Abstract
Transforming growth factor-β (TGF-β) is a major regulator of collagen gene expression in human skin fibroblasts. Cellular responses to TGF-β are mediated primarily through its cell surface type I (TβRI) and type II (TβRII) receptors. Ultraviolet (UV) irradiation impairs TGF-β signaling largely due to reduced TβRII gene expression, thereby decreasing type I procollagen synthesis, in human skin fibroblasts. UV irradiation does not alter either TβRII mRNA or protein stability, indicating that UV reduction of TβRII expression likely results from transcriptional or translational repression. To understand how UV irradiation regulates TβRII transcription, we used a series of TβRII promoter-luciferase 5′-deletion constructs (covering 2kb of the TβRII proximal promoter) to determine transcriptional rate in response to UV irradiation. We identified a 137bp region upstream of the transcriptional start site that exhibited high promoter activity, and was repressed 60% by UV irradiation. Whereas, all other TβRII promoter reporter constructs exhibited either low promoter activities or no regulation by UV irradiation. Mutation of potential transcription factor binding sites within the promoter region revealed that an inverted CCAAT box (−81bp from transcription start site), is required for promoter activity. Mutation of the CCAAT box completely abolished UV irradiation regulation of the TβRII promoter. Protein binding assay, as determined by EMSA using the inverted CCAAT box as probe (−100/−62), demonstrated significantly enhanced protein-binding in response to UV irradiation. Super shift experiments indicated that nuclear factor Y (NFY) is able to binding to this sequence, but NFY binding was not altered in response to UV irradiation, indicating additional protein(s) are capable of binding this sequence in response to UV irradiation. Taken together, these data indicate that UV irradiation reduces TβRII expression, at least partially, through transcriptional repression. This repression is mediated by a 38bp sequence in TβRII promoter, in human skin fibroblasts.
Keywords: Ultraviolet, TGF-ß, Transcription
Introduction
The most abundant structural protein in human skin is type I procollagen, which is responsible for the skin’s strength and resiliency. Dermal fibroblasts are primary cellular source for type I collagen synthesis. Alteration of skin collagen content and organization impair wound healing, contributes to skin cancer, and are responsible for skin fragility in the elderly (1-4).
Transforming growth factor-β (TGF-β) is a major regulator of collagen gene expression in human skin fibroblasts. Cellular responses to TGF-β are mediated primarily through its cell surface type I receptor (TβRI) and type II receptor (TβRII). Binding of TGF-β to TβRII activates the intrinsic serine/threonine kinase activity of TβRI, which phosphorylates transcription factors Smad2 and Smad3. Phosphorylated Smad2 and Smad3 combine with Smad4, and translocate into the nucleus, where they function to regulate transcription of specific genes that possess TGF-β response elements in their promoters (5-7).
UV irradiation from the sun is a potent environmental hazard capable of damaging cellular DNA and causing mutations (8, 9). In addition, solar UV irradiation is the primary cause of premature skin aging (photoaging). We have reported previously that UV irradiation reduces type I procollagen production through impairment of TGF-β signal transduction pathway. This reduction is largely due to repression of TβRII gene expression (10, 11). We observed that UV irradiation significantly represses TβRII mRNA and protein, but not TβRI, in cultured human skin fibroblasts. In the same study, we also observed that neither TβRII mRNA stability nor TβRII protein stability was altered after UV exposure, indicating that UV repression of TβRII mRNA and protein must result from reduced mRNA transcription or protein synthesis.
We report here that UV irradiation reduces TβRII expression, at least partially, through transcriptional repression. This repression is mediated by a 38bp sequence in TβRII promoter, in human skin fibroblasts.
Materials and methods
Materials
Dulbecco’s Modified Eagle’s Medium, fetal calf serum, trypsin solution, penicillin/streptomycin, and L-glutamine were purchased from Gibco (Invitrogen, Carlsbad, CA). [γ-32p]ATP was obtained from Perkin Elmer (Boston, MA). Type I procollagen, TβRII and NF-Y antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All other reagents were purchased from Sigma-Aldrich Company (St. Louis, MO).
Cell culture and UV irradiation
Adult human skin primary dermal fibroblasts were isolated from punch biopsy of healthy adult normal human skin, as described previously (10). Cells were cultured at 37°C under 5% CO2 in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum. Cells were used between passages 5 and 10. UV irradiation of human skin primary dermal fibroblasts was performed as described previously (10-12). Briefly, sub-confluent cells were irradiated with a UVB/A2 source (50mJ/cm2) using an Ultralite Panelite lamp containing six FS24T12 UVB-HO bulbs. The UV irradiation intensity was monitored with an IL1400A phototherapy radiometer and a SED240/UVB/W photodetector (International Light, Newbury, MA). A Kodacel filter was used in order to remove UVC (wavelengths below 290nm).
