Abstract
Stereotactic body radiation therapy (SBRT) has a local control rate of 95% at 2 years for non-small cell lung cancer (NSCLC) and should improve the prognosis of inoperable patients, elderly patients, and patients with significant comorbidities who have early-stage NSCLC. The safety of SBRT is being confirmed in international, multi-institutional Phase II trials for peripheral lung cancer in both inoperable and operable patients, but reports so far have found that SBRT is a safe and effective treatment for early-stage NSCLC and early metastatic lung cancer. Radiation pneumonitis (RP) is one of the most common toxicities of SBRT. Although most post-treatment RP is Grade 1 or 2 and either asymptomatic or manageable, a few cases are severe, symptomatic, and there is a risk for mortality. The reported rates of symptomatic RP after SBRT range from 9% to 28%. Being able to predict the risk of RP after SBRT is extremely useful in treatment planning. A dose-effect relationship has been demonstrated, but suggested dose-volume factors like mean lung dose, lung V20, and/or lung V2.5 differed among the reports. We found that patients who present with an interstitial pneumonitis shadow on computed tomography scan and high levels of serum Krebs von den Lungen-6 and surfactant protein D have a high rate of severe radiation pneumonitis after SBRT. At our institution, lung cancer patients with these risk factors have not received SBRT since 2006, and our rate of severe RP after SBRT has decreased significantly since then.
Keywords: Radiation pneumonitis, Stereotactic radiation therapy, Dose-volume factors, Krebs von den Lungen-6, Surfactant protein D, Computed tomography changes
Core tip: Radiation pneumonitis (RP) is one of the most common toxicities after stereotactic body radiation therapy (SBRT). Although most RP is Grade 1 or 2 and either asymptomatic or manageable, a few cases are severe and there is a risk for mortality. A dose-effect relationship has been demonstrated that can be used for treatment planning. Other prognostic indicators of severe radiation pneumonitis after SBRT are an interstitial pneumonitis shadow on computed tomography scan and high levels of serum Krebs von den Lungen-6 and surfactant protein D before treatment.
INTRODUCTION
Stereotactic body radiotherapy (SBRT) is becoming standard of care therapy for patients with inoperable early-stage non-small cell peripheral lung cancer or lung cancer with limited demarcated metastases. The delivery of higher doses to smaller lung planning target volumes (PTVs) limits toxicity in the normal lung tissue surrounding the tumor to very limited areas. Local control of up to 95% at 2 years has been reported[1-4].
Given the improved local control and toxicity results reported by recent lung SBRT studies[1-4], future directions for this technique include the treatment of larger lesions. Palma et al[5] and Diot et al[6] suggest that toxicity might increase with lesion size, and this possibility should be anticipated and closely monitored.
Symptomatic lung toxicity with SBRT is typically less than 10%[7]; however, occurrences up to 25% have been reported[8], highlighting the necessity of developing SBRT treatment parameters that ensure consistently low toxicity levels.
Radiation-induced pneumonitis is the most frequent acute pulmonary toxicity. The majority of patients develop asymptomatic Grade 1 pneumonitis. Clinically symptomatic pneumonitis develops in less than 10% of patients, but most patients develop late pulmonary toxicity characterized by localized pulmonary fibrosis in the high-dose region[9]. Because this fibrosis is usually asymptomatic, we considered post-SBRT pulmonary function changes the clinically relevant endpoint of our review.
A dose-effect relationship has been demonstrated for SBRT that is similar to that observed in conventionally fractionated radiation therapy[10,11]; but a study by Guckenberger et al[12] failed to demonstrate this relationship in early-stage non-small cell lung cancer. Gluckenberger’s results were based on a large number of patients[12] and confirmed the findings in other studies that post-SBRT pulmonary function was either stable[13-17] or almost asymptomatic[18,19]. These data were encouraging and further supported the safety of SBRT.
SBRT has been widely used as a safe and effective treatment for primary or metastatic lung tumors for a number of years[20]. According to the protocol of the Japan Clinical Oncology Group (JCOG) 0403 study[21,22], the only absolute contraindication to SBRT is pregnancy.
In Japan, medical service fees officially cover SBRT treatment for primary and metastatic lung cancers only if the tumor is under 5 cm in size, there are no more than three tumors, and there is no metastatic disease in other organs. In Japan, surgery is generally the first-line treatment for early primary lung cancer, so primary lung cancer patients who undergo SBRT are in poor condition and either have multiple primary cancers or co-morbidities such as serious cardiovascular disease. Most Japanese patients with primary lung tumors who received SBRT had low pulmonary function from chronic obstructive pulmonary disease due to long smoking histories.
Contraindications to SBRT were (1) a history of irradiation to the concerned site; (2) severe interstitial pneumonitis or pulmonary fibrosis; (3) severe diabetes or connective tissue disease; and (4) common use of steroids.
LOCAL CONTROL RATE OF SBRT
SBRT with 3D conformal or intensity-modulation techniques is an effective treatment for localized early-stage lung cancer, with local control rates of 85.5% to 100% 2 to 3 years following treatment[1-4]. SBRT is also being employed to treat metastatic lung cancer, although the survival rates are not comparable to those for early-stage (T1 - T2, N0) disease[2].
