Abstract
For many years, it has been recognized that hypertension tends to cluster with various anthropometric and metabolic abnormalities including abdominal obesity, elevated triglycerides, reduced high-density lipoprotein cholesterol, glucose intolerance, insulin resistance and hyperuricemia. This constellation of various conditions has been transformed from a pathophysiological concept to a clinical entity, which has been defined metabolic syndrome (MetS). The consequences of the MetS have been difficult to assess without commonly accepted criteria to diagnose it. For this reason, on 2009 the International Diabetes Federation, the American Heart Association and other scientific organizations proposed a unified MetS definition. The incidence of the MetS has been increasing worldwide in parallel with an increase in overweight and obesity. The epidemic proportion reached by the MetS represents a major public health challenge, because several lines of evidence showed that the MetS, even without type 2 diabetes, confers an increased risk of cardiovascular morbidity and mortality in different populations including also hypertensive patients. It is likely that the enhanced cardiovascular risk associated with MetS in patients with high blood pressure may be largely mediated through an increased prevalence of preclinical cardiovascular and renal changes, such as left ventricular hypertrophy, early carotid atherosclerosis, impaired aortic elasticity, hypertensive retinopathy and microalbuminuria. Indeed, many reports support this notion, showing that hypertensive patients with MetS exhibit, more often than those without it, these early signs of end organ damage, most of which are recognized as significant independent predictors of adverse cardiovascular outcomes.
Keywords: Arterial hypertension, Metabolic syndrome, Target organ damage, Cardiovascular risk
Core tip: Several lines of evidence suggest that metabolic syndrome (MetS) may amplify hypertension-related target organ damage (TOD). Some of MetS components, when considered individually may have little or no influence on TOD, but when taken together may synergistically interact promoting the development of left ventricular hypertrophy, aortic stiffness and microalbuminuria. The marked tendency of hypertensive patients with MetS to develop these manifestations of subclinical organ damage, that are well-known predictors of cardiovascular events, largely explain the increased morbidity and mortality associated with the syndrome. Therefore, identifying MetS in hypertensive patients may enable the clinician to better assess the cardiovascular risk.
INTRODUCTION
For many years, it has been recognized that high blood pressure is often associated with various anthropometric and metabolic abnormalities including abdominal obesity, elevated triglycerides, reduced high-density lipoprotein (HDL) cholesterol, glucose intolerance and insulin resistance.
Several lines of evidence support the notion that these traits occur simultaneously to a greater degree than would be expected by chance alone. This evidence supports the existence of a discrete disorder meriting in the appellation as a “metabolic syndrome”. A variety of clinical and biohumoral alterations may co-exist with the main components of the metabolic syndrome: hyperuricemia, increases in apolipoprotein B and small dense low-density lipoprotein cholesterol, prothrombotic factors, chronic low grade inflammation, non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis, obstructive sleep apnoea and polycystic ovarian disease. Many of these conditions may contribute to explain why the metabolic syndrome (MetS) conveys an increased risk of developing subclinical and overt cardiovascular and renal diseases.
METABOLIC SYNDROME DEFINITIONS
In the effort to introduce the MetS into clinical practice, several scientific organizations have attempted to formulate working definition of the syndrome. The first proposals came in 1998[1] and in 1999[2] from a consultation group on the definition of diabetes for the World Health Organization (WHO)[2]. Alternative definitions have been proposed subsequently by the European Group for the Study of Insulin Resistance (EGIR)[3], the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III)[4] the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI)[5], the American Society of Clinical Endocrinologists (AACE)[6] and the International Diabetes Federation (IDF)[7]. Recently, IDF, AHA/NHLBI and other scientific societies, in an attempt to unify discordant criteria between previous definitions of MetS, proposed a new “harmonizing” definition of this syndrome[8]. All definitions include a measure of blood pressure (BP), triglycerides, HDL cholesterol, and fasting glucose. They differ with respect to the selection of cutoff points and a measure of obesity. In contrast to the gluco-centric approach of the WHO and EGIR definitions, in which the presence of insulin resistance is the starting point, the ATP III definition is based on the number of abnormalities only, whereas the AACE definition considers the number of abnormalities in selected subjects with high risk of insulin resistance. The ATP III and WHO definitions implicitly include type 2 diabetes (T2DM) as syndrome traits. Not all experts agree that T2DM should be part of the definition, as the importance of the syndrome is that it identifies patients at increased risk for the development of diabetes[8,9].
Among the various definitions proposed the most widely used is that of the ATP III or the AHA/NHLBI version, that slightly revised the former by lowering the threshold for fasting glucose from 110 to 100 mg/dL.
The wide use of these definitions is due primarily because they provide a relatively simple approach for diagnosing MetS by employing easily measurable risk factors.
In the revised ATP III definition[5], MetS is diagnosed when at least three or more of the following abnormalities are present: BP ≥ 130/85 mmHg (or drug treatment for hypertension), HDL < 1.0 mmol/L (40 mg/dL) in men or < 1.3 mmol/L (50 mg/dL) in women (or drug treatment for reduced HDL); fasting glucose ≥ 5.6 mmol/L (100 mg/dL) (or drug treatment for elevated glucose); triglycerides > 1.7 mmol/L (150 mg/dL) (or drug treatment for elevated triglycerides); and waist circumference > 102 cm in men or > 88 cm in women[5].
On 2005 the IDF has proposed a set of criteria that are similar to those of the updated ATP III criteria. In fact, thresholds are identical for triglycerides, HDL-C, BP, and plasma glucose. The major difference is that the IDF considered central obesity, as assessed by waist circumference (WC), essential for diagnosis. Moreover, in this obesity-centric definition the WC cutoffs were adjusted for different ethnic groups, taking into account that the risk associated with a particular waist measurement (especially for diabetes development) will differ in different populations. But despite the attempt to standardize clinical definition of MetS, this has led to some confusion on the part of clinicians regarding how to identify patients with the syndrome.
Thus came the initiative of the IDF and the AHA/NHLBI, joined by the World Heart Federation, International Atherosclerosis Society, and International Association for the Study of Obesity[8], to develop one unified definition.
The main difference between the ATP III and the IDF diagnostic criteria is that in the IDF definition abdominal obesity is a prerequisite of the diagnosis of MetS. As a major step in consensus, this obligation has been reversed; therefore the “harmonizing” definition is identical to the revised ATP III definition except that IDF waist circumference cut points were used[8]. In Europids (white people of European origin, regardless of where they live in the world) WC thresholds were the same as that used by the EGIR (≥ 94 cm for males and ≥ 80 cm for females), and lower than the ATP III recommendations[7]. For the Asian people IDF adopted even lower cutpoints for men (≥ 90 cm) and the same as for Europids for women.
Similarly to adults, no general consensus exists regarding the definition of MetS in children and adolescents[9,10]. Furthermore, studies published so far have used their own set of variables, number of criteria (three or four) and different cut-off points to define risk factors associated with MetS. In 2007, a consensus report was published by the IDF group[10], including three age groups: 6 to < 10, 10 to < 16 and 16 + years (adult criteria).
Based on this report, obesity is defined as WC ≥ 90th percentile, or adult cut-off if lower, while all other parameters are defined based, rather than percentiles, on absolute numbers, that are the same used for adults[10].
A scientific statement from the AHA and other scientific societies, published on 2009, called attention to the fact that, especially during adolescence, a marked instability exists in the categorical diagnosis of MetS. This instability, which includes both gain and loss of the diagnosis, suggests that the syndrome has reduced clinical utility in adolescence[9].
IS METABLIC SYNDROME REALLY A SYNDROME?
Some controversy also exists about whether the MetS is a true syndrome or a mixture of unrelated phenotypes.
Two major health organizations in Europe (the European Association for the Study of Diabetes) and the United States (American Diabetes Association) have claimed that the MetS is not a single pathophysiological entity, that its identification has no clinical utility, and that clinical emphasis should rather be placed on effectively treating any cardiovascular (CV) risk factor that is present[11,12]. We believe, together with many experts on CV risk[8,13,14], that this clustering of interrelated metabolic risk factors is a useful construct, and although it needs to be better defined, represents a good basis for calling this as a syndrome. The rationale supporting use of the Mets includes the following: (1) the label of MetS seems to be an important way to educate patients about the connection between their lifestyle, health risks, and medical outcomes; (2) it provides a framework for research exploring a possible unifying pathophysiological basis for the observed cluster of risk factors; (3) it quantifies chronic disease risk within populations and facilitates between-country comparisons; (4) it can guide relative risk prediction and clinical management decisions; (5) it results from the association of individuals components that are often defined by values that are lower than those meeting the definition of risk factors by many guidelines, which may thus fail to detect the presence of a high CV risk in several subjects with MetS; and (6) it provides an easily comprehensible public health message and reminds health professionals of the need to assess related risk factors when one risk factor is detected ultimately helping implementation of CV prevention[8,9].
On the other hand, the criteria used to diagnose the MetS have major limitations including: the dichotomization of risk factors; the attribution of relative as opposed to absolute risk; the differing predictive value of risk factor combinations; the inclusion of individuals with established diabetes and heart disease[9,10].
PREVALENCE OF METABOLIC SYNDROME IN GENERAL POPULATION AND IN HYPERTENSIVE PATIENTS
The prevalence of the MetS is at least in part dependent on the definition of the syndrome and its components and on the composition (sex, age, race, and ethnicity) of the population studied[14-27]. However, there is a strong epidemiological evidence that, regardless of the criteria used, the prevalence of MetS is high and rising in all western society and in Asia, very likely as a result of obesity epidemic[14-20]. In general, it has been estimated that approximately 10%-30% of the world’s adult population has the MetS[14]. A very consistent finding in all of these studies is that the prevalence of the MetS is highly age-dependent[14-19]. Data regarding gender effect on MetS prevalence are conflicting with the majority of the studies finding the highest prevalence in women compared to men[14-19]. The conflicting results with respect to gender effect may partly be explained by the application of different definitions for the MetS. The application of the modified WHO criteria tends to increase the prevalence of MetS in men[18,19].
Since high BP is a key component of MetS, it is not surprising that in MetS patients arterial hypertension is highly prevalent. The Pressioni Arteriose Monitorate E Loro Associazioni (PAMELA) population study revealed that high normal BP values and hypertension were present in 80% of individuals with MetS[21]. Conversely, the prevalence of MetS is more elevated in hypertensive patients than in general population[18-20,22-36].
In a large French population, the prevalence of MetS was 5.4% (n = 1181) among normotensive men and 2.8% (n = 360) among normotensive women, and rose to 19.3% (n = 3490) for hypertensive men and 14.8% (n = 1200) for hypertensive women. Much higher prevalences were reported in other studies performed only in hypertensive patients[23-36].
In the Progetto Ipertensione Umbria Monitoraggio Ambulatoriale (PIUMA) study, a prospective observational investigation of 1742 Italian adult subjects with essential hypertension, MetS, defined according to ATP III criteria, was diagnosed in 34% of the population[25].
Similar data were obtained in our cross-sectional study conducted in 353 essential hypertensives and 37% of whom had MetS[26]. In our study population, prevalence of MetS was higher in women than it was in men. This greater proportion of women with MetS was explained by a higher prevalence of visceral obesity and of low HDL values in females when compared to males[26].
