Abstract
Aldosterone is principally synthesized in the zona glomerulosa of the adrenal by a series of enzymatic reactions leading to the conversion of cholesterol to aldosterone. Angiotensin II (Ang II) is the major physiological regulator of aldosterone production acting acutely to stimulate aldosterone biosynthesis and chronically to increase the capacity of the adrenals to produce aldosterone. We previously defined eight transcription factors that are rapidly induced following Ang II treatment using three in vitro adrenocortical cell models. Herein, we investigated the function of these transcription factors in the regulation of the enzymes needed for aldosterone production. H295R adrenal cells were co-transfected with expression vectors for each transcription factor and promoter/reporter constructs prepared for genes encoding the enzymes needed for aldosterone production. NGFI-B family members induced promoter activity of 3-beta-hydroxysteroid-dehydrogenase type 2 (HSD3B2), 21-hydroxylase (CYP21), and aldosterone synthase (CYP11B2). The importance of NGFI-B in the regulation of CYP11B2 was also demonstrated by reduced CYP11B2 transcription in the presence of a dominant-negative (DN)-NGFI-B. A pharmacological approach was used to characterize the Ang II pathways regulating transcription of NGFI-B family genes. Transcription of NGFI-B members were decreased following inhibition of Ang II type 1 receptor (AT1R), protein kinase C (PKC), calcium/calmodulin-dependent kinases (CaMK), and Src tyrosine kinase (SRC). Taken together, these results suggest that Ang II binding to the AT1R increases activity of PKC, CaMK, and SRC, which act to increase expression of the family of NGFI-B genes as well as CYP11B2. Ang II induction of the NGFI-B family members represents an important pathway to increase the capacity of adrenal cells to produce aldosterone.
Keywords: Adrenal cortex, angiotensin, aldosterone, transcription factors, NGFI-B, CYP11B2
Introduction
Ang II is a major regulator of salt and water balance through regulation of aldosterone synthesis in the zona glomerulosa of the adrenal cortex. Aldosterone biosynthesis is regulated at two steps: 1) an early rate-limiting step that involves the transport of cholesterol into the mitochondria via the action of steroidogenic acute regulatory (StAR) protein and, 2) a late rate-limiting step that involves conversion of corticosterone to aldosterone by the enzyme CYP11B2 (Muller 1995, Quinn & Williams 1988). StAR is stimulated by Ang II both in vitro (Betancourt-Calle et al. 2001, Cherradi et al. 1997, Clark et al. 1995, Tremblay et al. 1992) and in vivo (Lehoux et al. 1999, Peters et al. 1998). Ang II also stimulates the expression of steroidogenic enzymes in vitro, which are involved in subsequent steps of aldosterone synthesis, including cholesterol side-chain cleavage (SCC or CYP11A1) (Bird et al. 1996), HSD3B2 (Rainey et al. 1991), and CYP21 (Bird et al. 1998). Finally, Ang II stimulates the last regulatory step in the control of aldosterone production: the transcription of CYP11B2, an enzyme almost uniquely localized in the zona glomerulosa of the adrenal gland (Adler et al. 1993, Clyne et al. 1997, Shibata et al. 1991).
Adrenal glomerulosa cell stimulation occurs predominantly through Ang II activation of AT1R via a q-subunit of G-protein coupled receptors (Gq), activation of inositol trisphosphate/Ca2+ signaling and PKC isoforms (Hunyady & Catt 2006). G protein stimulation also leads to activation of CaMK (Chabre et al. 1995, Cote et al. 1998), and mitogen-activated protein kinase (MAPK) members, such as mitogen-activated protein kinase kinase - extracellular signal-regulated kinase 1 and 2 (MEK-ERK1/2). Ang II can also act through G protein-independent pathways including β-arrestins, tyrosine kinases, and Janus kinase (JAK)/signal transduction and activators of transcription (STAT) pathways (Miura et al. 2004, pathways culminates with rapid induction of adrenal cell transcription for numerous response genes (Nogueira et al. 2007, Romero et al. 2004). Herein, we extend our earlier study that defined the transcription factors regulated by Ang II stimulation in adrenocortical cell models from three different species: human, bovine, and rat (Nogueira et al. 2007). The common genes across these three species include eight transcription factors: members of the nuclear receptor superfamily/nerve growth factor-induced (NGFI-B) family [NGFI-B (also named NR4A1), NURR-1 (also named NR4A2), and NOR-1 (also named NR4A3)], members of the AP-1 complex (FOS, FOSB, and JUNB), the early growth response gene 1 (EGR1), and the activating transcription factor 3 (ATF3) (Nogueira et al. 2007). These findings confirm with the studies performed by Romero and colleagues, who have also studied Ang II responsive genes in H295R cells (Romero et al. 2004). In the present study we examined the role of these transcription factors in the regulation of the enzymes involved in adrenal aldosterone synthesis. In addition, we investigated the Ang II intracellular signaling pathways that control transcription of the NGFI-B genes. We showed that Ang II, through activation of PKC, CaMK, and SRC signaling increased expression of the three members of the NGFI-B transcription factor family which were able to enhance transcription of enzymes needed for the final three steps of the aldosterone biosynthesis pathway.
Materials and Methods
Cell culture and treatments
H295R human adrenocortical tumor cells were cultured in DME/Ham’s F12 medium (Invitrogen, Grand Island, NY) and supplemented with 10% cosmic calf serum (Hyclone, Logan, UT), 1% penicillin/streptomycin (Invitrogen) and 0.01% gentamicin (Invitrogen). Cells were maintained in a 37°C humidified atmosphere (5% CO2). For experiments, cells were subcultured onto 12 well culture dishes (Corning Costar, Corning, NY) at a density of 4 × 105 cells/well for subsequent transfection.
