Abstract
After viewing short video clips of individuals with Parkinson’s disease (PD) who varied in the symptoms of facial masking (reduced expressivity) and abnormal bodily movement (ABM: including tremor and related movement disorders), older adult observers provided their first impressions of targets’ social positivity. Impressions of targets with higher masking or ABM were more negative than impressions of targets with lower masking or ABM. Furthermore, masking was more detrimental for impressions of women and when observers considered emotional relationship goals, whereas ABM was more detrimental for instrumental relationship goals. This study demonstrated the stigmatizing effects of both reduced and excessive movement.
Keywords: social perception, facial masking, tremor, Parkinson’s disease, stigma
Most research addressing how nonverbal behavior contributes to first impressions has been conducted with young, healthy students (Feldman & Tyler, 2006). This work demonstrates that many communication channels contribute to first impressions (Patterson & Manusov, 2006), and that more expressivity in these channels typically leads to more positive impressions (Bar, Neta, & Linz, 2006; Riggio & Friedman, 1986; Shrout & Fiske, 1981). However, less research has considered how aging may affect first impressions based on nonverbal behavior. Chronic conditions associated with aging can lead to atypical nonverbal expressivity by creating a dearth or excess of movement, such as reduced smiling and blinking; tremor; or uncoordinated, or uncontrollable movements. Atypical expressivity could compromise social relationships by reducing positive first impressions.
This study focused on Parkinson’s disease (PD), one of the most common chronic neurodegenerative disorders (Willis, Evanoff, Lian, Criswell, & Racette, 2010). PD represents natural variability in nonverbal behavior: individuals with PD may be indistinguishable from healthy controls or may have severe difficulties with nonverbal behavior. This study extends the literature by assessing the stigmatizing influence of two PD symptoms, facial masking and abnormal bodily movement (ABM), on older adults’ impressions of individuals with PD as potential friends and acquaintances. This study also tested the moderating role of gender and relationship goals (emotional vs. instrumental/physical supportiveness).
With increasing facial masking in PD, facial movement becomes slower and more effortful, and expressions of emotion and social engagement become less spontaneous (Simons, Pasqualini, Reddy, & Wood, 2004; Smith, Smith, & Ellgring, 1996; Spielman, Borod, & Ramig, 2003). Higher facial masking has been perceived more negatively than typical expressivity (Pentland, 1991; Pentland et al., 1987; Pentland et al., 1988; Tickle-Degnen, Zebrowitz, & Ma, 2011), but only one study to date has investigated how age peers perceive masking (Hemmesch, Tickle-Degnen, & Zebrowitz, 2009).
This study is among the first to examine how ABM, including tremor and jerky uncoordinated bodily movements, affects first impressions. ABM can jeopardize nonverbal communication (Pentland, 1991; Pitcairn et al., 1990) and may be associated with impressions of physical disability, although this hypothesis has not yet been tested.
Facial masking has been found to be more detrimental to impressions of the social supportiveness of women with PD than men (Hemmesch et al., 2009; Tickle-Degnen et al., 2011). ABM may be more harmful for observers’ impressions of men with PD than women because physical ability is more closely associated with male gender stereotypes (Wood & Eagly, 2002). Moreover, because the face is important for emotional expression (Darwin, 1889), higher facial masking may lead observers to question individuals’ ability to engage in the emotional tasks that support relationships, whereas ABM may lead observers to question individuals’ ability to perform instrumental tasks that support relationships, such as helping out with shopping or doing physical recreation activities with others.
Method
Observers
A sample of 59 non-institutionalized older adults (35 women) were recruited from the Boston area to view and form impressions of videotaped targets with PD. Observers were at least 55 years of age (M = 76.37, SD = 6.49) and able to communicate in English. Experience with PD was not required for participation, nor were individuals with personal (n = 29) or professional experience (n = 14) with PD excluded.
Target Stimuli
Twenty-four Caucasian Americans with PD (12 women; age M = 69.08, SD = 7.64) at Hoehn &Yahr Stage 2–3 (bilateral, mild-to-moderate symptoms) were selected as targets from a database of 106 participants without depression or dementia who were videotaped during interviews about their daily lives and who agreed to have their data used for other research (Tickle-Degnen, Ellis, Saint-Hilaire, Thomas, & Wagenaar, 2010). Targets were ‘on’ medication during their interviews, and were selected to not overlap with those from Hemmesch et al. (2009). Six targets had lower masking and lower ABM, six had lower masking and higher ABM, six had higher masking and lower ABM, and six had higher masking and higher ABM (with three men and women in each cell). Targets were matched on apparent age, attractiveness, and self-reported social positivity.