An even greater prevalence of MetS was observed in the Global Cardiometabolic Risk Profile in Patients with hypertension disease (GOOD) study[33]. This was an observational, cross-sectional survey conducted in 305 sites in 12 European countries-Among the 3370 outpatients included in the analyses 58% had the MetS. This very high prevalence is probably explained by the older age (61 years) of the study population when compared to those of the other investigations conducted in hypertensive subjects. In the same survey it was noticed that the proportion of patients with uncontrolled BP was significantly higher among the subjects with MetS compared with those without it (P < 0.001)[33]. Analogous results were found among the hypertensive population of the Korean National Health and Nutrition Examination Survey[34] and in the Renal Dysfunction in Hypertension (REDHY) study. In the latter investigation, where a total of 1856 Sicilian hypertensive individuals, free from diabetes mellitus were enrolled[35], a significantly higher (P < 0.001) percentage of patients with uncontrolled BP (> 140/90 mmHg) were found in the group with MetS (79%) as compared to the subjects without MetS (71%).
It has been also reported a high frequency of resistant hypertension among individuals with MetS[36], that can be attributed to a number of pathophysiological mechanisms[36] that will be described in the following section.
The prevalence of the MetS is growing worldwide[14-17]. Between 2008 and 2010, the proportion of the hypertensive population with MetS was forecast to increase to 78%, 45% and 43% in Germany, Spain and Italy respectively[16]. All MetS components were forecast to rise with the prevalence of abdominal obesity and impaired fasting glucose increasing the most. Total annual costs of hypertension with MetS amounted to €24427, €1909 and €4877 million respectively in Germany, Spain and Italy in 2008. By 2020, keeping costs set at 2008 prices, these annual costs of hypertension with MetS were forecast to rise by 59%, 179%, 157% in Germany, Spain and Italy respectively. The largest component of the total annual economic burden of hypertensive patients with MetS was the treatment and management of the consequence of disease rather than the management of hypertension itself including physician and drug costs[16].
Pathophysiology of hypertension in metabolic syndrome
Several mechanisms have been hypothesized to explain why the MetS may be considered as a prohypertensive state[32] (Figure 1).
Figure 1.
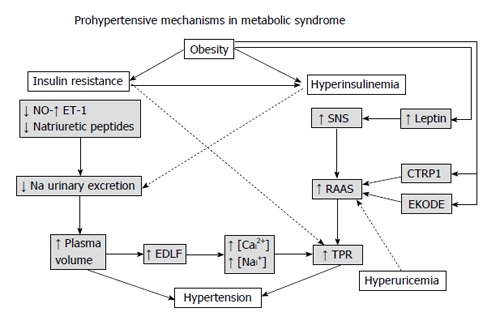
Hypothetical mechanisms by which the metabolic syndrome may lead to high blood pressure. NO: Nitric oxide; ET-1: Endothelin-1; SNS: Sympathetic nervous system; RAAS: Renin-angiotensin-aldosterone system; CTRP1: Complement-C1q tumor necrosis factor-related protein 1; EKODE: Epoxy-keno derivative of linoleic acid; TPR: Total peripheral resistance; EDLF: Endogenous digoxin-like factor; [Cai2+]: Intracellular concentration of calcium; [Nai+]: Intracellular concentration of sodium.
Although further research is required to better understand the pathophysiology behind the syndrome and the gene-environment interactions that increase susceptibility, there is general agreement that visceral obesity and insulin resistance (IR) are at the core of most cases of MetS[1-6,37,38]. It is widely believed that IR results from a combination of genetic and environmental factors[1-6]. Resistance to insulin-mediated glucose disposal determines a compensatory hyperinsulinemia, which serves to maintain glucose homeostasis. However, this initially adaptive mechanism ultimately may promote hypertension and various atherogenic processes. It is only after the pancreas is unable to meet the increased demand for insulin necessary to overcome IR that glucose control becomes abnormal. Therefore, hyperglycemia signifies a more advanced stage in the loss of normal glucose homeostasis[1-6].
About 50% of patients with essential hypertension are insulin-resistant[18-20,39-41]. Independently of body mass index, hypertensive individuals, when compared with healthy normotensive controls, have higher fasting and postprandial insulin levels, and greater reductions in insulin sensitivity[39-42].
Insulin, in response to states of over-nutrition, stimulates the sympathetic nervous system (SNS) to promote thermogenesis and to minimize weight gain. The insulin-mediated hyperadrenergic state, however, leads to an increase in heart rate, and BP[37,43,44].
Other factors may contribute to the sympathetic activation occurring in MetS. They include leptin, which increases in obesity and has been shown to act as a powerful sympathostimulator[36,43-46]. Sleep apnoea, which frequently occurs in obesity, may also play a role because its sympathoexcitatory effect via the hypoxic activation of the chemoreceptor reflex[44,46,47].
The enhanced SNS activity and insulin and leptin per se[48] stimulate renal sodium absorption leading to volume expansion and further elevation of BP[36,43-46] (Figure 1).
Moreover, insulin can cause an upregulation of angiotensin II type I receptors by post-transcriptional mechanisms such as stabilization of receptor mRNA and prolongation of its half life[49]. The increase in angiotensin II type I receptors potentiates the physiologic actions of angiotensin II that include peripheral vasoconstriction and plasma volume expansion. Furthermore, overexpression of systemic as well as local adipose tissue renin-angiotensin-aldosterone system (RAAS) has been documented in obese persons[36,45,46,50,51].
The increased activity of RAAS in subjects with MetS may be related also to vitamin D deficiency. Indeed, vitamin D status has been inversely associated with MetS[52,53].
Experimental studies have suggested that vitamin D may exert its beneficial effects by stimulating the expression of insulin receptor to improve insulin responsiveness for glucose transport or by controlling calcium influx, which is essential for the insulin mediated intracellular process in insulin responsive tissues[53].
In some studies, increased plasma aldosterone concentrations (PAC) have been reported in obese subjects. These elevated aldosterone levels are often out of proportion to the increase in renin activity[46,54-58]. Indeed, it has been demonstrated that a variety of adipose tissue-derived factors can stimulate aldosterone synthesis[36,46,55,57]. Goodfriend et al[55] reported that an epoxy-keto derivative of linoleic acid (EKODE), one of the oxidized products of fatty acids, stimulates aldosterone secretion in rat adrenal cells. More recently, in vitro experiments documented that human adipocytes secrete potent mineralcorticoid releasing factors. Among these a Complement-C1q tumor necrosis factor-related protein 1 (CTRP1) is able to increase aldosterone production in cultured human adrenal cortical cells, and serum CTRP1 expression was higher in a small number of hypertensive patients compared with healthy volunteers[57,58].
On the other hand, the low levels of plasma natriuretic peptides observed in individuals with obesity and MetS might predispose to increased adrenal production of aldosterone, because the stimulatory effect of EKODE on aldosteronogenesis is inhibited by natriuretic peptides[57] Another putative mechanism explaining augmented PAC may be endothelin 1[59-61], which is increased in insulin resistance states[59-61].
Moreover, insulin resistance and the accompanying compensatory hyperinsulinemia may contribute toward increasing PAC, because insulin is known to stimulate aldosterone synthesis in vitro[62] and reciprocal relationships between aldosterone, insulin resistance and hyperinsulinemia have been described in clinical studies[36,63,64] (Figure 1).
Adipocytes appear to have all the components of the RAAS and thus may produce locally generated angiotensin II and aldosterone[36,46,50,51]. On the other hand, increased intrarenal pressure accompanying perirenal fat deposition in obesity contributes to the increased activity of RAAS[45].
Insulin resistance/hyperinsulinemia and visceral obesity appear to predispose patients to impaired peripheral glucose utilization and nitric oxide (NO) production[65,66]. Indeed, insulin is a mediator of important vasodilatory functions on the vasculature. In obese individuals with insulin resistance, these functions are lost or even reversed leading to impaired vascular relaxation and hypertension[65,66]. The underlying mechanism may be an impairment of NO-mediated vasodilation and a relative increase in the activity of endothelins[59,60,65,66]. Cellular response to insulin is mediated by means of 2 pathways: phosphatidylinositol (PI3) 3-kinase and mitogen-activated protein (MAP) kinase[65,66]. Activation of the PI3 kinase pathway is associated with the metabolic effects of insulin, including glucose transport, and NO-synthesis, whereas MAP kinase activation is associated with mitogenic effects, such as cell growth and proliferation. It has been demonstrated that in the setting of insulin resistance or T2DM, insulin had reduced effects on PI3 kinase-mediated pathways, while maintaining MAP kinase activity[65,66].
Furthermore, the increased peripheral vascular resistance that often accompanies insulin resistance may be due in part to altered divalent cation metabolism (“cation imbalance”) of vascular smooth muscle cells (VSMC)[39,67]. One mechanism by which insulin, and its homologous peptide insulin-like growth factor-1 (IGF-1), attenuate vascular contractility is through effects on VSMC divalent cation metabolism[67]. These hormones reduce Ca2+ influx into VSMCs in conjunction with reductions in VSMC contractile responses. It is thought that the mechanism by which insulin and IGF-1 decreases VSMC intracellular Ca2+ vasoconstriction is through stimulation of the Na+/K+-ATPase pump[67]. It has been demonstrated that insulin/IGF-1 activation of the PI3-kinase pathway is critical for the ability of these peptides to stimulate the pump[67]. Thus, altered PI3-kinase responses to insulin/IGF-1, described in insulin resistance states, may explain the decreased ability of those peptides to mediate vasodilation in insulin-resistant patients[67]. As angiotensin II has been shown to interfere with PI3 activation in VSMC and cardiomyocytes, overexpression of the tissue RAAS may be one of the major factors in cardiovascular insulin/IGF-1 resistance[50,51,67,68].
Other factors may contribute to reduce the activity of Na+/K+-ATPase pump in patients with MetS, such as the increased production of the endogenous digoxin-like factor[68,69]. Elevated plasma levels of this substance have been documented in obese hypertensives with glucose intolerance[69] and in several circumstances characterized by volume expansion[68]. This Na+/K+-ATPase inhibitor promotes natriuresis but also produces accumulation of intracellular sodium, reducing in turn the sodium-calcium exchange system, and increasing cytosolic free calcium[68]. The cation imbalance may lead to enhanced VSMC contraction and to an elevation of peripheral vascular resistance. Moreover, reducing the sodium pump activity may exaggerate neural stimulation and norepinephrine overflow, which might contribute to increase BP[68].
On the other hand, the low levels of plasma natriuretic peptides observed in obese and overweight individuals, especially in those with IR[70] may also predispose to salt retention and increased activation of the sympathetic and renin-angiotensin systems, leading to persistent BP elevations in patients with MetS[36,46,70] (Figure 1).
