Abstract
BACKGROUND
Although many patients with chronic myeloid leukemia (CML) respond well to imatinib therapy, a significant proportion loses their initial response. Loss of response on imatinib is often because of BCR-ABL mutations. Dasatinib is a 325-fold more potent inhibitor of Bcr-Abl than imatinib and has been associated with high rates of durable responses in patients with CML in chronic phase (CP) after imatinib failure.
METHODS
To determine the optimal time for initiating dasatinib after loss of response on imatinib, data from dasatinib trials in CML-CP were analyzed. Patients were grouped according to whether they received early intervention with dasatinib (ie, after cytogenetic recurrence on imatinib), rather than after both cytogenetic and hematologic recurrence.
RESULTS
Overall, 72% of patients who received dasatinib after loss of a major cytogenetic response (MCyR) on imatinib achieved a complete cytogenetic response (CCyR) compared with 42% of patients who were treated after loss of both MCyR and complete hemato-logic response (CHR). Event-free survival (EFS) also was higher after earlier dasatinib treatment (24-month EFS rates: 89% after loss of MCyR on imatinib vs 29% after loss of both MCyR and CHR). Among patients who were treated after loss of CHR on imatinib with no prior MCyR, 26% achieved a CCyR with dasatinib, and the 24-month EFS rate was 64%. In all 3 groups, CCyR rates were similar in patients with or without pre-existing BCR-ABL mutations.
CONCLUSIONS
The results of the current study suggested that optimal outcomes are achieved when dasatinib is administered early after imatinib resistance.
Keywords: dasatinib, chronic myeloid leukemia, imatinib, antineoplastic drug resistance
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder characterized by a reciprocal t(9;22)(q34;q11.2) chromosomal translocation that gives rise to the Philadelphia (Ph) chromosome and the BCR-ABL fusion gene. The resulting Bcr-Abl oncoprotein is a constitutively active tyrosine kinase that promotes the growth advantage of leukemia cells through enhanced proliferation and reduced apoptosis and promotes genomic instability through inhibition of DNA repair.1 Greater than 80% of patients are diagnosed with CML during the initial chronic phase (CP).2 Without effective therapeutic intervention, patients with CML-CP inevitably progress to advanced phases of disease and have a short survival.
Currently, treatment of CML is based on efficient blockade of Bcr-Abl activity. Imatinib mesylate (Glivec [Gleevec in the US]; Novartis Pharmaceuticals, Basel, Switzerland) was the first Bcr-Abl–targeted agent approved for the treatment of CML.3,4 Although most patients respond well to imatinib therapy, an estimated 31% of patients who were treated during the phase 3 International Randomized Study of Interferon and STI571 or IRIS trial did not achieve a complete cyto-genetic response (CCyR) within 12 months, and 0.4% to 5.5% of patients who achieved a CCyR experienced treatment failure each year.3 Resistance to imatinib most commonly is a result of mutations that cause conformational changes or that alter critical residues within the imatinib-binding region of the Bcr-Abl oncoprotein.5
Dasatinib (Sprycel; Bristol-Myers Squibb, New York, NY) is a second-generation Bcr-Abl inhibitor that is indicated for the treatment of adults with CML in all phases who have resistance or intolerance to imatinib. In vitro, dasatinib is 325-fold more potent than imatinib.6 Dasatinib treatment has induced a high rate of durable responses among patients with both resistance and intolerance to imatinib, including patients with all BCR-ABL mutations examined, except for the threonine-to-isoleucine mutation at codon 315 (T315I).7-9
The timing of second-line treatment after failure of initial treatment may have a significant impact on long-term outcome. Among patients who received imatinib after failure on prior interferon-α (IFN) therapy, improved responses and higher rates of survival were achieved if imatinib was initiated at the time of cytogenetic recurrence rather than after hematologic recurrence.10,11 Similarly, a recent analysis of patients with imatinib failure identified the administration of second-line treatment after hematologic resistance or recurrence as an independent prognostic factor for poor survival compared with treatment after cytogenetic resistance/recurrence (P = .01; 3-year overall survival (OS) rate, 57% compared with 92%, respectively).12 The objective of the analysis presented here was to assess the impact of earlier intervention with dasatinib (ie, after cytogenetic recurrence rather than after hematologic recurrence on imatinib) on subsequent response rates and long-term outcomes.