H295R-TR, an adrenal cell line containing a tetracycline response system, was generously provided by Dr. Enzo Lalli (Doghman et al. 2007). DN-NGFI-B was produced by truncating the 5′ transactivation region of the human NGFI-B sequence. DN-NGFI-B, lacking 78 bp to 1099 bp from the full-length NGFI-B cDNA, encoded a variant protein without the transactivation domain. This protein exhibits normal DNA binding capacity without mediating transcriptional activation, thus acting as an inhibitor for all three subfamily members: NGFI-B, NURR-1, and NOR-1. For generation of H295R-TR stably expressing DN-NGFI-B (H295R-TR-DN), cDNA of DN-NGFI-B was amplified by PCR with forward and reverse primers: 5″-CACCATGTCAACCAAGGCCCG-3′ and 5′-GAGAGTGCGCATGTGCACACG-3′, respectively. The PCR product was ligated into the Invitrogen pLENTI6/V5 TOPO vector (Invitrogen) and packaged into a virus construct using Invitrogen’s ViraPower™ Lentiviral Gateway Expression System (Invitrogen). Briefly, 293T cells were co-transfected with the pLENTI6/V5 TOPO vector with ligated DN-NGFI-B gene and packaging vectors for 6 h using Transfast (Promega, Madison, WI) according to manufacturer instructions. Transfection was stopped by adding 2X low serum medium (0.2% cosmic calf serum + 2% penicillin/streptomycin and 0.02% gentamicin). Twelve hours later medium was substituted with growth medium for 2 d to allow virus packaging. Medium was then collected for further infection. H295R-TR cells were infected overnight with a 1:2 dilution of virus containing medium harvested from 293T cells and stayed in antibiotic-free growth medium for another 24 h. Further antibiotic selection with 200 μg/ml zeocin (Gemini, West Sacramento, CA) was done for three weeks. In this study, we used a mixed population of cells containing a tetracycline-regulated DN-NGFI-B. Doxycycline (tetracycline analog) was used at a concentration of 0.25 μg/ml to control the expression of integrated genes. H295R-TR-DN cells were cultured in DME/Ham’s F12 medium (Invitrogen) and supplemented with 10% cosmic calf serum (Hyclone), 1% penicillin/streptomycin (Invitrogen), 0.01% gentamicin (Invitrogen), 5 μg/ml blasticidin (Invitrogen), 1% insulin-transferrin-selenous acid (BD Biosciences, Bedford, MA). Cells were subcultured onto 24 well culture dishes (Corning Costar) at a density of 2 × 105 cells/well for subsequent treatment. Cells were incubated for 6 h with or without 10 nM Ang II. Twelve hours prior to treatment, the H295-TR cells were maintained overnight in 1x low serum medium (DMEM/F12 medium supplemented with 0.1% Cosmic Calf serum medium, 1% penicillin/streptomycin, and 0.01% gentamicin). Experiments were repeated five times.
In order to determine if the transcription factors studied were direct targets of Ang II, H295R cells were pre-treated with 35 μM cycloheximide for 30 min, followed by treatment with 10 nM Ang II for 1 h. RNA was isolated from these cells and used for microarray analysis.
For analysis of Ang II intracellular signaling pathways, H295R cells were sub-cultured onto 24 well dishes at a density of 2 × 105 cells/well. For experiments cells were incubated for 30 min with the following cell signaling inhibitors: 10 μM PD98059 (ERK1/2 inhibitor), 1 μM GFX (or GF103209X - PKC inhibitor), 3 μM KN93 (CAMK inhibitor), SRC-I (SRC inhibitor), and 50 μM AG490 (Janus kinase 2 inhibitor), 10 μM losartan (AT1R inhibitor), 10 μM PD123319 (AT2R inhibitor). The inhibitor’s concentrations were selected based on screening experiments where three different concentrations were tested on H295R cells. KN93, SRC-I, AG490, and PD98059 were purchased from Calbiochem (Gibbstown, NJ); Losartan (DUP753) was purchased from DuPont Pharmaceutical (Wilmington, DE); PD123319 was purchased from Sigma-Aldrich (St. Louis, MO); and GFX was purchased from Invitrogen. After pre-treatment with inhibitors, cells were treated with 10 nM Ang II for 1 or 6 h.
Microarray analysis
RNA from H295R cells was hybridized to an Affymetrix human HG_U133 + 2 oligonucleotide microarray set containing 54,675 probe sets representing approximately 40,500 independent human genes. The arrays were scanned at high resolution in the microarray core facility at the Medical College of Georgia in Augusta, GA. Results were analyzed using GeneSpring software version 7.3 (Silicon Genetics, Redwood City, CA) to identify differences in gene expression after incubation with cycloheximide (30 min) followed by Ang II (1 h) comparing to cycloheximide (30 min) alone.
Adrenal cell transfection
H295R cells were transfected using Transfast reagent (Promega Corporation) following the manufacturer’s protocols. 1 μg of the pGL3-basic (pGL3b) plasmids containing luciferase reporter vectors linked to the promoter region of each of the steroidogenic enzymes involved in aldosterone synthesis, namely: StAR, CYP11A1, CYP21, HSD3B2, or CYP11B2, was used for each transfection experiment. The pGL3b vectors were individually co-transfected with 0.1 μg of the following transcription factors: ATF3, EGR1, NGFI-B, NURR-1, or NOR-1 in pCMV-XL5; and FOS, FOSB, or JUNB in pcDNA3.1-zeo. All experiments were repeated a minimum of five times.
In addition, H295R cells were co-transfected with combinations of transcription factors. In these experiments, 1 μg of the reporter vectors for the enzymes investigated in our study (StAR, CYP11A1, HSD3B2, CYP21, and CYP11B2) was co-transfected with different combinations of expression vectors for the transcription factors using a dose of 0.1 μg for each expression vector. In this study the expression vector for JUNB was co-transfected with the expression vector for FOS, FOSB, or ATF3.
In the DN-NGFIB and NURR-1 co-transfection study, H295R cells were co-transfected under similar conditions with the following doses: 0.1 μg of NURR-1, 0.5 μg of DN-NGFI-B vectors, and 1 μg of CYP11B2 reporter vector.
Luciferase-mediated bioluminescence assay
Cell lysate from transfected H295R cells was plated in a 20 μl/well volume followed by immediate addition of 50 μl of the luciferase reagent (Promega Corporation) following the manufacturer’s instructions. Microplate was read using the FLUOstar Optima instrument (BMG Labtech, Durham, NC). The empty vector containing pGL3b was used as control to normalize the measurements of luciferase activity.
