Abstract
Fractures related to osteoporosis are associated with $20 billion in cost in the United States, with the majority of cost born by federal health-care programs, such as Medicare and Medicaid. Despite the proven fracture reduction benefits of several osteoporosis treatments, less than one-quarter of patients older than 65 years of age who fracture receive such care. A postfracture liaison service (FLS) has been developed in many health systems but has not been widely implemented in the United States. We developed a Markov state-transition computer simulation model to assess the cost-effectiveness of an FLS using a health-care system perspective. Using the model, we projected the lifetime costs and benefits of FLS, with or without a bone mineral density test, in men and women who had experienced a hip fracture. We estimated the costs and benefits of an FLS, the probabilities of refracture while on osteoporosis treatment, as well as the utilities associated with various health states from published literature. We used multi-way sensitivity analyses to examine impact of uncertainty in input parameters on cost-effectiveness of FLS. The model estimates that an FLS would result in 153 fewer fractures (109 hip, 5 wrist, 21 spine, 17 other), 37.43 more quality-adjusted life years (QALYs), and save $66,879 compared with typical postfracture care per every 10,000 postfracture patients. Doubling the cost of the FLS resulted in an incremental cost-effectiveness ratio (ICER) of $22,993 per QALY. The sensitivity analyses showed that results were robust to plausible ranges of input parameters; assuming the least favorable values of each of the major input parameters results in an ICER of $112,877 per QALY. An FLS targeting patients post-hip fracture should result in cost savings and reduced fractures under most scenarios.
Keywords: OSTEOPOROSIS, COST-EFFECTIVENESS, FRACTURE LIAISON SERVICE, HIP FRACTURE
Introduction
The cost of the Medicare program is projected to grow approximately 50% from 2012 to 2020.(1) Although these cost increases may be contained under various proposed policies, the growth of the 65 years and older population will translate into greater cost containment pressures on Medicare. Concurrent with these forces is the growth of accountability and quality measures in health care. Providers and health systems have numerous reporting requirements, assessing performance on an increasing number of quality measures, including postfracture care. Even though postfracture care measures are included in most reporting programs, postfracture treatment rates in the Medicare population are less than 25%.(2)
The reasons for poor performance on postfracture treatment quality measures are complex and vary by health-care setting, but the fragmented nature of postfracture care contributes to this problem in most health-care delivery systems in the United States. Emergency department physicians often diagnose patients who sustain fractures, and orthopaedic surgeons treat the fractures. However, after the acute fracture management, a minority of patients undergo a diagnostic work-up for osteoporosis or receive osteoporosis treatment.(2) Although long-term safety concerns raise caution about bisphosphonates,(3) strong evidence demonstrates their beneficial effects on reducing subsequent fractures by approximately 50%, at least in the first 5 years of treatment.(4) Several health systems have developed postfracture care programs aimed at improving the diagnosis and treatment of osteoporosis.(5,6) These programs identify postfracture patients, triage them to a fracture liaison service (FLS), and ensure appropriate work-up and treatment. The basic elements of such a program consist of an allied health professional under the supervision of a physician who works to ensure diagnosis, indicated treatments, fall prevention education, and appropriate use of calcium and vitamin D. Although approximately 10% of patients who experience a hip fracture are on osteoporosis treatment at the time of fracture,(7) these FLS programs have been associated with postfracture treatment rates much higher than the typical rates.(5,6)
Although several analyses suggest that an FLS program may have favorable economics, (5,6,8) they have not been subjected to a formal cost-effectiveness analysis from the perspective of the US health-care system.
Materials and Methods
Overview
We constructed a state-transition patient-level micro-simulation to project fracture outcomes, life expectancy, and osteoporosis-related costs among patients with an index hip fracture treated under one of three strategies: 1) usual care; 2) universal FLS; and 3) targeted FLS, including bone mineral density (BMD) testing by dual-energy X-ray absorptiometry (DXA) followed by FLS for those with osteopenia or osteoporosis. The model took a health-care systems perspective, including all health-care costs.(9) We projected cost-effectiveness of universal and targeted FLS over a lifetime horizon. There is uncertainty around several model inputs; thus, base case assumptions were set to bias against an FLS, such that its costs would be high and effectiveness low.
Model structure
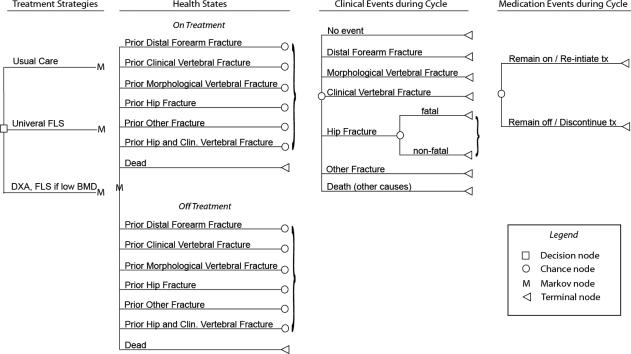
This model, adapted from a previously validated model(10) (Fig. 1), simulates the progression of patients as they move through a series of states describing health status (acute fracture, post-hip fracture, post-distal forearm fracture, post-clinical vertebral fracture, post-morphological vertebral fracture, post-other fracture, and dead) and treatment with generic alendronate. Movement between these states is governed by a series of parameters describing probabilities of fractures, mortality, and treatment discontinuation and reinitiation. Transitions are modeled as occurring at the end of each 3-month cycle. All costs were taken from 2010 estimates. Our primary analysis was conducted among patients with an index hip fracture; in a sensitivity analysis, we evaluated a population with a mixture of index fractures types.