The excellent local control rates for early-stage lung cancer treated with SBRT are leading to extensive use of this technique in clinical practice and to randomized trials comparing surgery to SBRT for Stage I non-small cell lung cancers in operable patients. Two randomized trials to compare SBRT to surgery for operable patients with Stage I lung cancer were launched in 2008: one in the Netherlands [the Randomized Clinical Trial of Either Surgery or Stereotactic Radiotherapy for Early Stage (IA) Lung Cancer trial] and one in the United States (testing the Cyberknife by Accuray Inc., Sunnyvale, CA, United States).
PREVIOUS REPORTS OF TOXICITIES AFTER SBRT
The safety of SBRT is being confirmed in multi-institutional Phase II trials for peripheral lung cancer in both inoperable[16,23] and operable patients[22]. In the Radiation Therapy Oncology Group (RTOG) trial 0236[23], protocol-specific, treatment-related Grade 3 and 4 adverse events occurred in 12.7% (7/59) and 3.6% (2/59) of cases, respectively. No Grade 5 adverse events were reported. In the Nordic Phase II study of SBRT[16]. Grade 3 toxicities were seen in 21% (12/57) of cases, but no Grade 4 or 5 toxicities were reported. Nishio et al[22] reported Grade 3 toxicities in 6.2% of operable patients in the JCOG 0403 trial.
Severe clinical toxicities after SBRT are fairly uncommon and occur more frequently in cases of centrally located tumors, such as those near the trachea, primary bronchus, major blood vessels and pericardium[3]. Rates of serious toxicities are low in most studies. Previous reports have described skin, chest wall, and brachial plexus toxicities with their associated risk factors[24-27].
This review documents clinically significant radiation pneumonitis (RP) rates for medically inoperable non-small cell lung cancer (NSCLC) patients treated with SBRT, adding to the sparse literature on pulmonary toxicity resulting from hypo-fractionated radiotherapy.
RP AFTER SBRT
RP is one of the most common toxicities after SBRT, as well as after conventional radiotherapy to the lung. Scoring systems should be considered when interpreting RP results. The reported rates of symptomatic RP after SBRT range from 9% to 28%[8,10,11,28-31]. Although most of the RP was Grade 1 or 2 and either asymptomatic or manageable, a few cases were severe and there was a risk for mortality[8]. It is very important to develop a method to predict the risk of RP after SBRT for lung cancer.
Grade 3 RP was observed in 3.6% of the overall patients in RTOG 0236[23] and in 3.1% of the operable patients in JCOG 0403[22]. Baumann et al[16] reported that no one developed Grade 3 pneumonitis in their Phase II trial of SBRT.
McGarry et al[31] reported that 2% (1/47) of patients developed circulating tumor cells and 6.4% (3/47) of patients developed Grade 2 and 3 RP in the updated Indiana University Phase I trial that included tumors up to 7 cm in size plus central lesions. G2 toxicity occurred at a dose of 48 Gy, and G3 toxicities developed after 54 Gy and 72 Gy in 3 fractions prescribed to the 80% iso-dose line[32]. Using similar criteria, Onishi reported 4.1% G2 (10/245), 1.2% G3 (3/245), and 1.2% G4 (3/245) RP in a multi-institutional trial of SBRT in Japan[33]. Nagata and colleagues reported no G3 or G4 RP using a slightly less potent dose of 48 Gy in 4 fractions delivered to the iso-center[1].
In the RTOG, Ricardi et al[32] treated 62 patients to 45 Gy in 3 fractions to the 80% iso-dose line and reported a 3.2% incidence of Grade 3 RP that required steroids or intermittent oxygen. When Stephans et al[34] treated (n = 56) patients to 50 Gy in 5 fractions and (n = 38) patients to 60 Gy in 3 fractions, there was a 2.3% incidence of RP that required steroids (for all 94 patients).
Grills et al[35] recently published a case–control study comparing SBRT to wedge resection. In that report, there was 11% G2 - 3 RP using a CTC vs a grading system based on the Common Terminology of Criteria of Adverse Events. Only 2% of these patients required temporary steroids for management. Finally, G3 RP occurred at a rate of 3.6% (2/55) in RTOG 0236[23].
Although the reported toxicities of lung SBRT have, for the most part, been minor, the dose constraints to use during treatment planning are based on extremely limited clinical data, most of which has not been validated[36]. Even the recent QUANTEC lung article devoted only one paragraph to the risk of pneumonitis in lung SBRT patients[7]. Baker et al[37] reported in QUANTEC that the greatest incidence of pneumonitis was Grade 1 (64.2%) (169/263), and there were 26 cases (9.9%) of Grade 2 pneumonitis and 3 cases (1.1%) of Grade 3 pneumonitis.
DOSE-VOLUME FACTORS FOR RP AFTER SBRT
Table 1 summarizes published reports that focused on the dose volumetrics associated with Grade 2 RP or worse after SBRT. The RP rates varied from 9.4% to 28.0%, and the suggested dose-volume factors for RP differed among the reports. This variation might be caused by differences in the PTV volume, dose fractionation schedule, or RP scoring system.
Table 1.