MATERIALS AND METHODS
Study Group
Patient data were analyzed retrospectively from 3 dasatinib trials in patients with CML-CP: CA180-013, CA180-017, and CA180-034 (Table 1). Study CA180-013 (START-C) is a phase 2 trial of dasatinib administered at a dose of 70 mg twice daily in patients with imatinib resistance or intolerance (N = 387).7 Study CA180-017 (START-R) is a phase 2 randomized trial of dasatinib versus imatinib dose escalation in patients who were resistant to imatinib at doses from 400 mg daily to 600 mg daily, including 101 patients who received dasatinib (70 mg twice daily).13 In both the CA180-013 study and the CA180-017 study, dose escalation to 90 mg twice daily was permitted for patients who were not responding or progressing, and dose interruption or reduction to 40 mg twice daily was permitted for adverse events. Study CA180-034 is a randomized, phase 3 dose-optimization trial in patients with imatinib resistance (including both suboptimal response and treatment failure) or intolerance (N = 670).14 Dasatinib was administered at 1 of 4 dosing schedules: 100 mg once daily (n = 167), 50 mg twice daily (n = 168), 140 mg once daily (n = 167), or 70 mg twice daily (n = 168). Escalation to 180 mg once daily or 90 mg twice daily was permitted for lack of response, and dose interruption or reduction to 80 mg once daily or 40 mg twice daily was permitted for adverse events. Each trial was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board/ ethics committee at each participating center. Written informed consent was obtained from all patients.
Table 1.
Summary of Study Populations Included in This Analysis
| Variable | CA180-013: START-C, n = 114* | CA180-017: START-R, n=41† | CA180-034, n=138‡ |
|---|---|---|---|
| Median duration of treatment, mo | 22.7 | 24.9 | 11.9 |
| Patient population | CML-CP | CML-CP | CML-CP |
| Imatinib resistance inclusion criteria | Primary or acquired resistance to imatinib >600 mg/d or acquired resistance to imatinib ≤600 mg/d with BCR-ABL mutation (high-level imatinib resistance)§ | Primary or acquired resistance to imatinib 400-600 mg/d; patients who had known BCR-ABL mutations with high-level imatinib resistance before study entry were excluded§ | Primary or acquired resistance or suboptimal response on imatinib ≥400 mg/d |
| No. of patients with loss of response to imatinib (%) | |||
| Total no. with loss of response | 114 | 41 | 138 |
| Loss of MCyR | 47 (41) | 19 (46) | 85 (62) |
| Loss of CHR | 50 (44) | 20 (49) | 39 (28) |
| Loss of MCyR and CHR | 17(15) | 2 (5) | 14 (10) |
| Median average dasatinib daily dose, mg | 112 | 128 | 100 |
CML-CP indicates chronic myeloid leukemia in chronic phase; MCyR, major cytogenetic response; CHR, complete hematologic response.
See Hochhaus 2008.7
See Kantarjian 2007.13
See Shah 2007.14
Leucine-to valine mutation at codon 248, glycine-to-glutamic acid mutation at codon 250, glutamine-to-histidine mutation at codon 252, tyrosine-to-histidine or phenylalanine mutation at codon 253, glutamic acid-to-lysine or valine mutation at codon 255, threonine-to-isoleucine or alanine acid mutation at codon 315, phenylalanine-to-leucine mutation at codon 317, and histidine-to-proline or arginine mutation at codon 396.
Response Definitions and Assessments
A complete hematologic response (CHR) was defined as a white blood cell count no higher than the institutional upper limit of normal, an absolute neutrophil count ≥1 × 109/L, a platelet count <450 × 109/L and no more than the institutional upper limit of normal, <5% myelocytes plus metamyelocytes in peripheral blood, <20% basophils in peripheral blood, and no extramedullary involvement, all maintained for at least 4 weeks. Cytogenetic responses (CyRs) were classified according the percentage of Ph-positive metaphases in bone marrow using the following standard definitions: 0% Ph-positive meta-phases, complete CyR (CCyR); and from 1% to ≤35% Ph-positive metaphases, partial CyR (PCyR). A major CyR (MCyR) was defined as the sum of CCyRs and PCyRs (ie, 35% Ph-positive metaphases). A major molecular response (MMR) was defined as a reduction in BCR-ABL transcripts to ≤0.1% compared with the international standardized baseline BCR-ABL:ABL ratio.
Assessments in the current analysis comprised rates of CHR, MCyR, CCyR, event-free survival (EFS), transformation-free survival (TFS), and overall survival (OS). All cytogenetic responses during dasatinib treatment were assigned using conventional cytogenetic assessment of Ph-positive metaphases in bone marrow samples. Rates of MMR were analyzed in the CA180-013 study only and are reported using all assessed patients as the denominator.
Events that were included in the EFS analysis were transformation to accelerated phase or blast phase, loss of CHR or MCyR, increasing white blood cell count (doubling of the count from the nadir to >20,000/mm3 or by >50,000/mm3, on 2 occasions at least 2 weeks apart) in patients who did not achieve CHR, or death. Events that were included in TFS analysis included transformation to accelerated phase or blast phase or death. For the OS analysis, patients in the CA180-017 study who were included in the current analysis but who had subsequently crossed over to imatinib treatment (n = 10) were censored at the time of crossover.
Patients were analyzed according to the presence of mutations in the BCR-ABL kinase domain before dasatinib therapy as measured by direct sequencing. The ATP phosphate-binding loop (P-loop) region of Bcr-Abl was defined as residues 248 through 256.