Promoter/reporter constructs
A series of TβRII promoter-luciferase 5′-deletion constructs (covering 2kb of TβRII proximal promoter) was kindly provided by Dr. Seong-Jin Kim (National Institute of Health, Bethesda, Maryland) (13). Luciferase reporters driven by wild-type and mutant TβRII promoter spanning −137 to −47 were generated by PCR using mutant templates, which were kindly provided by Dr. James W. Freeman (Department of Medicine, University of Texas Health Service Center, San Antonio, Texas) (14). PCR primers were: forward primer 5′-CGCTCGAGTGAGGGGCAGCTGAAAGTC-3′; reverse primer to generate wild-type and mutants (Sp1A, Sp1C and inverted CCAAT) reporters was: 5′-GCAAGCTTACGTCCAGCCCCTAG-3′; reverse primer for Sp1D mutant was: 5′-GCAAGCTTACGTCGAATTCCTAG-3′; Wild type and mutant TβRII promoter luciferase reporters, spanning from −100 to −62, were generated by PCR using wild type and mutant templates described above. PCR primers were: forward primer: 5′-CGCTCGAGGGCTGGTCTAGGAAAC-3′; reverse primer: 5′-GCCTCGAGCAGCTACGAGAGAGC-3′. The PCR products were cloned into pGL-3 luciferase reporter using Hind III and Xho I restriction sites.
Transient transfection of TβRII promoter constructs and luciferase assays
Human skin fibroblasts were transiently transfected co-transfected with a β-galactosidase, to provide an internal control for transfection efficiency, and the luciferase reporters described above. Transfection was performed by electroporation, according to the manufacturer’s protocol (Amaxa Biosystems, Gaithersburg, MD). Aliquots containing identical β-galactosidase activity were used for each luciferase assay. Luciferase activity was measured using an enhanced luciferase assay kit (PharMingen International, San Diego, CA) according to the manufacturer’s protocol.
Electrophoretic mobility-shift assay and supershift assay
Electrophoretic mobility-shift assays (EMSAs) were performed as described previously (10, 15). Nuclear extracts were prepared by Nuclear and Cytoplasmic Extraction reagents (Pierce, Rockford, IL). Double-stranded oligonucleotide probes for EMSAs were as follows: wild type TβRII promoter probe spanning −100 to −62 (38 base pair), 5′-GGCTGGTCTAGGAAACATGATTGGCA-GCTACGAGAGAG-3′, mutant TβRII promoter probe, 5′-GGCTGGTCTAGGAAACATGGTGTACAGCTACGAGAGAG-3′. Consensus wild type and mutant NFY probes were purchased from Santa Cruz Biotechnology. All other oligonucleotides were synthesized by Invitrogen. For competition experiments, a 10- to 50-fold molar excess of non-radioactive competitor probes were pre-incubated with the nuclear extract for 30 minutes on ice before [32P] probes were added. For antibody supershift assays, reactions were performed by preincubating nuclear extracts (20 μg) with antibody (2 μg) on ice for 30 minutes and then incubated with [32P] probes. Gels were transferred to 3MM Whatman paper, vacuum-dried, and scanned using the STORM MolecularImager (Molecular Dynamics).
RNA Isolation and Quantitative Real-Time RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Total RNA (100ng) was reverse transcribed using Taqman Reverse Transcription kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed on a 7900 Sequence Detector (Applied Biosystems) using Taqman Universal PCR Master Mix Reagents (Applied Biosystems). PCR primers and probes were purchased from Applied Biosystems. Type I procollagen, TβRII and 36B4 primers and probes were described previously (15). Target gene levels were normalized to the housekeeping gene 36B4, as an internal control for quantification.
Western Blot Analysis
Western analysis was performed as previously described (10). Briefly, whole cell extracts were prepared from cells using whole cell extraction buffer (25mM HEPES, 0.3M NaCl, 1.5mM MgCl2, 0.2mM EDTA, 0.1% Triton X-100, 0.5mM DTT, 20mM β-glycerolphosphate, 0.1mM Na3VO4, 2μg/ml leupeptin, and 100μg/ml PMSF). Protein concentrations were measured by Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Proteins were resolved on 6% or 10% SDS-PAGE, transferred to PVDF membrane, and reacted with primary antibodies. Protein bands were visualized and quantified with enhanced chemifluorescence (ECF) (Vistra ECF Western Blotting System, GE Healthcare, Piscataway, NJ) following the manufacturer’s protocol. The intensities of each band were quantified by STORM MolecularImager (Molecular Dynamics, Sunnyvale, CA) and normalized using β-actin as loading control.