Summary of reports on Grade 2 radiation pneumonitis after stereotactic body radiation therapy
| First author | Ref. | Year | Gy | Pt No. | Median PTV, cc | Median follow-up in months | G2- RP | G3 RP | G4 RP | G5 RP | RP factor 1 | RP factor 2 |
| Onishi | [32] | 2004 | 18-75Gy/1-25Fr | 245 | NA | 24 | 6.50% | 1.20% | 1.20% | 0% | ||
| McGarry | [31] | 2005 | 24Gy/3Fr | 47 | NA | NA | 8.40% | 4.30% | 2.10% | 0% | ||
| Takahashi | [40] | 2006 | 15-30Gy | 32 | NA | 18 | 12.50% | 0% | 6% | |||
| Yamashita | [8] | 2007 | 48Gy/4-6Fr | 25 | 43.9 | 17 | 28.00% | 4% | 4% | 12% | CI | |
| Baumann | [16] | 2008 | 45Gy/3Fr | 60 | NA | 23 | NA | 21% | 0% | 0% | ||
| Ricardi | [27] | 2009 | 45Gy/3Fr or 26Gy/1Fr | 60 | NA | 30.9 | 14.30% | 3.20% | 0% | 0% | MLD | |
| Borst | [10] | 2009 | 35-60Gy/4-8Fr | 128 | 9.6 | 16.1 | 10.90% | 0.80% | 0% | 0% | MLD | |
| Stephans | [13] | 2009 | 60Gy/3Fr or 50Gy/5Fr | 86 | 39.9/30.4 | 15.3 | 2.30% | 0% | 0% | 0% | ||
| Rusthoven | [2] | 2009 | 48-60Gy/3Fr | 7 | NA | 15.4 | 2.60% | |||||
| Yamashita | [39] | 2010 | 48Gy/4Fr | 117 | NA | 14.7 | NA | 1.70% | 1.70% | 6.00% | KL-6 and SP-D | IP-shadow |
| Timmerman | [23] | 2010 | 60Gy/3Fr or 54Gy/3Fr | 55 | NA | 34.4 | NA | 12.70% | 3.60% | 0% | ||
| Nagata | [20] | 2010 | 48Gy/4Fr | 104 | NA | 46.8 | NA | 6.20% | 0% | 0% | ||
| Guckenberger | [9] | 2010 | 26Gy/1Fr or 37.5Gy/3Fr | 59 | 33 | 13 | 18.60% | 0% | 0% | 0% | MLD | V2.5-50 |
| Ong | [28] | 2010 | 55Gy/5Fr or 60Gy/8Fr | 18 | 137 | 12.8 | 27.80% | 11.10% | 0% | 0% | V5 | |
| Grills | [35] | 2010 | 48Gy/4Fr or 60Gy/5Fr | 58 | NA | 30 | 11% | 2% | 0% | 0% | ||
| Stauder | [30] | 2011 | 32-60Gy/3-5Fr | 74 | 42.9 | 15.8 | 12.50% | 2.30% | 0% | 1.10% | Max dose | |
| Matsuo | [53] | 2012 | 48Gy/4Fr | 74 | 32.5 | 31.4 | 20.30% | 1.40% | 0% | 0% | V25 | PTV volume |
| Barriger | [29] | 2012 | 24-66Gy/3-5Fr | 84 | 48.3 | 17 | 9.40% | 2% | 0.40% | 0% | MLD (4Gy) | V20 (4%) |
| Baker | [37] | 2013 | Multiple | 240 | 37.6 | 15.6 | 11% | 1.10% | 0% | 0% | MLD (6Gy) | V20 (10%) |
MLD: Mean lung dose; NA: Not available.
Since most patients with pulmonary metastases had residual or recurrent disease after first-line treatment with chemotherapy, it appeared appropriate to consider a V20 of 30% as the dose restriction in SBRT for metastatic lung cancer.
Borst et al[10] evaluated the relationship between the mean lung dose (MLD) and the incidence of RP after SBRT. They calculated the MLD in the normalized total dose form, using the linear-quadratic model with a α/β ratio of 3. A significant dose-response relationship was found between RP and MLD.
According to Baker et al[10], the data from their center and the Japanese group demonstrate that a V20 of less than 10% is readily achievable, and at those levels pneumonitis is not statistically predictable. Analogously, an MLD of approximately 5 - 6 Gy is achievable, and at this dosage pneumonitis should not develop. Therefore, dosimetric guidelines of a V20 of less than 10% and an MLD of less than 6 Gy are a reasonable way to reduce the occurrence of Grades 2 - 4 RP. A German article suggested that a higher MLD or higher V2.5 - V50 (V2.5 in particular) was associated with symptomatic RP[9].
The Mayo Clinic recently reported on a series of patients treated at their institution with consecutive daily fractions of SBRT[32]. There was a 12.5% overall incidence of Grade 2 pneumonitis, but a 14.3% incidence in patients treated either with 54 Gy in three fractions for peripheral lesions or 48 Gy in four fractions for central lesions. In a univariate analysis, a PTV maximum dose greater than 60 Gy was predictive of RP (P = 0.016), although the overall number of events was small (n = 4). No other factors were statistically significant[32]. The decline in pulmonary function seemed to be transient, similar to the initial experience at Indiana University[38].