Patient Groups
The main objective of this analysis was to assess whether, in patients with CML-CP and imatinib failure, treatment with dasatinib after loss of MCyR (ie, earlier intervention) compared with after loss of MCyR and CHR (ie, later intervention) resulted in improved outcomes. For this analysis, patients with CML-CP and acquired resistance to imatinib because of loss of either CHR or MCyR were identified from dasatinib trials. No patients with imatinib intolerance were included. Eligible patients were divided into 3 groups according to patient histories provided by the recruiting investigator in terms of best response to imatinib and loss of response during prior imatinib treatment (cytogenetic and/or hematologic) before initiating treatment with dasatinib. Group 1 (loss of MCyR) comprised patients who had achieved an MCyR on imatinib and subsequently had lost their MCyR. Loss of MCyR was defined as no longer meeting the MCyR criteria and a ≥30% increase in the percentage of Ph-positive meta-phases. None of these patients were recorded by the investigator as having lost their CHR. Group 2 (loss of MCyR and CHR) comprised patients who had achieved both an MCyR and a CHR and subsequently had lost both. Group 3 (loss of CHR) comprised patients who had achieved a CHR and had lost their CHR without ever achieving an MCyR. Patients who had lost their CHR and who also had achieved an MCyR for whom the investigator did not document a loss of MCyR (n = 37) were excluded from the analysis.
RESULTS
Patient Characteristics
In total, 293 patients with loss of MCyR or CHR were eligible for the current analysis. There were 151 patients in Group 1 (loss of MCyR), 33 patients in Group 2 (loss of MCyR and CHR), and 109 patients in Group 3 (loss of CHR without prior MCyR) (Table 2). Among the 33 patients who had lost MCyR and CHR during imatinib treatment (Group 2), the simultaneous loss of both responses was recorded in 4 patients, and all other patients had loss of MCyR followed eventually by loss of CHR.
Table 2.
Baseline Characteristics*
| Variable | Group 1: Loss of MCyR, Early Intervention (n=151) | Group 2: Loss of CHR and MCyR, Late Intervention (n=33) | Group 3: Loss of CHR (n=109) |
|---|---|---|---|
| Sex, men/women, % | 55/45 | 52/48 | 54/46 |
| Median age (range), y | 55 (18-81) | 60 (28-85) | 59 (21-81) |
| Median duration of CML [range], mo | 64 (9-211) | 67 (13-150) | 79 (4-251) |
| Highest imatinib dose in mg/d, % | |||
| 400-600 | 48 | 49 | 37 |
| >600 | 52 | 51 | 63 |
| Prior interferon-α, % | 71 | 64 | 63 |
| Prior stem cell transplantation, % | 8 | 6 | 10 |
| Prior imatinib treatment, % | |||
| <1y | 1 | 3 | 5 |
| 1-3 y | 33 | 27 | 35 |
| >3y | 66 | 70 | 60 |
| Baseline response at initiation of dasatinib, %† | |||
| CHR | 49 | 6 | 12 |
| MCyR | 20 | 0 | 5 |
| BCR-ABL mutation, no. (%)‡ | 60 (44) | 20 (67) | 77 (75) |
| T315I | 2 (1) | 1 (3) | 1 (1) |
| F317L | 1 (1) | – | 4 (4) |
| F359C/I/V | 8 (6) | – | 6 (6) |
| P-loop region§ | 19 (14) | 7 (23) | 30 (29) |
| L248V | 2 (1) | – | 6 (6) |
| G250E | 6 (4) | 4 (13) | 12 (12) |
| Q252H | 1 (1) | – | 2 (2) |
| Y253F/H | 8 (6) | 1 (3) | 8 (8) |
| E255K/V | 2 (1) | 2 (7) | 4 (4) |
MCyR indicates major cytogenetic response; CHR, complete hematologic response; CML, chronic myeloid leukemia; T315I, threonine-to-isolucine mutation at codon 315; F317L, phenylalanine-to-leucine mutation at codon 317; F359C/I/V, phenylalanine-to-cysteine, valine, or isoleucine mutation at codon 359; P-loop, ATP phosphate-binding loop; L248V, leucine-to-valine mutation at codon 248; G250E, glycine-to-glutamic acid mutation at codon 250; Q252H, glutamine-to-histidine mutation at codon 252; Y253F/H, tyrosine-to-phenylalanine or valine mutation at codon 253; E255K/V, glutamic acid-to-phenylalanine or valine mutation at codon 255.
Eligible patients were grouped according to the patient history provided by the recruiting investigator on loss of treatment response during prior imatinib treatment.
As measured during dasatinib trials.
Patients who had samples available for baseline mutation analysis: Group 1, n=135; Group 2, n=30; Group 3, n=103.
Amino acids 248 through 256.