Transfection and siRNA
Human skin fibroblasts were transiently transfected with control siRNA (5′-UUCUCCGAACGUGUCACGU-3′, Qiagen, Chatsworth, CA), or NFY-A siRNA (5′-CCAUCGUCUAUCAACCAGUUA-3′), designed to target exon 6 of NFY-A (16) (Sigma-Aldrich Company, St Louis, MO), as described previously (17). All siRNA were transiently transfected into dermal fibroblasts by electroporation (Amaxa Biosystems, Koeln, Germany) as described above. Forty-eight hours after transfection, whole cell extract and total RNA were prepared. Protein and mRNA levels were determined by Western blot analysis and quantitative real-time RT-PCR respectively, as described above.
Statistical analysis
Comparisons between groups were determined with the Student’s t-test. All P-values are two-tailed and considered significant when p<0.05.
Results
UV irradiation represses T β RII promoter activity in human skin fibroblasts
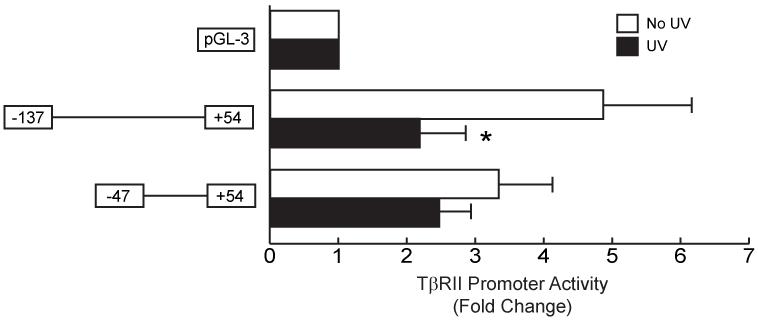
UV irradiation significantly reduces type I procollagen gene expression through impairment of TGF-β signal transduction pathway, largely due to repression of TβRII gene expression (11). This impairment is not due to reduced stability of either TβRII mRNA or protein, indicating that UV repression of expression likely results from reduced synthesis of TβRII mRNA or protein. To determine whether UV irradiation alters TβRII mRNA transcription, we transiently transfected a series of TβRII promoter-luciferease 5′-deletion constructs (covering 2kb of the TβRII proximal promoter) into cultured skin fibroblasts. Twenty-four hours after transfection, cells were exposured to UV (50mJ/cm2). Twenty-four hours after UV irradiation, luciferase activity was determined. These studies identified a 137 base pair region upstream of the transcriptional start site that exhibited high promoter activity, and was significantly repressed 60% (n=8, p<0.05) by UV irradiation (Fig. 1). The reporter construct pTβRII-47/+54 also exhibited high promoter activity but was not altered by UV irradiation. These data indicate that the TβRII promoter from −137 to −46 contains transcriptional regulation element(s) that is required for TβRII promoter activity and regulated by UV irradiation.
Figure 1.
UV irradiation represses TβRII promoter activity in human skin fibroblasts. Human skin fibroblasts were transiently transfected with TβRII promoter (sequence from −137 to +54 and −47 to +54) luciferase reporter constructs and β-galactosidase expression vector. Cells were exposed to UV irradiation (50mJ/cm2) 24 hours after transfection, and cell lysates were prepared 24 hours post UV irradiation. Luciferase activities were normalized to β-galactosidase activity. Data are means ± SEM for fold change in luciferase activity relative to activity in cells transfected with control vector, pGL-3 luciferase reporter. n=8. *p<0.05 versus non-UV-irradiated cells.
TβRII promoter activity requires inverted CCAAT box in human skin fibroblasts
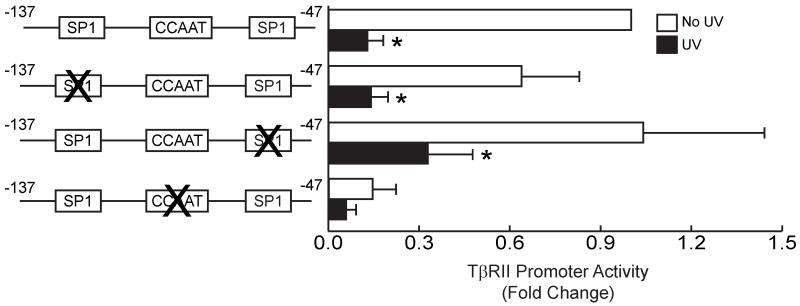
Analysis of the TβRII promoter from −137 to −47 revealed three potential SP1-binding sites and one inverted CCAAT transcription regulation site. To determine which of these sites are required for promoter activity and confer regulation by UV irradiation, we generated a series of mutant TβRII promoter luciferase reporter constructs (covering the −137 to −47 base pair region). Mutation of each SP1 site, did not alter either promoter activity or repression by UV irradiation (Fig. 2). In contrast, mutation of the inverted CCAAT box significantly reduced both TβRII promoter activity and responsiveness to UV irradiation (Fig. 2). These results indicate that the inverted CCAAT box is critical element for TβRII promoter regulation.
Figure 2.