RNA extraction, cDNA synthesis, and real-time RT-PCR
Total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA) according to protocols from the manufacturer. Purity and integrity of the RNA were checked spectroscopically using Nanodrop instrument (NanoDrop Technologies, Wilmington, DE). Total RNA was reverse transcribed using the high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). Primers for the amplification of the target sequences were designed using Primer Express 3.0 (Applied Biosystems). Primer sequences for the transcription factors used in this study are listed in the supplementary data of our previous article (Nogueira et al. 2007). The DN-NGFI-B primer sequences were F: 5′-CGACCTGCTCTCCGGTTCT-3′, R: 5′-CAGCAAAGCCAGGGATCTTC-3′. PCR amplifications were performed using the ABI 7500 Fast Real-Time PCR System (Applied Biosystems) following the reaction parameters recommended by the manufacturer, using 20 μl total volume consisting of Fast Reagent Master Mix (Applied Biosystems), primers/probes mix, and cDNA. 18S (Applied Biosystems) was used as the normalization gene. Negative controls contained water instead of cDNA.
In all experiments, the relative gene expression was calculated by the ΔΔCt method. Briefly, the resultant mRNA was normalized to a calibrator; in each case, the calibrator chosen was the basal sample. Final results were expressed as n fold difference in gene expression relative to 18s rRNA and calibrator as follows: n fold = 2−(ΔCt sample − ΔCt calibrator), where ΔCt values of the sample and calibrator were determined by subtracting the average Ct value of the transcript under investigation from the average Ct value of the 18s rRNA gene for each sample.
Aldosterone measurement
Aldosterone content of the medium recovered from each well was determined with aldosterone standards prepared in low-serum medium using aldosterone radioimmunoassays (Siemens, Los Angeles, CA). Results of aldosterone essay were normalized to cellular protein and expressed as pmol per mg cell protein.
Statistical analysis
All values were expressed as a mean ± S.E.M. One way ANOVA was used to compare groups in the transfection study as well as in the inhibitor study. P values lower than 0.05 were considered statistically significant.
Results
Ang II directly induces expression of transcription factors
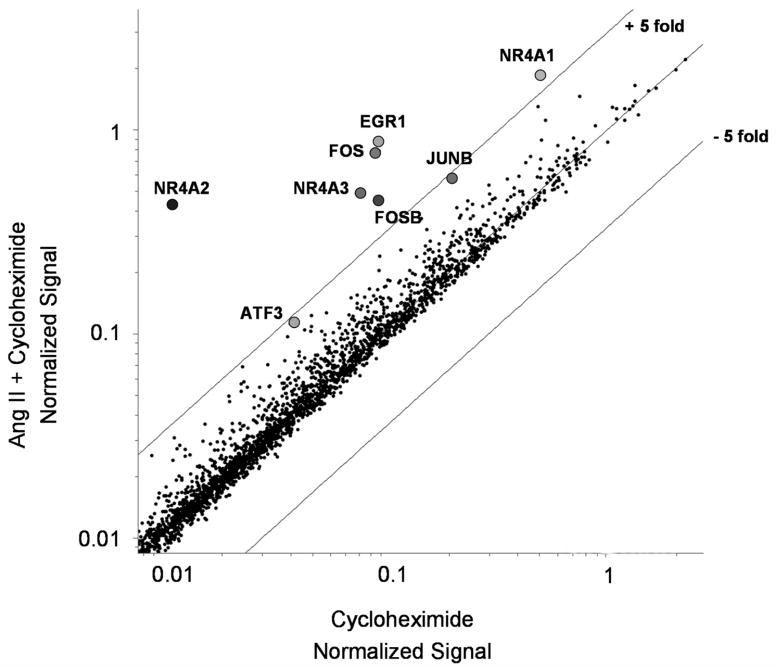
Inhibition of protein synthesis by pre-incubating H295R cells with cycloheximide (35 μM) allowed us to better define the direct versus indirect Ang II gene targets. In the presence of CX, Ang II (10 nM) treatment for 1 h increased the expression of ATF3, EGR1, FOS, FOSB, JUNB, NGFI-B, NURR-1, and NOR-1 (FIG. 1A) as was previously observed in the absence of cycloheximide (Nogueira et al. 2007). This data suggests that these genes are direct targets of Ang II in H295R cells.
Fig. 1.
Microarray scatter plot for Ang II plus cycloheximide versus cycloheximide alone. Total RNA was isolated from H295R cells that were incubated with 35 μM cycloheximide alone or followed by a combination with 10 nM Ang II for 1 h. Labeled genes represent the transcription factors previously shown to be commonly up-regulated by Ang II treatment in human, bovine, and rat adrenocortical cells (Nogueira et al. 2007).
Effects of transcription factors on aldosterone synthesis pathway
AP-1 complex
Members of the AP-1 complex are known to regulate of gene transcription as homo or heterodimers with members of its own family (Mukai et al. 1998). In the present study, H295R cells were co-transfected with vectors for each individual member of the AP-1 complex and the reporter vectors for StAR, CYP11A1, HSD3B2, CYP21, and CYP11B2. The significance was determined by comparing the promoter activity in the presence of the empty vector, pDNA3.1zeo, to the promoter activity in the presence of the transcription factor. In Fig. 2A and B, we demonstrate that FOS and FOSB caused a reduction of approximately 50% in the promoter activity of CYP21. No other significant effects were observed.
Fig. 2.