Facial masking
Categorization into lower or higher facial masking was based on ratings by six trained raters (see Hemmesch et al., 2009 for more information regarding the ratings). Ratings showed high inter-rater reliability, ICC = .97. Targets with higher masking were significantly less expressive (M = 1.86, SD = .28) than those with lower masking (M = 3.21, SD = .29; t(22) = 11.63, p < .001, r = .93). Although targets were matched as carefully as possible, there was a difference in masking across ABM cells (F(1, 16) = 6.64, p = .02). In the higher masking condition, targets with lower ABM showed more facial masking than targets with higher ABM (lower ABM: M = 1.68, SD = .11; higher ABM: M = 2.04, SD = .11; t(22) = 2.26, p = .04, r = .43). However facial expressivity did not differ significantly in the targets with lower versus higher ABM for the lower masking condition (t(22) = 1.39, p = .18, r = .28). There were no differences in facial masking across Target Gender cells (F < .10).
Abnormal bodily movement (ABM)
Categorization into lower or higher ABM was determined using the same raters during a separate session. Raters responded to items modeled after the Unified Parkinson’s Disease Rating Scale (UPDRS) motor examination (Fahn, Marsden, Calne, & Goldstein, 1987) to assess the severity of tremor/excessive abnormal movement in the trunk and each extremity. ABM ratings had high inter-rater reliability, ICC = .88. Targets with higher ABM showed significantly more abnormal movement (M = 1.98, SD = .51) than targets with lower ABM (M = 1.10, SD = .11; t(22) = 5.79, p < .001, r = .78). There were no differences in ABM across Facial Masking and Target Gender cells (F’s < 2.00).
Self-reported social positivity
Factor analysis was used to select items from the Parkinson’s Disease Questionnaire 39-item version (Peto, Jenkinson, Fitzpatrick, & Greenhal, 1995), the Ten Item Personality Inventory (Gosling, Rentfrow, & Swann, 2003), and an activity preference form (adapted from Clark & Bond, 1995) to estimate interest/ability to engage in emotionally and instrumentally supportive and straining behaviors. The 8-item support and 4-item strain scales demonstrated acceptable internal consistency, Cronbach’s α = .78 and α = .63, respectively. A composite was created by subtracting targets’ self-reported strain from their self-reported support. Self-reported social positivity did not differ by Masking (t(22) = −.14, p = .89) or ABM categories (t(22) = .31, p = .76), or by Target Gender (t(22) = −.90, p = .38).
Observer Measures
Observer demographics
General information such age, sex, income, and personal and professional experience with PD was obtained.
Perceived social positivity
A perceived social positivity composite was created from items measuring perceived supportiveness, caregiving expectations, and estimated reciprocity because these three measures were moderately correlated (rs > .32, ps < .05) and showed similar results (Table 1). Perceived supportiveness was calculated by subtracting perceived strain from perceived support ratings, which were measured with 12 items based on the Positive and Negative Social Exchange Assessment (Newsom, Rook, Nishishiba, Sorkin, & Mahan, 2005) that were modified for this study (Cronbach’s αs = .74–.90). Caregiving expectations were calculated by subtracting perceived burden from perceived reward ratings, which included 4 items that asked whether observers felt each target would be likely to be emotionally draining or taxing to provide care for, physically draining or taxing to provide care for, emotionally satisfying or rewarding to provide care for, and easy to provide care for physically. Estimated reciprocity was calculated by subtracting observers expected contributions (how much emotional and instrumental support they thought they would provide each target) from targets’ perceived supportiveness. These measures were combined so that a positive perceived social positivity score indicated that targets were rated as more supportive or rewarding as friends, whereas a negative score indicated that targets were rated as more straining or difficult. The emotional and instrumental positivity composites had good reliabilities: Cronbach’s α = .72 & .79, respectively.
Table 1.