Patients with MetS have often raised levels of serum uric acid (SUA)[6,71]. Hyperuricemia has been usually attributed to hyperinsulinemia and IR in MetS[6] and is not acknowledged as a main mediator of MetS, and CV diseases (CVD) development. However, investigations conducted in the last decades have changed this traditional view, supporting the concept of an independent link between hyperuricemia and increased risk of MetS, diabetes, hypertension, kidney disease and CV disorders[71-74]. Pharmacologically induced mild-to-moderate hyperuricemia, via oxonic acid administration, in rats resulted in the development of high BP[72,73]. Experimental studies suggest that SUA might play a role in initiating hypertension through multiple mechanisms, including induction of oxidative stress, activation of RAAS and inhibition of NO[72]. A plausible common pathway for the above mechanisms is the development of renal arteriolar disease with interstitial macrophage and T-cell infiltration, eventually leading to renal vasoconstriction and ischemia[72]. Subsequent studies showed that the hypertension developed in 2 phases. Initially, reducing SUA with either xanthine oxidase inhibitors or uricosuric agents could directly reverse the hypertension. Hypertension during this (salt-resistant) phase was mediated by uric acid-dependent activation of the RAAS, by the induction of oxidative stress, and by the reduction in endothelial NO levels[72]. Over time the animals developed significant renal microvascular disease and tubulointerstitial inflammation, and the hypertension became kidney-dependent and salt-sensitive and persisted despite allowing uric acid levels to return to baseline levels[73].
Lowering SUA with either allopurinol or probenecid has been shown to markedly reduce BP in pilot studies of adolescents with hypertension or prehypertension, whereas effects on adults with primary hypertension are less prominent[72,73]. More recently, in 2045 participants of the PAMELA study, elevated SUA levels predicted new-onset home and ambulatory hypertension as well as cardiovascular and all-cause mortality[74]. There are also studies that suggest SUA may not play a role in hypertension and related disorders[73,75,76]. One of the strongest arguments is based on gene wide association studies, which have been able to link polymorphisms in urate transporters with hyperuricemia and gout but not with high BP[73]. Therefore, despite the findings obtained in animals studies and in adolescents, the question regarding the exact role of uric acid in inducing hypertension and CV diseases remains unanswered.
All the above-described prohypertensive mechanisms may provide the explanation of a common pathophysiological feature observed in patients with MetS that is sodium sensitivity. Chen et al[77] evaluated the association between MetS and salt sensitivity of BP in 1906 subjects (with and without MetS). Study participants received a low-sodium diet (3 g sodium chloride per day) for 7 d, followed by a high-sodium diet (18 g sodium chloride per day) for an additional 7 d[77]. They found that: multivariable-adjusted mean changes in BP were significantly greater in participants with MetS than in those without on both low-sodium and high-sodium diets[77].
These results support the notion that patients with MetS, especially those with obesity, are very sensible to sodium intake[36,45,46].
METABOLIC SYNDROME AND CARDIOVASCULAR RISK
The high prevalence of the MetS is of considerable concern because several studies suggest that people with the MetS are at increased risk for developing T2DM[18-20,78] and CV events[18-22,25,27,29-32,78-82].
The ability of MetS to predict the development of T2DM has been examined in numerous studies; it was estimated that the MetS approximately quintuples the risk for incident T2DM[14,78].
About a hundred longitudinal studies were performed in order to assess the CV prognostic impact of the MetS[18-22,25,27,29-32,82] and the vast majority of them were included in the four meta-analyses carried out up to now summarizing this issue[78-81].
The most recent and largest of them was that of Mottillo et al[81] that included near one million patients (total n = 951083). The MetS, defined according to the ATPIII criteria, was associated with a 2-fold increase in risk of CVD, CV mortality, myocardial infarction and stroke, and a 1.5-fold increase in risk of all-cause mortality[81].
Whether or not the prognostic significance of the MetS exceeds the risk associated with the sum of its individual components is still a matter of debate. Even if a number of studies support the notion that diagnosing the MetS adds nothing beyond each individual risk factor for predicting CVD[11,12], other investigations[8,20], such as the METS-GREECE Multicentre study, seem to suggest the opposite[82]. More recently, in the 19257 hypertensive patients randomized in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm MetS was significantly associated with coronary outcomes, stroke, and all-cause mortality after adjusting for age, sex, and ethnicity. However, when the model was further adjusted for the individual components, MetS was associated with significantly increased risk of stroke and all-cause mortality but not coronary disease (Table 1)[32].
Table 1.
Prospective studies exploring the association of metabolic syndrome with cardiovascular events and all-cause mortality in hypertensive subjects
| Ref. | No. of subjects (population) | Mean follow-up (yr) | Mean age (yr) | MetS (%) | MetS definition | T2DM (%) | Risk of all-cause mortality | Risk of CV events |
| Schillaci et al[25] | 1742 (Italian hypertensives without CVD at baseline) | 4.1 | 50 | 34.0 | Modified ATP III | 6.0 | Not reported | HR = 1.73 (1.25-2.38) Cardiac events: HR = 1.48, (1.01-2.27). Cerebrovascular events: HR = 2.11 (1.27-3.50) After exclusion of T2DM HR = 1.43 (1.02-2.08) |
| Pierdomenico et al[27] | 802 (Italian hypertensives without T2DM, TOD and CVD at baseline) | 6.9 | 53 | 27.2 | Modified ATP III | 0 | Not assessed | HR = 2.64 (1.52-4.58) |
| Andreadis et al[29] | 1007 (Greek hypertensives without CVD at baseline) | 2.1 | 59 | 42.1 | Modified ATP III | 13.2 | Not assessed | HR = 1.75 (1.15-2.66) Cardiac events: HR = 1.73 (1.00-3.00). Cerebrovascular events: HR = 1.91 (1.01-3.58) After exclusion of T2DM: HR = 1.67 (1.01-2.74) |
| Zanchetti et al[28] | 2034 (European hypertensives participating in the ELSA study) | 3.7 | 56 | 33.3 | Modified ATP III | 4.5 | Not assessed | Incidence of CV events not different (about 6% in subjects with and in those without MetS) |
| Pannier et al[22] | 26447 French hypertensives without CVD at baseline | 4.1 | 50 | 17.8 | ATP III | Not reported | HR = 1.40 (1.13-1.74) | Not assessed |
| de Simone et al[30] | 8243 hypertensives with EKG-LVH participating in the LIFE study | 4.8 | 67 | 19.3 | Modified ATP III | 12.5 | Not assessed | HR = 1.47 (1.27-1.71) CV death: HR = 1.73 (1.38-2.17) |
| Vlek et al[31] | 1815 hypertensives with CVD at baseline and without T2DM | 3.9 | 61 | 42.7 | ATP III | 0 | Not assessed | HR = 1.24 (0.95-1.62) CV death: HR = 1.41 (1.01-1.98) |
| Gupta et al[32] | 19257 hypertensives participating in the ASCOT-BPLA study | 5.5 | 63 | 43.8 | ATP III | 27.0 | HR = 1.35 (1.16-1.58)1 | Stroke: HR = 1.34 (1.07-1.68)1 MI: HR = 1.16 (0.95-1.43)1 |
HR are adjusted for the individual components of MetS. MetS: Metabolic syndrome; CVD: Cardiovascular disease; ATP III: Third report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults; T2DM: Type 2 diabetes mellitus; TOD: Target organ damage; CV: Cardiovascular; MI: Myocardial infarction; EKG-LVH: Left ventricular hypertrophy detected by electrocardiography; ELSA: European Lacidipine Study on Atherosclerosis; LIFE: Losartan Intervention For Endpoint reduction; ASCOT-BPLA: Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm.
The adverse prognostic impact of the MetS, in hypertensive patients was also observed in other six investigations (Table 1)[22,25,27,29-31]. In the aforementioned PIUMA study, hypertensive participants with MetS had an increased risk of developing cardiac and cerebrovascular events, independently of traditional CV risk factors, including left ventricular (LV) hypertrophy (LVH) and 24-h BP[25]. Most notably, the association between the MetS and future CV morbidity also held in patients without diabetes mellitus at the baseline examination[25].
In contrast with these studies, in the European Lacidipine Study on Atherosclerosis study, a large cohort of well-treated hypertensive subjects, outcomes were not different between patients with MetS and those without it[28], probably because an effective antihypertensive treatment may largely counteract the detrimental influence of Mets (Table 1).
It is conceivable that the increased CV risk conferred by MetS in hypertensive subjects may in part be mediated through preclinical cardiac and renal organ damage. Indeed, major CV events in most hypertensive patients are preceded by the development of asymptomatic cardiovascular and renal structural and functional abnormalities[83]; most of which are recognized as significant independent predictors of adverse cardiovascular outcomes[84-87].
METABOLIC SYNDROME AND HYPERTENSIVE TARGET ORGAN DAMAGE
The very frequent occurrence of BP values in the high normal or frankly hypertension range in subjects with the MetS[18-21] may explain the increased prevalence of hypertension-related preclinical (or asymptomatic) organ damage, such as LVH, elevated urinary albumin excretion rate and arterial stiffening[18-21,23,24,26]. Some of these markers of organ damage, however, are frequently observed also in individuals who have the MetS without a BP elevation, or also in hypertensive individuals after adjustment for BP values in multivariate analyses, suggesting that other components of this condition play a role independently of BP[20] (Table 2).
Table 2.
Cross-sectional studies investigating the association of metabolic syndrome with various markers of subclinical organ damage
| Ref. | No. of subjects | LVM | LV diastolic | Carotid IMT | Micro- | CKD | Arterial |
| (population) | function | and plaques | albuminuria | stiffness | |||
| Mancia et al[21] | 2051 (Italian GP) | ↑ | - | - | - | - | - |
| Cuspidi et al[23] | 447 (Italian hypertensives) | ↑ | - | ↑ | ↑ | - | - |
| Leoncini et al[24] | 354 (Italian hypertensives) | ↑ | - | ↑ | ↑ | - | - |
| Mulè et al[26] | 353 (Italian hypertensives) | ↑ | Impaired | - | ↑ | - | - |
| Mulè et al[91] | 475 (Italian hypertensives) | ↑ | Impaired | - | - | - | - |
| Schillaci et al[92] | 618 (Italian hypertensives) | ↑1 | Impaired1 | - | - | - | - |
| Nicolini et al[93] | 200 (Italian hypertensives) | ↑1 | Impaired1 | - | - | - | - |
| Aijaz et al[94] | 2042 (United States GP) | ↑1 | Impaired1 | - | - | - | - |
| Sundström et al[96] | 820 (elderly Swedish GP) | ↑ | |||||
| de Simone et al[97] | 2758 (American Indian GP) | ↑ | Impaired | - | - | - | - |
| Burchfiel et al[98] | 1572 (United States Black GP) | ↑ | - | - | - | - | - |
| de las Fuentes et al[99] | 607 (United States GP) | ↑ | Impaired | - | - | - | - |
| Hwang et al[100] | 1599 (South Korean GP) | ↑ | Impaired | - | - | - | - |
| Kim et al[101] | 1886 (South Korean GP) | Impaired | = | - | - | ↑ | |
| Ingelsson et al[102] | 1945 (United States GP) | ↑ | - | ↑ | ↑ | - | - |
| Ferrara et al[103] | 340 (Italian hypertensives) | ↑ | = | ||||
| Aksoy et al[105] | 90 (Turkish subjects) | ↑ | Impaired | - | - | - | - |
| Mulè et al[88] | 93 (Italian hypertensives) | - | - | - | - | - | ↑ |
| Schillaci et al[119] | 169 (Italian hypertensives) | - | - | - | ↑ | - | ↑ |
| Scuteri et al[120] | 20750 (9 cohorts from Europe and United States) | - | - | - | - | ↑ | |
| Scuteri et al[121] | 6148 (Italian GP aged 14-102 years) | - | - | ↑ | - | - | ↑ |
| Scuteri et al[122] | 471 (United States GP) | - | - | ↑ | - | - | ↑ |
| Zanchetti et al[28] | 2034 (European hypertensives) | - | - | ↑ | - | - | - |
| Kawamoto et al[124] | 760 (Japanese patients) | - | - | ↑ | - | - | - |
| Irace et al[125] | 1853 (Italian GP) | = | |||||
| Chen et al[110] | 6217 (United States GP) | - | - | - | ↑ | ↑ | - |
| Chen et al[109] | 15160 (Chinese GP) | - | - | - | ↑ | ↑ | - |
| Navarro et al[111] | 8425 (Spanish hypertensives) | - | - | - | - | ↑ | - |
| Johns et al[112] | 574 (United States non-diabetic GP) | - | - | - | - | ↑ | - |
Only in women. LVM: Left ventricular mass; IMT: Intima-media thickness; CKD: Chronic kidney disease; =: No difference; ↑: Increased; -: Not evaluated; LV: Left ventricular; GP: General population.