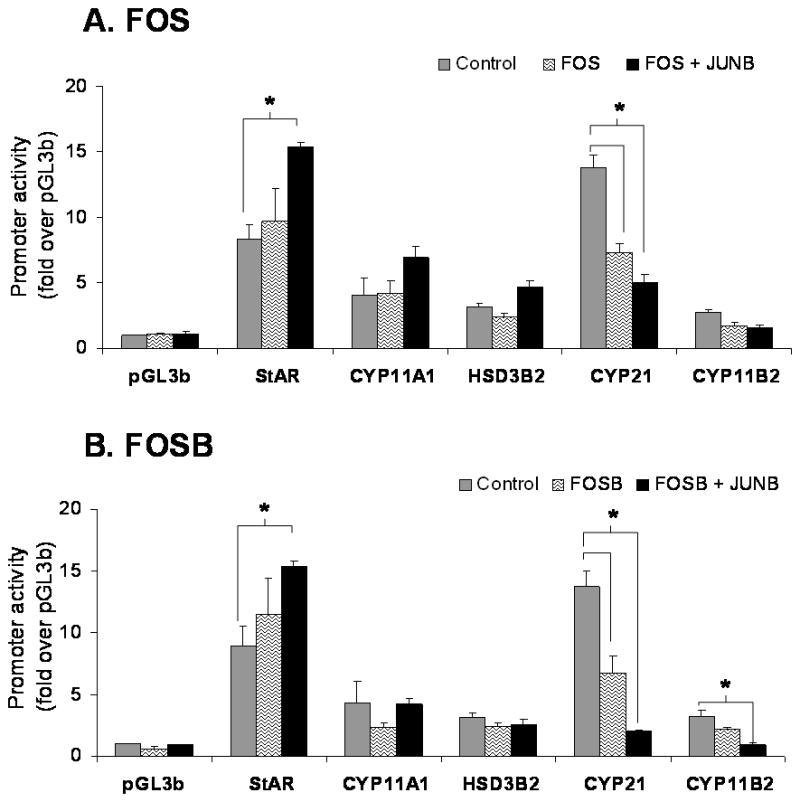
Effects of AP-1 complex members (FOS and FOSB) on the promoter activity of the genes encoding the enzymes involved in aldosterone synthesis. H295R cells were co-transfected with each transcription factor (0.1 μg/ml) and each of the indicated steroidogenic enzyme promoter/reporter vectors (1 μg/ml). For analysis of significance, comparison was made with the promoter activity in the presence of the control vector (empty vector = pcDNA3.1zeo) shown in gray bars versus promoter activity in the presence of the transcription factor (black bars). Results represent ± S.E.M. for five independent experiments. P<0.05 was considered significant.
Co-transfection of JUNB alone did not significantly change the promoter activity of the enzymes tested in our study. However, the combination of JUNB with FOS caused an approximate 2 fold increase in StAR and a 65% decrease in CYP21 promoter activity. Similar effects were seen with a combination of JUNB with FOSB, which increased the promoter activity of StAR by 2 fold and decreased the promoter activity of CYP21 by 85%. JUNB and FOSB also decreased CYP11B2 reporter activity by 70% (Fig. 2A and 2B).
ATF3 and EGR1
The transfection of H295R cells with expression vector for ATF3 did not significantly change the promoter activity of StAR, CYP11A1, CYP21, HSD3B2, or CYP11B2. Because ATF3 is known to heterodimerize with JUNB (Nilsson et al. 1997), we also carried out co-transfection experiments. Interestingly, co-transfection of ATF3 with JUNB significantly increased the promoter activity of StAR and CYP11A1 respectively by 2 and 3 fold (Fig. 3A).
Fig. 3.
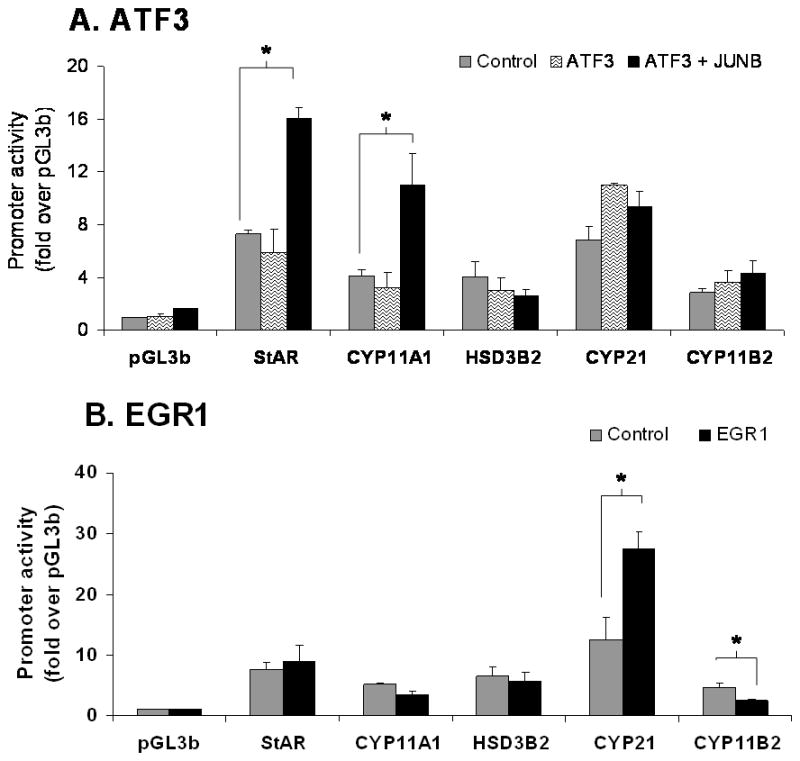
Effects of ATF3 (A) and EGR1 (B) on the promoter activity of the genes encoding the enzymes involved in aldosterone synthesis. H295R cells were co-transfected with each transcription factor (0.1 μg/ml) and each of the steroidogenic enzyme promoter/reporter vectors (1 μg/ml). For analysis of significance, comparison was made with the promoter activity in the presence of the control vector (empty vector = CMV-XL5) shown in gray bars versus promoter activity in the presence of the transcription factor (black bars). Results represent ± S.E.M. for five independent experiments. P<0.05 was considered significant.
The role of EGR1 has not been studied for the transcription of adrenal steroidogenic enzymes. Co-transfection of EGR1 with the selected steroidogenic promoters caused a 2 fold increase in the promoter activity of CYP21. However, EGR1 reduced the promoter activity of CYP11B2 by 50% as shown in Fig. 3B. StAR, CYP11A1, and HSD3B2 were not affected.