Summary of hypothesis tests from the social positivity subscales. The effect size ‘r’ is drawn from repeated measures ANOVAS.
| Social support | Social strain | Caregiving reward | Caregiving burden | Reciprocity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Observers perceive… | Effect Size | Supported? | Effect Size | Supported? | Effect Size | Supported? | Effect Size | Supported? | Effect Size | Supported? |
| Higher masking more negatively than lower masking | .81* | Y | .68* | Y | .71* | Y | .59* | Y | .32* | N |
| Higher ABM more negatively than lower ABM | .67* | Y | .74* | Y | .61* | Y | .69* | Y | .58* | Y |
| Higher masking as more detrimental for women than men | .26* | Y | .53* | Y | .48* | Y | .49* | Y | .00 | N |
| Higher ABM as more detrimental to men than women | .06 | N | .03 | N | .21+ | N | .24+ | N | .27* | Y |
| Higher masking as more detrimental for emotional than instrumental goals | .63* | Y | .47* | Y | .53* | Y | .51* | Y | .30* | Y |
| Higher ABM as more detrimental for instrumental than emotional goals | .86* | Y | .75* | Y | .68* | Y | .55* | Y | .26* | Y |
p < .05
Procedure
After providing informed consent, observers completed the protocol individually or in small groups. Observers were informed that each target they would see was diagnosed with PD. Each observer saw all targets in one of two randomized presentation orders. Observers watched a target’s 20-second clip in its entirety, then completed their ratings for that target while watching the clip on repeat for approximately three minutes.
Results
Analysis Plan
Hypotheses were tested using a 2 (Facial Masking: Lower vs. Higher) × 2 (Abnormal Bodily Movement: Lower vs. Higher) × 2 (Target Gender: Male vs. Female) repeated measures ANOVA for the relationship interest impressions. For social positivity impressions, a 2 (Facial Masking: Lower vs. Higher) × 2 (Abnormal Bodily Movement: Lower vs. Higher) × 2 (Target Gender: Male vs. Female) × 2 (Relationship Goal: Emotional vs. Instrumental) repeated measures ANOVA was conducted. Contrast analyses were calculated to compare targets who showed both higher ABM and masking against the other targets to test for amplifying effects of multiple symptoms. Effect sizes were measured with the r (Rosenthal & Rosnow, 1991). A higher r indicates a stronger effect, with r = .50 signifying a large effect (Cohen, 1992). Observer variables (e.g., gender, experience with PD) were not significantly correlated with the dependent measures (Pearson rs < .25; most Pearson rs < .15), so they were excluded from the ANOVAs.
Facial Masking
There were significant main effects for facial masking for both relationship interest (F(1, 58) = 81.25, p < .001, r = .76) and social positivity (F(1, 58) = 45.38, p < .001, r = .66). Observers expressed less interest in relationships with targets with higher masking (M = 3.15, SE = .08) than lower masking (M = 3.77, SE = .08), and perceived targets with higher masking as less positive social partners (M = −.18, SE = .05) than those with lower masking (M = .18, SE = .04).
The masking effects were qualified by significant two-way interactions with target gender (relationship interest: F(1, 58) = 10.84, p = .002, r = .40; social positivity: F(1, 58) = 29.19, p < .001, r = .58). Observers showed less interest in relationships with women than men in the higher masking condition (men: M = 3.27, SE = .09; women: M = 3.04, SE = .09; t(58) = 3.22, p = .002, r = .39), but did not differentiate between men and women in the lower masking condition (men: M = 3.76, SE = .09; women: M = 3.77, SE = .09; t(58) = .19, p = .85, r = .02). Similarly, there was a larger detrimental masking effect for observers’ impressions of women’s social positivity: impressions of men and women differed more in the higher masking condition (men: M = −.07, SE = .06; women: M = −.29, SE = .06; t(58) = 5.57, p < .001, r = .59; Figure 1) than in the lower masking condition (men: M = .14, SE = .05; women: M = .22, SE = .05; t(58) = 2.07, p = .04, r = .26), although both were significant.
Figure 1.