It is unclear exactly how RP correlates with changes in pulmonary function testing. This is an area that requires further research. It is interesting to note, however, that a dose of radiation higher than V10 was predictive of RP in the Indiana University patients, at least on univariate analysis. The same findings were reported by the Cleveland Clinic[34]. The conformity index was not predictive under univariate or multivariate modeling.
At our institution, we suppress the patient’s respiration during SBRT using abdominal compression in order to reduce lung V20 and MLD[8,39]. We had used irradiation dose of 48 Gy in 4 fractions (Figure 1). We also used 4D cone beam computed tomography (CT) to evaluate internal target volume and tumor motion just before SBRT, with the result that the margin between the internal target volume and the PTV became narrower[40,41].
Figure 1.
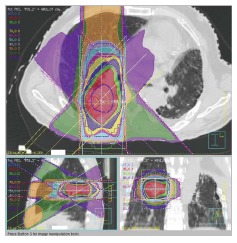
Dose distribution of stereotactic body radiation therapy of 48 Gy in 4 fractions.
EXPRESSSION OF KREBS VON DEN LUNGEN-6 IS A PREDICTOR OF RP AFTER SBRT
High levels of the glycoprotein Krebs von den Lungen-6 (KL-6) indicate interstitial pneumonitis (IP), and the levels rise significantly with physical activity in IP cases. In the human body, KL-6 only develops in type II alveolus epithelial cells, bronchial epithelial cells, and bronchus gland cells. A small quantity of KL-6 is present in the liquid coating the alveoli in normal lungs, but it occurs in higher levels in hyperplastic type II alveolus epithelial cells when IP is present. Inflammation also occurs in IP, which increases the permeability of the blood vessels and allows KL-6 to move into the blood where it can be measured. Blood levels of KL-6, surfactant protein D (SP-D), surfactant protein A (SP-A), and monocyte chemoattractant protein-1 are evaluated whenever there is an injury to the lung stroma, and KL-6 is the most sensitive (93.9%) and specific (96.3%) of these measures where the detection of RP is concerned[42]. SP-D levels at 50 to 60 Gy (midway through radiation therapy) showed greater sensitivity and positive predictive values for RP detection (74% and 68%, respectively) than SP-A (26% and 21%, respectively)[43].
Factors other than dose volumetrics also affect the incidence of pneumonitis after SBRT. Hara et al[44] evaluated 16 patients who received single-fraction SBRT from 20 to 35 Gy. Serum KL-6 levels rose significantly between pretreatment presentation and two months after SBRT was administered, and it was significantly correlated with Grade 3 RP by the RTOG criteria.
Iwata et al[45] reported that pretreatment serum KL-6 levels, gender, and PTV volume were associated with symptomatic RP in a univariate analysis, and pretreatment KL-6 levels remained significant in a multivariate analysis. They concluded that patients with pretreatment KL-6 levels ≥ 300 μ/mL should be followed carefully for RP. CT or X-ray imaging of the lung before and after SBRT should also help to predict severe RP.
To limit the risk of severe RP, we recommended to everyone prescreening for interstitial pneumonitis with CT scans and checking serum KL-6 and SP-D levels. After introducing these measures, we reported that the incidence of Grade 4 and 5 RP decreased from 18.8% to 3.5%[45].
Takeda et al[46] reported that the sooner RP appeared on chest X-ray after SBRT was administered, the more severe it was. The radiographic appearance of RP during the initial 2 mo after SBRT indicated a 40% risk for Grade 3 RP. The risk was only 1.2% when radiologic changes appeared 3 mo after SBRT.
Evaluating KL-6 and SP-D levels, radiologic imaging before and after treatment, and adjusting dose-volume factors during treatment planning helps lower the risk of severe pneumonitis after SBRT. While the biomarkers are both sensitive and specific for RP, the pathophysiological mechanisms underlying their predictive value are unclear, which makes some clinicians hesitate to use them.
At our institution, Grades 4 and 5 RP occurred in 6 out of 32 patients (18.8%) who received SBRT treatment for lung cancer before 2005 and only 3 out of 85 patients (3.5%) between 2006 and 2013[8,39]. We believe that the significant reduction in the occurrence of Grades 4 - 5 RP is due to our use of prognostic biomarkers and radiography to select appropriate patients for SBRT treatment. After 2006, patients were excluded from SBRT if they had an obvious IP shadow on their CT scan (slice thickness 3.0 mm), and/or if serum KL-6 and SP-D levels were high[39].
RP AFTER SBRT FOR METASTATIC LUNG CANCER
SBRT is also used to treat pulmonary metastases in selected patients and treatment results seem comparable to those obtained by surgical metastasectomy[47]. In this setting, the literature reports that Grade 3 RP occurred in 3% to 5% of cases[2,47,48]. According to Inoue[48], the incidence of G3/4 adverse respiratory events after SBRT for pulmonary metastases was 10%.
GENE EXPRESSION CLASSIFIER
It was reported that radiation pneumonitis after SBRT treatment for lung cancer was associated with pro-inflammatory genes such as TGFB1 or CD44. Accumulating the number of cases appropriate for statistically significant gene analysis might be difficult, and there is no information about predisposing genetic risk factors for lung cancer.