NGFI-B family
H295R cells respond to Ang II acutely with changes in gene transcription including the induction of all members of the NGFI-B family of nuclear hormone receptors (Bassett et al. 2004c, Kelly et al. 2005, Nogueira et al. 2007, Romero et al. 2004). In the present study, H295R cells were co-transfected with NGFI-B, NURR-1, or NOR-1 plasmids and luciferase reporter-vectors linked to the promoter region of StAR, CYP11A1, CYP21, HSD3B2, or CYP11B2. In agreement with the sequence analysis, NGFI-B significantly increased promoter activity of HSD3B2, CYP21, and CYP11B2 respectively, by approximately 2, 3, and 5 fold (Fig. 4A); NURR-1 significantly induced promoter activity of these enzymes respectively by 2, 3, and 4 fold (Fig. 4B); and NOR-1 significantly increased promoter activity of the same enzymes respectively by 2, 2, and 3 fold (Fig. 4C). An examination of the proximal promoter regions for each of these genes indicated the presences of putative NGFI-B binding cis- elements (AAAGGTCA) in CYP21, HSD3B2 and CYP11B2 (Fig. 4D). The statistical significance was determined by comparing the promoter activity in the presence of empty vector with the promoter activity in the presence of the transcription factor. These results suggest that NGFI-B family members increase aldosterone production by inducing expression of the enzymes involved in the last three steps of aldosterone synthesis: HSD3B2, CYP21, and CYP11B2.
Fig. 4.
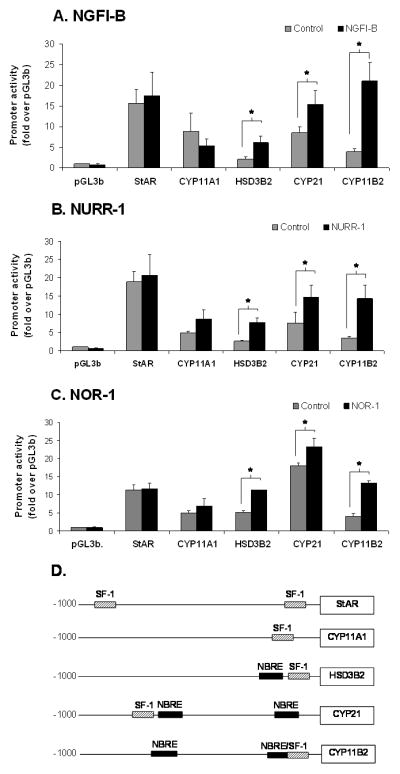
Effects of NGFI-B family members (NGFI-B, NURR-1, NOR-1) on the promoter activity of the genes encoding the enzymes needed for aldosterone synthesis (A, B, and C). H295R cells were co-transfected with each transcription factor (0.1 μg/ml) and each of the steroidogenic enzyme promoter/reporter vectors (1 μg/ml). Identification of NGFIB and SF-1 binding elements in the promoter region of the genes needed for aldosterone synthesis (D). The consensus DNA NGFI-B binding site is distinguished from the SF-1 binding site by 3 base pairs located 5′ to the nuclear receptor half-site: A-A-A for NGFI-B and T/A-C/A-A for SF-1. For analysis of significance, comparison was made with the promoter activity in the presence of the control vector (empty vector = CMV-XL5) shown in gray bars versus promoter activity in the presence of the transcription factor (black bars). Results represent ± S.E.M. for five independent experiments. P<0.05 was considered significant.
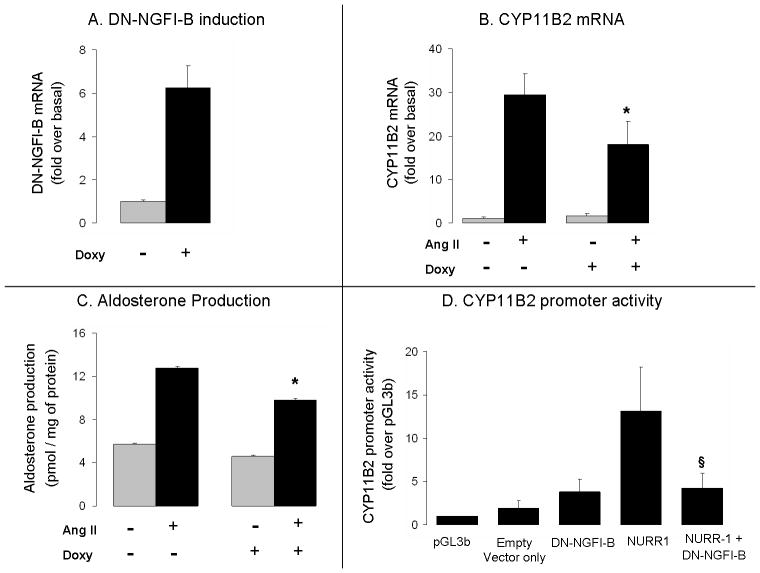
Expression of DN-NGFI-B decreased transcription of CYP11B2
The truncation present in the activator function 1 (AF-1) domain of the DN-NGFI-B decreases the function of endogenous NGFI-B members through competition for its NGFI-B regulatory element binding site (NBRE) (Cheng et al. 1997, Song et al. 2001). DN-NGFI-B H295TR cells contain a tetracycline responsive system that induces the transcription of this truncated protein after treatment with doxycycline (tetracycline analog). After incubation with doxycycline for 24 h, cells were treated with 10 nM Ang II for 6 h. As shown in Fig. 5A, DN-NGFI-B mRNA levels were 6 fold higher in doxycycline treated cells. This increase in DN-NGFI-B expression was concomitant with a 40% reduction in CYP11B2 mRNA and a 25% reduction in aldosterone levels (Fig. 5B and C). Treatment with doxycycline did not affect basal levels of CYP11B2 (Fig. 5B - gray bars). In addition, 11β-hydroxylase (CYP11B1) mRNA levels were not modified by DN-NGFI-B expression (data not shown).
Fig. 5.