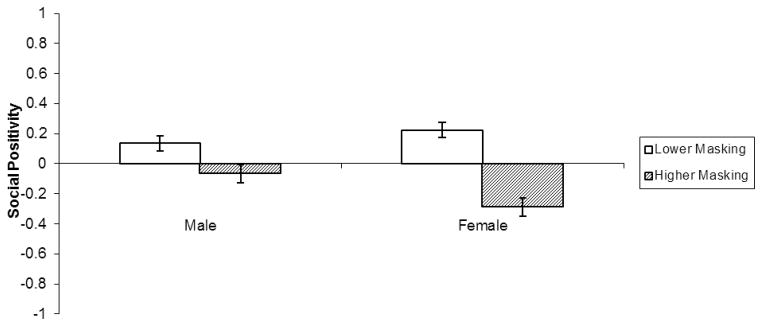
Facial Masking by Target Gender two-way interaction for observers’ perceptions of targets’ social positivity
For social positivity, the masking effect was also qualified by a two-way interaction with relationship goals (F(1, 58) = 38.64, p < .001, r = .63; Figure 2): the negative masking effect was larger when observers considered emotional goals (lower masking: M = .27, SE = .05; higher masking: M = −.26, SE = .07; t(58) = 7.33, p < .001, r = .69) than instrumental goals (lower masking: M = .09, SE = .06; higher masking: M = −.09, SE =.06; t(58) = 4.13, p < .001, r = .48).
Figure 2.
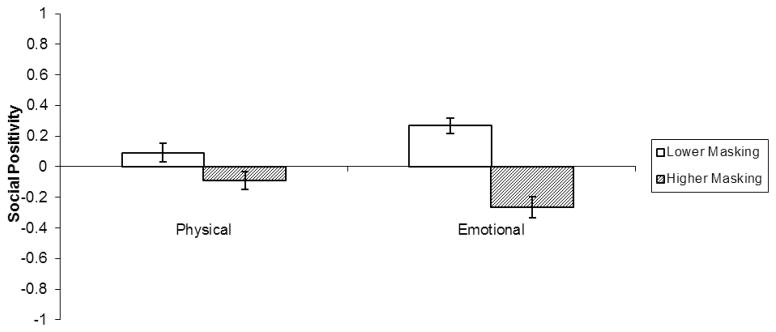
Facial Masking by Relationship Goal two-way interaction for observers’ perceptions of targets’ social positivity
ABM
Observers perceived targets with higher ABM as less positive social partners (M = −.19, SE = .04) than those with lower ABM (M = .20, SE = .05; F(1, 58) = 101.45, p < .001, r = .80). However, there was no main effect for ABM on relationship interest (F(1, 58) = 1.56, p = .22, r = .16; lower ABM: M = 3.43, SE = .07; higher ABM: M = 3.48, SE = .08), nor did the interaction between ABM and target gender approach significance for relationship interest (F(1, 58) = .002, p = .96, r = .01) or social positivity (F(1, 58) = .002, p = .97, r = .01).
For social positivity ratings, the ABM effect was qualified by a significant two-way interaction with relationship goals (F(1, 58) = 131.25, p < .001, r = .83; Figure 3): the negative ABM effect was larger when observers considered instrumental goals (lower ABM: M = .34, SE = .06; higher ABM: M = −.34, SE = .05; t(58) = 12.19, p < .001, r = .85) than when considering emotional goals (lower ABM: M = .05, SE = .06; higher ABM: M = −.04, SE = .05; t(58) = 2.79, p = .009, r = .33).
Figure 3.
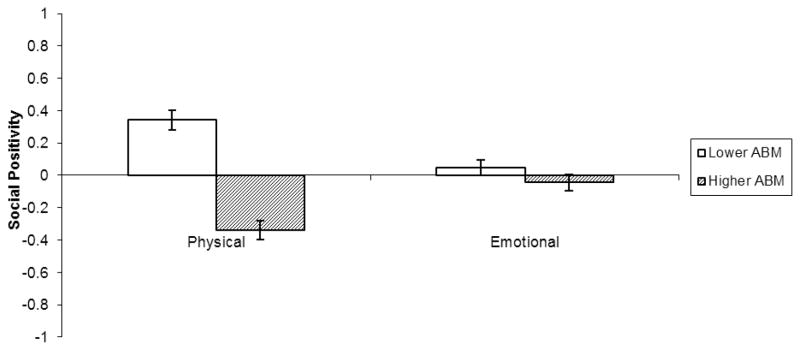
ABM by Relationship Goal two-way interaction for observers’ perceptions of targets’ social positivity
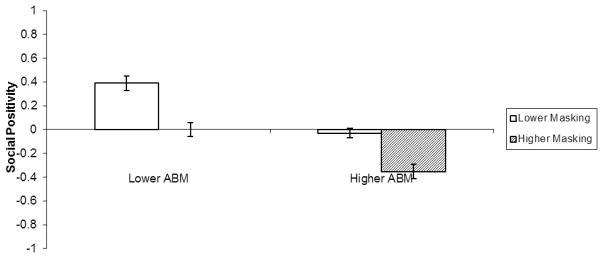
Interactions between masking and ABM suggest that symptoms may have a multiplicative effect on relationship interest (F(1, 58) = 6.19, p < .05, r = .31), but not social positivity (F(1, 58) = 1.23, p = .27, r = .14). Contrast analyses revealed that observers were least interested in relationships with targets high in both facial masking and ABM than targets in the three other groups combined (t(58) = 7.87, p < .01, r = .72), and that those targets were perceived as the most negative social partners (t(58) = 10.38, p < .01, r = .33; Figure 4).