TβRII promoter activity requires inverted CCAAT box in human skin fibroblasts. Human skin fibroblasts were transiently transfected with wild-type TβRII promoter (sequence from −137 to −47) luciferase reporter construct, or mutant TβRII promoter luciferase constructs in which Sp1 or inverted CCAAT box were mutated. Cells were co-transfected with the β-galactosidase expression vector. Cells were exposed to UV irradiation (50mJ/cm2) 24 hours after transfection, and cell lysates were prepared 24 hours post UV irradiation. Luciferase activities were normalized to β-galactosidase activity. Data are means ± SEM for fold change in luciferase activity relative to activity in cells transfected with wild-type TβRII promoter luciferase construct. n=6. *p<0.05 versus non-UV-irradiated cells.
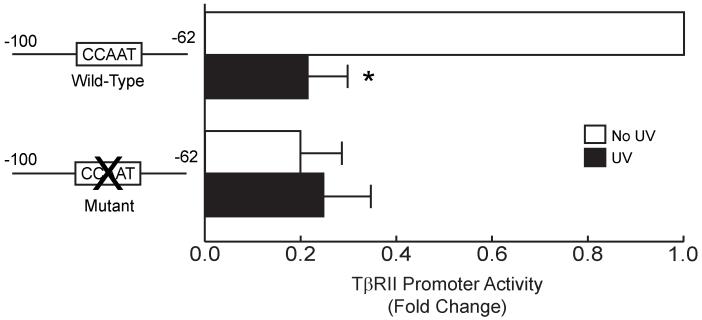
To further examine the activity of the inverted CCAAT site, we generated a 38 base pair TβRII promoter construct (−100/−62) containing the inverted CCAAT site, but excluding the SP1 sites. After transfection and UV irradiation, we found that this 38 base pair region retained full promoter, i.e. activity similar the −137/−47 construct (Fig. 2), and was repressed by UV irradiation (Fig. 3). Mutation of the inverted CCAAT site caused loss of promoter activity. Taken together, these data indicate that the inverted CCAAT sequence located between −100 to −62 base pairs is required and sufficient for TβRII proximal promoter activity and confers responsiveness to UV irradiation.
Figure 3.
Inverted CCAAT box confers UV irradiation repression to TβRII promoter in human skin fibroblasts. Human skin fibroblasts were transiently transfected with the wild-type TβRII promoter (sequence from −100 to −62) luciferase reporter construct, or mutant TβRII promoter luciferase reporter construct in which the inverted CCAAT box was mutated. Cells were co-transfected with the β-galactosidase expression vector. Cells were exposed to UV irradiation (50mJ/cm2) 24 hours after transfection, and cell lysates were prepared 24 hours post UV irradiation. Luciferase activities were normalized to β-galactosidase activity. Data are means ± S.E. for fold change in luciferase activity relative to activity of wild-type TβRII promoter luciferase reporter construct. n=4. *p<0.05 versus non-UV-irradiated cells.
UV irradiation increases protein binding to inverted CCAAT box in the TβRII proximal promoter in human skin fibroblasts
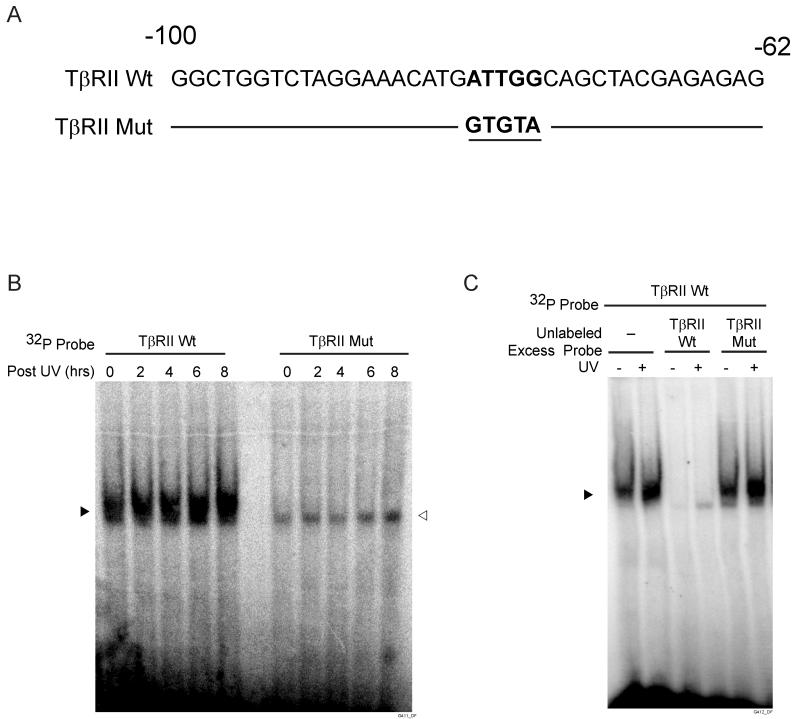
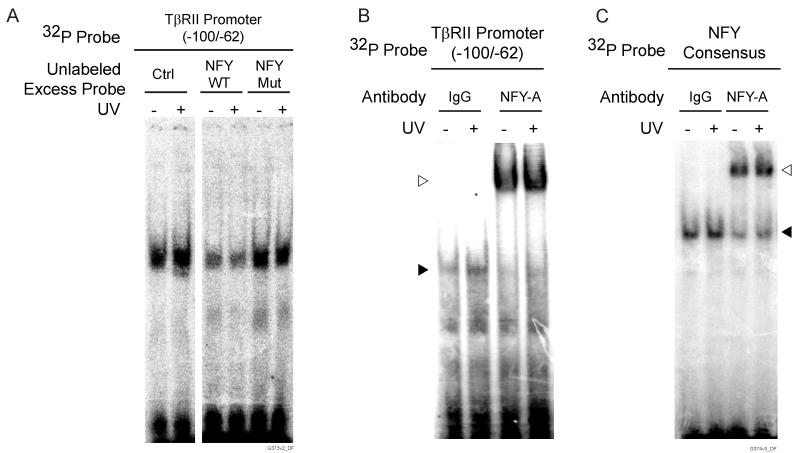
UV irradiation repression of the TβRII promoter may reflect altered protein binding to the inverted CCAAT region of the promoter. To examine this possibility, we performed electrophoretic mobility-shift assays (EMSA) using TβRII promoter −100/−62 as probe (Fig. 4A). UV irradiation substantially increased the amount of the retarded DNA-protein complex (Fig. 4B). This increased protein-binding occurred two hours post UV irradiation and remained increased for at least eight hours post UV exposure. No DNA-protein complex was detected with mutant CCAAT probe (Fig. 4B, right panel). To confirm the specificity of the retarded complexes formed with the wild-type probe, we performed competition EMSA. DNA-protein complex with the labeled −100/−62 probe was completely abolished by excess unlabeled the wild-type probe, but not by the mutant CCAAT probe (Fig. 4C).