We performed a cross-sectional study to assess the impact of MetS, defined according to the NCEP-ATPIII criteria, on some cardiac, renal and retinal markers of target organ damage (TOD), in 353 non-diabetic young and middle aged essential hypertensives without clinical or laboratory evidence of CV and renal diseases[26].
In a subset of untreated subjects of the same population, we also explored the carotid-femoral pulse wave velocity (PWV), a measure of aortic stiffness, in patients with and without MetS[88].
Hypertensive patients with MetS exhibited higher LV mass on echocardiography [either normalized by body surface area (BSA) or by height elevated by a power of 2.7], relative wall thickness, left atrial size, and greater prevalence of LV hypertrophy, lower mid-wall fractional shortening and a longer E-wave deceleration time than subjects without MetS[26]. These results were maintained even after correction for several confounding variables, such as age, gender distribution, severity and duration of hypertension and previous antihypertensive therapy. In particular, after adjustment for these covariates, the likelihood of LV hypertrophy was 2.89-fold (95% interval, 1.68 to 4.98) higher in subjects with MetS than in those without it, when LV mass was indexed by height2.7 (LVMH2.7)[26]. Moreover, the higher the number of components of the MetS, the greater the LVMH2.7[18]. It is noteworthy that the relationship between MetS and LV mass was confirmed in multivariate regression models, including MetS together with its individual components, as independent variables[26]; this seems to suggest that MetS may have a deleterious effect on cardiac structure over and above the potential contribution of each single component of this syndrome, and that the confluence of abnormalities that comprise MetS may have a synergistic negative impact on LV mass.
We obtained similar results also when the influence of Mets on cardiac mass was evaluated in white coat hypertensives[89]. and in a subgroup of overweight and obese hypertensive patients[90]. On the other hand we did not observe a significant effect modifier of gender on the association between MetS and LV mass[91], at variance with the results reported in some[92-94], but not all[95,96], investigations exploring this issue.
Indeed, we found similar differences, regarding LV mass, in females and males with MetS when compared to their counterparts without the MetS (Figure 2)[91]. Moreover, in a two-factor ANOVA model, the analysis of the interaction term “gender × MetS” revealed no significant effect of sex on the association between MetS and LV mass, either normalized for BSA or height2.7[91].
Figure 2.
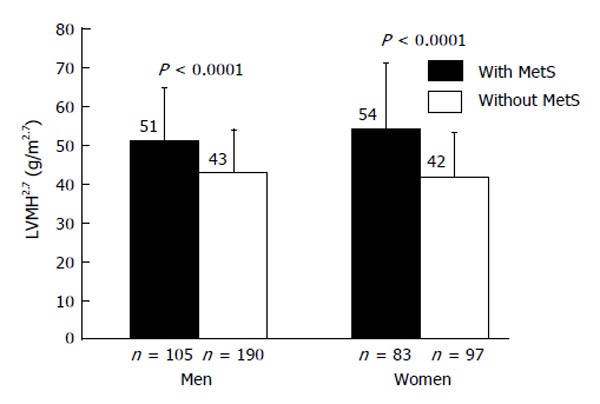
Mean values of left ventricular mass indexed for height2.7 in hypertensive men and women with and without the metabolic syndrome[91]. LVMH2.7: Left ventricular mass indexed for height2.7; MetS: Metabolic syndrome.
The unfavorable impact of the MetS on cardiac structure was confirmed in a large number of cross-sectional studies, conducted in different ethnic groups, in general populations[97-102], as well as in hypertensive patients[18-20,103] (Table 2). Moreover, it was even more convincingly demonstrated by the population based PAMELA study, in which the subjects with MetS had a three fold risk to develop LVH, than those without it, during a ten years follow-up period[104].
The putative mechanisms by which MetS promotes LVH[18,19] are summarized in Figure 3. It is interesting to note that a variety of studies suggest that LV diastolic function may be adversely influenced by the MetS per se[99-101], even in the absence of diabetes and hypertension and in part independently of age and left ventricular mass[105].
Figure 3.
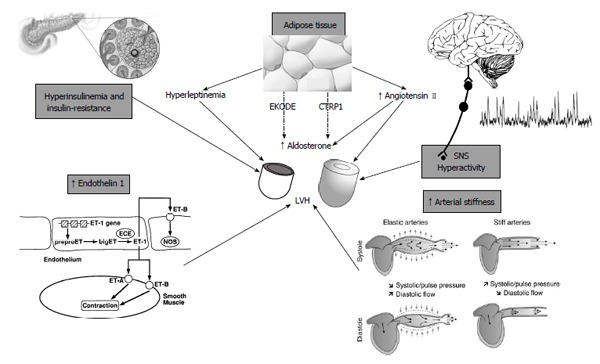
Putative mechanisms by which metabolic syndrome promotes left ventricular hypertrophy. SNS: Sympathetic nervous system; CTRP1: Complement-C1q Tumor necrosis factor-related protein 1; EKODE: Epoxy-keto derivative of linoleic acid; LVH: Left ventricular hypertrophy.
The asymptomatic changes in cardiac structure and function induced by the Mets largely explain why this syndrome is a powerful independent predictor of subsequent heart failure (HF), even after adjustment for established risk factors for HF[106,107]. This increased HF risk may be in part promoted by insulin resistance and accompanying hyperinsulinemia that may have direct myocardial effects in addition to its proatherosclerotic effects. Indeed, in the Uppsala Longitudinal Study of Adult Men, insulin resistance, measured with the reference standard euglycaemic insulin clamp technique, was an independent risk factor for HF, taking diabetes, obesity and other potential confounding factors into account[108].
There are other important findings from our study that deserve special mention: hypertensive subjects with Mets compared to those without it showed greater level of albumin excretion rate and consequently higher prevalence of microalbuminuria[26], that is nowadays considered, not only a predictor of renal complications, but also a harbinger of premature CVD[86,87]. These results, that we confirmed in the larger population of the above described REDHY study[35] (Figure 4), are consistent with the findings of other investigations conducted in hypertensive patients[23,24] and in general populations[102,109]. In some of these studies[109] and in other ones[110-113], with cross-sectional and longitudinal design, a relationship between the MetS and chronic kidney disease was also observed (Table 2).
Figure 4.
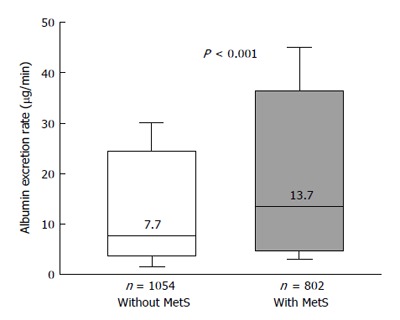
Box plots showing urinary albumin excretion rates in nondiabetic hypertensives participating in the Renal Dysfunction in Hypertension study[39], divided in subjects with and without the metabolic syndrome. In the Box-and-Whisker plots, the central boxes represent the interquartile range (25th to 75th percentile). The middle lines, and the numbers above these lines, represent the median values. Lower and upper whiskers extend to 5th and 95th percentile. MetS: Metabolic syndrome.
Another result of our study merits a comment. In keeping with other reports[114,115], we noted an increased prevalence of grade I and grade II hypertensive retinopathy in subjects with MetS when compared to persons without MetS[26]. However, because the prognostic implications of early hypertensive retinopathy grades are unclear[116], the clinical significance of these findings remains undefined.
Unlike the milder forms of hypertensive retinopathy, prognostic value of increased aortic stiffness seems to be more soundly demonstrated; there is an extensive and very consistent body of evidence showing that large artery stiffening is a powerful predictor of CV morbidity and mortality[85]. Because a fundamental principle states that pulse waves travel faster in stiffer arteries, PWV is the most widely used measure of arterial stiffness. PWV measured along the aortic and aorto-iliac pathway is the most clinically relevant since the aorta and its first branches are responsible for most of the pathophysiological effects of arterial stiffness[85]. Therefore, aortic PWV is regarded as the gold standard measurement of arterial stiffness.
When we assess the influence of MetS on aortic PWV in a sample of never treated non-diabetic patients with essential hypertension, we found more elevated PWV in subjects with MetS when compared to those without it[88].
These data, that we recently replicated in a wider group of hypertensive patients (Figure 5), are in line with the results we observed in another cross sectional study carried out in 528 nondiabetic patients (age 18 to 72 years) with essential hypertension[116]. We found that, when compared with subjects without MetS, hypertensive patients with MetS exhibited more elevated clinic and 24-h pulse pressures that may be considered as a proxy for arterial stiffness, especially in older subjects. The difference held even after correction for age, sex, stroke volume, mean pressures, and total cholesterol[116]. The regression line relating PP with age was steeper in patients with MetS than in those without MetS (Figure 6), suggesting that arterial aging is faster in the former as compared to the latter[117].
Figure 5.
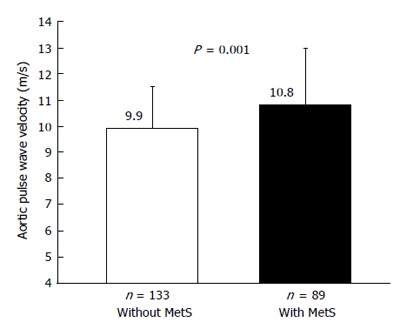
Mean values of aortic pulse wave velocity in untreated hypertensive subjects with and without the metabolic syndrome[76]. MetS: Metabolic syndrome.
Figure 6.
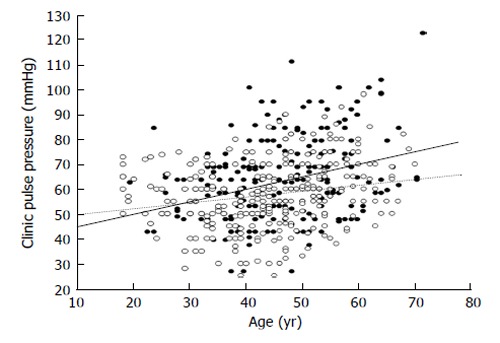
Scatterplot showing the relationship between age and pulse pressure in subjects with (black circles) and in those without (white circles) metabolic syndrome. The calculated regression lines for the former (continuous line) and the latter patients (dotted line) were also shown. The difference regarding the slopes of the two regression lines was statistically significant (P = 0.01).
