Abstract
Background
Postoperative cognitive dysfunction is a clinical entity that is associated with poor outcome. We determined the effectiveness of amantadine in reducing surgery-induced cognitive impairment and the role of glial cell line-derived neurotrophic factor (GDNF) in this effect.
Methods
Four-month old male Fischer 344 rats were subjected to right carotid exposure under intravenous anesthesia. Some rats received intraperitoneal injection of 25 mg/kg/day amantadine for three days with the first dose at 15 min before the surgery or intracerebroventricular injection of GDNF or an anti-GDNF antibody at the end of surgery. One week later, rats were started to be tested by Barnes maze and fear conditioning. Hippocampus was harvested at 6 h, 24 h or 10 days after the surgery for biochemical analysis. C8-B4 cells, a microglial cell line, were pretreated with 1 ng/ml GDNF for 30 min before being exposed to 5 ng/ml lipopolysaccharide for 2 h.
Results
Surgery increased the time to identify the target box in the Barnes maze when tested 1 day [22 (median) (11–66) (interquartile range) of control group vs. 158 (29–180) of surgery group, n = 15, P = 0.022) or 8 days after the training sessions and reduced context-related freezing behavior in the fear conditioning test. These effects were attenuated by amantadine (25 (14–90), n = 15, P = 0.029 compared with surgery group at 1 day after the training sessions in Barnes maze) and intracerebroventricular GDNF. Amantadine increased GDNF that was co-localized with glial fibrillary acidic protein, an astrocytic marker, in the hippocampus. Intracerebroventricular injection of an anti-GDNF antibody but not the denatured antibody blocked the effects of amantadine on cognition. Surgery induced neuroinflammation that was inhibited by amantadine. Lipopolysaccharide increased interleukin 1β production from C8-B4 cells. This effect was inhibited by GDNF.
Conclusions
Our results suggest that amantadine attenuated surgery-induced learning and memory impairment. This effect may be mediated by GDNF via inhibition of neuroinflammation.
Introduction
About 26 millions of Americans received surgery in 2010.* Post-operative cognitive dysfunction (POCD) is a documented clinical entity that is attracting significant attention from the public and professionals in recent years.1 Studies have shown that POCD can occur at one week or months after cardiac and non-cardiac surgeries.2–4 POCD can be associated with a substantial increase of morbidity, mortality and cost of care.3,5 Thus, it is urgent to identify effective strategies to prevent or reduce the occurrence of POCD.
Amantadine was initially used as an antiviral agent. Its clinical use has been expanded into many areas, such as in patients with Parkinson’s disease and traumatic brain injury, to improve patient outcome.6,7 Amantadine has been found to have significant neuroprotective effects.7,8 One of the mechanisms for these effects as shown in cell culture studies is to enhance the production of glial cell line-derived neurotrophic factor (GDNF).9 GDNF can inhibit microglial activation and neuroinflammation.8,9 Neuroinflammation is a basic neuropathological process for many neurodegenerative diseases.10 Neuroinflammation may also be the underlying pathology for POCD.11,12
Based on the above information, we hypothesize that amantadine can reduce POCD via increasing GDNF. To test this hypothesis, adult rats were subjected to right carotid artery exposure, a surgical procedure that is part of commonly performed carotid endarterectomy. Their learning and memory were tested. The expression of GDNF in the brain and neuroinflammation were examined.
Materials and Methods
The animal protocol was approved by the institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80–23) revised in 2011.
Animal groups
Four-month old male Fischer 344 rats weighing 290 – 330 g from Charles River Laboratories International, Inc. (Wilmington, MA) were randomly assigned to: 1) control group (not being exposed to surgery or any drugs), 2) amantadine group, 3) surgery group (right carotid artery exposure), and 4) surgery plus amantadine group in the first experiment. Each group had 15 rats. In the second experiment, the rats were assigned to: 5) control group, 6) anti-GDNF antibody group, 7) surgery plus amantadine plus boiled anti-GDNF antibody group, and 8) surgery plus amantadine plus anti-GDNF antibody group. Each group had 8 rats. In the third experiment, the rats were randomly assigned to: 7) control group, 8) anesthesia only group, and 9) surgery plus GDNF group. Each group had 8 rats. GDNF and the anti-GDNF antibody were injected intracerebroventricularly. One week later, these rats were started to be tested in Barnes maze and then fear conditioning. Separate rats were assigned to 1) control group, 2) surgery group, and 3) surgery plus amantadine group (n = 6 per condition) and sacrificed at 6 h, 24 h or 10 days after the surgery for Western blotting and immunohistochemistry.
Anesthesia and surgery
The surgery was right carotid artery exposure. Briefly, a rat was placed in an animal restrainer. The left or right lateral tail vein was cannulated with a 24-gauge intravenous catheter. Propofol (16GB0042, AAP Pharmaceuticals, LLC, Schaumburg, IL) at 15 mg/kg was infused intravenously to induce anesthesia and then at a rate of 60 mg/kg/h to maintain anesthesia. After loss of righting reflex was achieved, 0.15 mg/kg buprenorphine hydrochloride (Recktt Benckiser Healthcare Ltd., Kingston-upon-Thames, United Kingdom) was injected subcutaneously. The rat was immediately intubated with a 14-gauge catheter and mechanically ventilated with 100% O2 by a CIV-101 ventilator (Columbus Instruments, Columbus, OH) to maintain normal end-tidal carbon dioxide concentrations as monitored by a Datex infrared analyzer (Capnomac, Helsinki, Finland). The front of neck was meticulously shaved and disinfected with povidone iodine. Ten minutes after the induction of anesthesia, a 2.2-cm midline neck incision was made and the soft tissues over the trachea were retracted gently. One-centimeter long right common carotid artery was carefully dissected free from other tissues. Particular care was taken to avoid damage to the vagus nerve. The wound was then irrigated with normal saline and closed with surgical suture. The surgery was performed under strict sterile conditions and lasted for 15 min. After the surgery, propofol infusion rate was reduced to 45 mg/kg/h and then decreased to 30 mg/kg/h in the final 0.5 h of the anesthesia. The total duration of anesthesia lasted for 2 h, a clinically relevant duration of anesthesia. No response to toe pinching that was performed every 15 min was observed during the whole anesthesia period. After propofol infusion was stopped, animals were allowed to breathe spontaneously and then extubated at the recovery of righting reflex. During anesthesia, rectal temperature was monitored and maintained at 37°C with the aid of servo-controlled warming blanket (TCAT-2LV, Physitemp instruments Inc., Clifton, NJ).
Amantadine application
Amantadine (A1260, Sigma-Aldrich, St Louis, MO) was dissolved in normal saline and injected intraperitoneally at 25 mg/kg/day for three days with the first dose at 15 min before surgery. Similar injections were performed in the amantadine only group except that no surgery and anesthesia were performed. The amantadine dose was chosen based on previous studies.13,14
Intracerebroventricular injection of an anti-GDNF antibody or GDNF
In the second experiment, some rats received intracerebroventricular injection of 10 µl (200 µg/ml) goat polyclonal anti-GDNF antibody (catalogue number: sc-328-G; Santa Cruz Biotechnology, Santa Cruz, CA) based on a previous study.9 Others received the injection of 10 µl heat-denatured (5 min at 100°C) anti-GDNF antibody. In the third experiment, some rats received intracerebroventricular injection of 0.5 µg recombinant rat GDNF (catalogue number: 512-GF; R&D Systems, Minneapolis, MN) in 10 µl phosphate buffered saline (PBS) as described in a previous study.15 Each rat received only one injection to the right lateral ventricle immediately at the end of the surgery or under a 20-min propofol anesthesia (for anti-GDNF antibody injection only group in the second experiment). A single injection was performed because proteins injected intracerebroventricularly can stay in the brain tissues for a long time. For example, GDNF injected intracerebroventricularly was detectable in the brain tissues at least 7 days after the injection.15 The intracerebroventricular injection was performed as described before16 with the aid of a stereotactic apparatus (SAS-5100, ASI Instruments, Inc., Warren, MI) using the following coordinates: 0.8 mm posterior to bregma, 1.6 mm lateral from midline and 4.5 mm ventral from the surface of the skull. After the injection, the needle was kept in place for 1 min to prevent backflow of the injected solution.