Figure 4.
UV irradiation increases protein binding to inverted CCAAT box in the TβRII proximal promoter in human skin fibroblasts. A) Sequence of TβRII promoter spanning nucleotides −100 to −62 used as probes for EMSAs. Wild-type and mutant inverted CCAAT sites are bolded and underlined, respectively. B) EMSAs were performed using [32P] TβRII wild-type or mutant probes. Nuclear extracts were prepared at indicated times after UV irradiation (50mJ/cm2). Closed triangle indicates specific retarded complex. Open triangle indicate nonspecific bands. C) Competition of protein binding to TβRII wild-type probe by 50-fold molar excess unlabeled wild-type or mutant probe. Nuclear extracts were prepared six hours after UV irradiation. Closed triangle indicate specific retarded complex. Results are representative of three experiments.
NFY binds to the TβRII promoter activity but binding is not altered by UV in human skin fibroblasts
The CCAAT box is one of the most common cis-elements present in eukaryotic promoters and can serve as a binding site for transcription factor nuclear factor Y (NFY). To determine whether NFY interacts with the inverted CCAAT box in the TβRII promoter, we performed competition EMSA with labeled wild-type TβRII promoter −100/−62 probe and excess unlabeled NFY consensus probe. As shown in figure 5A, addition of excess NFY consensus probe reduced protein-binding to the TβRII probe. Similar reduction of binding was observed in samples from both non-irradiated and UV-irradiated dermal fibroblasts. In contrast, competition with excess mutant NFY probe did not alter formation of the retarded complexes, indicating that competition with NFY consensus probe was specific. To further confirm NFY-binding, we performed super shift assays with antibody that specifically recognizes the NFY-A subunit of NFY. Super shifted DNA-protein-antibody complexes were detected in samples from both UV-irradiated and non-UV-irradiated dermal fibroblasts (Fig. 5B). These data indicate that NFY binds to the −100/−62 sequence in the TβRII proximal promoter.
Figure 5.
NFY binds to the inverted CCAAT box in the TβRII promoter in human skin fibroblasts. Fibroblasts were mock or UV-irradiated (50mJ/cm2) and nuclear extracts were prepared six hours after irradiation. A) Competition of protein binding to TβRII promoter probe (spanning from −100 to −62) by 50-fold molar excess of unlabeled wild-type or mutant NFY probe. B) EMSA with [32P] TβRII promoter probe (spanning from −100 to −62) super shifted with control IgG or anti-NFYA antibody. Closed triangle indicates retarded complex; open triangle indicates super shifted complex. C) EMSA with [32P] NFY consensus probe super shifted with control IgG or anti-NFY-A antibody. Closed triangle indicates retarded complex; open triangle indicates super shifted complex. Results are representative of three experiments.