Yuan et al[49] reported in 2009 that he and his colleagues at the M.D. Anderson Cancer Center in Houston, Texas, United States, performed an association analysis between pneumonitis onset risk in both Black and White patients who received radiotherapy and/or chemotherapy for NSCLC (94% in both cases). The polymorphism marker on the THFB1 and T869C genes was associated with a low risk of developing RP or IP.
In recent years, many studies have found that SBRT for early stage lung cancer is both effective and safe[1,50], but there are only a few reports about the development of dangerous and lethal radiation pneumonitis that can result from SBRT[8], or the pulmonary fibrosis that may also appear[50].
TIMELINE AND PATTERN OF CT CHANGES
There have been few studies on CT findings in radiation-induced lung disease after SBRT for lung cancer[50]. Due to the differences in dose delivery and distribution, biologic effects, and overall treatment time, it is reasonable to expect that any CT changes that occur after SBRT will not have the same appearance, geographic extent, and progression timeline as those following CRT for lung cancers[51].
Like CRT-induced CT changes, CT findings after SBRT have two stages: early acute radiation pneumonitis that occurs within 6 mo of treatment and radiation fibrosis that occurs 6 mo or more after treatment[51,52]. In most cases, radiologic changes in normal lung tissue do not occur until at least 3 mo after SBRT. Clinical symptoms of acute radiation-induced lung injury develop approximately 3 to 6 mo after treatment. All of the severe RP cases in our institution consisted of the acute exacerbation of IP that was spread out over the radiation field (Figure 2)[39].
Figure 2.
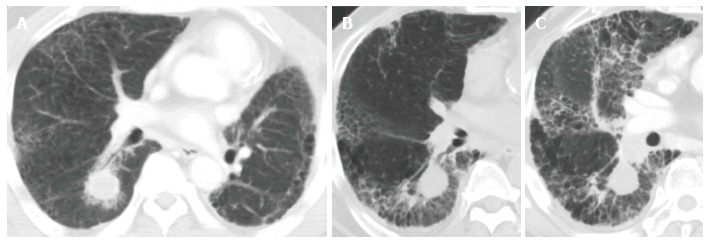
Computed tomography image. A: Before stereotactic body radiation therapy (SBRT) but after surgery for a left lung cancer. The nodule in the lower lobe of the right lung (S6) increased to 23 mm; B: Taken at post-SBRT month 5; C: Taken at post-SBRT month 7. Acute exacerbation of interstitial pneumonitis was found.
The analysis of SBRT-induced normal lung density changes by Diot et al[6] indicates that self-limiting acute effects in normal lung tissue are more pronounced than late effects, and acute CT changes in patients treated with 3 fractions were considerably less than those in patients treated with 4 or 5 fractions. The changes seemed to be explained by either increased low-dose exposure in normal lung tissue or differences in tumor volume.
CONCLUSION
On the basis of this review, radiation-induced pneumonitis is the most frequent acute pulmonary toxicity following SBRT for lung cancer. The majority of patients develop asymptomatic Grade 1 or asymptomatic and/or manageable Grade 2 pneumonitis, and clinically symptomatic pneumonitis is observed in less than 10%. A dose-effect relationship has been demonstrated that is useful in treatment planning. Since patients with an IP shadow on CT scan and high levels of serum KL-6 and SP-D before SBRT treatment develop severe radiation pneumonitis at a high rate after treatment, they should not receive SBRT.
Footnotes
P- Reviewer: Can MM, Storto G, Sun Z S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
References
- 1.Nagata Y, Takayama K, Matsuo Y, Norihisa Y, Mizowaki T, Sakamoto T, Sakamoto M, Mitsumori M, Shibuya K, Araki N, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005;63:1427–1431. doi: 10.1016/j.ijrobp.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Rusthoven KE, Kavanagh BD, Burri SH, Chen C, Cardenes H, Chidel MA, Pugh TJ, Kane M, Gaspar LE, Schefter TE. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27:1579–1584. doi: 10.1200/JCO.2008.19.6386. [DOI] [PubMed] [Google Scholar]
- 3.Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, Ewing M, Abdulrahman R, DesRosiers C, Williams M, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 4.Xia T, Li H, Sun Q, Wang Y, Fan N, Yu Y, Li P, Chang JY. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable Stage I/II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;66:117–125. doi: 10.1016/j.ijrobp.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Palma DA, van Sörnsen de Koste J, Verbakel WF, Vincent A, Senan S. Lung density changes after stereotactic radiotherapy: a quantitative analysis in 50 patients. Int J Radiat Oncol Biol Phys. 2011;81:974–978. doi: 10.1016/j.ijrobp.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Diot Q, Kavanagh B, Schefter T, Gaspar L, Stuhr K, Miften M. Regional normal lung tissue density changes in patients treated with stereotactic body radiation therapy for lung tumors. Int J Radiat Oncol Biol Phys. 2012;84:1024–1030. doi: 10.1016/j.ijrobp.2011.11.080. [DOI] [PubMed] [Google Scholar]