Figure 4.
ABM by Facial Masking two-way interaction for observers’ perceptions of targets’ social positivity
Additional analyses
A main effect for target gender was also observed for social positivity ratings: observers perceived men as more positive social partners (M = .04, SE = .05) than women (M = −.03, SE = .04; F(1, 58) = 4.94, p = .03, r = .28). Target gender interactions are described above.
As a preliminary step toward addressing the relative effects of masking and ABM using continuous symptom severity ratings, regressions were conducted using targets as the unit of analysis (n = 24). Regressions included masking, ABM, and target gender in the first step; interaction terms were entered in the second step. Standardized betas revealed that masking (β = −.81, p = .00) was more strongly associated with relationship interest than ABM (β = .18, p = .37) or target gender (β = −.11, p =.48), and the addition of masking by target gender, ABM by target gender, and masking by ABM interaction terms failed to reach statistical significance (β = .12, p = .76, β = .02, p = .94, β = .27, p = .19, respectively). For the social positivity ratings, both masking (β = −.50, p = .02) and ABM (β = −.45, p = .046) were significantly associated with ratings, but target gender was not (β = −.09, p = .62), nor was the addition of masking by target gender, ABM by target gender, and masking by ABM interaction terms (β = .17, p = .33, β = .19, p = .36, β = −.03, p = .89, respectively). These analyses suggest that masking may have broader social implications than ABM, although both symptoms showed moderate relationships with social positivity ratings.
Discussion
The current findings regarding the differential and detrimental effects of atypical nonverbal behavior in PD highlight the role of facial and bodily expressivity across the lifespan, and the moderating roles of gender and relationship goals. The relatively small magnitude differences in facial masking and ABM severity in this sample had powerful effects on older adults’ first impressions of social quality, and this stigma was amplified when targets showed both higher masking and ABM – the least typical nonverbal behavior. Atypical nonverbal expressivity may disqualify individuals with PD from being considered as positive social partners regardless of their actual supportiveness, which may contribute to the social complaints in PD (Ellgring et al., 1993; Nijhof, 1995).
This study provided the first experimental examination of the influence of ABM on first impressions of social positivity in PD. The ABM effect represents a stigma against atypical bodily expressivity. The discrepancy between self-reported and perceived positivity for individuals with higher ABM is consistent with work suggesting that many types of atypical movement in PD may be perceived negatively (Pentland, 1991). The stronger effect of ABM than facial masking on perceptions of social positivity suggest that observers are more concerned about ABM when considering the quality of potential relationships.
Diminished facial expressivity also appears to violate observers’ expectations for social interaction, and can lead observers to believe that individuals with higher facial masking are less socioemotionally competent (Hemmesch et al., 2009; Tickle-Degnen et al., 2011). Many observers in this study reported that smiling was important for their first impressions. However, diminished smiling was often misattributed to targets’ personality, not to PD, or was mistaken for dementia or depression. The misattribution of facial masking is consistent with studies documenting the social impact of PD (Schrag et al., 2007; Shulman et al., 2002; Tickle-Degnen & Lyons, 2004). This study suggests that reduced smiling due to PD (Katsikitis & Pilowsky, 1991) or smiles that are perceived as less sincere (Pitcairn, Clemie, Gray, and Pentland, 1990), may have negative implications for relationships with age peers. When compared to age-matched controls, individuals with PD have been found to experience similarly intense emotions, but to be aware that their emotional expressivity was decreased (Mikos et al., 2009). Thus, negative reactions to facial masking may reflect stigma against reduced expressivity. The stronger masking effect for relationship interest than social positivity suggests that masking-related stigma may threaten initial decisions about whom to approach or avoid. Furthermore, the combination of higher masking and ABM reveal that multiple forms of atypical behavior – whether dearth or excess – can interact to create stronger negative impressions.