Across the 3 groups, >50% of patients had received imatinib at a dose >600 mg daily, and ≥60% had received imatinib for > 3 years (Table 2). The median duration of CML was shorter in the loss of MCyR group (Group 1, 64 months) and in the loss of MCyR/CHR group (Group 2, 67 months) compared with the loss of CHR without prior MCyR group (Group 3, 79 months). A smaller proportion of patients in the loss of MCyR group (Group 1) had developed BCR-ABL mutations during imatinib treatment (44%) compared with the loss of MCyR/CHR group (67%) and the loss of CHR group (75%). No major differences were observed in the relative frequencies of individual BCR-ABL mutations among the 3 groups.
Efficacy
After dasatinib treatment and after a median duration of therapy with dasatinib of 13.8 months (range, 0.2-30.6 months), 90% of the total analysis group achieved a CHR, 62% achieved an MCyR, and 51% achieved a CCyR. The overall MMR rate was 40% among the assessed patients from Study CA180-013 who were included in the current analysis. Within each group, similar response rates were observed in patients who were recruited to each of the 3 trials (Table 3). No obvious differences in responses were observed among patients who received alternative dasatinib dosing regimens, although the numbers were small in some cases. The proportion of patients who achieved a CCyR with dasatinib after loss of MCyR on imatinib (Group 1) was similar for the patients who received 70 mg twice daily or 100 mg once daily. In Groups 2 and 3, few patients were treated with dasatinib 100 mg once daily, preventing any comparison.
Table 3.
Rates of Complete Hematologic Response, Major Cytogenetic Response (All Studies), and Major Molecular Response (CA180-013 Study Only) in Patients Treated With Dasatinib After Loss of Response to Prior Imatinib
| Patients, n/N (%) | |||
|---|---|---|---|
| Variable | Group 1: Loss of MCyR on Imatinib | Group 2: Loss of MCyR and CHR on Imatinib | Group 3: Loss of CHR on Imatinib Without Prior MCyR |
| CHR: All patients | 139/151 (92) | 28/33 (85) | 98/109 (90) |
| MCyR: All patients | 123/151 (81) | 21/33 (64) | 38/109 (35) |
| CCyR | |||
| All patients | 108/151 (72) | 14/33 (42) | 28/109 (26) |
| CCyR data by dosing schedule | |||
| 70 mg BID population | 67/89 (75) | 10/25 (40) | 20/78 (26) |
| 100 mg QD population | 21/28 (75) | 1/1 (100) | 1/9 (11) |
| Other regimens, 50 mg BID or 140 mg QD | 20/34 (59) | 3/7 (43) | 7/22 (32) |
| CCyR data by study | |||
| Study CA180-013, 70 mg BID | 36/47 (77) | 6/17 (35) | 13/50 (26) |
| Study CA180-017, 70 mg BID | 12/19 (63) | 1/2 (50) | 5/20 (25) |
| Study CA180-034 | |||
| 100 mg QD | 21/28 (75) | 1/1 (100) | 1/9 (11) |
| 50 mg BID | 11/17 (65) | 1/2 (50) | 2/10 (20) |
| 140 mg QD | 9/17 (53) | 2/5 (40) | 5/12 (42) |
| 70 mg BID | 19/23 (83) | 3/6 (50) | 2/8 (25) |
| MMR: Study CA180-013, 70 mg BID* | 28/47 (60) | 5/17 (29) | 13/50 (26) |
MCyR indicates major cytogenetic response; CHR, complete hematologic response; CCyR, complete cytogenetic response; BID, twice daily; QD, once daily; MMR, major molecular response.
In all assessed patients.
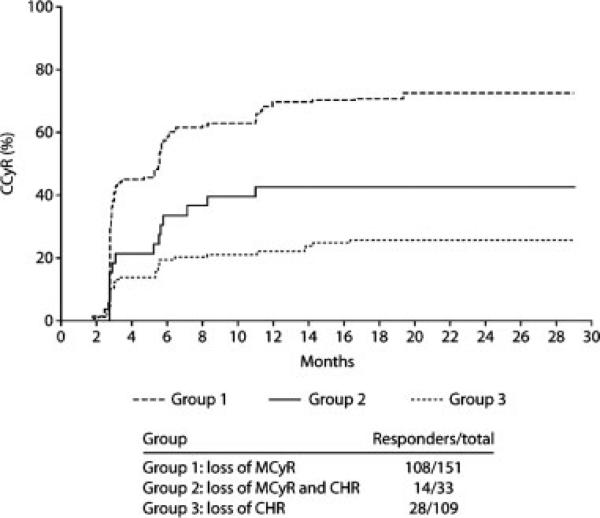
Among patients with loss of MCyR on imatinib (Group 1; early intervention), 72% achieved a CCyR with dasatinib treatment compared with 42% of patients who were treated after loss of both MCyR and CHR (Group 2; late intervention) (Table 3). Rates of MMR (data from Study CA180-013 only) were 60% in Group 1 (n = 47 evaluable patients) and 29% in Group 2 (n = 17 evaluable patients). Among the patients who were treated after loss of CHR and without prior MCyR to imatinib (Group 3), 26% achieved a CCyR, and 26% achieved an MMR (n = 50 evaluable patients) (Table 3). The time to CCyR among responding patients was similar in the 3 groups (Fig. 1). The median time to CCyR (95% confidence intervals [95% CI]) among responding patients was 2.9 months (95% CI, 2.8-3.1 months) in Group 1, 4.2 months (95% CI, 2.8-5.8 months) in Group 2, and 3.2 months (95% CI, 2.8-5.5 months) in Group 3. The majority of patients (80%-85%) who achieved a CCyR did so within the first 6 months of treatment.
