood, seen in schizophrenia patients, is critical for behavioral and synaptic deficits in mice. This study suggests that relevant schizophrenia may benefit from therapeutic intervention to restore NRG1 signaling.
Acknowledgment
This work is supported in part by grants from NARSAD and National Institute of Mental Health, NIH. Dr. Lin Mei is a NARSAD Distinguished Investigator. Dr. Dong-Min Yin and Dr.Yi-Sheng Lu are NARSAD Young Investigators. We are grateful to Dr. Douglas Falls for original NRG1 constructs, Dr. Joe Tsien for the pMM400 plasmid, Dr. Richard Huganir for antibodies against GluR1, GluR2/3, NR2A and NR2B, and Dr. Cary Lai for the anti-ErbB4 antibody. We thank Dr. Kathleen Yee and Dr. Martin Gassmann for providing ErbB4 null mutant mice, Dr. Alvin Terry and Dr. Patrick Callahan of the Small Animal Behavioral Core at GRU for assistance in behavioral analysis, and Dr. Alexis Stranaha for advice and assistance in stereology analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declared that they have no conflict of interest.
References
- Abe Y, Namba H, Kato T, Iwakura Y, Nawa H. Neuregulin-1 signals from the periphery regulate AMPA receptor sensitivity and expression in GABAergic interneurons in developing neocortex. J Neurosci. 2011;31:5699–5709. doi: 10.1523/JNEUROSCI.3477-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci. 2001;4:367–373. doi: 10.1038/86011. [DOI] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- Barros CS, Calabrese B, Chamero P, Roberts AJ, Korzus E, Lloyd K, Stowers L, Mayford M, Halpain S, Muller U. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci U S A. 2009;106:4507–4512. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABA(A) receptor subunit expression in schizophrenia. Cereb Cortex. 2011;21:999–1011. doi: 10.1093/cercor/bhq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram I, Bernstein HG, Lendeckel U, Bukowska A, Dobrowolny H, Keilhoff G, Kanakis D, Mawrin C, Bielau H, Falkai P, et al. Immunohistochemical evidence for impaired neuregulin-1 signaling in the prefrontal cortex in schizophrenia and in unipolar depression. Ann N Y Acad Sci. 2007;1096:147–156. doi: 10.1196/annals.1397.080. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, Sigmundsson A, Firth DR, Nielsen B, Stefansdottir R, et al. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/- knock-outs compared with wild-type mice. J Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Muller T, Wende H, Stassart RM, Nawaz S, Humml C, Velanac V, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill ME, Jones KA, Rafalovich I, Xie Z, Barros CS, Muller U, Penzes P. Control of interneuron dendritic growth through NRG1/erbB4-mediated kalirin-7 disinhibition. Mol Psychiatry. 2012;17(1):99–107. doi: 10.1038/mp.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Fischbach GD. An acute effect of neuregulin 1 beta to suppress alpha 7-containing nicotinic acetylcholine receptors in hippocampal interneurons. J Neurosci. 2006;26:11295–11303. doi: 10.1523/JNEUROSCI.1794-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Johnson MA, Lieberman MD, Goodchild RE, Schobel S, Lewandowski N, Rosoklija G, Liu RC, Gingrich JA, Small S, et al. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008;28:6872–6883. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Zhang M, Yin DM, Wen L, Ting A, Wang P, Lu YS, Zhu XH, Li SJ, Wu CY, et al. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci U S A. 2010;107:21818–21823. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong VZ, Thompson M, Beltaifa S, Webster MJ, Law AJ, Weickert CS. Elevated neuregulin-1 and ErbB4 protein in the prefrontal cortex of schizophrenic patients. Schizophr Res. 2008;100:270–280. doi: 10.1016/j.schres.2007.12.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophr Res. 1995;17:5–13. doi: 10.1016/0920-9964(95)00024-g. [DOI] [PubMed] [Google Scholar]