Our observations are in agreement with several lines of evidence[101,118-122], suggesting that the Mets accelerates the age-related rise in arterial stiffness, leading to a condition defined as early vascular aging (EVA)[123]. Premature arterial senescence in MetS is biologically plausible. The structural changes occurring during aging in large arteries include extensive impairment of the elastin fiber network, increase in collagen content, calcification of the media, and accumulation and migration of VSMC in the arterial walls[121,123]. In subjects with MetS, these modifications may occur earlier, especially in the aorta, for several reasons: (1) activation of the RAAS, that is involved in regulating the turnover of extracellular matrix proteins and that is a strong regulator of matrix metalloproteinase and tissue inhibitor of metalloproteinases; (2) increase in oxidative stress and chronic low grade inflammation; (3) increased glycation of matrix proteins; (4) decreased endothelial bioavailability of nitric oxide associated with insulin resistance; (5) endothelin-1 increase; (6) elevation in leptin; and (7) hypoadiponectinemia[18-20].
EVA, as well as other indices of preclinical organ damage, reflects cumulative damaging effects from risk factors and entails an enhanced risk of CV events and of cognitive dysfunction[123]. Schillaci et al[119] in 169 newly diagnosed non-diabetic hypertensive subjects, observed a greater aortic PWV in the subgroup with MetS, whereas upper limb PWV did not differ in the groups with and without MetS. Very recently, Scuteri et al[120] studied 20570 subjects from 9 cohorts representing 8 different European countries and the United States, participating in the Metabolic syndrome and Arteries REsearch (MARE) Consortium. In this large-scale observational study any cluster of MetS components identified as MetS, with the exception of low HDLc (H) + high triglycerides (T) + abdominal obesity (W), was associated with stiffer arteries than in control subjects[120]. Overall, the combinations T + elevated BP (B) + W, elevated fasting glucose (G) + B + W, and G + T + B + W were consistently associated with significantly stiffer arteries to an extent similar or greater than observed in subjects with alteration in all the five MetS components, even after adjustment for multiple confounders. Differences in BP levels amongst the clusters of MetS components do not seem to explain the reported difference in the odds of having stiffer arteries[120]. The results attained in the MARE Consortium concur with those obtained in the SardiNIA Project[121] and in the Baltimore Longitudinal Study on Aging[122] where the subject with MetS showed an increased carotid stiffness when compared with subjects without it.
Moreover, the results of these studies support the notion that the MetS accelerates arterial ageing over and above the predicted power of its individual components, in marked contrast with the concept that the MetS does not provide further information in addition to the sum of its components. In the same investigations an association between MetS and carotid intima-media thickness has been observed[121,122], in accordance with some[23,24,28,102,124], but not all studies[101,125].
A significant association between carotid atherosclerosis and MetS has been reported in participants in the Framingham Offspring study with MetS[102]. In the same study a greater prevalence of various indices of subclinical CVD (left ventricular hypertrophy by electrocardiography or echocardiography, carotid ultrasound abnormalities, reduced ankle-brachial index, microalbuminuria) was described in subjects with MetS[102].
Interestingly, individuals with MetS with evidence of subclinical disease experienced a risk of CV events nearly threefold that of participants without subclinical disease. The presence of subclinical disease conferred approximately a two-fold risk of overt CVD even in those without either MetS or diabetes (compared with their counterparts without subclinical disease). Adjustment for subclinical disease presence markedly attenuated the association of MetS with CVD risk[102]. This observation emphasizes the important role of subclinical disease in mediating the CV risks associated with MetS.
METABOLIC SYNDROME AND HYPERTENSION: THERAPEUTIC IMPLICATIONS
Effective CVD prevention requires that multiple risk factors be addressed simultaneously to obtain the most significant reduction of morbidity and mortality in a given population. From this point of view, the identification of patients with the MetS offers a unique chance of practicing preventive medicine.
Once identified, aggressive treatment of the MetS is crucial to reduce the increased CV risk. Medications are targeted to individual components of the syndrome (Table 3). However, although pharmacological therapy is often necessary, the cornerstone of treating the MetS remains lifestyle modification[5,20], that represents the only truly holistic therapeutic approach that can reduce insulin resistance and visceral obesity. It involves behavioral counseling, education, dietary changes and increased physical activity, with a goal of ≥ 30 min of moderate-intensity activity on most days of the week[5,20]. Even modest weight loss (7% to 10% of body weight) results in decreased fat mass, BP, glucose, and triglyceride levels[5,20]. These benefits can also translate into improved long-term outcome, especially if weight loss and lifestyle alterations are maintained.
Table 3.
Therapeutic approaches in patients with metabolic syndrome
| Metabolic syndrome component | Goal of therapy | Drugs | Diet | Physical exercise |
| Arterial hypertension | BP < 140/90 mmHg | ACEI or ARBs and/ or Ca-antagonists and/ or alpha-blockers1 Limit diuretics and beta-blockers | Salt restriction and hypocaloric | Regular exercise |
| Hyperglycemia | HbA1c < 7%-6.5% | Metformin GLP-1-Agonists DPP-4-inhibitors | Hypocaloric | Regular exercise |
| Obesity | Weight loss 7%-10% | Orlistat Bariatric Surgery | Hypocaloric | Regular exercise |
| Dyslipidemia | LDL < 100-70 mg/dL TG < 150 mg/dL HDL: Men > 40/ Women > 50 mg/dL | Statins ± ezetimibe. PUFA-n-3, Fibrates | Hypocaloric | Regular exercise |
Not first choice. BP: Blood pressure; ACEI: Angiotensin converting enzyme inhibithors; ARBs: Angiotensin II receptor blockers; GLP-1: Glucagon-like peptide-1; DPP-4: Dipeptidyl peptidase-4; LDL: Low-density lipoprotein; TG: Triglycerides; HDL: High-density lipoprotein; PUFA-n-3: Omega-3-Polyunsaturated.
A meta-analysis of 50 studies and 534906 individuals showed that adherence to the Mediterranean diet protect against the development of the MetS and its individual components. This dietary pattern, that can be easily followed by various cultures with small modifications, is characterized by the frequent consumption of olive oil, fruits, tree nuts, legumes, whole grains, weekly consumption of fish and poultry, a relatively low consumption of red meat, as well as a moderate consumption of alcohol normally with meal and usually in the form of red wine[126]. However, the remaining challenge is how to promote long-term adherence to a healthier, more active lifestyle and avoid reversion to old habits.
The more recent European guidelines for the management of hypertension do not recommend prescribing antihypertensive drugs in subjects with high normal BP, because no evidence is available[127]. The same guidelines do point out that beta-blockers (except for vasodilating beta-blockers) and diuretics (especially when combined together) may facilitate the development of new onset diabetes and therefore should be avoided as first line therapy in hypertensives with MetS[127]. When diuretics are employed, low doses should be used, preferably in association with a potassium-sparing drug, because hypokalemia may worsen glucose metabolism[127].
Unlike beta-blockers and diuretics, newer antihypertensive medications are associated with a reduced (or not increased) risk of incident diabetes[20,51,127] and they are also associated with better adherence to therapy[128]. In addition, it has been demonstrated that obese hypertensive patients during weight loss therapy show significantly better weight reduction and improvement of insulin resistance when treated with newer antihypertensive medications compared to the older BP lowering drugs (especially beta-blockers)[20,65,127]. Of the newer antihypertensive treatments angiotensin receptor blockers (ARBs) have been found to be associated with lowest rate of discontinuation of therapy[128] and with lowest incidence of new onset diabetes[129]. Moreover, specific ARBs (telmisartan and to a lesser extent irbesartan) seem to allow a superior control of BP over 24 h, documented also in subjects with MetS[130] and also a partial peroxisome proliferator-activated receptor-γ agonism not present in other ARBs or ACE-inhibitors (ACEI)[20,127,131]. However, the clinical relevance of these differences seem to be negligible or uncertain, since in the Ongoing Telmisartan Alone and in Combination with Ramipril Trial, telmisartan was not more effective than rampiril in preventing CV events or delaying onset of diabetes[127].
The choice of the newer BP lowering drugs, such as the RAAS-blockers and the long-acting calcium antagonists, seems to be particularly recommended in hypertensive patients with MetS, in the light of the abovementioned marked tendency of these subjects to the development of LVH and stiffening of the large arteries[18-26]. As a matter of fact, the efficacy of these drugs, in reducing LV mass and arterial stiffness[127] is greater than the older ones.
Although meta-analyses suggest antihypertensive drugs have a similar effect on reducing CV events[127], no randomized clinical trial has been specifically performed in hypertensive patients with the MetS, having the aim to test the superiority of one class of BP lowering drugs over another. However, very recently, in the Cardiovascular Health Study, a community-based prospective cohort study conducted by the National Heart Lung and Blood Institute, the association between the use of ACEI/ARBs and incident CV events was evaluated in elderly people with hypertension and MetS[132]. ACEI/ARBs use was associated with a lower risk of CVD events, primarily due to a reduction in coronary events[132]. Pending validation from prospective clinical trials, it seems reasonable to say that ACEI/ARBs may be the preferred treatment for hypertension management in patients with MetS.
Newer antihypertensive agents lead to better control of BP in part brought about by better adherence, thereby reducing the risk of CVD. Needless to say that CV events and new onset T2DM are associated with significant social and health costs. Therefore, in patients with hypertension and MetS, some of the drug costs of newer antihypertensive medications will be balanced by costs saved from reducing these negative outcomes.
CONCLUSION
An extensive body of evidence suggests that the MetS may accelerate arterial aging and amplify hypertension-related cardiac and renal changes. Some of the MetS components, when considered individually may have little or no influence on TOD, but when taken together may synergistically interact promoting the development of LV hypertrophy, LV diastolic dysfunction, aortic stiffness and microalbuminuria. The marked tendency of the hypertensive patients with the MetS to develop these preclinical manifestations of end-organ damage, may largely explain why the MetS entails an increased risk of CV morbidity and mortality, since these markers of TOD are well-known predictors of CV events. Therefore, identifying the MetS in hypertensive patients may enable the clinician to better assess the CV risk. Once this syndrome is properly identified, aggressive implementation of therapeutic lifestyle changes and appropriate medications, able to decrease insulin resistance, hyperinsulinemia and weight gain can greatly reduce its adverse prognostic impact.