Fig. 1.
Model structure. Patients enter the model with a prior hip fracture (primary analysis) or distributed between prior fracture types (secondary analysis), with the percent “on treatment” determined by treatment strategy. In each simulated 3-month cycle, patients may experience no clinical event, a fracture, or death. Patients who remain alive and have been on treatment can remain on treatment or discontinue treatment; patients who have been off treatment can remain off treatment or reinitiate treatment. Patients begin the next cycle in a state determined by their clinical event history and most recent medication events. The process is repeated until all patients are dead.
The outcomes of the model include estimates of life expectancy, quality-adjusted life expectancy expressed in quality-adjusted life years (QALY), and osteoporosis-related costs. A QALY is a measure of disease burden that includes the duration and quality of life, where quality is measured by utilities (see below). Incremental cost-effectiveness ratios (ICERs) for the FLS-based strategies (with or without BMD testing) versus usual care are calculated as a difference in lifetime costs divided by a difference in lifetime QALYs. Future costs and life years are discounted at an annual rate of 3%. Discounting refers to the reduction in value for future events. All analyses were conducted with Data TreeAge Pro (TreeAge, Williamstown, MA, USA).
Model inputs
Rates of treatment discontinuation and reinitiation were derived from an analysis of insurance claims data.(11) The cost of the FLS program was derived from the experience of another US FLS program (personal communication, Rick Dell; 2013). Remaining model inputs were derived from the published literature describe below (Table 1).
Table 1.
Model Parameter Estimates
| Base case value | Range | Source | |
|---|---|---|---|
| Transition probabilities | |||
| Mortality | |||
| All-cause mortality rate, per 100 person-yearsa | 5.00 | N/A | US life tables(57) |
| Increase in mortality during 6 months after hip fracture | 6.28 times all-cause mortality rate | 4.9–6.7 | Tosteson(26) |
| Refracture rates, per 100 person-yearsa | ±10% | Johnell,(16) Melton(17) | |
| Hip fracture | 1.8 | ||
| Clinical vertebral fracture | 1.4 | ||
| Distal forearm fracture | 0.6 | ||
| Other fracture | 2.9 | ||
| Any fracture | 8.8 | ||
| Effect of bisphosphonates on fracture ratesb | |||
| Vertebral fracture, men | 0.36 | 0.17–0.77 | Sawka(19) and Lyles(49) |
| Nonvertebral fracture, men | 0.73 | 0.32–1.67 | Sawka(19) and Lyles(49) |
| Vertebral fracture, women | 0.55 | 0.43–0.69 | Wells(4) and Lyles(49) |
| Hip fracture, women | 0.47 | 0.26–0.85 | Wells(4) and Lyles(49) |
| Nonvertebral, non-hip fracture, women | 0.77 | 0.64–0.92 | Wells(4) and Lyles(49) |
| Adherence | |||
| Probability of bisphosphonate discontinuation, per 90-day interval | 32% 1st 90 days after initiation, declining to 9% after 2 years | PACE data (see text) | |
| Probability of bisphosphonate reinitiation, per 90-day interval | 38% 1st 90 days after discontinuation, declining to 2% after 2 years | PACE data (see text) | |
| Costs | |||
| Fracture liaison service | $105 | to $205 | |
| DXA | $100 | ||
| Bisphosphonate treatment, annual | $84 | to $250 | Red book, Drugstore.com(32) |
| Acute event costsa | |||
| Hip fracture | |||
| Inpatient | $29,488 | $26,338 | Burge,(12) Gabriel(34) |
| Long-term care (year of fracture) | $14,628 | $12,488 | Burge,(12) Leibson,(35) Genworth Financial(37) |
| Clinical vertebral fracture | $2122 | $10,772 | Burge,(12) Gabriel(34) |
| Morphologic vertebral fracture | $0 | $0 | Assumption |
| Distal forearm | $1984 | $5847 | Burge,(12) Gabriel(34) |
| Other fracture | $4682 | $8465 | Burge,(12) Gabriel(34) |
| Ongoing, postfracture costs (annual)a | |||
| Hip fracture | $4705 | $8906 | Burge,(12) Leibson,(35) Genworth Financial(37) |
| Vertebral, distal forearm, other fractures | $0 | $0 | Assumption |
| Quality of life weights (utilities) | |||
| Disutilities associated with prior fracture | |||
| Morphologic vertebral fracture—1st year | 0.126 | Oleksik(40) | |
| Years 1–6 | 0.06 | Oleksik(40) | |
| Years >6 | 0 | Oleksik(40) | |
| Distal forearm fracture—1st year | 0.06 | Borgstrom(38) | |
| Subsequent years | 0.001 | Borgstrom(38) | |
| Other fracture | 0.073 | Kanis(39) | |
| Subsequent years | 0.023 | Kanis(39) | |
| Clinical vertebral fracture | 0.23 | Borgstrom(38) | |
| Subsequent years | 0.064 | Borgstrom(38) | |
| Hip fracture | 0.17 | Borgstrom(38) | |
| Subsequent years | 0.131 | Borgstrom(38) | |
| Clinical vertebral and hip fracture—1st year | 0.36 | Tosteson(41) | |
| Subsequent years | 0.2 | Tosteson(41) |
Vary by age and sex. Values presented are those for women ages 80 years, the mean age of patients presenting with an index fracture.
Bisphosphonate effects apply to patients with osteopenia and osteoporosis. Patients with normal BMD are assumed to derive no benefit.