We next investigated whether NFY DNA-binding is altered by UV irradiation. To examine this question, we performed EMSA and super shift EMSA with [32P] NFY consensus probe and anti-NFY-A antibody. As shown in figure 5C, retarded complexes and super shifted bands were readily detected in nuclear extracts from non-irradiated skin fibroblasts. Notably, the intensities of both retarded and super shifted bands were not altered by treatment of fibroblasts with UV irradiation.
Taken together, these data indicate that NFY binds to the 38 base pair region of the TβRII promoter and this binding is not regulated by UV irradiation. Therefore, increased NFY DNA-binding is not responsible for increased DNA-protein complex formation with the TβRII promoter, which is observed following UV irradiation.
Discussion
Transcriptional regulation of TβRII gene expression plays a key role in modulating TGF-β responsiveness. Reduced expression of TβRII has been identified in several types of tumor cells. Transfection of wild-type TβRII construct into such tumor cells is able to restore their sensitivity to TGF-β and suppress cell growth (18, 19). In skin dermis, TGF-β is a major mediator of fibroblast function and extracellular matrix production. Our previous studies have demonstrated that UV irradiation significantly reduces type I procollagen gene expression, in cultured skin fibroblasts, through impairment of TGF-β signaling, largely due to reduction of TβRII (11). Restoration of TβRII gene expression overcomes the inhibitory effect of UV on type I procollagen production. In addition, knock down of TβRII by siRNA reduces type I procollagen expression, similar to UV irradiation.
In this report, we have investigated regulation of TβRII transcription by UV irradiation in human dermal fibroblasts. We identified a 38 base pair sequence (−100/−62), which harbors an inverted CCAAT box, in the proximal TβRII promoter that is necessary for promoter activity and confers inhibition by UV irradiation. Reduction of TβRII promoter activity by UV irradiation was associated with enhanced protein-binding to the 38 base pair sequence. NFY binds to the 38 base pair promoter region, however its binding was not altered in response to UV irradiation, implying additional protein(s) are capable of binding to this sequence in response to UV irradiation.
Previous studies have identified several cis-regulatory elements in the TβRII gene promoter (13, 20) However, the role of these cis-elements in the regulation of TβRII gene transcription appears to be complex and cell-type dependent. We found that human skin fibroblasts, transcription of the TβRII proximal promoter is dependent on a 38 base pair sequence from −100 to −62. This sequence also confers regulation by UV irradiation, which inhibits transcription and induces protein-binding.
CCAAT box is one the most common cis-elements present in eukaryotic promoters. Various DNA binding proteins interact with this element, including NFY. NFY is a heteromeric complex composed of three subunits, NFY-A, NFY-B and NFY-C, all required for CCAAT binding. NFY have been shown to exert both positive and negative gene regulation (21-24). We observed that knock down of NFY-A by siRNA resulted in repression of TβRII promoter activity, indicating NFY function as a positive regulator of TβRII gene expression, in cultured human skin fibroblasts. This conclusion is consistent with reports from Park and others, who studied different cell types (25, 26).
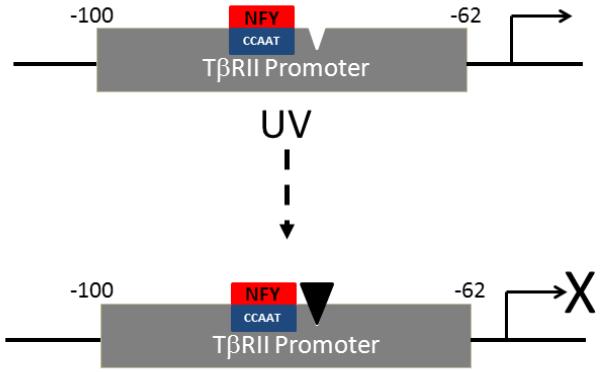
NFY functions via both cooperative and antagonistic interactions with other transcription factors (27-29). Cooperative interactions enhance NFY activity, which could act to repress or stimulate promoter activity, depending on promoter context (30-32). Conversely, antagonistic interactions act to diminish NFY function (33-35). Our data demonstrate that NFY binds to an inverted CCAAT box, which is contained within a 38 base pair sequence in the TβRII proximal promoter. This sequence is required for promoter activity, and repression of activity by UV irradiation is associated with increased protein-binding to this sequence. Given that NFY is required for promoter activity, a likely scenario is that increased protein(s)-binding in response to UV irradiation antagonizes NFY function, thereby reducing transcription of the TβRII gene (Fig. 6). The identity of the antagonistic protein(s) remains to be determined.
Figure 6.