Effects of DN-NGFI-B on CYP11B2 expression and aldosterone production in H295R cells. Panel A) Doxycycline (2.5 μg/ml) induction of DN-NGFI-B mRNA. Panel B) Ang II (10 nM) effects on CYP11B2 mRNA levels with and without induction of DN-NGFI-B. Panel C) Ang II effects on aldosterone production with or without induction of DN-NGFI-B. D) Effects of DN-NGFI-B on NURR-1 stimulated CYP11B2 promoter activity. Fold change was calculated considering pGL3b as control. Empty vector only = pGL3b + pcDNA3.1zeo. For all panels the results represent ± S.E.M. for four independent experiments. P<0.05 was considered significant.
We also used the DN-NGFI-B expression vector to study the regulation of CYP11B2 transcription. In Fig. 5D, transfection of H295R cells with NURR-1 expression vector induced a 7 fold increase in the promoter activity of CYP11B2 in comparison to its empty vector (CMV-XL5). However, when NURR-1 was co-transfected with the DN-NGFI-B expression vector, activity of CYP11B2 was reduced by 68% in comparison to control levels (NURR-1 + vector only).
Ang II signaling pathways that regulate NGFI-B family
Ang II activates several intracellular signaling cascades that modulate cellular function. It has been documented that Ang II regulates aldosterone production through AT1R, PKC, CaMK, and SRC signaling pathways (Spat & Hunyady 2004). Here we investigated signaling pathways that regulate Ang II induction of NGFI-B family members. Fig. 6 shows the mRNA levels for NGFI-B members and CYP11B2 after Ang II-incubation for 1 h in the presence (black bars) or absence (gray bars) of selective inhibitors of Ang II intracellular signaling pathways. As seen in Fig. 6A, inhibition of AT1R signaling by losartan reduced transcription of the 3 members of NGFI-B family and CYP11B2 to basal levels; whereas PD123319 (AT2R blocker) did not significantly change the expression of these genes. As shown in Fig. 6B, PKC inhibition by GFX reduced the mRNA levels of NGFI-B members by more than 50%. In contrast, this inhibitor caused a small but significant 1.25 fold increase in CYP11B2 transcription. Inhibition of CaMK caused a significant reduction of approximately 20% in NGFI-B, 40% in NURR-1 and NOR-1, and 70% in CYP11B2 mRNA levels. Inhibition of SRC signaling with SRC-I was also able to significantly decrease expression of NGFI-B (20%), NURR-1 (50%), NOR-1 (50%), and CYP11B2 (60%). Inhibition of JAK2 signaling (using AG490) and ERK1/2 (using PD98059) did not significantly change expression of CYP11B2 in our study (data not shown). Finally, alterations in aldosterone production were found to correlate well with inhibitor effects on CYP11B2 mRNA expression (data not shown).
Fig. 6.
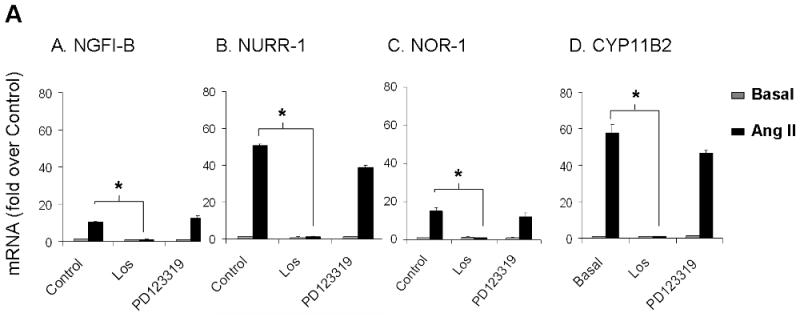
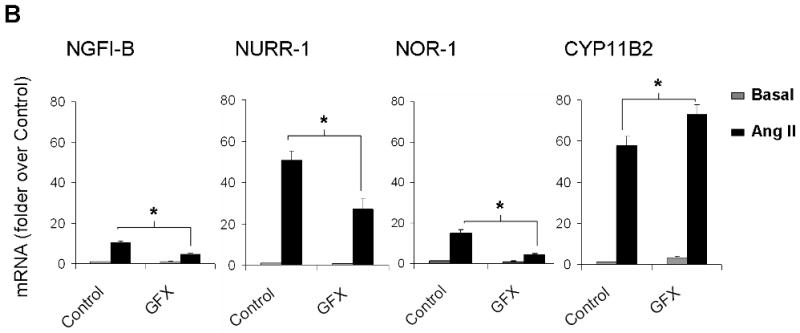
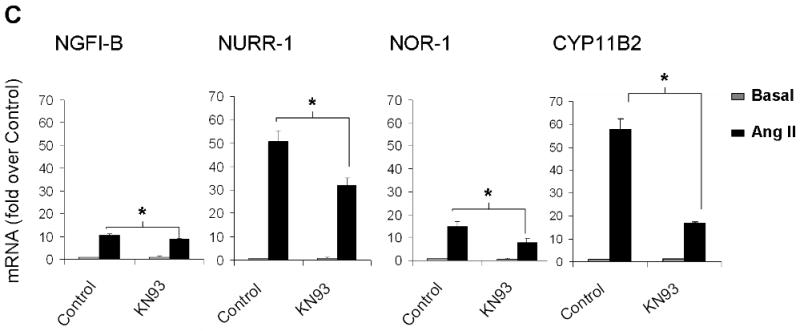
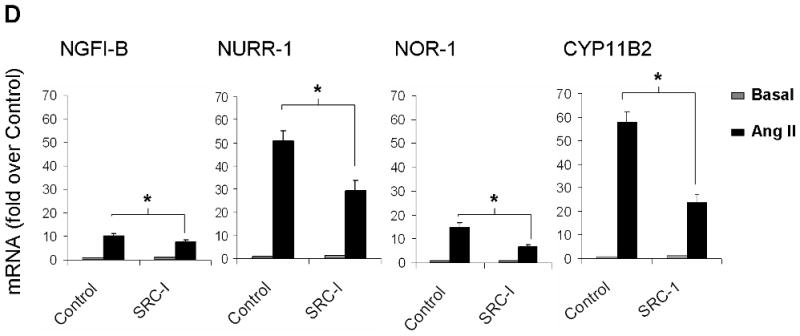
Investigation of Ang II-activated signaling pathways that regulate expression of the NGFI-B family members and CYP11B2. H295R cells were pre-treated with inhibitors for 30 min followed by Ang II incubation for 1 h. Transcript levels for NGFI-B, NURR-1, NOR-1 and CYP11B2 were measured using qPCR. LOS (losartan = AT1R inhibitor); PD123319 (AT2R inhibitor), GFX (or GF103209X = PKC inhibitor); KN93 (CaMK inhibitor); SRC-I (SRC inhibitor). * = p<0.05 (in Ang II-treated cells in the presence versus absence of inhibitors). Results represent ± S.E.M. for five independent experiments. P<0.05 was considered significant.