Even though greater expressivity is often linked to more positive impressions of both men and women (Riggio & Friedman, 1986; Shrout & Fiske, 1981), the larger masking effect for women is consistent with gender norms that women should be more expressive than men (Briton & Hall, 1995; DePaulo, 1992; Diekman & Goodfriend, 2006). Stronger negative impressions of women with masking may contribute to women with PD reporting more problems with close relationships and stigma than men (Huang, 2009; Solimeo, 2008). This study did not support the new hypothesis that higher ABM would be more discrediting for men than women with PD. ABM may violate gender norms for both men and women with PD. Although gender stereotypes often align physical ability more closely with men (Wood & Eagly, 2002), ABM can threaten women’s ability to engage in gender stereotypical behaviors, such as personal grooming and housework (Caap-Ahlgren, et al., 2002).
Relationship goals also moderated the effects of atypical expressivity, demonstrating that facial and bodily cues may have different social implications for older adults. The larger masking effect observed when observers considered emotional goals may stem from masking being interpreted as hostility, coldness, depression, or aloofness (Pentland, 1991; Pentland et al., 1987; 1988). In contrast, higher ABM was more detrimental when observers considered instrumental goals, suggesting that higher ABM is a cue of physical disability. There was also a small negative effect of higher ABM when observers considered emotional goals, possibly because ABM may be perceived as anxiety or mental illness (Pentland, 1991).
This study included a few limitations. Notifying observers that all of the targets they would see were diagnosed with PD may have influenced their impressions. However, this did not remove the relative disadvantage for targets who displayed higher masking or ABM, suggesting that atypical nonverbal expressivity affects impressions above and beyond the effects of diagnosis alone. Although targets were matched on disease severity and self-reported social supportiveness (which included items assessing interest and ability to provide emotional and instrumental support), this study did not directly assess disability. Finally, target characteristics unrelated to facial masking and ABM, including attractiveness, perceived age, or attire, may have influenced the results of the current study. Nonetheless, the large effect sizes observed for higher facial masking and ABM make it unlikely that other target characteristics could completely explain the current findings. Future research could extend the current findings by exploring other cues that might be more valid indicators of social positivity in PD, and by examining the influence of atypical expressivity on existing relationships.
Acknowledgments
This research was supported by the 2009 APA Divisions 20 & 40 Walter G. McMillen Memorial Award for Parkinson’s Disease Research awarded to Amanda Hemmesch. This research was also partially supported by NIA Grant NAG21152 (PI: Robert Wagenaar; Co-I: Linda Tickle-Degnen; Research Associate: Terry Ellis), and NIH-, NIA-, & DHHS-NRSA Training Grant T32-AG00204-16. I would like to thank Linda Tickle-Degnen, Leslie Zebrowitz, Kathleen Bogart, the Health Quality of Life Lab at Tufts University, and the Face Perception Lab at Brandeis University for their assistance with research design and manuscript revisions.