- Deakin IH, Law AJ, Oliver PL, Schwab MH, Nave KA, Harrison PJ, Bannerman DM. Behavioural characterization of neuregulin 1 type I overexpressing transgenic mice. Neuroreport. 2009;20:1523–1528. doi: 10.1097/WNR.0b013e328330f6e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin IH, Nissen W, Law AJ, Lane T, Kanso R, Schwab MH, Nave KA, Lamsa KP, Paulsen O, Bannerman DM, et al. Transgenic overexpression of the type I isoform of neuregulin 1 affects working memory and hippocampal oscillations but not long-term potentiation. Cereb Cortex. 2012;22:1520–1529. doi: 10.1093/cercor/bhr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, Lerma J, Marin O, Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marin O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Pisacane P, Erickson S. Heregulin, but not ErbB2 or ErbB3, heterozygous mutant mice exhibit hyperactivity in multiple behavioral tasks. Behav Brain Res. 2000;109:219–227. doi: 10.1016/s0166-4328(99)00175-8. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABA(A) receptor alpha1 subunit messenger RNA expression in schizophrenia. Neuropsychopharmacology. 2011;36:2103–2110. doi: 10.1038/npp.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Chub N, Wenner P. NKCC1 and AE3 appear to accumulate chloride in embryonic motoneurons. J Neurophysiol. 2009;101:507–518. doi: 10.1152/jn.90986.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J Neurosci. 2005;25:4974–4984. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR. Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry. 2004;9:299–307. doi: 10.1038/sj.mp.4001434. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges H. Maze procedures: the radial-arm and water maze compared. Brain Res Cogn Brain Res. 1996;3:167–181. doi: 10.1016/0926-6410(96)00004-3. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, Uzunov DP, Smalheiser NR, Davis JM, Pandey GN, et al. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Kasai A, Mizuno M, Fengyi L, Shintani N, Maeda S, Yokoyama M, Ozaki M, Nawa H. Phenotypic characterization of transgenic mice overexpressing neuregulin-1. PLoS One. 2010;5:e14185. doi: 10.1371/journal.pone.0014185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Krakowski MI, Czobor P, Citrome L, Bark N, Cooper TB. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63:622–629. doi: 10.1001/archpsyc.63.6.622. [DOI] [PubMed] [Google Scholar]
- Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Harrison PJ, Kleinman JE, Weinberger DR. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc Natl Acad Sci U S A. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KX, Lu YM, Xu ZH, Zhang J, Zhu JM, Zhang JM, Cao SX, Chen XJ, Chen Z, Luo JH, et al. Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat Neurosci. 2012;15:267–273. doi: 10.1038/nn.3006. [DOI] [PubMed] [Google Scholar]
- Liu X, Bates R, Yin DM, Shen C, Wang F, Su N, Kirov SA, Luo Y, Wang JZ, Xiong WC, et al. Specific regulation of NRG1 isoform expression by neuronal activity. J Neurosci. 2011;31:8491–8501. doi: 10.1523/JNEUROSCI.5317-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Takahashi H, Meng J, Zhang Y, Lu G, Asrar S, Nakamura T, Jia Z. Regulation of ADF/cofilin phosphorylation and synaptic function by LIM-kinase. Neuropharmacology. 2004;47:746–754. doi: 10.1016/j.neuropharm.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, Janus C, Cruz L, Jackson M, Lu WY, MacDonald JF, Wang JY, Falls DL, et al. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron. 2002;35:121–133. doi: 10.1016/s0896-6273(02)00758-4. [DOI] [PubMed] [Google Scholar]
- Morales M, Colicos MA, Goda Y. Actin-dependent regulation of neurotransmitter release at central synapses. Neuron. 2000;27:539–550. doi: 10.1016/s0896-6273(00)00064-7. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Murray C, Lopez A. The Global Burden of Disease. Harvard School of Public Health; 1996. [Google Scholar]
- O'Tuathaigh CM, Babovic D, O'Sullivan GJ, Clifford JJ, Tighe O, Croke DT, Harvey R, Waddington JL. Phenotypic characterization of spatial cognition and social behavior in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007;147:18–27. doi: 10.1016/j.neuroscience.2007.03.051. [DOI] [PubMed] [Google Scholar]
- Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABA(A) receptor alpha-1 subunit messenger RNA and human GABA transporter-1 (HGAT-1) messenger RNA expression. Neuroscience. 1999;93:441–448. doi: 10.1016/s0306-4522(99)00189-x. [DOI] [PubMed] [Google Scholar]
- Okada M, Corfas G. Neuregulin1 downregulates postsynaptic GABAA receptors at the hippocampal inhibitory synapse. Hippocampus. 2004;14:337–344. doi: 10.1002/hipo.10185. [DOI] [PubMed] [Google Scholar]
- Parlapani E, Schmitt A, Wirths O, Bauer M, Sommer C, Rueb U, Skowronek MH, Treutlein J, Petroianu GA, Rietschel M, et al. Gene expression of neuregulin-1 isoforms in different brain regions of elderly schizophrenia patients. World J Biol Psychiatry. 2010;11:243–250. doi: 10.3109/15622970802022376. [DOI] [PubMed] [Google Scholar]