Discussion
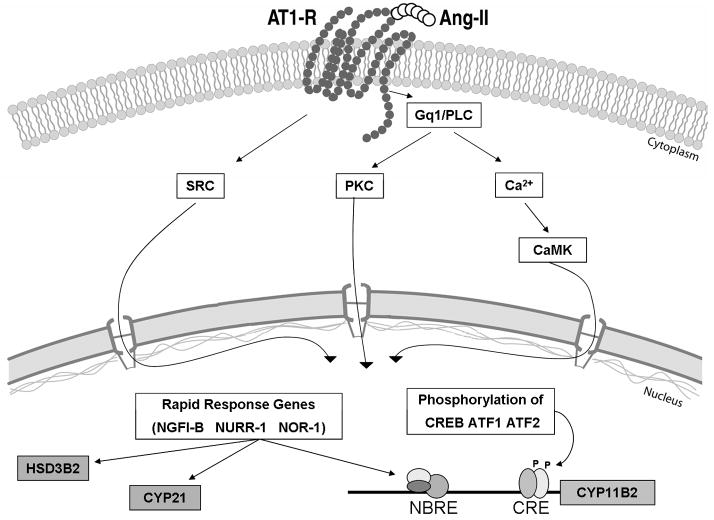
One of the chronic actions of Ang II is its ability to increase the capacity for aldosterone biosynthesis in the adrenal cortex. This occurs through an increase in the proteins need for aldosterone synthesis including StAR, CYP11A1, HSD3B2, CYP21, and CYP11B2. The transcription of these genes is regulated through the activation of signaling cascades that influence the activity of already present transcription factors as well as by increasing expression of new transcription factors. We recently defined a group of transcription factors that are rapidly induced following Ang II treatment of adrenocortical cells. In the current study, we demonstrated that these genes are direct targets of Ang II. In addition, we assessed the function of these factors in the transcriptional control of genes encoding the steroidogenic enzymes needed for aldosterone production (Fig. 7).
Fig. 7.
Schematic of Ang II induction of aldosterone production by adrenal glomerulosa cells. Ang II binding to its type 1 receptor (AT1R) activates diverse intracellular signaling pathways, including Src kinase (SRC), protein kinase C (PKC), and calcium-dependent calmodulin kinases (CaMK). This cascade of events induces transcription of rapid response genes such as members of the NGFI-B family, which act as transcription factors for downstream genes including those that are needed for aldosterone synthesis: HSD3B2, CYP21, and CYP11B2. These kinases may also phosphorylate and activate several other transcription factors already present in the cell, such as the activator factor 1 and 2 (ATF1, ATF2), and the cAMP-response element binding protein (CREB). Physiologically, Ang II action on the adrenal culminates with increased size and capacity of the zona glomerulosa to produce aldosterone.
AP-1 complex transcription factors have been shown to play a role in the trophic effects of Ang II in several tissues including the adrenal cortex (Hattori et al. 2007, Suzuki et al. 2003, Watanabe et al. 1996). The induction of AP-1 complex members by Ang II in adrenal cells and their role in adrenal steroidogenesis have also been reported previously (Guo et al. 2007, Naville et al. 2001, Romero et al. 2007). Importantly, members of this family commonly need to homo- and/or hetero-dimerize among themselves or with other transcription factors to induce transcription (Guo et al. 2007, Mukai et al. 1998). In the present study, we investigated the effect of FOS, FOSB, and JUNB individually and in combination (FOS + JUNB or FOSB + JUNB) on the promoter activity of selected steroidogenic enzymes. A previous study of these genes using transfection of H295R cells suggested a greater role of the AP-1 members in CYP11B1 (Romero et al. 2007). This observation is supported by the presence of a putative AP-1 site in the CYP11B1 but not CYP11B2 gene promoter region (Mukai et al. 1995, Rainey 1999). In our study, AP-1 members alone had no significant effect on genes needed for aldosterone production with the exception of an inhibition of CYP21. However, combination of these vectors (FOS + JUNB and FOSB + JUNB) stimulated the promoter activity of StAR (early regulatory step in aldosterone production). Intriguingly, the combination of FOS and JUNB further decreased the promoter activity of CYP21 when compared to the effect of FOS and FOSB alone. In summary, it is important to consider that the ratios of AP-1 complex members may be important in determining the ultimate cellular phenotype and the role of these transcription factors in steroidogenesis.
Although other members of the ATF family, such as ATF1 and ATF2, have been implicated in the regulation of CYP11B2 (Bassett et al. 2000, Wang et al. 2000), the effects of ATF3 on the genes needed for aldosterone synthesis have not been described previously. Herein, we investigated the effects of ATF3 alone and in combination with JUNB, since these two transcription factors have been shown to interact (Nilsson et al. 1997). Interestingly, this combination resulted in a stimulatory effect of the genes needed to complete the early steps of aldosterone synthesis, specifically StAR and CYP11A1. Once again, the ratios of transcription factors stimulated by Ang II are likely to be important in determining the steroidogenic enzyme expression pattern in adrenocortical cells. Further studies will be needed to define the binding sites that are involved in ATF3 regulation of these genes.