Characteristics of FLS
In the base case analysis, we assumed the FLS is offered to all patients hospitalized with an index hip fracture. We assumed 75% of postfracture patients are female with an age distribution similar to that reported in several large studies of patients with an index fracture.(12–14) We assumed 80% have low BMD—osteopenia or osteoporosis—based on data from a Canadian postfracture study.(15)
The universal FLS strategy assumed that all patients receive care, whereas the targeted DXA strategy assumed FLS follow-up only for patients with low BMD. We assumed the FLS is staffed by a nurse practitioner, who sees each patient for an average of three half-hour sessions and spends an additional 15 minutes per patient on administrative activities. (personal communication, Rick Dell; 2013). However, because such a nurse practitioner may not work at full capacity, we varied this assumption by doubling the amount of time that she or he may spend with patients. In addition, we estimate that 1 in 5 patients requires an extra moderate-complexity physician visit that may not be required in usual care (personal communication, Rick Dell; 2013). Rates of bisphosphonate initiation are estimated at 21% under usual care.(2) Based on data from several FLS quality-improvement programs in the US that have observed treatment rates between 40% and 80%,(5,6) we made the assumption that 42% (doubling) would receive a bisphosphonate in the base case FLS assumption; higher rates of treatment initiation in the FLS program, 66% and 88%, were evaluated in sensitivity analyses. We assumed bisphosphonate treatment is prescribed for 5 years but incorporated nonadherence, such that 58% of patients are adherent at 1 year, 50% at 2 years, and 43% at 5 years, based on the results of a primary data analysis described below.(11)
Refracture rates
Rates of refracture across age strata, sex, and index fracture type are not well described in the literature. We derived relative risks of fracture for subjects with an index fracture compared with the general population from a large Swedish cohort study.(16) These relative risks were then applied to a general community-dwelling elderly population by age, sex, and anatomic fracture site using data from the Rochester Epidemiologic Project Study(17) and previously described methods.(18)
Effect of bisphosphonate treatment on fracture outcomes
Based on a meta-analysis of randomized controlled trials of alendronate in women, bisphosphonate therapy was assumed to reduce the rate of vertebral fracture by 45%, hip fracture by 53%, and nonvertebral, non-hip fracture by 23% for women under full adherence.(4) A 64% vertebral fracture rate reduction and 27% nonvertebral rate reduction were assumed for men based on data from the two published RCTs of alendronate in men.(19) We applied the same relative risk reductions to patients with osteoporosis and osteopenia and assumed no benefit of bisphosphonate treatment in patients with normal BMD.(16,20) The fracture reduction benefit was assumed to begin 3 months after the start of treatment, persist through the fifth year of treatment, and then decline linearly to no effect at 10 years.(21,22) For patients discontinuing treatment before the end of 5 years, we assumed that the length of the treatment offset period was equal to the duration of treatment, based on data suggesting that the rate of bone loss after treatment discontinuation is dependent on the duration of therapy.(23)
Patterns of bisphosphonate use
Probabilities of bisphosphonate discontinuation and reinitiation under usual care were estimated from a cohort of patients aged 65 years or older dually enrolled in Medicare and a Pennsylvania pharmaceutical benefit program for the elderly.(11) The model accounts for reinitiation based on prior work that found 30% of patients will restart osteoporosis treatments during the 6 months after stopping treatment.(24) Estimates of bisphosphonate adherence derived from this population are similar to those reported in the literature for patients initiating bisphosphonate treatment in other settings.(25) These estimates were applied to the study cohort.
Mortality rates
Background mortality rates by age and sex were obtained from current US life tables. Mortality during the 6 months after a hip fracture was assumed to be 6.28 times the background rate.(26) In the primary analysis, no excess mortality was assumed beyond 6 months or after fractures at other anatomic sites.(26,27) We conducted sensitivity analyses where we included a 23%(28) and 90%(29) increased risk of mortality in remaining years of life after vertebral and hip fractures, respectively. In addition, we varied the short-term increase in mortality after hip fracture from a 4.9-fold to 6.7-fold increase.(30)
Costs
Based on a physician visit cost of $104(31) and national average annual compensation of $99,439 in wage plus benefits for a registered nurse, our base case FLS cost was $105 per patient. This was increased to $205 in sensitivity analyses. The cost of a DXA was assumed to be $56.40 after current Medicare reimbursement.
We used retail pharmacy data to estimate the cost of bisphosphonate treatment.(32,33) In the base case analysis, we assumed that treatment would consist of 70 mg of generic alendronate taken on a weekly basis. The direct medical costs associated with fractures by age and clinical site were obtained from a recent study of fracture-related costs in the United States.(12) For hip fractures, ongoing costs associated with nursing home placement were included. In a sensitivity analysis, an alternative set of cost estimates were applied. (34–37) In the primary analysis, we excluded medical costs arising in added years of life; these were included in a sensitivity analysis.
All costs are expressed in 2010 US dollar values. Where necessary, older estimates were inflated to current dollar values using the Medical Care Component of the Consumer Price Index.
Quality of life adjustment (utilities)
Quality of life adjustments are incorporated through utilities, which vary from 1.0 (perfect health) to 0 (death). Utility values for postfracture states (Table 1) were derived from the published literature.(38–41) In a sensitivity analysis, we included a bisphosphonate treatment disutility based on reported rates of gastrointestinal and bone adverse effects and reported disutilities associated with these effects. In this analysis, bisphosphonate treatment was associated with an increased incidence of dyspepsia (8%),(42) osteonecrosis of the jaw (ONJ, 0.02%),(43) and atypical femur fractures (0.039%).(44) Atypical femur fractures were incorporated by increasing hip fracture rates under bisphosphonate treatment. Dyspepsia was assigned a disutility of 0.08 and an annual cost of $57 based on the cost of 20 mg generic omeprazole taken once a week, and ONJ was assigned a onetime cost of $1546 and a disutility of 0.13, based on weighting stage-specific disutilities reported in the literature.(45–47)
Model validation
The model was validated against external estimates of fracture risk and medication adherence (Supplemental Material).