Footnotes
P- Reviewer: Aggarwal S, Beltowski J, Cicero AFG, Omboni S, Salles GF, Turiel M S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
References
- 1.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO Consultation. Part 1: diagnosis and classification of diabetes mellitus. Geneva, Switzerland: World Health Organization; 1999. Available from: http: //whqlibdoc.who.int/hq/1999/WHO_NCD_NCS_99.2.pdf. [Google Scholar]
- 3.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 4.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr. Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and Management of the Metabolic Syndrome. An American Heart Association/ National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 6.Einhorn D, Reaven GM, Cobin RH, Ford E, Ganda OP, Handelsman Y, Hellman R, Jellinger PS, Kendall D, Krauss RM, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003;9:237–252. [PubMed] [Google Scholar]
- 7.Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 8.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 9.Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, Mietus-Snyder ML; American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–647. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 10.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 11.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 12.Kahn R. Metabolic syndrome: is it a syndrome? Does it matter? Circulation. 2007;115:1806–1810; discussion 1811. doi: 10.1161/CIRCULATIONAHA.106.658336. [DOI] [PubMed] [Google Scholar]
- 13.Beaser RS, Levy P. Metabolic syndrome: a work in progress, but a useful construct. Circulation. 2007;115:1812–1818; discussion 1818. doi: 10.1161/CIRCULATIONAHA.106.673616. [DOI] [PubMed] [Google Scholar]
- 14.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 15.Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999-2010. J Am Coll Cardiol. 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scholze J, Alegria E, Ferri C, Langham S, Stevens W, Jeffries D, Uhl-Hochgraeber K. Epidemiological and economic burden of metabolic syndrome and its consequences in patients with hypertension in Germany, Spain and Italy; a prevalence-based model. BMC Public Health. 2010;10:529. doi: 10.1186/1471-2458-10-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen J, Goyal A, Sperling L. The emerging epidemic of obesity, diabetes, and the metabolic syndrome in china. Cardiol Res Pract. 2012;2012:178675. doi: 10.1155/2012/178675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulé G, Cottone S, Nardi E, Andronico G, Cerasola G. Metabolic syndrome in subjects with essential hypertension: relationships with subclinical cardiovascular and renal damage. Minerva Cardioangiol. 2006;54:173–194. [PubMed] [Google Scholar]
- 19.Mulè G, Cerasola G. The metabolic syndrome and its relationship to hypertensive target organ damage. J Clin Hypertens (Greenwich) 2006;8:195–201. doi: 10.1111/j.1524-6175.2006.04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redon J, Cifkova R, Laurent S, Nilsson P, Narkiewicz K, Erdine S, Mancia G; Scientific Council of the European Society of Hypertension. The metabolic syndrome in hypertension: European society of hypertension position statement. J Hypertens. 2008;26:1891–1900. doi: 10.1097/HJH.0b013e328302ca38. [DOI] [PubMed] [Google Scholar]
- 21.Mancia G, Bombelli M, Corrao G, Facchetti R, Madotto F, Giannattasio C, Trevano FQ, Grassi G, Zanchetti A, Sega R. Metabolic syndrome in the Pressioni Arteriose Monitorate E Loro Associazioni (PAMELA) study: daily life blood pressure, cardiac damage, and prognosis. Hypertension. 2007;49:40–47. doi: 10.1161/01.HYP.0000251933.22091.24. [DOI] [PubMed] [Google Scholar]
- 22.Pannier B, Thomas F, Bean K, Jégo B, Benetos A, Guize L. The metabolic syndrome: similar deleterious impact on all-cause mortality in hypertensive and normotensive subjects. J Hypertens. 2008;26:1223–1228. doi: 10.1097/HJH.0b013e3282fd9936. [DOI] [PubMed] [Google Scholar]
- 23.Cuspidi C, Meani S, Fusi V, Severgnini B, Valerio C, Catini E, Leonetti G, Magrini F, Zanchetti A. Metabolic syndrome and target organ damage in untreated essential hypertensives. J Hypertens. 2004;22:1991–1998. doi: 10.1097/00004872-200410000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Leoncini G, Ratto E, Viazzi F, Vaccaro V, Parodi D, Parodi A, Falqui V, Tomolillo C, Deferrari G, Pontremoli R. Metabolic syndrome is associated with early signs of organ damage in nondiabetic, hypertensive patients. J Intern Med. 2005;257:454–460. doi: 10.1111/j.1365-2796.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- 25.Schillaci G, Pirro M, Vaudo G, Gemelli F, Marchesi S, Porcellati C, Mannarino E. Prognostic value of the metabolic syndrome in essential hypertension. J Am Coll Cardiol. 2004;43:1817–1822. doi: 10.1016/j.jacc.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 26.Mulè G, Nardi E, Cottone S, Cusimano P, Volpe V, Piazza G, Mongiovì R, Mezzatesta G, Andronico G, Cerasola G. Influence of metabolic syndrome on hypertension-related target organ damage. J Intern Med. 2005;257:503–513. doi: 10.1111/j.1365-2796.2005.01493.x. [DOI] [PubMed] [Google Scholar]
- 27.Pierdomenico SD, Lapenna D, Di Tommaso R, Di Carlo S, Caldarella MP, Neri M, Mezzetti A, Cuccurullo F. Prognostic relevance of metabolic syndrome in hypertensive patients at low-to-medium risk. Am J Hypertens. 2007;20:1291–1296. doi: 10.1016/j.amjhyper.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Zanchetti A, Hennig M, Baurecht H, Tang R, Cuspidi C, Carugo S, Mancia G. Prevalence and incidence of the metabolic syndrome in the European Lacidipine Study on Atherosclerosis (ELSA) and its relation with carotid intima-media thickness. J Hypertens. 2007;25:2463–2470. doi: 10.1097/HJH.0b013e3282f063d5. [DOI] [PubMed] [Google Scholar]
- 29.Andreadis EA, Tsourous GI, Tzavara CK, Georgiopoulos DX, Katsanou PM, Marakomichelakis GE, Diamantopoulos EJ. Metabolic syndrome and incident cardiovascular morbidity and mortality in a Mediterranean hypertensive population. Am J Hypertens. 2007;20:558–564. doi: 10.1016/j.amjhyper.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 30.de Simone G, Olsen MH, Wachtell K, Hille DA, Dahlöf B, Ibsen H, Kjeldsen SE, Lyle PA, Devereux RB. Clusters of metabolic risk factors predict cardiovascular events in hypertension with target-organ damage: the LIFE study. J Hum Hypertens. 2007;21:625–632. doi: 10.1038/sj.jhh.1002203. [DOI] [PubMed] [Google Scholar]
- 31.Vlek AL, van der Graaf Y, Spiering W, Visseren FL; SMART study group. Effect of metabolic syndrome or type II diabetes mellitus on the occurrence of recurrent vascular events in hypertensive patients. J Hum Hypertens. 2008;22:358–365. doi: 10.1038/jhh.2008.5. [DOI] [PubMed] [Google Scholar]
- 32.Gupta AK, Dahlof B, Sever PS, Poulter NR. Metabolic syndrome, independent of its components, is a risk factor for stroke and death but not for coronary heart disease among hypertensive patients in the ASCOT-BPLA. Diabetes Care. 2010;33:1647–1651. doi: 10.2337/dc09-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kjeldsen SE, Naditch-Brule L, Perlini S, Zidek W, Farsang C. Increased prevalence of metabolic syndrome in uncontrolled hypertension across Europe: the Global Cardiometabolic Risk Profile in Patients with hypertension disease survey. J Hypertens. 2008;26:2064–2070. doi: 10.1097/HJH.0b013e32830c45c3. [DOI] [PubMed] [Google Scholar]
- 34.Lee SR, Cha MJ, Kang DY, Oh KC, Shin DH, Lee HY. Increased prevalence of metabolic syndrome among hypertensive population: ten years’ trend of the Korean National Health and Nutrition Examination Survey. Int J Cardiol. 2013;166:633–639. doi: 10.1016/j.ijcard.2011.11.095. [DOI] [PubMed] [Google Scholar]
- 35.Cerasola G, Mulè G, Cottone S, Nardi E, Cusimano P. Hypertension, microalbuminuria and renal dysfunction: the Renal Dysfunction in Hypertension (REDHY) study. J Nephrol. 2008;21:368–373. [PubMed] [Google Scholar]
- 36.Chaudhary K, Buddineni JP, Nistala R, Whaley-Connell A. Resistant hypertension in the high-risk metabolic patient. Curr Diab Rep. 2011;11:41–46. doi: 10.1007/s11892-010-0155-x. [DOI] [PubMed] [Google Scholar]
- 37.Mulé G, Cerasola G. The metabolic syndrome as a prohypertensive state. Am J Hypertens. 2008;21:8. doi: 10.1038/ajh.2007.22. [DOI] [PubMed] [Google Scholar]
- 38.Sesti G, Capaldo B, Cavallo Perin P, Del Prato S, Frittitta L, Frontoni S, Hribal ML, Marchesini G, Paolisso G, Piatti PM, Solini A, Bonora E; Group of Italian Scientists of Insulin Resistance. Correspondence between the International Diabetes Federation criteria for metabolic syndrome and insulin resistance in a cohort of Italian nondiabetic Caucasians: the GISIR database. Diabetes Care. 2007;30:e33. doi: 10.2337/dc06-2394. [DOI] [PubMed] [Google Scholar]
- 39.Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S. Insulin resistance in essential hypertension. N Engl J Med. 1987;317:350–357. doi: 10.1056/NEJM198708063170605. [DOI] [PubMed] [Google Scholar]
- 40.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Targher G, Alberiche M, Bonadonna RC, Muggeo M. Prevalence of insulin resistance in metabolic disorders: the Bruneck Study. Diabetes. 1998;47:1643–1649. doi: 10.2337/diabetes.47.10.1643. [DOI] [PubMed] [Google Scholar]
- 41.Dengel DR, Pratley RE, Hagberg JM, Goldberg AP. Impaired insulin sensitivity and maximal responsiveness in older hypertensive men. Hypertension. 1994;23:320–324. doi: 10.1161/01.hyp.23.3.320. [DOI] [PubMed] [Google Scholar]
- 42.Andronico G, Ferrara L, Mangano M, Mulè G, Cerasola G. Insulin, sodium-lithium countertransport, and microalbuminuria in hypertensive patients. Hypertension. 1998;31:110–113. doi: 10.1161/01.hyp.31.1.110. [DOI] [PubMed] [Google Scholar]
- 43.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities--the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 44.Mancia G, Bousquet P, Elghozi JL, Esler M, Grassi G, Julius S, Reid J, Van Zwieten PA. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007;25:909–920. doi: 10.1097/HJH.0b013e328048d004. [DOI] [PubMed] [Google Scholar]
- 45.Hall JE, Hildebrandt DA, Kuo J. Obesity hypertension: role of leptin and sympathetic nervous system. Am J Hypertens. 2001;14:103S–115S. doi: 10.1016/s0895-7061(01)02077-5. [DOI] [PubMed] [Google Scholar]