Barnes maze
One week after surgery, animals were subjected to Barnes maze as we previously described17,18 to test their spatial learning and memory. Animals were first placed in the middle of a circular platform with 20 equally spaced holes (SD Instruments, San Diego, CA). One of these holes was connected to a dark chamber called target box. Aversive noise (85 dB) and bright light (200 W) shed on the platform was used to encourage rats to find the target box. They had a spatial acquisition phase that lasted for 4 days with 3 min per trial, 4 trials per day and 15 min between each trial. Animals then went through the reference memory phase to test the short-term retention on day 5 and long-term retention on day 12. No test or handling was performed from day 5 to day 12. The latency to find the target box during each trial was recorded with the assistance of ANY-Maze video tracking system (SD Instruments).
Fear conditioning
One day after Barnes maze test, rats were subjected to fear conditioning test as we previously described.17,18 Each rat was placed into a test chamber wiped with 70% alcohol and exposed to 3 tone-foot shock pairings (tone: 2000 Hz, 85 db, 30 s; foot shock: 1 mA, 2 s) with an intertrial interval 1 min in a relatively dark room. The rat was removed from this test chamber 30 s after the conditioning stimuli. The animal was placed back to the same chamber without the tone and shock 24 h later for 8 min. The animal was placed 2 h later into another test chamber that had different context and smell from the first test chamber in a relatively light room. This second chamber was wiped with 1% acetic acid. Freezing was recorded for 3 min without the tone stimulus. The tone then was turned on for 3 cycles, each cycle for 30 s followed by 1-min inter-cycle interval (4.5 min in total). Animal behavior in these two chambers was video recorded. The freezing behavior in the 8 min in the first chamber (context-related) and 4.5 min in the second chamber (tone-related) was scored in an 8 s interval by an observer who was blind to group assignment.
Brain tissue harvest
Rats were deeply anesthetized with 5% isoflurane for 2 min and perfused transcardially with saline at 6 or 24 h after anesthesia and surgery. Their left hippocampus was dissected out immediately for Western blotting and the right cerebral hemisphere from Bregma −3 to −6 mm was used for immunohistochemistry.
Preparation of cytoplasmic and nuclear proteins from hippocampus
The cytoplasmic and nuclear proteins were prepared as we described before.19 Briefly, hippocampus was homogenized in a buffer containing 10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol and 0.05% NP40 (pH 7.9) as well as protease inhibitor cocktail (10 mg/ml aproteinin, 5 mg/ml peptastin, 5 mg/ml leupeptin, and 1 mM phenylmethanesulfonylfluoride) and incubated on ice for 10 min. They were then centrifuged at 3000 rpm for 10 min at 4°C. The supernatant was stored as cytoplasmic fraction. The resulted pellet was resuspended in a buffer containing 5 mM HEPES, 300 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol and 26% glycerol (v/v) (pH 7.9) and was homogenized with 20 full strokes in a glass homogenizer. They were left on ice for 30 min and then centrifuged at 24,000 g for 20 min at 4°C. The supernatant was kept as the nuclear protein fraction. The protein concentration was determined by the Bradford assay.
Western blot analysis
Fifty microgram proteins per lane were separated on a polyacrylamide gel and then blotted onto a polyvinylidene difluoride membrane. The membranes were blocked with Protein-Free T20 Blocking Buffer (catalogue number: 37573, Thermo Scientific, Logan, UT) and incubated with the following primary antibodies overnight at 4°C: rabbit monoclonal anti-nuclear factor (NF)-κB p65 antibody (1:1000 dilution, catalogue number: 8242; Cell signaling Technology, Danvers, MA), rabbit polyclonal anti-histone H3 antibody (1:1000 dilution, catalogue number: 9715; Cell signaling Technology), goat polyclonal anti-GDNF antibody (1:200 dilution, catalogue number: sc-328-G; Santa Cruz Biotechnology), rabbit polyclonal anti-interleukin (IL)-6 antibody (1:1000 dilution, catalogue number: ab6672; Abcam, Cambridge, MA), rabbit polyclonal anti-IL-1β antibody (1:1000 dilution, catalogue number: ab15077; Abcam) and rabbit polyclonal anti-glyceraldehyde 3-phosphate dehydrogenase antibody (1:5000 dilution, catalogue number: G9545; Sigma Aldrich). Appropriate secondary antibodies were used. Protein bands were visualized by Genesnap version 7.08 and quantified by Genetools version 4.01. The relative protein expression of nuclear p65 and cytoplasmic GDNF, IL-1β and IL-6 was normalized to H3 and glyceraldehyde 3-phosphate dehydrogenase, respectively. The results from animals under various experimental conditions then were normalized by the mean values of the corresponding control animals.
Immunohistochemistry
As we described,12 brains were harvested and postfixed in 4% paraformaldehyde in 0.1 M PBS at 4°C for 18 h, rehydrated, and embedded in paraffin. Coronal 5-µm sections of the cerebral hemisphere were cut sequentially and mounted on Superfrost plus microscope slides. Antigen retrieval with Tris/EDTA buffer (10 mM Tris Base, 1 mM EDTA, 0.05% Tween 20, pH 9.0) was performed at 95 – 100°C for 20 min. After being washed in Tris-buffered saline (TBS) containing 0.025% triton-X 100, sections were blocked in 10% donkey serum plus 1% bovine serum albumin in TBS for 2 h at room temperature and then incubated at 4°C overnight with the following primary antibodies: goat polyclonal anti-GDNF antibody (1:100 dilution, catalogue number: sc-328-G; Santa Cruz Biotechnology), mouse monoclonal anti-glial fibrillary acidic protein (GFAP) antibody (1:500 dilution, catalogue number: MAB360; Millipore, Billerica, MA), rabbit polyclonal anti-ionized calcium binding adapter molecule 1 (Iba-1) antibody (1:500 dilution, catalogue number: 019-19741; Wako Chemicals USA, Richmond, VA), mouse monoclonal anti-neuronal nuclei (NeuN) antibody (1:250 dilution, catalogue number: MAB377; Millipore), and mouse monoclonal anti-p65 antibody (1:250 dilution, catalogue number: #6956; Cell signaling Technology). Sections were rinsed in TBS with 0.025% triton-X 100. The donkey anti-mouse IgG antibody conjugated with NL493 (1:200 dilution, catalogue number: NL009; R&D Systems), donkey anti-rabbit IgG antibody conjugated with NL557 (1:200 dilution, catalogue number: NL004; R&D Systems), donkey anti-goat IgG antibody conjugated with NL637 (1:200 dilution, catalogue number: NL002; R&D Systems), or donkey anti-goat IgG antibody conjugated with Alexa Fluor 488 (1:200 dilution, catalogue number: A11055; Invitrogen) were incubated with the sections for 1 h at room temperature in the dark. After washed in TBS, sections were mounted and coverslipped with Vectashield mounting medium.
A second but different staining for Iba-1 was also performed. The antigen retrieval, section washing and blocking, as well as incubation with the primary antibody were performed as described above. Sections were then incubated with 0.3% H2O2 in TBS for 30 min to block endogenous peroxidase, followed by the incubation with biotinylated goat anti-rabbit IgG antibody at room temperature for 1 h. The staining was visualized with diaminobenzidine method. The sections were dehydrated and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA).
Images of immunostaining were acquired with a fluorescence microscope equipped with a charge-coupled device camera. A negative control without the incubation with the primary antibody was performed in all experiments. The quantification was performed as described previously.20 Briefly, images of 3 non-overlapping fields in the hippocampal CA3 or dentate gyrus area per section were randomly acquired using a counting frame size of 0.4 mm2. Three sections per rat were imaged. The number of pixels per image with intensity above a predetermined threshold level was considered as positively stained areas and quantified using the Image J 1.47n software (National Institutes of Health, Bethesda, MD). The immunoreactivity to a protein was quantified by percentage area with positive staining to the total area of the imaged field. All quantitative analyses were performed in a blinded manner.