Sensitivity analyses
A series of one-way sensitivity analyses, examining one variable at a time, around the characteristics of the FLS and other model parameters was conducted (Table 1). We increased the FLS cost to $205, increased treatment initiation rates under the FLS to 66% and 88%, and broadened the patient population to include patients with vertebral and nonvertebral non-hip fractures. In addition, we increased the bisphosphonate treatment cost to $250 to reflect a mix of branded and generic formulations that was observed for a typical cohort of patients (personal communication, John Schousboe; 2013). To account for the uncertainty in refracture rates, we varied them by 10% around the base case values, and the relative benefits of treatment were varied based on a more recent trial of IV zoledronic acid among post-hip fracture patients.(48,49)
We conducted a threshold cost analysis for annual IV zoledronic acid, assuming 100% adherence. The cost of zoledronic acid has begun to decrease after its patent expiration date in May 2013. We tested different annual costs to determine the thresholds where use of zoledronic acid has 1) ICERs less than $100,000 per QALY and 2) ICERs that demonstrate cost savings compared with generic alendronate.
Finally, we conducted a multi-way sensitivity analysis varying all assumptions to values least favorable for an FLS.
Results
Over the remaining life span (mean 8.6 years) of 10,000 men and women who had experienced an index hip fracture and received usual care, the model projected 5579 subsequent fractures: 1958 hip fractures, 453 distal forearm fractures, 998 vertebral fractures, and 2170 other fractures. Implementation of a universal FLS was projected to reduce rates of subsequent hip fracture by 109 per 10,000. In addition, we projected 21 fewer spine fractures, 5 fewer distal forearm fractures, and 17 fewer other osteoporotic fractures. This reduction in fracture rate was associated with a $66,879 reduction in costs and an increase in quality adjusted life expectancy (QALE) of 37.4 years. Because we modeled bisphosphonate treatment as having no effect among patients with a normal BMD, the two FLS strategies evaluated—universal follow-up by an FLS and BMD testing by DXA followed by FLS for those with osteopenia or osteoporosis—were identical in efficacy. Under base case assumptions, the targeted FLS strategy with DXA component was more costly and therefore dominated by universal FLS program.
Several variables were assessed in one-way and multi-way sensitivity analyses (Table 2). Increasing the FLS cost estimate by $100, from the base case of $105 to $205, resulted in an ICER of $22,993 per QALY for the FLS program compared with usual care. Similarly, increasing the annual cost of osteoporosis treatment from the base case of $84 to $250 resulted in an ICER of $14,513 per QALY. When both costs were set at the higher values, the DXA strategy became less costly than universal follow-up through an FLS, with an ICER compared with usual care of $37,729 per QALY. In a “worst case” analysis, where the FLS cost was increased to $205 per participant, the bisphosphonate cost was increased to $250 per year, bisphosphonate side effects were incorporated, bisphosphonate efficacy was reduced, fracture rates were reduced by 10%, and alternate fracture cost estimates were used, the targeted FLS with DXA strategy dominated universal FLS and had an ICER of $112,877 per QALY relative to usual care.
Table 2.
Base Case and Sensitivity Analyses
| Usual care |
Fracture liaison service (FLS)a |
FLS versus usual care |
|||||
|---|---|---|---|---|---|---|---|
| Cost | QALE | Cost | QALE | Δ Cost | Δ QALE | ICER | |
| Base case | $40,810 | 4.032 | $40,803 | 4.036 | $-7 | 0.004 | Cost-saving |
| One-way sensitivity analyses | |||||||
| FLS cost increased to $205 | $40,810 | 4.032 | $40,896 | 4.036 | $86 | 0.004 | $22,993 |
| FLS bisphosphonate initiation rate increased to 66% | $40,810 | 4.032 | $40,670 | 4.040 | $-140 | 0.008 | Cost-saving |
| FLS bisphosphonate initiation rate increased to 88% | $40,810 | 4.032 | $40,555 | 4.044 | $-255 | 0.012 | Cost-saving |
| Osteoporosis medication cost increased to $250 | $40,871 | 4.032 | $40,925 | 4.036 | $54 | 0.004 | $14,513 |
| Patients with non-hip fractures included | $19,203 | 4.683 | $19,056 | 4.69 | $-147 | 0.007 | Cost-saving |
| Fracture rates reduced by 10% | $40,216 | 4.045 | $40,233 | 4.05 | $17 | 0.005 | $4072 |
| Fracture rates increased by 10% | $41,365 | 4.017 | $41,342 | 4.021 | $-23 | 0.004 | Cost-saving |
| Discount rate reduced to 0% | $50,513 | 4.965 | $50,490 | 4.969 | $-23 | 0.004 | Cost-saving |
| Discount rate increased to 5% | $36,000 | 3.561 | $36,003 | 3.564 | $3 | 0.003 | $1165 |
| Alternative fracture cost estimates applied | $74,155 | 4.032 | $74,168 | 4.036 | $13 | 0.004 | $3344 |
| Bisphosphonate treatment disutility includedb | $40,822 | 4.031 | $40,833 | 4.033 | $11 | 0.003 | $3971 |
| Treatment benefits reduced per Lyles | $40,830 | 4.031 | $40,869 | 4.034 | $39 | 0.003 | $11,603 |
| Multi-way sensitivity analyses | |||||||
| FLS cost at $205, medication cost at $250c | $40,871 | 4.032 | $41,012 | 4.036 | $141 | 0.004 | $37,729 |
| FLS cost at $205, initiation rate at 66%c | $40,810 | 4.032 | $40,770 | 4.040 | $-40 | 0.008 | Cost-saving |
| Non-hip fractures included, FLS bisphosphonate initiation rate at 66% | $19,203 | 4.683 | $18,812 | 4.699 | $-391 | 0.016 | Cost-saving |
| “Worst case” analysis 1c,d | $73,646 | 4.044 | $73,853 | 4.047 | $207 | 0.003 | $68,124 |
| “Worst case” analysis 2c,d | $73,687 | 4.044 | $73,913 | 4.046 | $226 | 0.002 | $112,877 |
Note: The incremental cost-effectiveness ratio (ICER) is the ratio of change in costs/change in effectiveness.