- 7.Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, El Naqa I, Hubbs JL, Lebesque JV, Timmerman RD, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76:S70–S76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamashita H, Nakagawa K, Nakamura N, Koyanagi H, Tago M, Igaki H, Shiraishi K, Sasano N, Ohtomo K. Exceptionally high incidence of symptomatic grade 2-5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat Oncol. 2007;2:21. doi: 10.1186/1748-717X-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang K, Dahele M, Senan S, Guckenberger M, Rodrigues GB, Ward A, Boldt RG, Palma DA. Radiographic changes after lung stereotactic ablative radiotherapy (SABR)--can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiother Oncol. 2012;102:335–342. doi: 10.1016/j.radonc.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Guckenberger M, Baier K, Polat B, Richter A, Krieger T, Wilbert J, Mueller G, Flentje M. Dose-response relationship for radiation-induced pneumonitis after pulmonary stereotactic body radiotherapy. Radiother Oncol. 2010;97:65–70. doi: 10.1016/j.radonc.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 11.Borst GR, Ishikawa M, Nijkamp J, Hauptmann M, Shirato H, Onimaru R, van den Heuvel MM, Belderbos J, Lebesque JV, Sonke JJ. Radiation pneumonitis in patients treated for malignant pulmonary lesions with hypofractionated radiation therapy. Radiother Oncol. 2009;91:307–313. doi: 10.1016/j.radonc.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Guckenberger M, Klement RJ, Kestin LL, Hope AJ, Belderbos J, Werner-Wasik M, Yan D, Sonke JJ, Bissonnette JP, Xiao Y, et al. Lack of a dose-effect relationship for pulmonary function changes after stereotactic body radiation therapy for early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85:1074–1081. doi: 10.1016/j.ijrobp.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Stephans KL, Djemil T, Reddy CA, Gajdos SM, Kolar M, Machuzak M, Mazzone P, Videtic GM. Comprehensive analysis of pulmonary function Test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol. 2009;4:838–844. doi: 10.1097/JTO.0b013e3181a99ff6. [DOI] [PubMed] [Google Scholar]
- 14.Bral S, Gevaert T, Linthout N, Versmessen H, Collen C, Engels B, Verdries D, Everaert H, Christian N, De Ridder M, et al. Prospective, risk-adapted strategy of stereotactic body radiotherapy for early-stage non-small-cell lung cancer: results of a Phase II trial. Int J Radiat Oncol Biol Phys. 2011;80:1343–1349. doi: 10.1016/j.ijrobp.2010.04.056. [DOI] [PubMed] [Google Scholar]
- 15.Fritz P, Kraus HJ, Blaschke T, Mühlnickel W, Strauch K, Engel-Riedel W, Chemaissani A, Stoelben E. Stereotactic, high single-dose irradiation of stage I non-small cell lung cancer (NSCLC) using four-dimensional CT scans for treatment planning. Lung Cancer. 2008;60:193–199. doi: 10.1016/j.lungcan.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Baumann P, Nyman J, Hoyer M, Gagliardi G, Lax I, Wennberg B, Drugge N, Ekberg L, Friesland S, Johansson KA, et al. Stereotactic body radiotherapy for medically inoperable patients with stage I non-small cell lung cancer - a first report of toxicity related to COPD/CVD in a non-randomized prospective phase II study. Radiother Oncol. 2008;88:359–367. doi: 10.1016/j.radonc.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Ohashi T, Takeda A, Shigematsu N, Kunieda E, Ishizaka A, Fukada J, Deloar HM, Kawaguchi O, Takeda T, Takemasa K, et al. Differences in pulmonary function before vs. 1 year after hypofractionated stereotactic radiotherapy for small peripheral lung tumors. Int J Radiat Oncol Biol Phys. 2005;62:1003–1008. doi: 10.1016/j.ijrobp.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 18.Henderson M, McGarry R, Yiannoutsos C, Fakiris A, Hoopes D, Williams M, Timmerman R. Baseline pulmonary function as a predictor for survival and decline in pulmonary function over time in patients undergoing stereotactic body radiotherapy for the treatment of stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;72:404–409. doi: 10.1016/j.ijrobp.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto T, Baba M, Yamamoto N, Koto M, Sugawara T, Yashiro T, Kadono K, Ezawa H, Tsujii H, Mizoe JE, et al. Curative treatment of Stage I non-small-cell lung cancer with carbon ion beams using a hypofractionated regimen. Int J Radiat Oncol Biol Phys. 2007;67:750–758. doi: 10.1016/j.ijrobp.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Nagata Y, Negoro Y, Aoki T, Mizowaki T, Takayama K, Kokubo M, Araki N, Mitsumori M, Sasai K, Shibamoto Y, et al. Clinical outcomes of 3D conformal hypofractionated single high-dose radiotherapy for one or two lung tumors using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2002;52:1041–1046. doi: 10.1016/s0360-3016(01)02731-6. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo Y, Takayama K, Nagata Y, Kunieda E, Tateoka K, Ishizuka N, Mizowaki T, Norihisa Y, Sakamoto M, Narita Y, et al. Interinstitutional variations in planning for stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys. 2007;68:416–425. doi: 10.1016/j.ijrobp.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Nishio T, Kunieda E, Shirato H, Ishikura S, Onishi H, Tateoka K, Hiraoka M, Narita Y, Ikeda M, Goka T. Dosimetric verification in participating institutions in a stereotactic body radiotherapy trial for stage I non-small cell lung cancer: Japan clinical oncology group trial (JCOG0403) Phys Med Biol. 2006;51:5409–5417. doi: 10.1088/0031-9155/51/21/002. [DOI] [PubMed] [Google Scholar]