Microglial cell culture and treatment
The C8-B4 cells (CRL-2540™), a rodent microglial clone, were purchased from the American Type Culture Collection (Manassas, VA) and cultured as we previously described.21,22 Briefly, they were cultured in Dulbecco's Modified Eagle's Medium (DMEM) containing 4 mM L-glutamine, 4500 mg/L glucose, 1 mM sodium pyruvate, 1500 mg/L sodium bicarbonate and 10% heat inactivated fetal bovine serum (FBS) in a humidified atmosphere of 95% air-5% CO2 at 37°C. The medium was changed every 3 days. The cells were plated at a density of 5×105 cells/well on 6-well culture plates for enzyme-linked immunosorbent assay (ELISA) of IL-1β or 1×105 cells/well on 6-well culture plates for immunofluorescent staining.
The cells were randomly assigned to four groups at 24 h after they were plated: control group, GDNF group, lipopolysaccharide group and GDNF plus lipopolysaccharide group. lipopolysaccharide was used to induce microglial activation, a critical component of neuroinflammation.23,24 In addition, we have shown systemic lipopolysaccharide application induced proinflammatory mediator production in rodent brain.21 For the immunostaining study of p65 nuclear translocation, the cells were pretreated with 1 ng/ml recombinant rat GDNF (catalogue number: 512-GF; R&D Systems) for 30 min and then stimulated with 5 ng/ml lipopolysaccharide (catalogue number: L4641; Sigma-Aldrich) for 1 h in the presence or absence of GDNF. The cells were immediately fixed for immunostaining. To measure the IL-1β production, the C8-B4 cells were pretreated with 1 ng/ml GDNF for 30 min and then stimulated with 5 ng/ml lipopolysaccharide (5 ng/ml) for 2 h in the presence or absence of GDNF. The culture medium was harvested at 24 h after the lipopolysaccharide stimulation for measuring IL-1β. In these experiments, cells were kept in their usual culture medium but without 10% FBS during the exposure to GDNF and lipopolysaccharide. This FBS-free medium was replaced by the usual culture medium once the exposure was finished.
Immunofluorescence staining of p65 in microglial cultures
As described previously,9,25 the cells were fixed with iced cold methanol for 20 min at −20 °C and washed with PBS for 5 min twice. The fixed cells were then permeabilized with 0.5% Triton X-100 in PBS for 1 h at room temperature. After being washed in PBS three times, the cells were incubated in 10% donkey serum and 1% bovine serum albumin in PBS for 2 h at room temperature and then at 4°C overnight with mouse monoclonal anti-p65 antibody (1:250 dilution, catalogue number: 6956; Cell signaling Technology). After rinsed in PBS for three times, the donkey anti-mouse IgG antibody conjugated with NL493 (1:200 dilution, catalogue number: NL009; R&D Systems) were incubated with the cells for 1 h at room temperature in the dark and then washed with PBS. Cells were counterstained with Hoechst 33342 (Catalogue number: 62249; Thermo Scientific), rinsed, and mounted with Vectashield mounting medium (H-1000, Vector labs, Burlingame, CA). The number of cells with p65 nuclear translocation was determined under a fluorescence microscope in a blinded fashion. Cell count was obtained from 5 microscopic fields randomly selected in one well. Three wells were counted for each experimental condition. In this way, more than 200 cells for each condition were counted in each experiment. The experiment was repeated 6 times with different sets of cells.
ELISA of IL-1β in the culture medium
IL-1β secreted into culture medium collected after 24 h of lipopolysaccharide treatment was measured with Quantikine ELISA kit (catalogue number: RLB00; R&D Systems) as we described before.22
Statistical analysis
Parametric results in normal distribution are presented as mean ± S.D. (n ≥ 6). The data from the training sessions of Barnes maze test within the same group were tested by one-way repeated measures analysis of variance followed by Tukey test. The data from the training sessions of Barnes maze test between groups were tested by two-way repeated measures analysis of variance followed by Tukey test. All other data were analyzed by one-way analysis of variance followed by the Tukey test if the data were normally distributed or by one-way analysis of variance on ranks followed by the Tukey test if the data were not normally distributed. These non-normally distributed data were presented as box plots in the figures. Differences were considered significant at P < 0.05 based on two-tailed hypothesis testing. All statistical analyses were performed with SigmaStat (Systat Software, Inc., Point Richmond, CA, USA).
Results
No animal died during the surgery or the intended observation period after the surgery. Data from all animals were included for analysis and reported here.
Amantadine attenuated surgery-induced learning and memory impairment
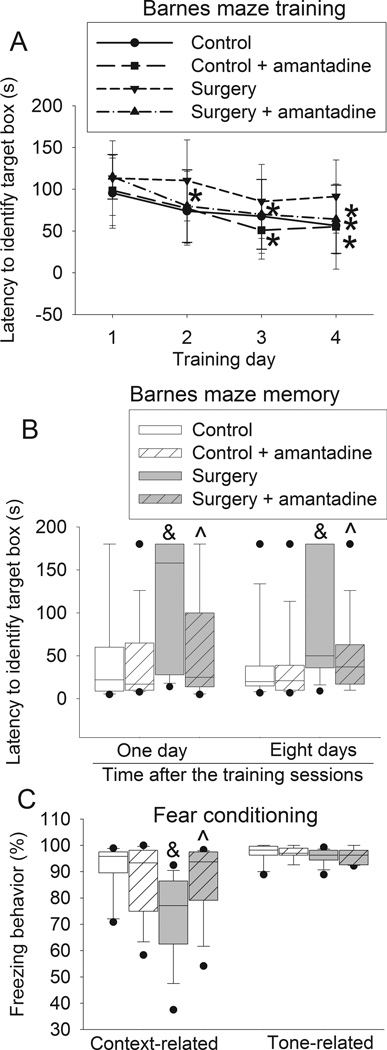
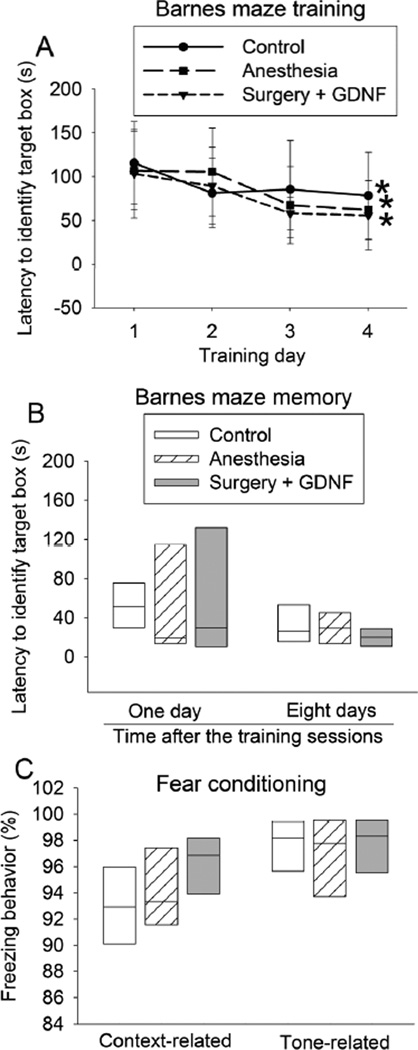
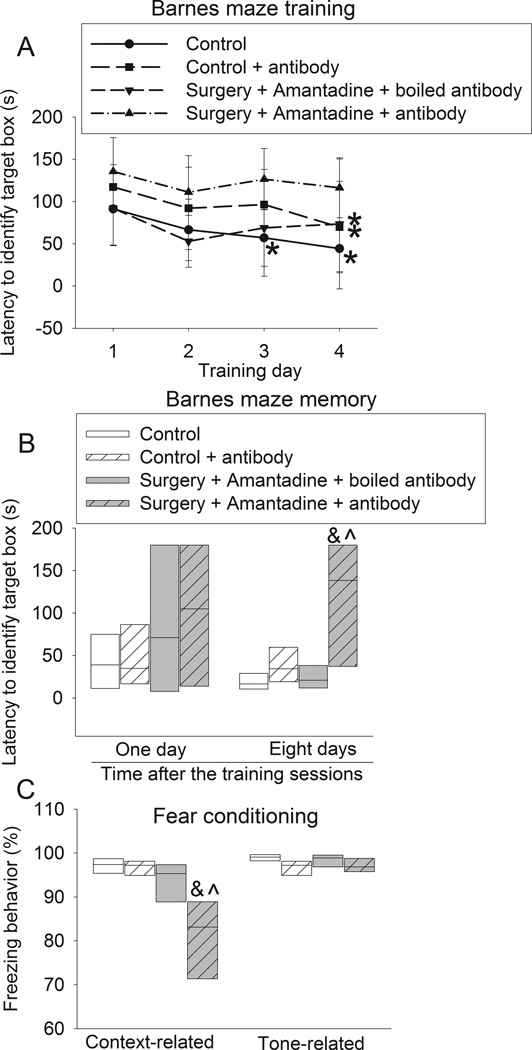
The time to identify the target box during the 4-day training sessions of Barnes maze test was reduced with increased training sessions in the control rats, rats received anesthesia only, rats received amantadine only and rats received surgery plus amantadine. This time on day 4 was significantly shorter than that on day 1 for these four groups of rats. This effect was not apparent in the rats after surgery alone. Surgery had a significant effect on the time needed to identify the target box in the training sessions [F(1,28) = 5.625, P = 0.025]. This effect was abolished by amantadine [F(1,28) = 0.840, P = 0.367; compared with control group]. Amantadine or anesthesia only did not have a significant effect on the time to identify the target box during the training sessions [F(1,28) = 0.063, P = 0.804; F(1, 14) = 0.074, P + 0.790] (Figs. 1 and 2). When the rats were tested 1 day after the training sessions, the time to identify the target box for the rats subjected to surgery was longer than that for the control rats. This prolongation was attenuated by amantadine. A similar change pattern occurred when the test was performed 8 days after the training sessions. However, anesthesia and amantadine alone did not affect the time to identify the target box whether the test was performed 1 day or 8 days after the training sessions (Fig. 1B and 2B).