FIGURE 1.
The time to complete cytogenetic response in patients who received dasatinib after loss of response to prior imatinib. MCyR indicates major cytogenetic response; CHR, complete hematologic response.
Response Rates in Patients With BCR-ABL Mutations
Across all 3 groups, the rates of CCyR did not appear to be affected by the presence of pre-existing BCR-ABL mutations, although patient numbers were relatively low in some cases (Table 4). For patients with and without any BCR-ABL mutation, the rates of CCyR were 77% versus 68% in Group 1 (loss of MCyR), respectively; 45% versus 50% in Group 2 (loss of MCyR and CHR), respectively; and 25% versus 31% in Group 3 (loss of CHR without prior MCyR). In Groups 1 and 3, similar CCyR rates also were observed in patients with mutations within the P-loop region compared with all patients in those groups. In Group 2, the CCyR rate was lower in patients with P-loop mutations, although the patient numbers were small (n = 7).
Table 4.
Rates of Complete Cytogenetic Response (All Studies) and Major Molecular Response (CA180-013 Study Only) in Patients With Pre-existing BCR-ABL Mutations Treated With Dasatinib After Loss of Response to Prior Imatinib
| Patients, n/N (%) | |||
|---|---|---|---|
| Variable | Group 1: Loss of MCyR | Group 2: Loss of MCyR and CHR | Group 3: Loss of CHR |
| CCyR | |||
| No BCR-ABL mutation | 51/75 (68) | 5/10 (50) | 8/26 (31) |
| Any BCR-ABL mutation | 46/60 (77) | 9/20 (45) | 19/77 (25) |
| T315I | 0/2 (0) | 0/1 (0) | 0/1 (0) |
| F317L | 1/1 (100) | – | 0/4 (0) |
| F359C/I/V | 8/8 (100) | – | 1/6 (17) |
| P-loop region* | 13/19 (68) | 2/7 (29) | 7/30 (23) |
| L248V | 1/2 (50) | – | 2/6 (33) |
| G250E | 5/6 (83) | 1/4 (25) | 1/12 (8) |
| Q252H | 0/1 (0) | – | 1/2 (50) |
| Y253F/H | 6/8 (75) | 1/1 (100) | 2/8 (25) |
| E255K/V | 1/2 (50) | 0/2 (0) | 1/4 (25) |
| Sample unavailable | 11/16 (69) | 0/3 (0) | 1/6 (17) |
| MMR | |||
| No BCR-ABL mutation | 13/24 (54) | 1/3 (33) | 5/12 (42) |
| Any BCR-ABL mutation | 13/21 (62) | 4/13 (31) | 8/35 (23) |
| Sample unavailable | 2/2 (100) | 0/1 (0) | 0/3 (0) |
MCyR indicates major cytogenetic response; CHR, complete hematologic response; CCyR, complete cytogenetic response; T315I, threonine-to-isoleucine mutation at codon 315; F317L, phenylalanine-to-leucine mutation at codon 317; F359C/I/V, phenylalanine-to-cysteine, valine, or isoleucine mutation at codon 359; P-loop, adenosine triphosphate phosphate-binding loop; L248V, leucine-to-valine mutation at codon 248; G250E, glycine-to-glutamic acid mutation at codon 250; Q252H, glutamine-to-histidine mutation at codon 252; Y253F/H, tyrosine-to-phenylalanine or histidine mutation at codon 253; E255K/V, glutamic acid-to-lysine or valine mutation at codon 255; MMR, major molecular response.
Residues 248 through 256.
In the loss of MCyR group (Group 1) and the loss of MCyR/CHR group (Group 2), MMR rates in evaluable patients with and without BCR-ABL mutations were similar (Group 1: 62% vs 54%, respectively; Group 2: 31% vs 33%, respectively). In the loss of CHR without prior MCyR group (Group 3), MMR rates appeared to be lower in patients with BCR-ABL mutations versus patients without mutations (24% vs 42%, respectively). Consistent with previous observations, no responses were observed in patients who had a T315I mutation.