In the base case and majority of analyses, the strategy of BMD testing by DXA followed by FLS for those with osteopenia or osteoporosis is dominated by (ie, more costly and no more effective than) universal follow-up by an FLS and has been omitted from this table for readability. For the scenarios noted by footnote c, the reverse is true and targeted FLS dominates universal FLS. Supplemental Table S1 includes both FLS strategies.
Under this scenario, the strategy of BMD testing by DXA followed by FLS for those with osteopenia or osteoporosis is more effective than universal follow-up by an FLS. Its ICER relative to universal FLS is $76,349/QALY.
Under this scenario, the strategy of BMD testing by DXA followed by FLS for those with osteopenia or osteoporosis is as effective and less costly than the strategy of universal follow-up by an FLS and therefore dominates it. The values reported here are those for DXA followed by FLS for patients with osteoporosis or osteopenia.
Under the “worst case” analysis 1 scenario, the FLS cost is increased to $205, bisphosphonate treatment is assumed to cost $250 per year, fracture rates are reduced by 10%, bisphosphonate side effects are incorporated, and alternative fracture cost estimates are applied. Under the “worst case” analysis 2, treatment efficacy was also reduced according to Lyles.(48)
We examined the results of increasing the effectiveness of the FLS program from a treatment rate of 44% to 66%. Under this assumption, the estimated savings for a cohort of 10,000 men and women treated under the FLS increased to $1,395,200 (Table 2). Expanding the fracture types included in the FLS to all typical osteoporotic fractures also resulted in cost savings of $1,467,900. Varying both of these variables simultaneously results in cost savings of $4,349,500.
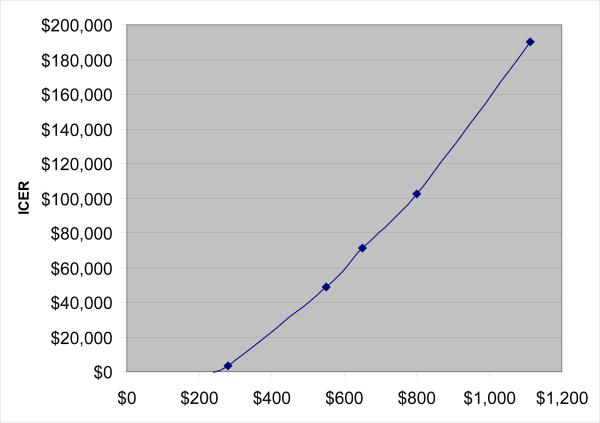
Finally, we performed a threshold analysis to examine how different annual costs for zoledronic acid affected the ICER for the FLS program comparing it with generic alendronate (Fig. 2). When the annual cost of zoledronic acid was reduced to $240, it resulted in cost savings; at approximately $800, its ICER was $100,000 per QALY.
Fig. 2.
This threshold analysis examined how different annual costs for zoledronic acid affected the ICER for the FLS program comparing it with generic alendronate. When the annual cost of zoledronic acid was reduced to $240, it resulted in cost savings; at approximately $800, its ICER was $100,000 per QALY.
Discussion
The care of patients who fracture from osteoporosis was estimated at $15 billion in 2010 and projected to be $25 billion by 2025.(12) Because approximately 20% of patients who fracture will experience a repeat fracture in the next 5 years,(16) they represent a high-risk group likely to benefit from treatment, but providers often neglect treating this group's osteoporosis.(2) Health systems that have employed an FLS program use osteoporosis treatments in a much higher proportion of fracture patients than has been demonstrated in typical patients.(5,6) It is important to formally assess the projected costs and benefits of an FLS before wide dissemination.
We examined the cost-effectiveness of an FLS compared with usual care and found that under base case assumptions, the FLS reduced fractures, increased QALYs, and reduced costs. With approximately 2.5 million osteoporotic fractures per year in the United States, if all these postfracture patients were cared for in an FLS, the cost savings might be as much as $16.7 million. Even under the most pessimistic assumptions, the ICER was $112,877 per QALY, less costly than many interventions deemed worth purchasing.