Fig. 1. Amantadine attenuated surgery-induced learning and memory impairment.
Four-month old Fischer 344 rats were subjected to right carotid exploration under intravenous anesthesia with or without the treatment of amantadine. Barnes maze training sessions started 1 week after the surgery. A: Barnes maze training sessions. Results are mean ± S.D. (n = 15). * P < 0.05 compared with the corresponding data on day 1. B: Barnes maze memory phase. C: fear conditioning. Results in panels B and C are in box plot format (n = 15). ●: lowest or highest score (the score will not show up if it falls in the 95th percentile); between lines: 95th percentile of the data; inside boxes: 25th to 75th percentile including the median of the data. & P < 0.05 compared with the control group, ^ P < 0.05 compared with surgery alone group.
Fig. 2. Glial cell line-derived neurotrophic factor (GDNF) attenuated surgery-induced learning and memory impairment.
Four-month old Fischer 344 rats were subjected to right carotid exploration under intravenous anesthesia. Some rats received intra-cerebroventricular injection of GDNF. Barnes maze training sessions started 1 week after the surgery. A: Barnes maze training sessions. Results are mean ± S.D. (n = 8). * P < 0.05 compared with the corresponding data on day 1. B: Barnes maze memory phase. C: fear conditioning. Results in panels B and C are in box plot format (n = 8). Inside boxes: 25th to 75th percentile including the median of the data.
Rats in the surgery group but not in the anesthesia only group or amantadine group had less context-related freezing behavior in the fear conditioning test than control rats. This surgical effect was abolished by amantadine (Fig. 1C). There was no difference in the tone-related freezing behavior among the control rats, rats received amantadine, rats received surgery and rats received surgery plus amantadine (Fig. 1C and 2C).
Amantadine attenuated surgery-induced neuroinflammation
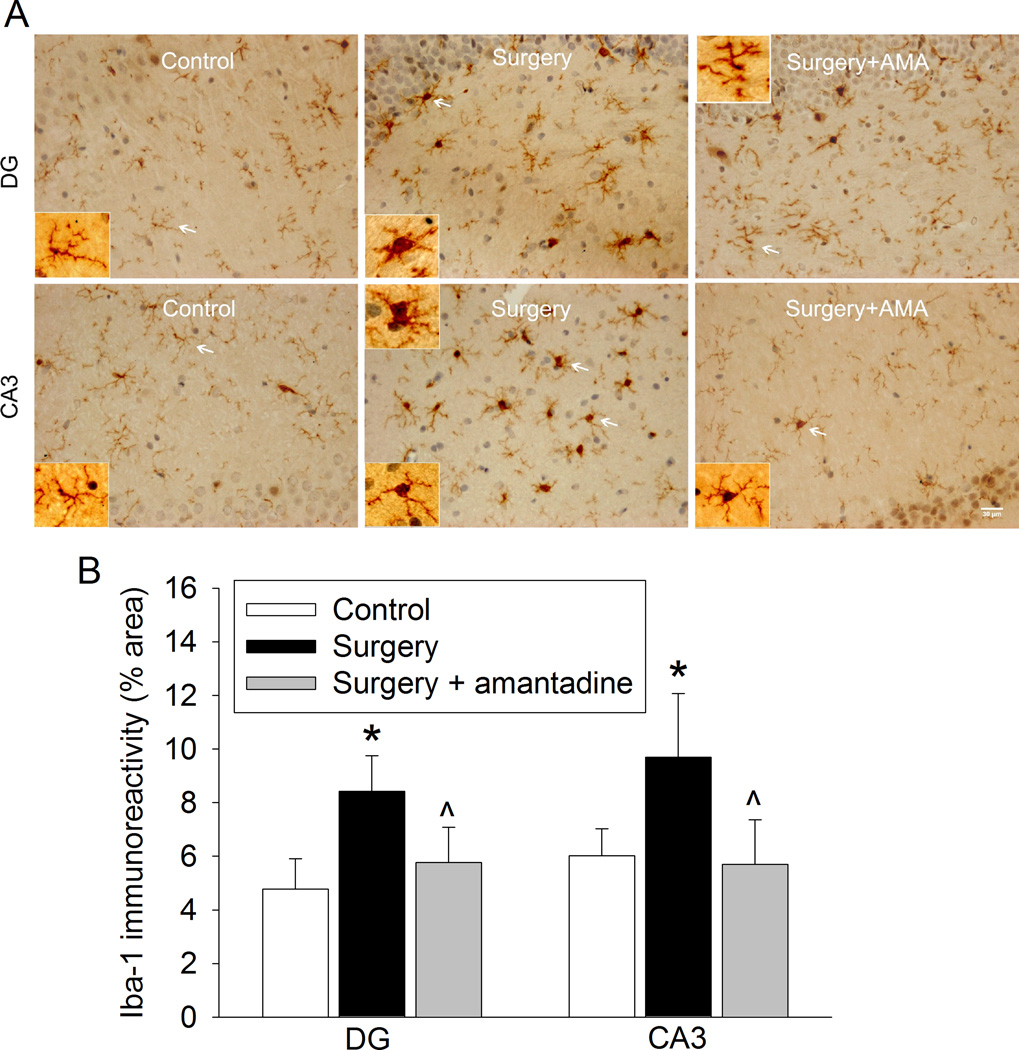
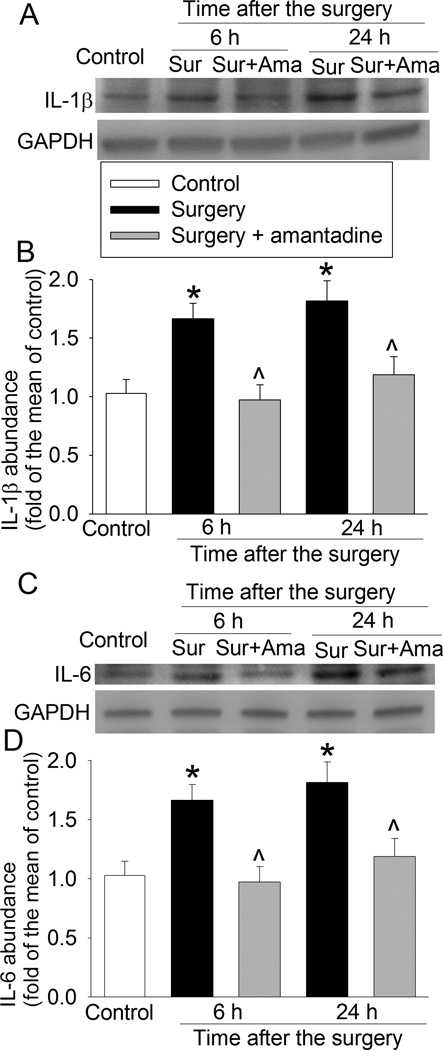
The expression of Iba-1 (a microglial marker), IL-1β and IL-6 in the hippocampus was significantly increased at 6 and 24 h after the surgery. These increases were abolished by amantadine (Figs. 3 and 4). Similarly, Iba-1 expression in the hippocampal dentate gyrus region was also increased at 10 days after the surgery and this increase was blocked by amantadine (Fig. 5). These results suggest that surgery induces neuroinflammation that was inhibited by amantadine.