References
- Bar M, Neta M, Linz H. Very first impressions. Emotion. 2006;6:269–278. doi: 10.1037/1528-3542.6.2.269. [DOI] [PubMed] [Google Scholar]
- Caap-Ahlgren M, Lannerheim L, Dehlin O. Older Swedish women’s experiences living with symptoms related to Parkinson’s disease. Journal of Advanced Nursing. 2002;39:87–95. doi: 10.1046/j.1365-2648.2002.02245.x. [DOI] [PubMed] [Google Scholar]
- Clark MS, Bond MJ. The Adelaide activities profile: A measure of the life-style activities of elderly people. Aging. 1995;7:174–184. doi: 10.1007/BF03324332. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Darwin C. The expression of emotions in man and animals. Oxford: Oxford University Press; 1889. [Google Scholar]
- DePaulo BM. Nonverbal behavior and self-presentation. Psychological Bulletin. 1992;111:203–243. doi: 10.1037/0033-2909.111.2.203. [DOI] [PubMed] [Google Scholar]
- Diekman AB, Goodfriend W. Rolling with the changes: A role congruity perspective on gender norms. Psychology of Women Quarterly. 2006;30:369–383. doi: 10.1111/j.1471-6402.2006.00312.x. [DOI] [Google Scholar]
- Ellgring H, Seiler S, Perleth B, Frings W, Gasser T, Oertel W. Psychosocial aspects of Parkinson’s disease. Neurology. 1993;43:S41–S44. [PubMed] [Google Scholar]
- Fahn S, Marsden C, Calne D, Goldstein M. Recent Developments in Parkinson’s Disease. Vol. 2. Florham Park, NJ: Macmillian; 1987. [Google Scholar]
- Feldman RS, Tyler JM. Factoring in age: Nonverbal communication across the lifespan. In: Manusov V, Patterson ML, editors. The Sage handbook of nonverbal communication. Thousand Oaks, CA: Sage Publications; 2006. pp. 191–199. [Google Scholar]
- Gosling SD, Rentfrow PJ, Swann WB., Jr A very brief measure of the Big Five personality domains. Journal of Research in Personality. 2003;37:504–528. doi: 10.1016/S0092-6566(03)00046-1. [DOI] [Google Scholar]
- Hemmesch AR, Tickle-Degnen L, Zebrowitz LA. The influence of facial masking and sex on age peers’ impressions of older adults with Parkinson’s disease. Psychology & Aging. 2009;24:542–549. doi: 10.1037/a0016105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P-C. Doctoral dissertation. Boston University; 2009. Social behavior, gender, and quality of life in Parkinson’s disease. 2009. [Google Scholar]
- Katsikitis M, Pilowsky I. A controlled quantitative study of facial expression in Parkinson’s disease and depression. The Journal of Nervous and Mental Disease. 1991;179:683–688. doi: 10.1097/00005053-199111000-00006. [DOI] [PubMed] [Google Scholar]
- Macht M, Schwarz R, Ellgring H. Patterns of psychological problems in Parkinson’s disease. Acta Neurologica Scandinavica. 2005;111:95–101. doi: 10.1111/j.1600-0404.2005.00375.x. [DOI] [PubMed] [Google Scholar]
- Mikos AE, Springer US, Nisenzon AN, Kellison IL, Fernandez HH, Okun MS, Bowers D. Awareness of expressivity deficits in non-demented Parkinson’s disease. The Clinical Neurologist. 2009;23:805–817. doi: 10.1080/13854040802572434. [DOI] [PubMed] [Google Scholar]
- Newsom JT, Rook KS, Nishishiba M, Sorkin D, Mahan TL. Understanding the relative importance of positive and negative social exchanges: Examining specific domains and appraisals. Journals of Gerontology: Psychological Sciences. 2005:P305–P312. doi: 10.1093/geronb/60.6.P304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhof G. Parkinson’s disease as a problem of shame in public appearance. Sociology of Health & Illness. 1995;17:193–205. doi: 10.1111/1467-9566.ep10933386. [DOI] [Google Scholar]
- Patterson ML, Manusov V. Nonverbal communication: Basic issues and future prospects. In: Manusov V, Patterson ML, editors. The Sage handbook of nonverbal communication. Thousand Oaks, CA: Sage Publications; 2006. pp. 522–532. [Google Scholar]
- Pentland B. Body language in Parkinson’s disease. Behavioural Neurology. 1991;4:181–187. doi: 10.3233/BEN-1991-4306. [DOI] [PubMed] [Google Scholar]
- Pentland B, Gray JM, Riddle WJR, Pitcairn TK. The effects of reduced non-verbal communication in Parkinson’s disease. British Journal of Disorders of Communication. 1988;23:31–34. doi: 10.3109/13682828809019874. [DOI] [PubMed] [Google Scholar]