- 23.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone D, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andolino DL, Forquer JA, Henderson MA, Barriger RB, Shapiro RH, Brabham JG, Johnstone PA, Cardenes HR, Fakiris AJ. Chest wall toxicity after stereotactic body radiotherapy for malignant lesions of the lung and liver. Int J Radiat Oncol Biol Phys. 2011;80:692–697. doi: 10.1016/j.ijrobp.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Dunlap NE, Cai J, Biedermann GB, Yang W, Benedict SH, Sheng K, Schefter TE, Kavanagh BD, Larner JM. Chest wall volume receiving & gt; 30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:796–801. doi: 10.1016/j.ijrobp.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 26.Hoppe BS, Laser B, Kowalski AV, Fontenla SC, Pena-Greenberg E, Yorke ED, Lovelock DM, Hunt MA, Rosenzweig KE. Acute skin toxicity following stereotactic body radiation therapy for stage I non-small-cell lung cancer: who’s at risk? Int J Radiat Oncol Biol Phys. 2008;72:1283–1286. doi: 10.1016/j.ijrobp.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 27.Ricardi U, Filippi AR, Guarneri A, Giglioli FR, Mantovani C, Fiandra C, Anglesio S, Ragona R. Dosimetric predictors of radiation-induced lung injury in stereotactic body radiation therapy. Acta Oncol. 2009;48:571–577. doi: 10.1080/02841860802520821. [DOI] [PubMed] [Google Scholar]
- 28.Ong CL, Palma D, Verbakel WF, Slotman BJ, Senan S. Treatment of large stage I-II lung tumors using stereotactic body radiotherapy (SBRT): planning considerations and early toxicity. Radiother Oncol. 2010;97:431–436. doi: 10.1016/j.radonc.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Barriger RB, Forquer JA, Brabham JG, Andolino DL, Shapiro RH, Henderson MA, Johnstone PA, Fakiris AJ. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2012;82:457–462. doi: 10.1016/j.ijrobp.2010.08.056. [DOI] [PubMed] [Google Scholar]
- 30.Stauder MC, Macdonald OK, Olivier KR, Call JA, Lafata K, Mayo CS, Miller RC, Brown PD, Bauer HJ, Garces YI. Early pulmonary toxicity following lung stereotactic body radiation therapy delivered in consecutive daily fractions. Radiother Oncol. 2011;99:166–171. doi: 10.1016/j.radonc.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 31.McGarry RC, Papiez L, Williams M, Whitford T, Timmerman RD. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: phase I study. Int J Radiat Oncol Biol Phys. 2005;63:1010–1015. doi: 10.1016/j.ijrobp.2005.03.073. [DOI] [PubMed] [Google Scholar]
- 32.Ricardi U, Filippi AR, Guarneri A, Giglioli FR, Ciammella P, Franco P, Mantovani C, Borasio P, Scagliotti GV, Ragona R. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer. 2010;68:72–77. doi: 10.1016/j.lungcan.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, Yamashita T, Niibe Y, Karasawa K, Hayakawa K, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101:1623–1631. doi: 10.1002/cncr.20539. [DOI] [PubMed] [Google Scholar]
- 34.Stephans KL, Djemil T, Reddy CA, Gajdos SM, Kolar M, Mason D, Murthy S, Rice TW, Mazzone P, Machuzak M, et al. A comparison of two stereotactic body radiation fractionation schedules for medically inoperable stage I non-small cell lung cancer: the Cleveland Clinic experience. J Thorac Oncol. 2009;4:976–982. doi: 10.1097/JTO.0b013e3181adf509. [DOI] [PubMed] [Google Scholar]
- 35.Grills IS, Mangona VS, Welsh R, Chmielewski G, McInerney E, Martin S, Wloch J, Ye H, Kestin LL. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. 2010;28:928–935. doi: 10.1200/JCO.2009.25.0928. [DOI] [PubMed] [Google Scholar]
- 36.Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18:215–222. doi: 10.1016/j.semradonc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Baker R, Han G, Sarangkasiri S, DeMarco M, Turke C, Stevens CW, Dilling TJ. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int J Radiat Oncol Biol Phys. 2013;85:190–195. doi: 10.1016/j.ijrobp.2012.03.041. [DOI] [PubMed] [Google Scholar]