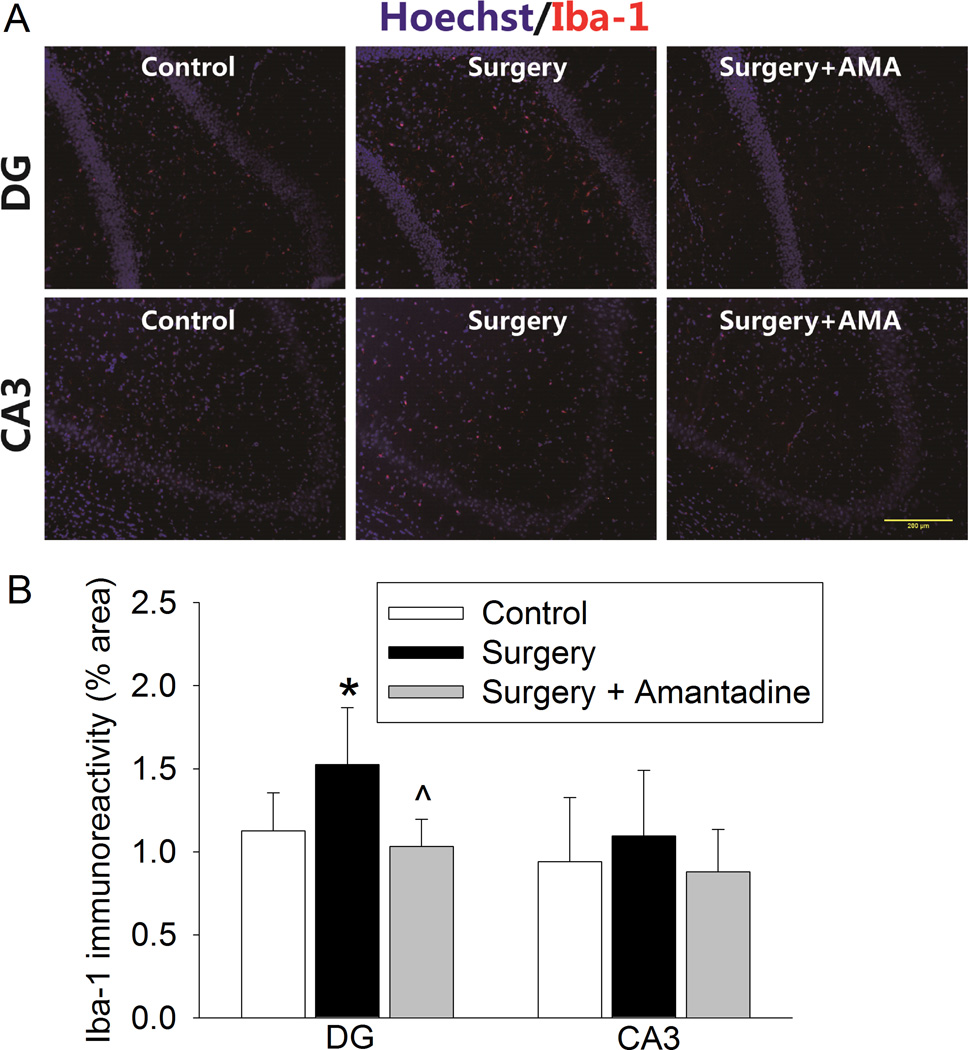
Fig. 3. Amantadine inhibited surgery-induced ionized calcium binding adapter molecule 1 (Iba-1) expression at one day after the surgery.
Four-month old Fischer 344 rats were subjected to right carotid exploration under intravenous anesthesia with or without the treatment of amantadine. Hippocampus was harvested at 24 h after the surgery. A: representative immunostaining images for Iba-1. Scale bars = 30 µm. B: graphic presentation of the percentage area that is Iba-1-postive staining in the CA3 and dentate gyrus (DG). Values are presented as mean ± S.D. (n = 6). * P < 0.05 compared with the control group, ^ P < 0.05 compared with surgery alone group.
Fig. 4. Amantadine inhibited surgery-induced interleukin (IL)-6 and IL-1β expression.
Four-month old Fischer 344 rats were subjected to right carotid exploration under intravenous anesthesia with or without the treatment of amantadine. Hippocampus was harvested at 6 h or 24 h after surgery. A: representative Western blot images of IL-1β. B: graphic presentation of IL-1β abundance. C: representative Western blot images of IL-6. D: graphic presentation of IL-6 abundance. Values are expressed as fold changes over the mean values of control rats and are presented as mean ± S.D. (n = 6). * P < 0.05 compared with the control group, ^ P < 0.05 compared with corresponding surgery only group. Ama: amantadine, GAPDH: glyceraldehyde 3-phosphate dehydrogenase, Sur: Surgery.
Fig. 5. Amantadine inhibited surgery-induced ionized calcium binding adapter molecule 1 (Iba-1) expression at ten days after the surgery.
Four-month old Fischer 344 rats were subjected to right carotid exploration under intravenous anesthesia with or without the treatment of amantadine. Hippocampus was harvested at 10 days after the surgery. A: representative immunostaining images. Scale bars = 200 µm. B: graphic presentation of the percentage area that is Iba-1-postive staining in the CA3 and dentate gyrus (DG). Values are presented as mean ± S.D. (n = 6). * P < 0.05 compared with the control group, ^ P < 0.05 compared with surgery alone group. AMA: amantadine.
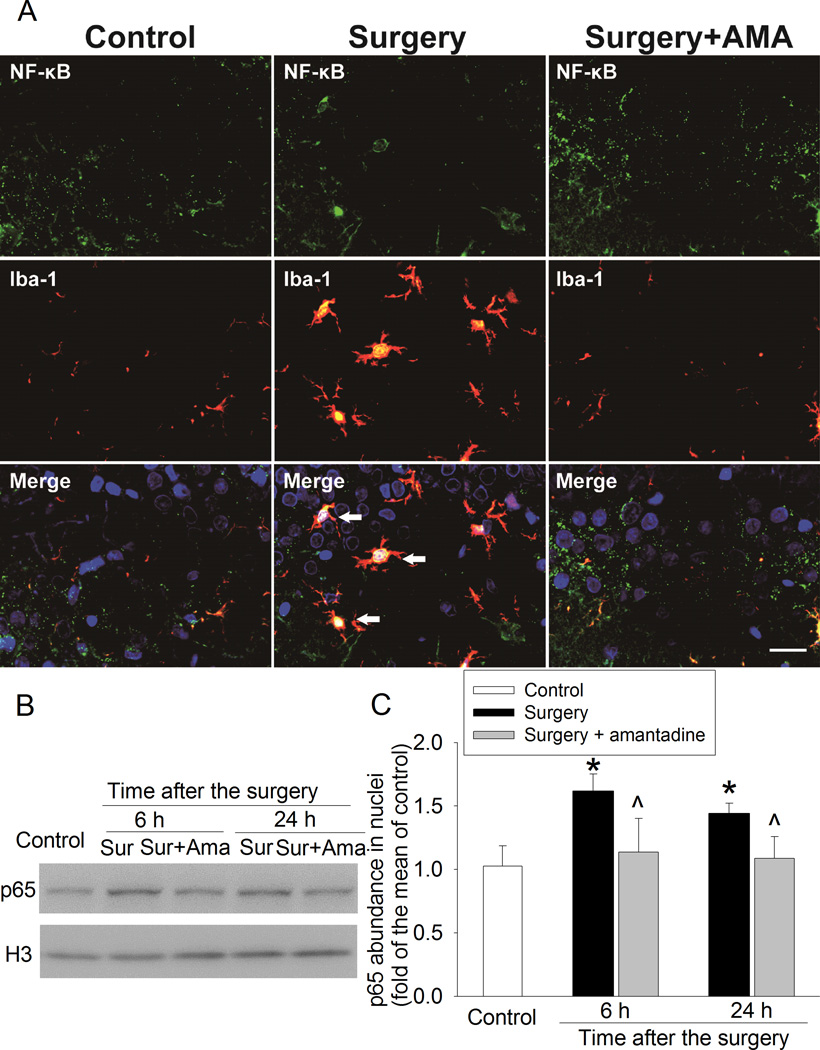
Surgery induced translocation of p65, a nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) component, into nuclei of the microglia in the hippocampus. This effect was inhibited by amantadine (Fig. 6).