Model of mechanism of inhibition of type II TGF-β receptor gene transcription by UV irradiation in human skin fibroblasts. An inverted CCAAT box, in the proximal promoter (−62-100) of the TGF-β type II receptor (TβRII) gene, is required for transcriptional activity. In adult human skin fibroblasts, NFY transcription factor complex binds to the inverted CCAAT box. UV irradiation induces binding of an unidentified protein (filled triangle) to the TβRII proximal promoter. Protein-binding antagonizes NFY function, thereby reducing transcription of the TβRII gene.
Acknowledgements
We would like to thank Diane Fiolek for assistance with graphics and administrative assistance. The work was supported, in part, by the National Institute of Health grant AG019364 to Gary Fisher.
Footnotes
Conflict of Interest: The authors have no conflict of interest that is directly relevant to the content of this article.
References
- 1.Eaglstein WH. Wound healing and aging. Dermatol Clin. 1986;4:481–484. [PubMed] [Google Scholar]
- 2.Holt DR, Kirk SJ, Regan MC, Hurson M, Lindblad WJ, Barbul A. Effect of age on wound healing in healthy human beings. Surgery. 1992;112:293–297. discussion 297-298. [PubMed] [Google Scholar]
- 3.Khorramizadeh MR, Tredget EE, Telasky C, Shen Q, Ghahary A. Aging differentially modulates the expression of collagen and collagenase in dermal fibroblasts. Mol Cell Biochem. 1999;194:99–108. doi: 10.1023/a:1006909021352. [DOI] [PubMed] [Google Scholar]
- 4.Weinstock MA. Epidemiology of nonmelanoma skin cancer: clinical issues, definitions, and classification. J Invest Dermatol. 1994;102:4S–5S. doi: 10.1111/1523-1747.ep12385720. [DOI] [PubMed] [Google Scholar]
- 5.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 6.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 7.Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- 8.Fisher GJ, Datta SC, Talwar HS, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 9.Bender K, Blattner C, Knebel A, Iordanov M, Herrlich P, Rahmsdorf HJ. UV-induced signal transduction. J Photochem Photobiol B. 1997;37:1–17. doi: 10.1016/s1011-1344(96)07459-3. [DOI] [PubMed] [Google Scholar]
- 10.Quan T, He T, Voorhees JJ, Fisher GJ. Ultraviolet irradiation blocks cellular responses to transforming growth factor-beta by down-regulating its type-II receptor and inducing Smad7. J Biol Chem. 2001;276:26349–26356. doi: 10.1074/jbc.M010835200. [DOI] [PubMed] [Google Scholar]
- 11.Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am J Pathol. 2004;165:741–751. doi: 10.1016/s0002-9440(10)63337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Connective tissue growth factor: expression in human skin in vivo and inhibition by ultraviolet irradiation. J Invest Dermatol. 2002;118:402–408. doi: 10.1046/j.0022-202x.2001.01678.x. [DOI] [PubMed] [Google Scholar]
- 13.Bae HW, Geiser AG, Kim DH, et al. Characterization of the promoter region of the human transforming growth factor-beta type II receptor gene. J Biol Chem. 1995;270:29460–29468. doi: 10.1074/jbc.270.49.29460. [DOI] [PubMed] [Google Scholar]
- 14.Zhao S, Venkatasubbarao K, Li S, Freeman JW. Requirement of a specific Sp1 site for histone deacetylase-mediated repression of transforming growth factor beta Type II receptor expression in human pancreatic cancer cells. Cancer Res. 2003;63:2624–2630. [PubMed] [Google Scholar]
- 15.Quan T, He T, Voorhees JJ, Fisher GJ. Ultraviolet irradiation induces Smad7 via induction of transcription factor AP-1 in human skin fibroblasts. J Biol Chem. 2005;280:8079–8085. doi: 10.1074/jbc.M409647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benatti P, Dolfini D, Vigano A, Ravo M, Weisz A, Imbriano C. Specific inhibition of NF-Y subunits triggers different cell proliferation defects. Nucleic Acids Res. 2011;39:5356–5368. doi: 10.1093/nar/gkr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quan T, Qin Z, Xu Y, et al. Ultraviolet irradiation induces CYR61/CCN1, a mediator of collagen homeostasis, through activation of transcription factor AP-1 in human skin fibroblasts. J Invest Dermatol. 2010;130:1697–1706. doi: 10.1038/jid.2010.29. [DOI] [PubMed] [Google Scholar]