Event-free Survival, Transformation-free Survival, and Overall Survival
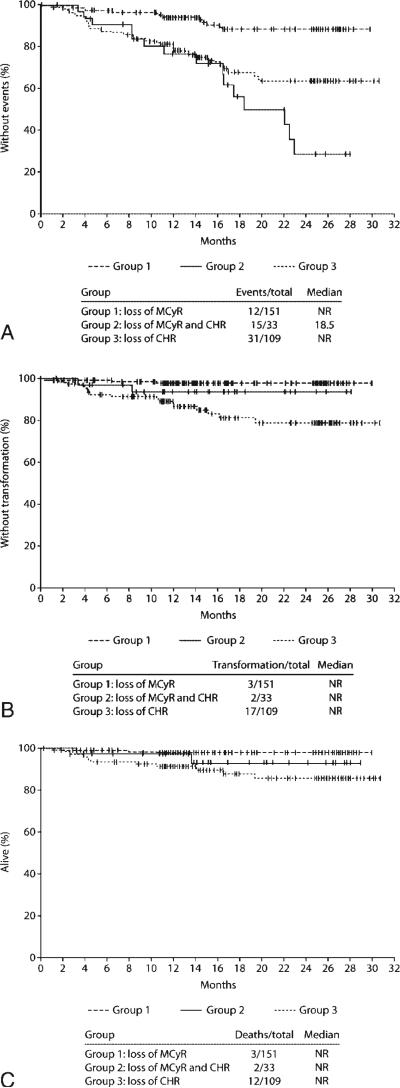
Treatment with dasatinib after loss of MCyR (Group 1) resulted in a lower rate of events (as defined above) compared with treatment after loss of both MCyR and CHR (Group 2) (Fig. 2A). The rates of EFS at 24 months was 89% in Group 1 and 29% in Group 2. In Group 2, the median EFS was 18.5 months (the median was not reached in Group 1). Rates of TFS and OS at 24 months were 98% (TFS) and 98% (OS) in Group 1, and 93% (TFS) and 93% (OS) in Group 2.
FIGURE 2.
Kaplan-Meier analysis of patients who received dasatinib after loss of response to prior imatinib. (A) Event-free survival. (B) Transformation-free survival. (C) Overall survival. MCyR indicates major cytogenetic response; CHR, complete hematologic response.
Patients who received dasatinib after loss of CHR without prior MCyR on imatinib (Group 3) had a lower 24-month EFS rate (64%) compared with patients who were treated after loss of MCyR (Group 1) but a higher rate compared with patients who were treated after loss of both MCyR and CHR (Group 2). However, patients in Group 3 had a lower 24-month TFS rate (79%) and OS rate (86%) than patients in both Groups 1 and 2.
DISCUSSION
Dasatinib is an effective treatment for patients who have experienced imatinib failure. The data in this analysis demonstrate that response rates, EFS, TFS, and OS were most favorable when dasatinib was administered early after imatinib failure, ie, after the loss of MCyR, rather than after the loss of both MCyR and CHR. These observations are in line with what has been reported previously for second-line treatment with imatinib after prior IFN failure.10,11 It is noteworthy that responding patients from each group in this analysis appeared to achieve their responses within a similar timeframe, as demonstrated by the overlapping CIs for the median time to CCyR, although this may have been caused in part to the smaller sample size in Group 2. This suggests the possibility that the improved outcomes with early intervention are not explained by a more rapid time to response, which may be prognostically favorable in CML.15 However, patients who received earlier intervention with dasatinib in Group 1 had the highest rate of CCyR. Similar CCyR rates were achieved by patients in this group who received dasatinib 70 mg twice daily or dasatinib 100 mg once daily.
In previous studies, BCR-ABL mutations have been detected in 50% to 90% of patients with acquired resistance to imatinib.16-19 In the current analysis, patients who had lost both MCyR and CHR on imatinib (Group 2) or who had lost their CHR without prior MCyR (Group 3) appeared to have a higher incidence of BCR-ABL mutations compared with patients who had lost MCyR only (Group 1). However, as reported previously,7,13 the rates of CCyR with dasatinib therapy were similar in patients with mutations and patients without mutations. Differences between the groups also did not appear to result from an increased incidence of patients with a T315I mutation or other mutations that have been associated with relative dasatinib insensitivity. However, mutation data in this analysis should be interpreted with caution given the relatively low number of patients, particularly in Group 2. In a report of patients with primary cytogenetic resistance to imatinib, the development of BCR-ABL mutations was associated with increased disease progression and shorter survival,20 although no survival difference was reported in an analysis that included patients with other categories of imatinib resistance, possibly reflecting the effectiveness of second-generation Bcr-Abl inhibitors.21 Mutations can be detected several months before hematologic or cytogenetic recurrence on imatinib,19 and patients who develop mutations are at significantly increased risk of disease recurrence or progression.19-20,22,23 However, mutations also have been detected in patients with stable cytogenetic remission.24,25 Patients who have a detectable BCR-ABL mutation during imatinib treatment who have any sign of loss of response or disease progression should receive second-line treatment at the earliest opportunity.