Fig. 6. Amantadine inhibited surgery-induced nuclear translocation of p65.
Four-month old Fischer 344 rats were subjected to right carotid exploration under intravenous anesthesia with or without the treatment of amantadine. Hippocampus was harvested at 6 h or 24 h after surgery. A: representative immunostaining images of the hippocampal CA3 region at 24 h after the surgery. Scale bars = 30 µm. B: representative Western blot images. C: graphic presentation of p65 abundance in nuclei. Values are expressed as fold changes over the mean values of control rats and are presented as mean ± S.D. (n = 6). * P < 0.05 compared with control group, ^ P < 0.05 compared with corresponding surgery group. Ama: amantadine, Iba-1: ionized calcium binding adapter molecule 1, NF-κB: nuclear factor κ-light-chain-enhancer of activated B cells, Sur: Surgery.
Amantadine increased the expression of GDNF that inhibited microglial activation
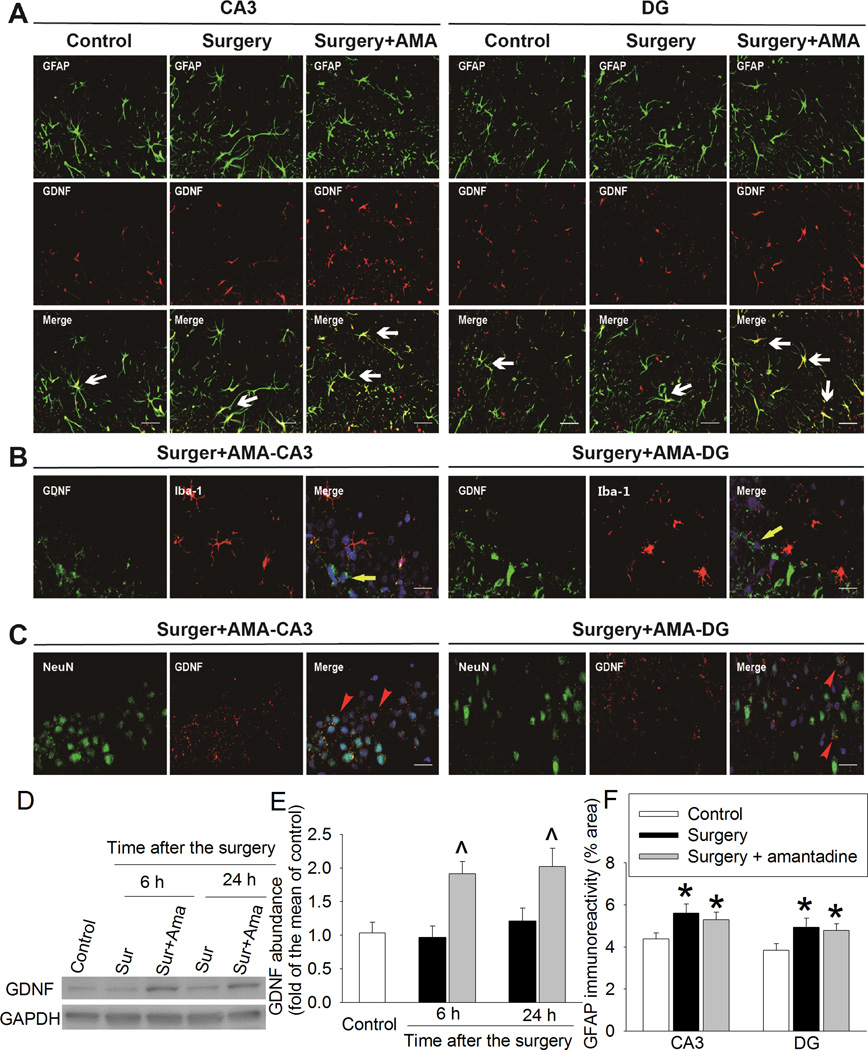
Amantadine significantly increased GDNF in the hippocampus (Fig. 7). GDNF was mainly co-localized with GFAP, an astrocytic marker, but was not co-localized with Iba-1 (Figs. 7A and 7B). Some GDNF appeared to be around NeuN, a neuronal marker (Fig. 7C). Surgery also increased GFAP but this increase was not affected by amantadine in the hippocampus (Figs. 7A and 7E).
Fig. 7. Amantadine increased glial cell line-derived neurotrophic factor (GDNF) expression.
Four-month old Fischer 344 rats were subjected to right carotid exploration under intravenous anesthesia with or without the treatment of amantadine. Hippocampus was harvested at 6 h or 24 h after surgery. A, B and C: representative immunostaining images of hippocampus harvested at 24 h after surgery. Scale bars = 30 µm. D: representative Western blot images. E: graphic presentation of GDNF abundance. Values are expressed as fold changes over the mean values of control rats and are presented as mean ± S.D. (n = 6). ^ P < 0.05 compared with corresponding surgery alone group. F: graphic presentation of the percentage area that is glial fibrillary acidic protein (GFAP)-positive staining in the CA3 and dentate gyrus (DG) at 24 h after the surgery. Values are presented as mean ± S.D. (n = 6). * P < 0.05 compared with the control group. Ama: amantadine, GAPDH: glyceraldehyde 3-phosphate dehydrogenase, Iba-1: ionized calcium binding adapter molecule 1, Sur: Surgery.