- 18.Inagaki M, Moustakas A, Lin HY, Lodish HF, Carr BI. Growth inhibition by transforming growth factor beta (TGF-beta) type I is restored in TGF-beta-resistant hepatoma cells after expression of TGF-beta receptor type II cDNA. Proc Natl Acad Sci U S A. 1993;90:5359–5363. doi: 10.1073/pnas.90.11.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun L, Wu G, Willson JK, et al. Expression of transforming growth factor beta type II receptor leads to reduced malignancy in human breast cancer MCF-7 cells. J Biol Chem. 1994;269:26449–26455. [PubMed] [Google Scholar]
- 20.Yu YS, Suzuki Y, Yoshitomo K, Muramatsu M, Yamaguchi N, Sugano S. The promoter structure of TGF-beta type II receptor revealed by “oligo-capping” method and deletion analysis. Biochem Biophys Res Commun. 1996;225:302–306. doi: 10.1006/bbrc.1996.1170. [DOI] [PubMed] [Google Scholar]
- 21.Boucher PD, Ruch RJ, Hines RN. Specific nuclear protein binding to a negative regulatory element on the human CYP1A1 gene. J Biol Chem. 1993;268:17384–17391. [PubMed] [Google Scholar]
- 22.Xu Y, Banville D, Zhao HF, Zhao X, Shen SH. Transcriptional activity of the SHP-1 gene in MCF7 cells is differentially regulated by binding of NF-Y factor to two distinct CCAAT-elements. Gene. 2001;269:141–153. doi: 10.1016/s0378-1119(01)00445-0. [DOI] [PubMed] [Google Scholar]
- 23.Kelly D, Kim SJ, Rizzino A. Transcriptional activation of the type II transforming growth factor-beta receptor gene upon differentiation of embryonal carcinoma cells. J Biol Chem. 1998;273:21115–21124. doi: 10.1074/jbc.273.33.21115. [DOI] [PubMed] [Google Scholar]
- 24.Bernadt CT, Rizzino A. Roles of the conserved CCAAT and GC boxes of the human and mouse type II transforming growth factor-beta receptor genes. Mol Reprod Dev. 2003;65:353–365. doi: 10.1002/mrd.10313. [DOI] [PubMed] [Google Scholar]
- 25.Park SH, Lee SR, Kim BC, et al. Transcriptional regulation of the transforming growth factor beta type II receptor gene by histone acetyltransferase and deacetylase is mediated by NF-Y in human breast cancer cells. J Biol Chem. 2002;277:5168–5174. doi: 10.1074/jbc.M106451200. [DOI] [PubMed] [Google Scholar]
- 26.Jin S, Scotto KW. Transcriptional regulation of the MDR1 gene by histone acetyltransferase and deacetylase is mediated by NF-Y. Mol Cell Biol. 1998;18:4377–4384. doi: 10.1128/mcb.18.7.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maity SN, de Crombrugghe B. Role of the CCAAT-binding protein CBF/NF-Y in transcription. Trends Biochem Sci. 1998;23:174–178. doi: 10.1016/s0968-0004(98)01201-8. [DOI] [PubMed] [Google Scholar]
- 28.Mantovani R. The molecular biology of the CCAAT-binding factor NF-Y. Gene. 1999;239:15–27. doi: 10.1016/s0378-1119(99)00368-6. [DOI] [PubMed] [Google Scholar]
- 29.Liu JX, Howell SH. bZIP28 and NF-Y transcription factors are activated by ER stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis. Plant Cell. 2010;22:782–796. doi: 10.1105/tpc.109.072173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi X, Metges CC, Seyfert HM. Interaction of C/EBP-beta and NF-Y factors constrains activity levels of the nutritionally controlled promoter IA expressing the acetyl-CoA carboxylase-alpha gene in cattle. BMC Mol Biol. 2012;13:21. doi: 10.1186/1471-2199-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito Y, Zhang Y, Dangaria S, Luan X, Diekwisch TG. NF-Y and USF1 transcription factor binding to CCAAT-box and E-box elements activates the CP27 promoter. Gene. 2011;473:92–99. doi: 10.1016/j.gene.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Amicis F, Giordano F, Vivacqua A, et al. Resveratrol, through NF-Y/p53/Sin3/HDAC1 complex phosphorylation, inhibits estrogen receptor alpha gene expression via p38MAPK/CK2 signaling in human breast cancer cells. FASEB J. 2011;25:3695–3707. doi: 10.1096/fj.10-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barberis A, Superti-Furga G, Busslinger M. Mutually exclusive interaction of the CCAAT-binding factor and of a displacement protein with overlapping sequences of a histone gene promoter. Cell. 1987;50:347–359. doi: 10.1016/0092-8674(87)90489-2. [DOI] [PubMed] [Google Scholar]
- 34.Superti-Furga G, Barberis A, Schaffner G, Busslinger M. The −117 mutation in Greek HPFH affects the binding of three nuclear factors to the CCAAT region of the gamma-globin gene. EMBO J. 1988;7:3099–3107. doi: 10.1002/j.1460-2075.1988.tb03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo W, Skalnik DG. CCAAT displacement protein competes with multiple transcriptional activators for binding to four sites in the proximal gp91phox promoter. J Biol Chem. 1996;271:18203–18210. doi: 10.1074/jbc.271.30.18203. [DOI] [PubMed] [Google Scholar]