The results presented here suggest that the higher level of insensitivity to dasatinib in Groups 2 and 3 compared with Group 1 is a result of resistance mechanisms other than BCR-ABL mutations. Several other potential mechanisms of imatinib resistance have been described, including increased expression of the BCR-ABL gene through gene amplification, decreased intracellular imatinib concentrations resulting from plasma-binding proteins, decreased drug influx, increased drug efflux, decreased oral bioavailability, and activation of Src-family kinases, such as Lyn.5,26,27 However, the high potency of dasatinib compared with imatinib and the lesser impact of transporters in dasatinib transport might be expected to overcome these types of imatinib resistance. A more relevant mechanism may be clonal evolution (CE), ie, the acquisition of additional chromosomal abnormalities in Ph-positive cells, which may lead to BCR-ABL independence. CE has been detected at a frequency similar to BCR-ABL mutation in patients with late CML-CP (after IFN) and hematologic resistance to imatinib.28 In a separate analysis in late CML-CP, CE was identified as an independent adverse prognostic factor for hematologic recurrence (P < .0001).29 During imatinib treatment, loss of MCyR indicates that imatinib no longer is providing effective inhibition of Bcr-Abl. Continuation of imatinib treatment until loss of CHR may create the opportunity for leukemic stem cells to accumulate additional genetic changes, potentially leading to a greater likelihood of resistance to all Bcr-Abl inhibitors. Data on the relative incidence of CE before dasatinib were not available in the current analysis, and this would be an interesting area for future study. It also is possible that other, unknown mechanisms of resistance may be involved in differential responses to dasatinib.
A proportion of patients who received dasatinib after loss of CHR without prior MCyR (Group 3) achieved a CCyR or an MMR, although their rates were lower than those in the other groups. Patients in Group 3 also had the lowest TFS and OS rates. The rate of EFS in Group 3, which was higher than the rate in Group 2 (loss of both MCyR and CHR), may have been affected by the definitions of events used in this analysis, particularly loss of MCyR. A much lower proportion of patients in Group 3 achieved an MCyR and could subsequently record loss of MCyR as an event, which may explain in part the lack of correlation with EFS in these patients. It is possible that patients within this group, who did not achieve an MCyR with imatinib, had a disease biology different from that in patients who did achieve a MCyR. Further studies will be required to investigate characteristics among patients who have different types of primary resistance to imatinib.
Overall, the results of the current analysis suggest that patients who have lost their response to imatinib are more likely to respond to dasatinib and to have prolonged survival without disease progression if they receive early intervention with dasatinib (at loss of MCyR). Regular monitoring is essential during imatinib treatment to determine when resistance first develops so that patients can derive maximal benefit from alternative treatment.
Acknowledgments
FUNDING STATEMENT:
ARTICLE ID : CNCR _24325
This article was supported by National Institute of Health (P30 CA016672).
Footnotes
Conflict of Interest Disclosures
Funding for clinical trials discussed in this report, statistical analysis, and medical writing assistance was provided by Bristol-Myers Squibb.
Drs. Cortes, O'Brien, and Kantarjian have received research support from Bristol-Myers Squibb and Novartis.
Dr. Ravandi has received research support and honoraria from Bristol-Myers Squibb.
Dr. Borthakur is a member of the Speaker's Bureau for Bristol-Myers Squibb and Novartis.
Drs. Liu, Bleickardt, and Chen are employees of Bristol-Myers Squibb.