- Petryshen TL, Middleton FA, Kirby A, Aldinger KA, Purcell S, Tahl AR, Morley CP, McGann L, Gentile KL, Rockwell GN, et al. Support for involvement of neuregulin 1 in schizophrenia pathophysiology. Mol Psychiatry. 2005;10:366–374. 328. doi: 10.1038/sj.mp.4001608. [DOI] [PubMed] [Google Scholar]
- Pitcher GM, Beggs S, Woo RS, Mei L, Salter MW. ErbB4 is a suppressor of long-term potentiation in the adult hippocampus. Neuroreport. 2008;19:139–143. doi: 10.1097/WNR.0b013e3282f3da10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher GM, Kalia LV, Ng D, Goodfellow NM, Yee KT, Lambe EK, Salter MW. Schizophrenia susceptibility pathway neuregulin 1-ErbB4 suppresses Src upregulation of NMDA receptors. Nat Med. 2011;17:470–478. doi: 10.1038/nm.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimer M, Barrett DW, Maldonado MA, Vock VM, Gonzalez-Lima F. Neuregulin-1 immunoglobulin-like domain mutant mice: clozapine sensitivity and impaired latent inhibition. Neuroreport. 2005;16:271–275. doi: 10.1097/00001756-200502280-00014. [DOI] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I, Dudbridge F, Holmans PA, Whittemore AS, Mowry BJ, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SH. Amphetamine psychosis: a “model” schizophrenia mediated by catecholamines. Am J Psychiatry. 1973;130:61–67. doi: 10.1176/ajp.130.1.61. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sarginson J, Kong A, Yates P, Steinthorsdottir V, Gudfinnsson E, Gunnarsdottir S, Walker N, Petursson H, Crombie C, et al. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet. 2003;72:83–87. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Jiam NT, Spiegel AM, Gallagher M. Aging reduces total neuron number in the dorsal component of the rodent prefrontal cortex. J Comp Neurol. 2012;520:1318–1326. doi: 10.1002/cne.22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GH, Liu YY, Hu XL, Yin DM, Mei L, Xiong ZQ. Neuregulin 1 represses limbic epileptogenesis through ErbB4 in parvalbumin-expressing interneurons. Nat Neurosci. 2012;15:258–266. doi: 10.1038/nn.3005. [DOI] [PubMed] [Google Scholar]
- Ting AK, Chen Y, Wen L, Yin DM, Shen C, Tao Y, Liu X, Xiong WC, Mei L. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci. 2011;31:15–25. doi: 10.1523/JNEUROSCI.2538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Frenzel KE, Wen D, Falls DL. Transmembrane neuregulins interact with LIM kinase 1, a cytoplasmic protein kinase implicated in development of visuospatial cognition. J Biol Chem. 1998;273:20525–20534. doi: 10.1074/jbc.273.32.20525. [DOI] [PubMed] [Google Scholar]
- Wang JY, Miller SJ, Falls DL. The N-terminal region of neuregulin isoforms determines the accumulation of cell surface and released neuregulin ectodomain. J Biol Chem. 2001;276:2841–2851. doi: 10.1074/jbc.M005700200. [DOI] [PubMed] [Google Scholar]
- Wang JY, Wigston DJ, Rees HD, Levey AI, Falls DL. LIM kinase 1 accumulates in presynaptic terminals during synapse maturation. J Comp Neurol. 2000;416:319–334. doi: 10.1002/(sici)1096-9861(20000117)416:3<319::aid-cne4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Tiwari Y, Schofield PR, Mowry BJ, Fullerton JM. Schizophrenia-associated HapICE haplotype is associated with increased NRG1 type III expression and high nucleotide diversity. Transl Psychiatry. 2012;2:e104. doi: 10.1038/tp.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, Yin DM, Lai C, Terry AV, Jr., Vazdarjanova A, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong XP, Wu J, Gassmann M, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Yang JZ, Si TM, Ruan Y, Ling YS, Han YH, Wang XL, Zhou M, Zhang HY, Kong QM, Liu C, et al. Association study of neuregulin 1 gene with schizophrenia. Mol Psychiatry. 2003;8:706–709. doi: 10.1038/sj.mp.4001377. [DOI] [PubMed] [Google Scholar]
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- Yildirim M, Janssen WG, Tabori NE, Adams MM, Yuen GS, Akama KT, McEwen BS, Milner TA, Morrison JH. Estrogen and aging affect synaptic distribution of phosphorylated LIM kinase (pLIMK) in CA1 region of female rat hippocampus. Neuroscience. 2008;152:360–370. doi: 10.1016/j.neuroscience.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YD, Lee S, Jin Z, Wright M, Smith SE, Anderson MP. Arrested maturation of excitatory synapses in autosomal dominant lateral temporal lobe epilepsy. Nat Med. 2009;15:1208–1214. doi: 10.1038/nm.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.