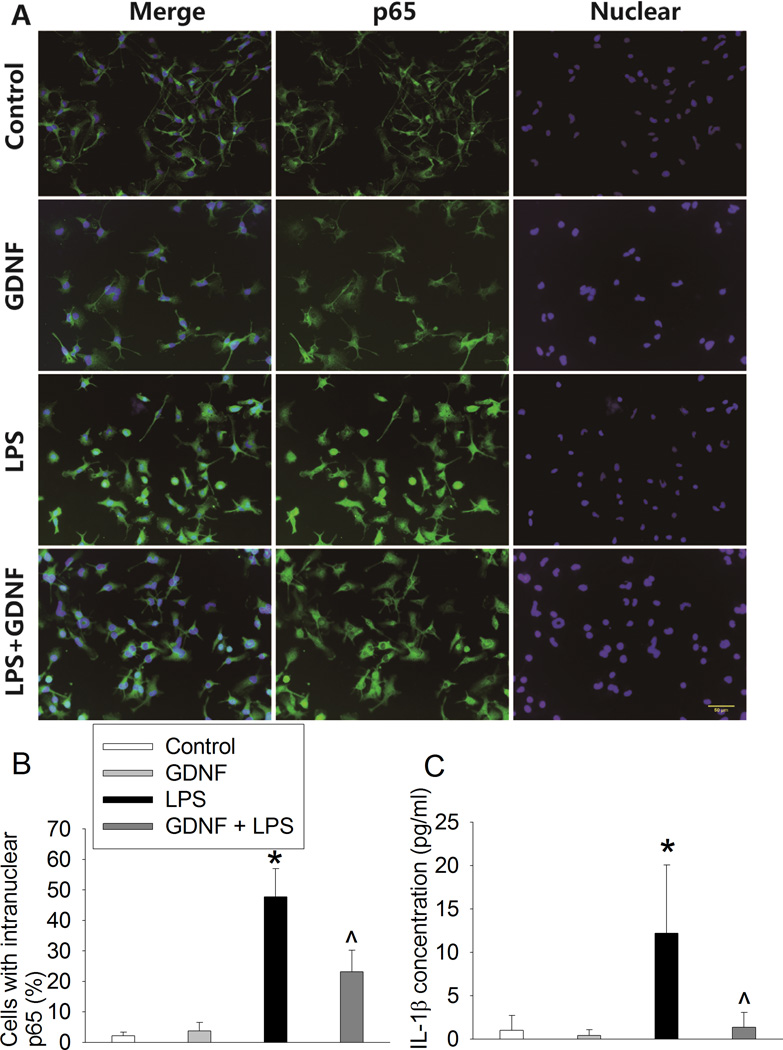
Similar to surgery, lipopolysaccharide also increased nuclear translocation of p65 in the C8-B4 cells and IL-1β production from these cells. These effects were attenuated by GDNF (Fig. 8).
Fig. 8. Glial cell line-derived neurotrophic factor (GDNF) inhibited lipopolysaccharide (LPS)-induced microglial activation.
C8-B4 cells were pretreated with 1 ng/ml GDNF for 30 min before being exposed to 5 ng/ml lipopolysaccharide. A: representative immunostaining images of the cells after being exposed to lipopolysaccharide for 1 h. Nuclei were stained by Hoechst 33342. Scale bars = 50 µm. B: graphic presentation of the percentage cells that are positive staining of intra-nuclear p65 after being exposed to lipopolysaccharide for 1 h. C: interleukin (IL)-1β concentrations in culture medium that was harvested at 24 h after the cells were incubated with lipopolysaccharide for 2 h. Values are presented as mean ± S.D. (n = 6). * P < 0.05 compared with the control group, ^ P < 0.05 compared with the group treated with lipopolysaccharide only.
Amantadine-induced attenuation of learning and memory impairment after surgery was inhibited by anti-GDNF antibody
Similar to the control rats, rats in antibody only group and the surgery plus amantadine plus boiled antibody group had a decreased time to find the target box with increased training sessions. This time on the training day 4 was shorter than that on training day 1 for these two groups of rats. This effect was not apparent for rats in the surgery plus amantadine plus anti-GDNF antibody group. The anti-GDNF antibody was found to have a significant effect on the time to identify the target box during the training sessions [F(1,14) = 19.009, P < 0.001; compared with control) (Fig. 9A). The time to identify the target box on day 1 after the training session was not different among control rats, rats received antibody, rates received surgery plus amantadine plus anti-GDNF antibody or rats received surgery plus amantadine plus boiled antibody. However, rats subjected to surgery plus amantadine plus anti-GDNF antibody required much longer time than control rats or rats received surgery plus amantadine plus boiled antibody to identify the target box on day 8 after the training sessions (Fig. 9B).
Fig. 9. Anti-glial cell line-derived neurotrophic factor (GDNF) antibody blocked the effects of amantadine on learning and memory after surgery.
Four-month old Fischer 344 rats were subjected to right carotid exploration under intravenous anesthesia with or without the treatment of amantadine. Some rats received intra-cerebroventricular injection of an anti-GDNF antibody. Barnes maze training sessions started 1 week after the surgery. A: Barnes maze training sessions. Results are mean ± S.D. (n = 8). * P < 0.05 compared with the corresponding data on day 1. B: Barnes maze memory phase. C: fear conditioning. Results in panels B and C are in box plot format (n = 8). Inside boxes: 25th to 75th percentile including the median of the data. & P < 0.05 compared with the control group, ^ P < 0.05 compared with surgery plus amantadine plus boiled antibody group.
Similarly, rats subjected to surgery plus amantadine plus anti-GDNF antibody also had less context-related freezing behavior than control rats or rats received surgery plus amantadine plus boiled antibody in the fear conditioning test. However, the tone-related freezing behavior was not different among the three groups (Fig. 9C).
Surgery-induced learning and memory impairment was blocked by GDNF
The time for rats received surgery plus GDNF to find the target box on training day 4 of Barnes maze test was shorter than that on training day 1 (Fig. 2A). The time for those rats to identify the target box on day 1 and day 8 after the training sessions was not different from that of control rats (Fig. 2B). Similarly, there was no difference between the control rats and rats received surgery plus GDNF in the context- and tone-related freezing behavior (Fig. 2C).
Discussion
We performed right carotid artery exposure, a necessary surgical step for carotid endarterectomy. We did not clamp the vessel and paid special attention not to damage the vagus nerve. This design is to simulate clinical surgical stimulation but avoid inducing brain ischemia. One week was elapsed between the surgery and the beginning of learning and memory tests to avoid influence of pain in these tests. Our study showed that surgery but not propofol-based general anesthesia induced learning and memory impairment because rats in the surgery group did not have a significant reduction in time to find the target box with the increased number of training sessions and took longer time than control rats to identify the target box in the long-term spatial reference memory test in the Barnes maze. They also had less freezing behavior than control rats in the fear conditioning test. Since the rats subjected to surgery had decreased context- and tone-related fear conditioning, surgery may have impaired hippocampus-dependent and hippocampus-independent learning and memory.26 These results validate our surgical model for use in studying POCD.
Our results clearly showed that amantadine abolished the learning and memory impairment caused by surgery. This finding is the initial evidence for a possible therapeutic effect of amantadine on POCD. Amantadine is known to have neuroprotective effects.6,7 Early studies focused on its effects on neurons, such as blocking N-methyl-D-aspartate receptors, to explain the neuroprotective effects of amantadine.27,28 Recent studies suggest that amantadine can reduce neuroinflammation induced by lipopolysaccharide in cell cultures or in mice.8,13 We and others have shown that neuroinflammation may be the underlying pathology for the cognitive impairment after anesthesia and surgery.11,12 Thus, we proposed that amantadine could reduce cognitive impairments after surgery and designed this study to test this possibility.
Consistent with the neuroinflammation theory, the surgery increased the amount of Iba-1 in the microglia of hippocampus, suggesting microglial activation. This increase still existed at 10 days after the surgery when learning and memory were found to be impaired in these rats. Surgery also increased the levels of IL-6 and IL-1β, two inflammatory cytokines, and the translocation of p65 into the nuclei of microglia. p65 is a component of NF-κB, a transcription factor. The components of NF-κB are usually in the cytosol when they bind with their inhibitors. NF-κB is activated when its components are separated from the inhibitors and translocated into nuclei to upregulate the expression of various inflammatory mediators including cytokines.19,29 Thus, our results suggest that surgery induces neuroinflammation in the hippocampus and that activation of NF-κB participates in this process. Our results also indicate that amantadine blocks surgery-induced neuroinflammation because the increased Iba-1, IL-6 and IL-1β as well as the nuclear translocation of NF-κB after surgery were abolished by amantadine.
Very little is known on how amantadine can reduce neuroinflammation. Amantadine can induce GDNF production8 and GDNF can reduce Zymosan A-induced activation of microglial cultures.9 Thus, GDNF may be a possible mediator for the anti-neuroinflammatory effects of amantadine. Consistent with this possibility, we showed that amantadine increased GDNF that was mostly from astrocytes in the rat hippocampus. GDNF reduced lipopolysaccharide-induced p65 nuclear translocation and IL-1β production in the microglial cultures. These results provide initial evidence for the role of GDNF in mediating the anti-neuroinflammatory effects of amantadine.