- Pentland B, Pitcairn TK, Gray JM, Riddle WJ. The effects of reduced expression in Parkinson’s disease on impression formation by health professionals. Clinical Rehabilitation. 1987;1:307–313. doi: 10.1177/026921558700100410. [DOI] [Google Scholar]
- Peto V, Jenkinson C, Fitzpatrick R, Greenhal R. The development and validation of a short measure of functioning and well-being for individuals with Parkinson’s disease. Quality of Life Research. 1995;4:241–248. doi: 10.1007/BF02260863. [DOI] [PubMed] [Google Scholar]
- Pitcairn TK, Clemie S, Gray JM, Pentland B. Non-verbal cues in the self-presentation of Parkinsonian patients. British Journal of Clinical Psychology. 1990;29:177–184. doi: 10.1111/j.2044-8260.1990.tb00867.x. [DOI] [PubMed] [Google Scholar]
- Riggio RE, Friedman HS. Impression formation: The role of expressive behavior. Journal of Personality and Social Psychology. 1986;50:421–427. doi: 10.1037/0022-3514.50.2.421. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Rosnow RL. Essentials of behavioral research: Methods and data analysis. 2. Boston: McGraw-Hill; 1991. [Google Scholar]
- Schrag A, Barone P, Brown RG, Leentjens AFG, McDonald WM, Starkstein S, et al. Depression rating scales in Parkinson’s disease: Critique and recommendations. Movement Disorders. 2007;22:1077–1092. doi: 10.1002/mds.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag A, Jahanshahi M, Quinn N. How does Parkinson’s disease affect quality of life? A comparison with quality of life in the general population. Movement Disorders. 2000;15:1112–1118. doi: 10.1136/jnnp.69.3.308. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fiske DW. Nonverbal behavior and social evaluation. Journal of Personality. 1981;49:115–128. doi: 10.1111/j.1467-6494.1981.tb00732.x. [DOI] [Google Scholar]
- Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms associated with Parkinson’s disease. Parkinsonism and Related Disorders. 2002;8:193–197. doi: 10.1016/S1353-8020(01)00015-3. [DOI] [PubMed] [Google Scholar]
- Simons G, Pasqualini MCS, Reddy V, Wood J. Emotional and nonemotional facial expressions in people with Parkinson’s disease. Journal of the International Neuropsychological Society. 2004;10:521–535. doi: 10.1017/S135561770410413X. [DOI] [PubMed] [Google Scholar]
- Smith MC, Smith MK, Ellgring H. Spontaneous and posed facial expression in Parkinson’s Disease. Journal of the International Neuropsychological Society. 1996;2:383–391. doi: 10.1017/S1355617700001454. [DOI] [PubMed] [Google Scholar]
- Solimeo S. Sex and gender in older adults’ experience of Parkinson’s disease. Journal of Gerontology: Social Sciences. 2008;63B:S42–S48. doi: 10.1093/geronb/63.1.S42. [DOI] [PubMed] [Google Scholar]
- Spielman JL, Borod JC, Ramig LO. The effects of intensive voice treatment on facial expressiveness in Parkinson disease: Preliminary data. Cognitive and Behavioral Neurology. 2003;16:177–188. doi: 10.1097/00146965-200309000-00005. [DOI] [PubMed] [Google Scholar]
- Tickle-Degnen L, Ellis T, Saint-Hilaire MH, Thomas CA, Wagenaar RC. Self-management rehabilitation and health-related quality of life in Parkinson’s disease: A randomized controlled trial. Movement Disorders. 2010;25:194–204. doi: 10.1002/mds.22940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickle-Degnen L, Lyons KD. Practitioner’s impressions of patients with Parkinson’s disease: The social ecology of the expressive mask. Social Science & Medicine. 2004;58:603–614. doi: 10.1016/S0277-9536(03)00213-2. [DOI] [PubMed] [Google Scholar]
- Tickle-Degnen L, Zebrowitz LA, Ma HI. Culture, gender, and health care stigma: Practitioners’ response to facial masking experience by people with Parkinson’s disease. Social Science & Medicine. 2011;73:95–102. doi: 10.1016/j.socscimed.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis AW, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson’s disease: A population-based study of US Medicare beneficiaries. Neuro-epidemiology. 2010;34:143–151. doi: 10.1159/000275491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W, Eagly AH. A cross-cultural analysis of the behavior of women and men: Implications for the origins of sex differences. Psychological Bulletin. 2002;128:699–727. doi: 10.1037/0033-2909.128.5.699. [DOI] [PubMed] [Google Scholar]