The strengths of this study are several. First, the simulation model has been validated in a prior study.(10) Second, all standard recommendations of the US Task Force on Cost Effectiveness studies were followed.(9) Third, we incorporated nonadherence into the model as has been suggested.(22) The probability of reinitiation of osteoporosis treatment after nonadherence was also incorporated. Fourth, we biased the analyses against an FLS (assuming no improvement in medication adherence) and still found favorable ICERS. Finally, we included treatment disutility from the oral bisphosphonate. Limitations of our methods derive primarily from a lack of precision with several important estimates, such as refracture rates and the cost of the FLS. More information is needed to better estimate these key assumptions. We performed wide sensitivity analyses on these key assumptions, as has been suggested,(50) and the results remained favorable for the FLS but not cost saving. Some data suggest that bisphosphonates results in a mortality benefit, but this assumption was not incorporated into the current model.(48) Furthermore, some data question the fracture benefit of bisphosphonates in patients with osteopenia.(51) We took a health-care systems perspective for these analyses, primarily because the indirect costs of fracture in older adults are poorly understood. It is likely that a societal perspective that was able to incorporate indirect costs of fracture would have been even more favorable for an FLS.
Net costs of the FLS depend on how efficiently nurse practitioners can deliver care. It is likely that an FLS nurse practitioner can manage 500 to 1000 postfracture patients annually. This volume allows her or him to earn a full-time salary performing this function. However, if a full-time nurse practitioner is hired for this role but serves far fewer patients, then the cost of the FLS born by the health system rises. Using a physician and not a nurse practitioner to run the FLS would increase the costs substantially without a clear benefit in outcomes, but such an approach may be considered in some health-care systems. Another important factor in the cost of the FLS is the system for identifying postfracture patients. Some health systems have integrated information systems that would allow for easy identification of such patients in real time, whereas other health systems would require much more person-time identifying such patients from inpatient, emergency department, and radiology records.
These analyses were not meant to justify treating patients postfracture. All professional societies already recommend treating patients after a hip fracture for osteoporosis, with or without an assessment of BMD.(52) Health policy makers and payers should consider these results when considering ways to stimulate improvement in care through system-level programs. Furthermore, one might anticipate that an FLS will enhance communication between members of the health-care team, leading to improved quality of care.
Health-care spending in the US Medicare program is expected to grow by 50% by 2020, (1) with a majority of these costs expended by older adults at risk of fractures. “Bending the cost curve” is a popular concept, but few interventions have actually been shown to improve care and reduce costs. We studied the economic consequences of a postfracture liaison service in the US health-care setting, the FLS, and found that under base case assumptions, this program would likely yield cost savings and possibly reduced fractures. Even under less favorable assumptions, the FLS would have favorable ICERs. The infrastructure for implementing an FLS has been developed,(53) and more than 100 health systems and orthopaedic surgery practices in the US have adopted some form of an FLS.(54) It is likely that with reimbursement for an FLS through designated Medicare payment codes, more health systems would adopt an FLS. Health-care systems around the globe are adopting similar systems of care.(55,56)
Supplementary Material
Acknowledgments
Support was provided by NIH (NIAMS AR P60047782). The funder had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Authors’ roles: Study design: DHS, ARP, JS, and EL. Study conduct: DHS and ARP. Data collection: DHS and ARP. Data analysis: DHS, ARP, and EL. Data interpretation: TC, DD, RF, and AF. Drafting manuscript: DHS and ARP. Revising and approving final manuscript: DHS, ARP, JS, and EL. DHS takes responsibility for the integrity of the data analysis.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosures
DHS receives salary support through research grants to BWH from Lilly and Amgen, unrelated to this research. DHS serves on the Multidisciplinary Board of the American Orthopaedics Association Own The Bone Program and receives an honorarium < $5,000. DHS receives royalties (<$10,000) from UpToDate for chapters unrelated to this work. Dr. Solomon also serves on the National Bone Health Alliance's Governance Board without compensation. Dr. Schousboe was a paid consultant to Merck, Inc., in 2011.
References
- 1.Board of Trustees FHITF Annual report. Services HaH. 2012:60. <zaq;2>. [Google Scholar]
- 2.National Committee for Quality Assurance . 2010 The state of health care quality. NCQA; Washington, DC: 2010. p. 94. [Google Scholar]
- 3.Recker RR, Lewiecki EM, Miller PD, Reiffel J. Safety of bisphosphonates in the treatment of osteoporosis. Am J Med. 2009;122(2 Suppl):S22–32. doi: 10.1016/j.amjmed.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Wells GA, Cranney A, Peterson J, et al. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;(1):CD001155. doi: 10.1002/14651858.CD001155.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Dell R. Fracture prevention in Kaiser Permanente Southern California. Osteoporos Int. 2011;22(Suppl 3):457–60. doi: 10.1007/s00198-011-1712-0. [DOI] [PubMed] [Google Scholar]