- 38.Timmerman R, Papiez L, McGarry R, Likes L, DesRosiers C, Frost S, Williams M. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124:1946–1955. doi: 10.1378/chest.124.5.1946. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita H, Kobayashi-Shibata S, Terahara A, Okuma K, Haga A, Wakui R, Ohtomo K, Nakagawa K. Prescreening based on the presence of CT-scan abnormalities and biomarkers (KL-6 and SP-D) may reduce severe radiation pneumonitis after stereotactic radiotherapy. Radiat Oncol. 2010;5:32. doi: 10.1186/1748-717X-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi W, Yamashita H, Kida S, Masutani Y, Sakumi A, Ohtomo K, Nakagawa K, Haga A. Verification of planning target volume settings in volumetric modulated arc therapy for stereotactic body radiation therapy by using in-treatment 4-dimensional cone beam computed tomography. Int J Radiat Oncol Biol Phys. 2013;86:426–431. doi: 10.1016/j.ijrobp.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa K, Haga A, Kida S, Masutani Y, Yamashita H, Takahashi W, Sakumi A, Saotome N, Shiraki T, Ohtomo K, et al. 4D registration and 4D verification of lung tumor position for stereotactic volumetric modulated arc therapy using respiratory-correlated cone-beam CT. J Radiat Res. 2013;54:152–156. doi: 10.1093/jrr/rrs058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohnishi H, Yokoyama A, Kondo K, Hamada H, Abe M, Nishimura K, Hiwada K, Kohno N. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am J Respir Crit Care Med. 2002;165:378–381. doi: 10.1164/ajrccm.165.3.2107134. [DOI] [PubMed] [Google Scholar]
- 43.Kong FM, Ao X, Wang L, Lawrence TS. The use of blood biomarkers to predict radiation lung toxicity: a potential strategy to individualize thoracic radiation therapy. Cancer Control. 2008;15:140–150. doi: 10.1177/107327480801500206. [DOI] [PubMed] [Google Scholar]
- 44.Hara R, Itami J, Komiyama T, Katoh D, Kondo T. Serum levels of KL-6 for predicting the occurrence of radiation pneumonitis after stereotactic radiotherapy for lung tumors. Chest. 2004;125:340–344. doi: 10.1378/chest.125.1.340. [DOI] [PubMed] [Google Scholar]
- 45.Iwata H, Shibamoto Y, Baba F, Sugie C, Ogino H, Murata R, Yanagi T, Otsuka S, Kosaki K, Murai T, et al. Correlation between the serum KL-6 level and the grade of radiation pneumonitis after stereotactic body radiotherapy for stage I lung cancer or small lung metastasis. Radiother Oncol. 2011;101:267–270. doi: 10.1016/j.radonc.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 46.Takeda A, Ohashi T, Kunieda E, Enomoto T, Sanuki N, Takeda T, Shigematsu N. Early graphical appearance of radiation pneumonitis correlates with the severity of radiation pneumonitis after stereotactic body radiotherapy (SBRT) in patients with lung tumors. Int J Radiat Oncol Biol Phys. 2010;77:685–690. doi: 10.1016/j.ijrobp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Hof H, Hoess A, Oetzel D, Debus J, Herfarth K. Stereotactic single-dose radiotherapy of lung metastases. Strahlenther Onkol. 2007;183:673–678. doi: 10.1007/s00066-007-1724-z. [DOI] [PubMed] [Google Scholar]
- 48.Norihisa Y, Nagata Y, Takayama K, Matsuo Y, Sakamoto T, Sakamoto M, Mizowaki T, Yano S, Hiraoka M. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys. 2008;72:398–403. doi: 10.1016/j.ijrobp.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Yuan X, Liao Z, Liu Z, Wang LE, Tucker SL, Mao L, Wang XS, Martel M, Komaki R, Cox JD, et al. Single nucleotide polymorphism at rs1982073: T869C of the TGFbeta 1 gene is associated with the risk of radiation pneumonitis in patients with non-small-cell lung cancer treated with definitive radiotherapy. J Clin Oncol. 2009;27:3370–3378. doi: 10.1200/JCO.2008.20.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeda A, Enomoto T, Sanuki N, Nakajima T, Takeda T, Sayama K, Kunieda E. Acute exacerbation of subclinical idiopathic pulmonary fibrosis triggered by hypofractionated stereotactic body radiotherapy in a patient with primary lung cancer and slightly focal honeycombing. Radiat Med. 2008;26:504–507. doi: 10.1007/s11604-008-0261-8. [DOI] [PubMed] [Google Scholar]
- 51.Guckenberger M, Heilman K, Wulf J, Mueller G, Beckmann G, Flentje M. Pulmonary injury and tumor response after stereotactic body radiotherapy (SBRT): results of a serial follow-up CT study. Radiother Oncol. 2007;85:435–442. doi: 10.1016/j.radonc.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 52.Kimura T, Matsuura K, Murakami Y, Hashimoto Y, Kenjo M, Kaneyasu Y, Wadasaki K, Hirokawa Y, Ito K, Okawa M. CT appearance of radiation injury of the lung and clinical symptoms after stereotactic body radiation therapy (SBRT) for lung cancers: are patients with pulmonary emphysema also candidates for SBRT for lung cancers? Int J Radiat Oncol Biol Phys. 2006;66:483–491. doi: 10.1016/j.ijrobp.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Matsuo Y, Shibuya K, Nakamura M, Narabayashi M, Sakanaka K, Ueki N, Miyagi K, Norihisa Y, Mizowaki T, Nagata Y, et al. Dose--volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys. 2012;83:e545–e549. doi: 10.1016/j.ijrobp.2012.01.018. [DOI] [PubMed] [Google Scholar]