- 46.Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, Sowers J. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of The Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich) 2013;15:14–33. doi: 10.1111/jch.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konecny T, Kara T, Somers VK. Obstructive sleep apnea and hypertension: an update. Hypertension. 2014;63:203–209. doi: 10.1161/HYPERTENSIONAHA.113.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975;55:845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nickenig G, Röling J, Strehlow K, Schnabel P, Böhm M. Insulin induces upregulation of vascular AT1 receptor gene expression by posttranscriptional mechanisms. Circulation. 1998;98:2453–2460. doi: 10.1161/01.cir.98.22.2453. [DOI] [PubMed] [Google Scholar]
- 50.Engeli S. Role of the renin-angiotensin- aldosterone system in the metabolic syndrome. Contrib Nephrol. 2006;151:122–134. doi: 10.1159/000095324. [DOI] [PubMed] [Google Scholar]
- 51.Putnam K, Shoemaker R, Yiannikouris F, Cassis LA. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H1219–H1230. doi: 10.1152/ajpheart.00796.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu L, Yu Z, Pan A, Hu FB, Franco OH, Li H, Li X, Yang X, Chen Y, Lin X. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care. 2009;32:1278–1283. doi: 10.2337/dc09-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muscogiuri G, Sorice GP, Ajjan R, Mezza T, Pilz S, Prioletta A, Scragg R, Volpe SL, Witham MD, Giaccari A. Can vitamin D deficiency cause diabetes and cardiovascular diseases? Present evidence and future perspectives. Nutr Metab Cardiovasc Dis. 2012;22:81–87. doi: 10.1016/j.numecd.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Andronico G, Cottone S, Mangano MT, Ferraro-Mortellaro R, Baiardi G, Grassi N, Ferrara L, Mulé G, Cerasola G. Insulin, renin-aldosterone system and blood pressure in obese people. Int J Obes Relat Metab Disord. 2001;25:239–242. doi: 10.1038/sj.ijo.0801483. [DOI] [PubMed] [Google Scholar]
- 55.Goodfriend TL, Ball DL, Egan BM, Campbell WB, Nithipatikom K. Epoxy-keto derivative of linoleic acid stimulates aldosterone secretion. Hypertension. 2004;43:358–363. doi: 10.1161/01.HYP.0000113294.06704.64. [DOI] [PubMed] [Google Scholar]
- 56.Mulè G, Nardi E, Cusimano P, Cottone S, Seddio G, Geraci C, Palermo A, Andronico G, Cerasola G. Plasma aldosterone and its relationships with left ventricular mass in essential hypertensive patients with the metabolic syndrome. Am J Hypertens. 2008;21:1055–1061. doi: 10.1038/ajh.2008.225. [DOI] [PubMed] [Google Scholar]
- 57.Calhoun DA, Sharma K. The role of aldosteronism in causing obesity-related cardiovascular risk. Cardiol Clin. 2010;28:517–527. doi: 10.1016/j.ccl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Byrd JB, Brook RD. A critical review of the evidence supporting aldosterone in the etiology and its blockade in the treatment of obesity-associated hypertension. J Hum Hypertens. 2014;28:3–9. doi: 10.1038/jhh.2013.42. [DOI] [PubMed] [Google Scholar]
- 59.Iglarz M, Clozel M. At the heart of tissue: endothelin system and end-organ damage. Clin Sci (Lond) 2010;119:453–463. doi: 10.1042/CS20100222. [DOI] [PubMed] [Google Scholar]
- 60.Andronico G, Mangano M, Ferrara L, Lamanna D, Mulé G, Cerasola G. In vivo relationship between insulin and endothelin role of insulin-resistance. J Hum Hypertens. 1997;11:63–66. doi: 10.1038/sj.jhh.1000386. [DOI] [PubMed] [Google Scholar]
- 61.Sarafidis PA, Bakris GL. Review: Insulin and endothelin: an interplay contributing to hypertension development? J Clin Endocrinol Metab. 2007;92:379–385. doi: 10.1210/jc.2006-1819. [DOI] [PubMed] [Google Scholar]
- 62.Petrasek D, Jensen G, Tuck M, Stern N. In vitro effects of insulin on aldosterone production in rat zona glomerulosa cells. Life Sci. 1992;50:1781–1787. doi: 10.1016/0024-3205(92)90062-t. [DOI] [PubMed] [Google Scholar]
- 63.Goodfriend TL, Egan B, Stepniakowski K, Ball DL. Relationships among plasma aldosterone, high-density lipoprotein cholesterol, and insulin in humans. Hypertension. 1995;25:30–36. doi: 10.1161/01.hyp.25.1.30. [DOI] [PubMed] [Google Scholar]
- 64.Colussi G, Catena C, Lapenna R, Nadalini E, Chiuch A, Sechi LA. Insulin resistance and hyperinsulinemia are related to plasma aldosterone levels in hypertensive patients. Diabetes Care. 2007;30:2349–2354. doi: 10.2337/dc07-0525. [DOI] [PubMed] [Google Scholar]
- 65.Deedwania P. Hypertension, dyslipidemia, and insulin resistance in patients with diabetes mellitus or the cardiometabolic syndrome: benefits of vasodilating β-blockers. J Clin Hypertens (Greenwich) 2011;13:52–59. doi: 10.1111/j.1751-7176.2010.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Rev Endocr Metab Disord. 2013;14:5–12. doi: 10.1007/s11154-012-9229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manrique C, Lastra G, Sowers JR. New insights into insulin action and resistance in the vasculature. Ann N Y Acad Sci. 2014;1311:138–150. doi: 10.1111/nyas.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adrogué HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med. 2007;356:1966–1978. doi: 10.1056/NEJMra064486. [DOI] [PubMed] [Google Scholar]
- 69.Andronico G, Mulé G, Mangano MT, Piazza G, Donatelli M, Cerasola G, Bompiani GD. Insulin resistance and endogenous digoxin-like factor in obese hypertensive patients with glucose intolerance. Acta Diabetol. 1992;28:203–205. doi: 10.1007/BF00778999. [DOI] [PubMed] [Google Scholar]
- 70.Khan AM, Cheng S, Magnusson M, Larson MG, Newton-Cheh C, McCabe EL, Coviello AD, Florez JC, Fox CS, Levy D, et al. Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community-based studies. J Clin Endocrinol Metab. 2011;96:3242–3249. doi: 10.1210/jc.2011-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grassi D, Desideri G, Di Giacomantonio AV, Di Giosia P, Ferri C. Hyperuricemia and cardiovascular risk. High Blood Press Cardiovasc Prev. 2014:Epub ahead of print. doi: 10.1007/s40292-014-0046-3. [DOI] [PubMed] [Google Scholar]
- 72.Feig DI, Madero M, Jalal DI, Sanchez-Lozada LG, Johnson RJ. Uric acid and the origins of hypertension. J Pediatr. 2013;162:896–902. doi: 10.1016/j.jpeds.2012.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson RJ, Sánchez-Lozada LG, Mazzali M, Feig DI, Kanbay M, Sautin YY. What are the key arguments against uric acid as a true risk factor for hypertension? Hypertension. 2013;61:948–951. doi: 10.1161/HYPERTENSIONAHA.111.00650. [DOI] [PubMed] [Google Scholar]
- 74.Bombelli M, Ronchi I, Volpe M, Facchetti R, Carugo S, Dell’oro R, Cuspidi C, Grassi G, Mancia G. Prognostic value of serum uric acid: new-onset in and out-of-office hypertension and long-term mortality. J Hypertens. 2014;32:1237–1244. doi: 10.1097/HJH.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 75.Mulè G, Nardi E, Costanzo M, Mogavero M, Guarino L, Viola T, Vario MG, Cacciatore V, Andronico G, Cerasola G, et al. Absence of an independent association between serum uric acid and left ventricular mass in Caucasian hypertensive women and men. Nutr Metab Cardiovasc Dis. 2013;23:715–722. doi: 10.1016/j.numecd.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 76.Mulè G, Riccobene R, Castiglia A, D’Ignoto F, Ajello E, Geraci G, Guarino L, Nardi E, Vaccaro F, Cerasola G, et al. Relationships between mild hyperuricaemia and aortic stiffness in untreated hypertensive patients. Nutr Metab Cardiovasc Dis. 2014;24:744–750. doi: 10.1016/j.numecd.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 77.Chen J, Gu D, Huang J, Rao DC, Jaquish CE, Hixson JE, Chen CS, Chen J, Lu F, Hu D, Rice T, Kelly TN, Hamm LL, Whelton PK, He J; GenSalt Collaborative Research Group. Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: a dietary intervention study. Lancet. 2009;373:829–835. doi: 10.1016/S0140-6736(09)60144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 79.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 80.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 81.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 82.Athyros VG, Mikhailidis DP, Papageorgiou AA, Didangelos TP, Ganotakis ES, Symeonidis AN, Daskalopoulou SS, Kakafika AI, Elisaf M. Prevalence of atherosclerotic vascular disease among subjects with the metabolic syndrome with or without diabetes mellitus: the METS-GREECE Multicentre Study. Curr Med Res Opin. 2004;20:1691–1701. doi: 10.1185/030079904x5599. [DOI] [PubMed] [Google Scholar]
- 83.Devereux RB, Alderman MH. Role of preclinical cardiovascular disease in the evolution from risk factor exposure to development of morbid events. Circulation. 1993;88:1444–1455. doi: 10.1161/01.cir.88.4.1444. [DOI] [PubMed] [Google Scholar]
- 84.Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J. 2001;141:334–341. doi: 10.1067/mhj.2001.113218. [DOI] [PubMed] [Google Scholar]
- 85.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cerasola G, Cottone S, Mulè G. The progressive pathway of microalbuminuria: from early marker of renal damage to strong cardiovascular risk predictor. J Hypertens. 2010;28:2357–2369. doi: 10.1097/HJH.0b013e32833ec377. [DOI] [PubMed] [Google Scholar]
- 87.Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, Iseki K, Jassal SK, Kimm H, Kronenberg F, et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 2013;346:f324. doi: 10.1136/bmj.f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mulè G, Cottone S, Mongiovì R, Cusimano P, Mezzatesta G, Seddio G, Volpe V, Nardi E, Andronico G, Piazza G, et al. Influence of the metabolic syndrome on aortic stiffness in never treated hypertensive patients. Nutr Metab Cardiovasc Dis. 2006;16:54–59. doi: 10.1016/j.numecd.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 89.Mulè G, Nardi E, Cottone S, Cusimano P, Incalcaterra F, Palermo A, Giandalia M, Geraci C, Buscemi S, Cerasola G. Metabolic syndrome in subjects with white-coat hypertension: impact on left ventricular structure and function. J Hum Hypertens. 2007;21:854–860. doi: 10.1038/sj.jhh.1002238. [DOI] [PubMed] [Google Scholar]
- 90.Mulè G, Nardi E, Cottone S, Cusimano P, Incalcaterra F, Giandalia ME, Palermo A, Mezzatesta G, Cerasola G. Impact of metabolic syndrome on left ventricular mass in overweight and obese hypertensive subjects. Int J Cardiol. 2007;121:267–275. doi: 10.1016/j.ijcard.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 91.Mulè G, Cusimano P, Nardi E, Cottone S, Geraci C, Palermo A, Costanzo M, Foraci AC, Cerasola G. Relationships between metabolic syndrome and left ventricular mass in hypertensive patients: does sex matter? J Hum Hypertens. 2008;22:788–795. doi: 10.1038/jhh.2008.69. [DOI] [PubMed] [Google Scholar]
- 92.Schillaci G, Pirro M, Pucci G, Mannarino MR, Gemelli F, Siepi D, Vaudo G, Mannarino E. Different impact of the metabolic syndrome on left ventricular structure and function in hypertensive men and women. Hypertension. 2006;47:881–886. doi: 10.1161/01.HYP.0000216778.83626.39. [DOI] [PubMed] [Google Scholar]