- 6.Newman ED, Ayoub WT, Starkey RH, Diehl JM, Wood GC. Osteoporosis disease management in a rural health care population: hip fracture reduction and reduced costs in postmenopausal women after 5 years. Osteoporos Int. 2003;14(2):146–51. doi: 10.1007/s00198-002-1336-5. [DOI] [PubMed] [Google Scholar]
- 7.Cadarette SM, Katz JN, Brookhart MA, et al. Trends in drug prescribing for osteoporosis after hip fracture, 1995–2004. J Rheumatol. 2008;35(2):319–26. [PMC free article] [PubMed] [Google Scholar]
- 8.McLellan AR, Wolowacz SE, Zimovetz EA, et al. Fracture liaison services for the evaluation and management of patients with osteoporotic fracture: a cost-effectiveness evaluation based on data collected over 8 years of service provision. Osteoporos Int. 2011;22(7):2083–98. doi: 10.1007/s00198-011-1534-0. [DOI] [PubMed] [Google Scholar]
- 9.Gold MR, Siegel JE, Russel LB, Weinstein M. Cost-effectiveness in health and medicine. Oxford University Press; New York: 1996. [Google Scholar]
- 10.Patrick AR, Schousboe JT, Losina E, Solomon DH. The economics of improving medication adherence in osteoporosis: validation and application of a simulation model. J Clin Endocrinol Metab. 2011;96(9):2762–70. doi: 10.1210/jc.2011-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patrick AR, Brookhart MA, Losina E, et al. The complex relation between bisphosphonate adherence and fracture reduction. J Clin Endocrinol Metab. 2010;95(7):3251–9. doi: 10.1210/jc.2009-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 13.Center JR, Bliuc D, Nguyen TV, Eisman JA. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA. 2007;297(4):387–94. doi: 10.1001/jama.297.4.387. [DOI] [PubMed] [Google Scholar]
- 14.Pike CT, Birnbaum HG, Schiller M, Swallow E, Burge RT, Edgell ET. Prevalence and costs of osteoporotic patients with subsequent non-vertebral fractures in the US. Osteoporos Int. 2011;22(10):2611–21. doi: 10.1007/s00198-010-1494-9. [DOI] [PubMed] [Google Scholar]
- 15.Majumdar SR, Lier DA, Beaupre LA, et al. Osteoporosis case manager for patients with hip fractures: results of a cost-effectiveness analysis conducted alongside a randomized trial. Arch Intern Med. 2009;169(1):25–31. doi: 10.1001/archinte.169.1.25. [DOI] [PubMed] [Google Scholar]
- 16.Johnell O, Kanis JA, Oden A, et al. Fracture risk following an osteoporotic fracture. Osteoporos Int. 2004;15(3):175–9. doi: 10.1007/s00198-003-1514-0. [DOI] [PubMed] [Google Scholar]
- 17.Melton LJ, 3rd, Crowson CS, O'Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos Int. 1999;9(1):29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]
- 18.Schousboe JT, Taylor BC, Fink HA, et al. Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men. JAMA. 2007;298(6):629–37. doi: 10.1001/jama.298.6.629. [DOI] [PubMed] [Google Scholar]
- 19.Sawka AM, Papaioannou A, Adachi JD, Gafni A, Hanley DA, Thabane L. Does alendronate reduce the risk of fracture in men? A meta-analysis incorporating prior knowledge of anti-fracture efficacy in women. BMC Musculoskelet Disord. 2005;6:39. doi: 10.1186/1471-2474-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melton LJ, 3rd, Ilstrup DM, Beckenbaugh RD, Riggs BL. Hip fracture recurrence. A population-based study. Clin Orthop Relat Res. 1982;(167):131–8. [PubMed] [Google Scholar]
- 21.Tosteson AN, Jonsson B, Grima DT, O'Brien BJ, Black DM, Adachi JD. Challenges for model-based economic evaluations of postmenopausal osteoporosis interventions. Osteoporos Int. 2001;12(10):849–57. doi: 10.1007/s001980170036. [DOI] [PubMed] [Google Scholar]
- 22.Strom O, Borgstrom F, Kanis JA, Jonsson B. Incorporating adherence into health economic modelling of osteoporosis. Osteoporos Int. 2009;20(1):23–34. doi: 10.1007/s00198-008-0644-9. [DOI] [PubMed] [Google Scholar]
- 23.Bagger YZ, Tanko LB, Alexandersen P, Ravn P, Christiansen C. Alendronate has a residual effect on bone mass in postmenopausal Danish women up to 7 years after treatment withdrawal. Bone. 2003;33(3):301–7. doi: 10.1016/s8756-3282(03)00112-1. [DOI] [PubMed] [Google Scholar]
- 24.Brookhart MA, Avorn J, Katz JN, et al. Gaps in treatment among users of osteoporosis medications: the dynamics of noncompliance. Am J Med. 2007;120(3):251–6. doi: 10.1016/j.amjmed.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Weycker D, Macarios D, Edelsberg J, Oster G. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007;18(3):271–7. doi: 10.1007/s00198-006-0230-y. [DOI] [PubMed] [Google Scholar]
- 26.Tosteson AN, Gottlieb DJ, Radley DC, Fisher ES, Melton LJ., 3rd Excess mortality following hip fracture: the role of underlying health status. Osteoporos Int. 2007;18(11):1463–72. doi: 10.1007/s00198-007-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Friesendorff M, Besjakov J, Akesson K. Long-term survival and fracture risk after hip fracture: a 22-year follow-up in women. J Bone Miner Res. 2008;23(11):1832–41. doi: 10.1359/jbmr.080606. [DOI] [PubMed] [Google Scholar]
- 28.Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159(11):1215–20. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- 29.Empana JP, Dargent-Molina P, Breart G. Effect of hip fracture on mortality in elderly women: the EPIDOS prospective study. J Am Geriatr Soc. 2004;52(5):685–90. doi: 10.1111/j.1532-5415.2004.52203.x. [DOI] [PubMed] [Google Scholar]