References
- 1.Melo JV, Barnes DJ. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer. 2007;7:441–453. doi: 10.1038/nrc2147. [DOI] [PubMed] [Google Scholar]
- 2.Cortes J. Natural history and staging of chronic myelogenous leukemia. Hematol Oncol Clin North Am. 2004;18:569–584. doi: 10.1016/j.hoc.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 4.Roy L, Guilhot J, Krahnke T, et al. Survival advantage from imatinib compared with the combination interferon-alpha plus cytarabine in chronic-phase chronic myelogenous leukemia: historical comparison between 2 phase 3 trials. Blood. 2006;108:1478–1484. doi: 10.1182/blood-2006-02-001495. [DOI] [PubMed] [Google Scholar]
- 5.Apperley JF. Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8:1018–1029. doi: 10.1016/S1470-2045(07)70342-X. [DOI] [PubMed] [Google Scholar]
- 6.O'Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 7.Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22:1200–1206. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- 8.Guilhot F, Apperley J, Kim DW, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109:4143–4150. doi: 10.1182/blood-2006-09-046839. [DOI] [PubMed] [Google Scholar]
- 9.Cortes J, Rousselot P, Kim DW, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2007;109:3207–3213. doi: 10.1182/blood-2006-09-046888. [DOI] [PubMed] [Google Scholar]
- 10.Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 11.Kantarjian HM, Cortes JE, O'Brien S, et al. Long-term survival benefit and improved complete cytogenetic and molecular response rates with imatinib mesylate in Philadelphia chromosome-positive chronic-phase chronic myeloid leukemia after failure of interferon-alpha. Blood. 2004;104:1979–1988. doi: 10.1182/blood-2004-02-0711. [DOI] [PubMed] [Google Scholar]
- 12.Kantarjian H, O'Brien S, Talpaz M, et al. Outcome of patients with Philadelphia chromosome-positive chronic myelogenous leukemia post-imatinib mesylate failure. Cancer. 2007;109:1556–1560. doi: 10.1002/cncr.22569. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian H, Pasquini R, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–5150. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 14.Shah NP, Kim DW, Kantarjian HM, et al. Dasatinib 50 mg or 70 mg BID compared to 100 mg or 140 mg once daily in patients with CML in chronic phase (CP) who are resistant or intolerant to imatinib: 1-year results of CA180034 [abstract]. J Clin Oncol. 2007;25(suppl):358s. Abstract 7004. [Google Scholar]
- 15.Quintas-Cardama A, Kantarjian HM, Jones D, et al. Delayed achievement of molecular responses is associated with increased risk of progression among patients with chronic myelogenous leukemia in chronic phase treated with imatinib [abstract]. Blood. 2006;108(suppl):613a. Abstract 432. [Google Scholar]
- 16.Soverini S, Colarossi S, Gnani A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. 2006;12:7374–7379. doi: 10.1158/1078-0432.CCR-06-1516. [DOI] [PubMed] [Google Scholar]
- 17.Hochhaus A, Kreil S, Corbin AS, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia. 2002;16:2190–2196. doi: 10.1038/sj.leu.2402741. [DOI] [PubMed] [Google Scholar]
- 18.Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 19.Ernst T, Erben P, Muller MC, et al. Dynamics of BCRABL mutated clones prior to hematologic or cytogenetic resistance to imatinib. Haematologica. 2008;93:186–192. doi: 10.3324/haematol.11993. [DOI] [PubMed] [Google Scholar]
- 20.Soverini S, Martinelli G, Rosti G, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: a study by the GIMEMA Working Party on Chronic Myeloid Leukemia. J Clin Oncol. 2005;23:4100–4109. doi: 10.1200/JCO.2005.05.531. [DOI] [PubMed] [Google Scholar]
- 21.Jabbour E, Kantarjian H, Jones D, et al. Characteristics and outcomes of patients with chronic myeloid leukemia and T315I mutation following failure of imatinib mesylate therapy. Blood. 2008;112:53–55. doi: 10.1182/blood-2007-11-123950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Branford S, Rudzki Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102:276–283. doi: 10.1182/blood-2002-09-2896. [DOI] [PubMed] [Google Scholar]
- 23.Khorashad JS, de Lavallade H, Apperley JF, et al. Finding of kinase domain mutations in patients with chronic phase chronic myeloid leukemia responding to imatinib may identify those at high risk of disease progression. J Clin Oncol. 2008;26:4806–4813. doi: 10.1200/JCO.2008.16.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherbenou DW, Wong MJ, Humayun A, et al. Mutations of the BCR-ABL-kinase domain occur in a minority of patients with stable complete cytogenetic response to imatinib. Leukemia. 2007;21:489–493. doi: 10.1038/sj.leu.2404554. [DOI] [PubMed] [Google Scholar]
- 25.Chu S, Xu H, Shah NP, et al. Detection of BCR-ABL kinase mutations in CD34+ cells from chronic myelogenous leukemia patients in complete cytogenetic remission on imatinib mesylate treatment. Blood. 2005;105:2093–2098. doi: 10.1182/blood-2004-03-1114. [DOI] [PubMed] [Google Scholar]
- 26.Li S. Src-family kinases in the development and therapy of Philadelphia chromosome-positive chronic myeloid leukemia and acute lymphoblastic leukemia. Leuk Lymphoma. 2008;49:19–26. doi: 10.1080/10428190701713689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, Meng F, Kong LY, et al. Association between imatinib-resistant BCR-ABL mutation-negative leukemia and persistent activation of LYN kinase. J Natl Cancer Inst. 2008;100:926–939. doi: 10.1093/jnci/djn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lahaye T, Riehm B, Berger U, et al. Response and resistance in 300 patients with BCR-ABL-positive leukemias treated with imatinib in a single center: a 4.5-year follow-up. Cancer. 2005;103:1659–1669. doi: 10.1002/cncr.20922. [DOI] [PubMed] [Google Scholar]
- 29.O'Dwyer ME, Mauro MJ, Blasdel C, et al. Clonal evolution and lack of cytogenetic response are adverse prognostic factors for hematologic relapse of chronic phase CML patients treated with imatinib mesylate. Blood. 2004;103:451–455. doi: 10.1182/blood-2003-02-0371. [DOI] [PubMed] [Google Scholar]