Since neuroinflammation may be the key process for surgery-induced cognitive impairment11,12 and GDNF may be a mediator for the amantadine effects on neuroinflammation, it is possible that GDNF plays a role in amantadine-induced attenuation of cognitive impairment after surgery. This possibility was supported by the results that an anti-GDNF antibody but not the boiled/denatured antibody reversed the effects of amantadine on learning and memory after the surgery. Also, antibody alone did not affect the learning and memory of the rats. In addition, GDNF blocked surgery-induced cognitive impairment. Taken together, our results support the theory that amantadine induces the production of GDNF that then inhibits neuroinflammation to reverse surgery-induced learning and memory impairments.
Our results showed that surgery increased GFAP and that this increase was not affected by amantadine. The significance of this increase is not known from our study.
Our study has limitations. We subjected rats to a relatively minor surgery. It is not known whether amantadine is effective to reduce neuroinflammation and cognitive impairment after a major surgery. Also, we used male animals in this study to reduce potential influence of fluctuating estrogen and progesterone concentrations on learning and memory in female rats. The effects of amantadine on surgery-induced cognitive impairment in females are not known. Finally, our results establish the role of GDNF in amantadine-induced attenuation of neuroinflammation and cognitive impairment after surgery. However, it is not known whether the direct protective effects of amantadine on neurons play a role in its improvement of cognitive impairment after surgery.
In summary, we have shown that adult male rats have learning and memory impairment after right carotid exposure. This surgery also induces neuroinflammation. Amantadine reduces these surgical effects and increases GDNF in the brain. GDNF reduces inflammatory cytokine production from microglia and antagonizing GDNF reverses the effects of amantadine on surgery-induced cognitive dysfunction. These results suggest that amantadine attenuates surgery-induced cognitive dysfunction via GDNF.
Acknowledgments
Grant support: This study was supported by grants (R01 GM065211 and R01 GM098308 to Z Zuo) from the National Institutes of Health, Bethesda, MD, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, OH, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, MD, and the Robert M. Epstein Professorship endowment, University of Virginia, Charlottesville, VA.
Footnotes
Competing interests: The authors declare no competing interests.
Centers for Disease Control and Prevention/National Center for Health Statistics: National hospital discharge survey, 2010 http://www.cdc.gov/nchs/fastats/insurg.htm, accessed on January 15, 2014.
References
- 1.Terrando N, Brzezinski M, Degos V, Eriksson LI, Kramer JH, Leung JM, Miller BL, Seeley WW, Vacas S, Weiner MW, Yaffe K, Young WL, Xie Z, Maze M. Perioperative cognitive decline in the aging population. Mayo Clin. Proc. 2011;86:885–893. doi: 10.4065/mcp.2011.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 3.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 4.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N. Engl. J. Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 5.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–555. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 6.Diaz NL, Waters CH. Current strategies in the treatment of Parkinson's disease and a personalized approach to management. Expert Rev Neurother. 2009;9:1781–1789. doi: 10.1586/ern.09.117. [DOI] [PubMed] [Google Scholar]
- 7.Giacino JT, Whyte J, Bagiella E, Kalmar K, Childs N, Khademi A, Eifert B, Long D, Katz DI, Cho S, Yablon SA, Luther M, Hammond FM, Nordenbo A, Novak P, Mercer W, Maurer-Karattup P, Sherer M. Placebo-controlled trial of amantadine for severe traumatic brain injury. N. Engl. J. Med. 2012;366:819–826. doi: 10.1056/NEJMoa1102609. [DOI] [PubMed] [Google Scholar]
- 8.Ossola B, Schendzielorz N, Chen SH, Bird GS, Tuominen RK, Mannisto PT, Hong JS. Amantadine protects dopamine neurons by a dual action: reducing activation of microglia and inducing expression of GDNF in astroglia [corrected] Neuropharmacology. 2011;61:574–582. doi: 10.1016/j.neuropharm.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocha SM, Cristovao AC, Campos FL, Fonseca CP, Baltazar G. Astrocyte-derived GDNF is a potent inhibitor of microglial activation. Neurobiol. Dis. 2012;47:407–415. doi: 10.1016/j.nbd.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann. Neurol. 2010;68:360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao L, Li L, Lin D, Zuo Z. Isoflurane induces learning impairment that is mediated by interleukin 1beta in rodents. PLoS One. 2012;7:e51431. doi: 10.1371/journal.pone.0051431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Lee HW, Hwang J, Kim J, Lee MJ, Han HS, Lee WH, Suk K. Microglia-inhibiting activity of Parkinson's disease drug amantadine. Neurobiol. Aging. 2012;33:2145–2159. doi: 10.1016/j.neurobiolaging.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Bido S, Marti M, Morari M. Amantadine attenuates levodopa-induced dyskinesia in mice and rats preventing the accompanying rise in nigral GABA levels. J. Neurochem. 2011;118:1043–1055. doi: 10.1111/j.1471-4159.2011.07376.x. [DOI] [PubMed] [Google Scholar]
- 15.Lapchak PA, Jiao S, Collins F, Miller PJ. Glial cell line-derived neurotrophic factor: distribution and pharmacology in the rat following a bolus intraventricular injection. Brain Res. 1997;747:92–102. doi: 10.1016/s0006-8993(96)01265-6. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Zyang X, Wang Y, Ji H, Du Y, Liu H. DAPT protects brain against cerebral ischemia by down-regulating the expression of Notch 1 and Nuclear factor kappa B in rats. Neurol Sci. 2012;33:1257–1264. doi: 10.1007/s10072-012-0948-6. [DOI] [PubMed] [Google Scholar]
- 17.Lin D, Cao L, Wang Z, Li J, Washington JM, Zuo Z. Lidocaine attenuates cognitive impairment after isoflurane anesthesia in old rats. Behav. Brain Res. 2012;228:319–327. doi: 10.1016/j.bbr.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin D, Zuo Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology. 2011;61:1354–1359. doi: 10.1016/j.neuropharm.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Yin J, Li L, Deng J, Feng C, Zuo Z. Isoflurane postconditioning reduces ischemiainduced nuclear factor-kappaB activation and interleukin 1beta production to provide neuroprotection in rats and mice. Neurobiol. Dis. 2013;54:216–224. doi: 10.1016/j.nbd.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, Jonsson Fagerlund M, Charo IF, Akassoglou K, Maze M. Resolving postoperative neuroinflammation and cognitive decline. Ann. Neurol. 2011;70:986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Li L, Zuo Z. Delayed treatment with isoflurane attenuates lipopolysaccharide and interferon γ-induced activation and injury of mouse microglial cells. Anesthesiology. 2009;111:566–573. doi: 10.1097/ALN.0b013e3181af5b3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong HJ, Lin D, Li L, Zuo Z. Delayed treatment with lidocaine reduces mouse microglial cell injury and cytokine production after stimulation with lipopolysaccharide and interferon gamma. Anesth. Analg. 2012;114:856–861. doi: 10.1213/ANE.0b013e3182460ab5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol. Neurobiol. 2003;27:325–355. doi: 10.1385/MN:27:3:325. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Kim JA, Zuo Z. Isoflurane preconditioning reduces mouse microglial activation and injury induced by lipopolysaccharide and interferon-gamma. Neurosci. 2008;154:1002–1008. doi: 10.1016/j.neuroscience.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang J, Zheng LT, Ock J, Lee MG, Kim SH, Lee HW, Lee WH, Park HC, Suk K. Inhibition of glial inflammatory activation and neurotoxicity by tricyclic antidepressants. Neuropharmacology. 2008;55:826–834. doi: 10.1016/j.neuropharm.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 26.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 27.Blanpied TA, Clarke RJ, Johnson JW. Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J. Neurosci. 2005;25:3312–3322. doi: 10.1523/JNEUROSCI.4262-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weller M, Marini AM, Finiels-Marlier F, Martin B, Paul SM. MK-801 and memantine protect cultured neurons from glutamate toxicity induced by glutamate carboxypeptidase-mediated cleavage of methotrexate. Eur. J. Pharmacol. 1993;248:303–312. doi: 10.1016/0926-6917(93)90004-a. [DOI] [PubMed] [Google Scholar]
- 29.Gilmore TD. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]