- 30.Haentjens P, Magaziner J, Colon-Emeric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380–90. doi: 10.1059/0003-4819-152-6-201003160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz JN, Losina E, Barrett J, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population [see comment]. J Bone Joint Surg Am. 2001;83-A(11):1622–9. doi: 10.2106/00004623-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Thomson Reuters . 2010 Red book. Thomson Reuters; Montvale, NJ: 2010. [Google Scholar]
- 33.Thomson Healthcare (Firm) 2007 Red book. Thomson PDR; Montvale, NJ: 2007. p. v. [Google Scholar]
- 34.Gabriel SE, Tosteson AN, Leibson CL, et al. Direct medical costs attributable to osteoporotic fractures. Osteoporos Int. 2002;13(4):323–30. doi: 10.1007/s001980200033. [DOI] [PubMed] [Google Scholar]
- 35.Leibson CL, Tosteson AN, Gabriel SE, Ransom JE, Melton LJ. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50(10):1644–50. doi: 10.1046/j.1532-5415.2002.50455.x. [DOI] [PubMed] [Google Scholar]
- 36.Schousboe JT, Nyman JA, Kane RL, Ensrud KE. Cost-effectiveness of alendronate therapy for osteopenic postmenopausal women. Ann Intern Med. 2005;142(9):734–41. doi: 10.7326/0003-4819-142-9-200505030-00008. [DOI] [PubMed] [Google Scholar]
- 37.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44(12):2737–45. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 38.Borgstrom F, Zethraeus N, Johnell O, et al. Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int. 2006;17(5):637–50. doi: 10.1007/s00198-005-0015-8. [DOI] [PubMed] [Google Scholar]
- 39.Kanis JA, Johnell O, Oden A, et al. The risk and burden of vertebral fractures in Sweden. Osteoporos Int. 2004;15(1):20–6. doi: 10.1007/s00198-003-1463-7. [DOI] [PubMed] [Google Scholar]
- 40.Oleksik A, Lips P, Dawson A, et al. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res. 2000;15(7):1384–92. doi: 10.1359/jbmr.2000.15.7.1384. [DOI] [PubMed] [Google Scholar]
- 41.Tosteson AN, Gabriel SE, Grove MR, Moncur MM, Kneeland TS, Melton LJ., 3rd Impact of hip and vertebral fractures on quality-adjusted life years. Osteoporos Int. 2001;12(12):1042–9. doi: 10.1007/s001980170015. [DOI] [PubMed] [Google Scholar]
- 42.Adachi JD, Faraawi RY, O’Mahony MF, et al. Upper gastrointestinal tolerability of alendronate sodium monohydrate 10 mg once daily in postmenopausal women: a 12-week, randomized, double-blind, placebo-controlled, exploratory study. Clin Ther. 2009;31(8):1747–53. doi: 10.1016/j.clinthera.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 43.Solomon DH, Mercer E, Woo SB, Avorn J, Schneeweiss S, Treister N. Defining the epidemiology of bisphosphonate-associated osteonecrosis of the jaw: prior work and current challenges. Osteoporos Int. 2013;24(1):237–44. doi: 10.1007/s00198-012-2042-6. [DOI] [PubMed] [Google Scholar]
- 44.Gedmintas L, Solomon DH, Kim SC. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: a systematic review and meta-analysis. J Bone Miner Res. 2013;28(8):1729–37. doi: 10.1002/jbmr.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groeneveld PW, Lieu TA, Fendrick AM, et al. Quality of life measurement clarifies the cost-effectiveness of Helicobacter pylori eradication in peptic ulcer disease and uninvestigated dyspepsia. Am J Gastroenterol. 2001;96(2):338–47. doi: 10.1111/j.1572-0241.2001.03516.x. [DOI] [PubMed] [Google Scholar]
- 46.Miksad RA, Lai KC, Dodson TB, et al. Quality of life implications of bisphosphonate-associated osteonecrosis of the jaw. Oncologist. 2011;16(1):121–32. doi: 10.1634/theoncologist.2010-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Najm M, Solomon D, Woo S, Treister N. Resource utilization in cancer patients with bisphosphonate-associated osteonecrosis of the jaw. Oral Dis. 2014;20(1):94–9. doi: 10.1111/odi.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid in reducing clinical fracture and mortality after hip fracture. N Engl J Med. 2007;357:nihpa40967. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture [see comment]. N Engl J Med. 2007;357(18):1799–809. doi: 10.1056/NEJMoa074941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gold MR. Cost-effectiveness in health and medicine. Oxford University Press; New York: 1996. p. xxiii. [Google Scholar]
- 51.Ryder KM, Cummings SR, Palermo L, et al. Does a history of non-vertebral fracture identify women without osteoporosis for treatment? J Gen Intern Med. 2008;23(8):1177–81. doi: 10.1007/s11606-008-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Osteoporosis Foundation . Clinician's guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation; Washington, DC: 2013. [Google Scholar]
- 53.Tosi LL, Gliklich R, Kannan K, Koval KJ. The American Orthopaedic Association's “own the bone” initiative to prevent secondary fractures. J Bone Joint Surg Am. 2008;90(1):163–73. doi: 10.2106/JBJS.G.00682. [DOI] [PubMed] [Google Scholar]
- 54.American Orthopaedic Association . Own the bone. American Orthopaedic Association; Rosemont, IL: 2013. [Google Scholar]
- 55.Chandran M, Tan MZ, Cheen M, Tan SB, Leong M, Lau TC. Secondary prevention of osteoporotic fractures—an “OPTIMAL” model of care from Singapore. Osteoporos Int. 2013;24(11):2809–17. doi: 10.1007/s00198-013-2368-8. [DOI] [PubMed] [Google Scholar]
- 56.Eisman JA, Bogoch ER, Dell R, et al. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res. 2012;27(10):2039–46. doi: 10.1002/jbmr.1698. [DOI] [PubMed] [Google Scholar]
- 57.Arias E. United States life tables, 2004. Natl Vital Stat Rep. 2007;56(9):1–39. [PubMed] [Google Scholar]
- 58.Tosteson AN, Gabriel SE, Grove MR, Moncur MM, Kneeland TS, Melton LJ., 3rd Impact of hip and vertebral fractures on quality-adjusted life years. Osteoporos Int. 2001;12(12):1042–9. doi: 10.1007/s001980170015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.