- 93.Nicolini E, Martegani G, Maresca AM, Marchesi C, Dentali F, Lazzarini A, Speroni S, Guasti L, Bertolini A, Venco A, et al. Left ventricular remodeling in patients with metabolic syndrome: influence of gender. Nutr Metab Cardiovasc Dis. 2013;23:771–775. doi: 10.1016/j.numecd.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 94.Aijaz B, Ammar KA, Lopez-Jimenez F, Redfield MM, Jacobsen SJ, Rodeheffer RJ. Abnormal cardiac structure and function in the metabolic syndrome: a population-based study. Mayo Clin Proc. 2008;83:1350–1357. doi: 10.4065/83.12.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schillaci G, Pucci G. Influence of gender on the relation between the metabolic syndrome and left ventricular mass. J Hum Hypertens. 2009;23:430; author reply 428–429. doi: 10.1038/jhh.2008.159. [DOI] [PubMed] [Google Scholar]
- 96.Sundström J, Arnlöv J, Stolare K, Lind L. Blood pressure-independent relations of left ventricular geometry to the metabolic syndrome and insulin resistance: a population-based study. Heart. 2008;94:874–878. doi: 10.1136/hrt.2007.121020. [DOI] [PubMed] [Google Scholar]
- 97.de Simone G, Devereux RB, Chinali M, Roman MJ, Lee ET, Resnick HE, Howard BV. Metabolic syndrome and left ventricular hypertrophy in the prediction of cardiovascular events: the Strong Heart Study. Nutr Metab Cardiovasc Dis. 2009;19:98–104. doi: 10.1016/j.numecd.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burchfiel CM, Skelton TN, Andrew ME, Garrison RJ, Arnett DK, Jones DW, Taylor HA. Metabolic syndrome and echocardiographic left ventricular mass in blacks: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2005;112:819–827. doi: 10.1161/CIRCULATIONAHA.104.518498. [DOI] [PubMed] [Google Scholar]
- 99.de las Fuentes L, Brown AL, Mathews SJ, Waggoner AD, Soto PF, Gropler RJ, Dávila-Román VG. Metabolic syndrome is associated with abnormal left ventricular diastolic function independent of left ventricular mass. Eur Heart J. 2007;28:553–559. doi: 10.1093/eurheartj/ehl526. [DOI] [PubMed] [Google Scholar]
- 100.Hwang YC, Jee JH, Kang M, Rhee EJ, Sung J, Lee MK. Metabolic syndrome and insulin resistance are associated with abnormal left ventricular diastolic function and structure independent of blood pressure and fasting plasma glucose level. Int J Cardiol. 2012;159:107–111. doi: 10.1016/j.ijcard.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 101.Kim NH, Park J, Kim SH, Kim YH, Kim DH, Cho GY, Baik I, Lim HE, Kim EJ, Na JO, et al. Non-alcoholic fatty liver disease, metabolic syndrome and subclinical cardiovascular changes in the general population. Heart. 2014;100:938–943. doi: 10.1136/heartjnl-2013-305099. [DOI] [PubMed] [Google Scholar]
- 102.Ingelsson E, Sullivan LM, Murabito JM, Fox CS, Benjamin EJ, Polak JF, Meigs JB, Keyes MJ, O’Donnell CJ, Wang TJ, et al. Prevalence and prognostic impact of subclinical cardiovascular disease in individuals with the metabolic syndrome and diabetes. Diabetes. 2007;56:1718–1726. doi: 10.2337/db07-0078. [DOI] [PubMed] [Google Scholar]
- 103.Ferrara LA, Guida L, Ferrara F, De Luca G, Staiano L, Celentano A, Mancini M. Cardiac structure and function and arterial circulation in hypertensive patients with and without metabolic syndrome. J Hum Hypertens. 2007;21:729–735. doi: 10.1038/sj.jhh.1002222. [DOI] [PubMed] [Google Scholar]
- 104.Mancia G, Bombelli M, Facchetti R, Madotto F, Corrao G, Trevano FQ, Giannattasio C, Grassi G, Sega R. Long-term risk of diabetes, hypertension and left ventricular hypertrophy associated with the metabolic syndrome in a general population. J Hypertens. 2008;26:1602–1611. doi: 10.1097/HJH.0b013e328302f10d. [DOI] [PubMed] [Google Scholar]
- 105.Aksoy S, Durmuş G, Ozcan S, Toprak E, Gurkan U, Oz D, Canga Y, Karatas B, Duman D. Is left ventricular diastolic dysfunction independent from presence of hypertension in metabolic syndrome? An echocardiographic study. J Cardiol. 2014;64:194–198. doi: 10.1016/j.jjcc.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 106.Ingelsson E, Arnlöv J, Lind L, Sundström J. Metabolic syndrome and risk for heart failure in middle-aged men. Heart. 2006;92:1409–1413. doi: 10.1136/hrt.2006.089011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Voulgari C, Tentolouris N, Dilaveris P, Tousoulis D, Katsilambros N, Stefanadis C. Increased heart failure risk in normal-weight people with metabolic syndrome compared with metabolically healthy obese individuals. J Am Coll Cardiol. 2011;58:1343–1350. doi: 10.1016/j.jacc.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 108.Ingelsson E, Sundström J, Arnlöv J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–341. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 109.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 110.Chen J, Gu D, Chen CS, Wu X, Hamm LL, Muntner P, Batuman V, Lee CH, Whelton PK, He J. Association between the metabolic syndrome and chronic kidney disease in Chinese adults. Nephrol Dial Transplant. 2007;22:1100–1106. doi: 10.1093/ndt/gfl759. [DOI] [PubMed] [Google Scholar]
- 111.Navarro J, Redón J, Cea-Calvo L, Lozano JV, Fernández-Pérez C, Bonet A, González-Esteban J. Metabolic syndrome, organ damage and cardiovascular disease in treated hypertensive patients. The ERIC-HTA study. Blood Press. 2007;16:20–27. doi: 10.1080/08037050701217817. [DOI] [PubMed] [Google Scholar]
- 112.Johns BR, Pao AC, Kim SH. Metabolic syndrome, insulin resistance and kidney function in non-diabetic individuals. Nephrol Dial Transplant. 2012;27:1410–1415. doi: 10.1093/ndt/gfr498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 114.Wong TY, Duncan BB, Golden SH, Klein R, Couper DJ, Klein BE, Hubbard LD, Sharrett AR, Schmidt MI. Associations between the metabolic syndrome and retinal microvascular signs: the Atherosclerosis Risk In Communities study. Invest Ophthalmol Vis Sci. 2004;45:2949–2954. doi: 10.1167/iovs.04-0069. [DOI] [PubMed] [Google Scholar]
- 115.Katsi V, Souretis G, Alexopoulos N, Vlachopoulos C, Dagalaki I, Sideris S, Tousoulis D, Stefanadis C, Kallikazaros I. Exploring the association of retinopathy with metabolic syndrome, ambulatory blood pressure and cardiac remodeling in hypertensive individuals. Int J Cardiol. 2013;166:764–766. doi: 10.1016/j.ijcard.2012.09.167. [DOI] [PubMed] [Google Scholar]
- 116.Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369:425–435. doi: 10.1016/S0140-6736(07)60198-6. [DOI] [PubMed] [Google Scholar]
- 117.Mulè G, Nardi E, Cottone S, Cusimano P, Incalcaterra F, Palermo A, Giandalia ME, Mezzatesta G, Andronico G, Cerasola G. Relationship of metabolic syndrome with pulse pressure in patients with essential hypertension. Am J Hypertens. 2007;20:197–203. doi: 10.1016/j.amjhyper.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 118.Safar ME, Lange C, Blacher J, Eschwège E, Tichet J, Balkau B; DESIR Study Group. Mean and yearly changes in blood pressure with age in the metabolic syndrome: the DESIR study. Hypertens Res. 2011;34:91–97. doi: 10.1038/hr.2010.180. [DOI] [PubMed] [Google Scholar]
- 119.Schillaci G, Pirro M, Vaudo G, Mannarino MR, Savarese G, Pucci G, Franklin SS, Mannarino E. Metabolic syndrome is associated with aortic stiffness in untreated essential hypertension. Hypertension. 2005;45:1078–1082. doi: 10.1161/01.HYP.0000165313.84007.7d. [DOI] [PubMed] [Google Scholar]
- 120.Scuteri A, Cunha PG, Rosei EA, Badariere J, Bekaert S, Cockcroft JR, Cotter J, Cucca F, De Buyzere ML, De Meyer T, et al. Arterial stiffness and influences of the metabolic syndrome: a cross-countries study. Atherosclerosis. 2014;233:654–660. doi: 10.1016/j.atherosclerosis.2014.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scuteri A, Najjar SS, Orru’ M, Usala G, Piras MG, Ferrucci L, Cao A, Schlessinger D, Uda M, Lakatta EG. The central arterial burden of the metabolic syndrome is similar in men and women: the SardiNIA Study. Eur Heart J. 2010;31:602–613. doi: 10.1093/eurheartj/ehp491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scuteri A, Najjar SS, Muller DC, Andres R, Hougaku H, Metter EJ, Lakatta EG. Metabolic syndrome amplifies the age-associated increases in vascular thickness and stiffness. J Am Coll Cardiol. 2004;43:1388–1395. doi: 10.1016/j.jacc.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 123.Kotsis V, Stabouli S, Karafillis I, Nilsson P. Early vascular aging and the role of central blood pressure. J Hypertens. 2011;29:1847–1853. doi: 10.1097/HJH.0b013e32834a4d9f. [DOI] [PubMed] [Google Scholar]
- 124.Kawamoto R, Tomita H, Oka Y, Kodama A, Kamitani A. Metabolic syndrome amplifies the LDL-cholesterol associated increases in carotid atherosclerosis. Intern Med. 2005;44:1232–1238. doi: 10.2169/internalmedicine.44.1232. [DOI] [PubMed] [Google Scholar]
- 125.Irace C, Cortese C, Fiaschi E, Carallo C, Sesti G, Farinaro E, Gnasso A. Components of the metabolic syndrome and carotid atherosclerosis: role of elevated blood pressure. Hypertension. 2005;45:597–601. doi: 10.1161/01.HYP.0000158945.52283.c2. [DOI] [PubMed] [Google Scholar]
- 126.Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57:1299–1313. doi: 10.1016/j.jacc.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 127.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 128.Corrao G, Zambon A, Parodi A, Poluzzi E, Baldi I, Merlino L, Cesana G, Mancia G. Discontinuation of and changes in drug therapy for hypertension among newly-treated patients: a population-based study in Italy. J Hypertens. 2008;26:819–824. doi: 10.1097/HJH.0b013e3282f4edd7. [DOI] [PubMed] [Google Scholar]
- 129.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369:201–207. doi: 10.1016/S0140-6736(07)60108-1. [DOI] [PubMed] [Google Scholar]
- 130.Kinoshita S, Ryuzaki M, Sone M, Nishida E, Nakamoto H; On behalf of FUJIYAMA Study Group. Effectiveness of using long-acting angiotensin II type 1 receptor blocker in Japanese obese patients with metabolic syndrome on morning hypertension monitoring by using telemedicine system (FUJIYAMA Study) Clin Exp Hypertens. 2014:Epub ahead of print. doi: 10.3109/10641963.2013.863325. [DOI] [PubMed] [Google Scholar]
- 131.Vitale C, Mercuro G, Castiglioni C, Cornoldi A, Tulli A, Fini M, Volterrani M, Rosano GM. Metabolic effect of telmisartan and losartan in hypertensive patients with metabolic syndrome. Cardiovasc Diabetol. 2005;4:6. doi: 10.1186/1475-2840-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zreikat HH, Harpe SE, Slattum PW, Mays DP, Essah PA, Cheang KI. Effect of Renin-Angiotensin system inhibition on cardiovascular events in older hypertensive patients with metabolic syndrome. Metabolism. 2014;63:392–399. doi: 10.1016/j.metabol.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