EGR1 has been extensively studied with regard to its role in cell growth and proliferation (Cao et al. 1990, Zhu et al. 2007). Despite the stimulatory effect of the EGR2 isoform on CYP11B2, Romero and colleagues reported no effect of EGR1 on CYP11B2 gene (Romero et al. 2007). We observed that, although EGR1 stimulated CYP21 transcription, it decreased CYP11B2 promoter activity. In other tissues, EGR1 synergizes with SF-1 to stimulate luteinizing hormone (LH) expression in gonadotrope cells (Dorn et al. 1999). SF-1 has been shown to inhibit CYP11B2 expression in adrenocortical cells (Bassett et al. 2002, Li et al. 2004, Shibata et al. 2004, Ye et al. 2008). Thus, the possibility of interaction between these two proteins may provide a potential mechanism contributing to zone-specific repression of CYP11B2.
Among the eight transcription factors studied, the three members of the NGFI-B family (NGFI-B, NURR-1, and NOR-1) up-regulated the transcription of genes responsible for the last three steps in aldosterone production: HSD3B2, CYP21, and CYP11B2. We investigated the presence of NBRE sites in the promoter regions of the enzymes needed for aldosterone synthesis. As shown in Fig. 4D, HSD3B2, CYP21, and CYP11B2 were the only enzymes involved in aldosterone biosynthesis that have consensus NBRE sites in their proximal promoter regions. This fact may explain the ability of the NGFI-B family to selectively stimulate the promoter activity of these genes. The present study confirms previous reports that indicated a role of the NGFI-B family in the regulation of adrenal-specific gene expression in vitro and in vivo (Bassett et al. 2004c, Crawford et al. 1995, Fernandez et al. 2000). Bassett and colleagues have described the binding of NGFI-B and NURR-1 to the NBRE and Ad5 cis-elements present in the 5′-flanking region of hCYP11B2. This study further demonstrated the selective effects of these transcription factors on the transcription of HSD3B2 and CYP11B2, whereas no effect was observed on the transcription of CYP11B1 or CYP17 (Bassett et al. 2004a). The NGFI-B members have also been reported by Romero et al. to have a stimulatory effect on CYP11B2 expression in H295R cells (Romero et al. 2007). Earlier studies suggested that NGFI-B (Nurr77 in mice) could regulate transcription of mouse Cyp21 (Chang & Chung 1995, Wilson et al. 1993). However, mice deficient in NGFI-B appeared to have similar adrenal responses and expression of Cyp21 to that seen in wild type mice (Crawford et al. 1995). Interpretation of this study is complicated by the possible compensation by other members of the NGFI-B family in mouse adrenocortical function (Crawford et al. 1995). To address this concern, we used a dominant negative form of NGFI-B that blocks transcriptional activity of all NGFI-B family members (Cheng et al. 1997, Song et al. 2001). Expression of DN-NGFI-B in H295R cells decreased the ability of Ang II to maximally stimulate aldosterone production and CYP11B2 transcription. However, DN-NGFI-B did not completely block Ang II stimulation. Partial effects may be due to: 1) the relatively low induction level of DN-NGFI-B (6 fold) compared to approximately 30 fold induction of endogenous NGFI-B members by Ang II or, 2) the NGFI-B-independent induction of CYP11B2 transcription, which occurs due to activation of already present CREB and ATF1 (Bassett et al. 2004b, Bassett et al. 2000). Thus, the NGFI-B family likely plays an important role in aldosterone production by the adrenals but it is likely that these transcription factors represent only one of several ways that Ang II increases the capacity to produce aldosterone.
The mechanisms through which Ang II regulates the expression of NGFI-B family members have not been well understood. Here, we showed for the first time that Ang II induction of NGFI-B family members occurred uniquely through the AT1R. Similarly, Ang II regulation of CYP11B2 transcription occurred through stimulation of the AT1R in H295R cells. Inhibition of PKC, SRC, as well as calcium signaling through CaMK, was able to partially reduce the expression of NGFI-B members. Inhibition of CaMK also partially blocked the expression of CYP11B2. The role of CaMK in aldosterone synthesis has been previously shown in H295R cells (Condon et al. 2002, Pezzi et al. 1997). SRC has been suggested to regulate aldosterone production by stimulation of HSD3B2 and inhibition of 17α-hydroxylase (Sirianni et al. 2001). Here, we reported for the first time that SRC signaling may regulate aldosterone production through the induction of CYP11B2.
The interpretation of PKC data is complicated by the large number of PKC family members that respond differently to the GFX compound. In addition, adrenal cell activation of different PKC isoforms is known to differentially regulate aldosterone synthesis (LeHoux & Lefebvre 1998, 2006). Interestingly, in our study, inhibition of PKC by GFX caused a small increase in CYP11B2 expression, a result that agrees with those of LeHoux et al. This group have also reported that diacylglycerol (DAG)-regulated PKC can suppress whereas the atypical PKC lambda can enhance the basal expression of CYP11B2 in H295R cells (LeHoux et al. 2001). Thus, the role of PKC isoforms in the regulation of gene expression is likely to be complicated by contrasting actions of different isoforms. Future studies including knockdown of selected PKC isoforms with techniques such as siRNA, may provide important information regarding the role of PKC in aldosterone synthesis.
Using adrenocortical cells, we have shown that large numbers of genes are increased within a short time of Ang II stimulation. Acutely, Ang II increases the expression of hundreds of genes, most of which have not been studied with regard to adrenal function. Our study confirms and extends previous reports related to the role of acutely responsive transcription factors in adrenal steroidogenesis, including the NGFI-B family that appears to increase expression of enzymes involved in aldosterone biosynthesis. In addition, we provide a broad examination of the role of these transcription factors in the transcription of all the genes needed for the synthesis of aldosterone. Based on the current study, Fig. 7 provides a summary of the molecular mechanisms involved in the regulation of aldosterone synthesis. Further studies that focus on the interactions between Ang II responsive transcription factors and those already present will help to better understand the mechanisms controlling the capacity of the adrenal to produce aldosterone.
Acknowledgments
This work was supported by National Institute of Health grant DK43140 to WER.
Footnotes
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
Disclaimer. This is not the definitive version of record of this article. This manuscript has been accepted for publication in Journal of Molecular Endocrinology, but the version presented here has not yet been copy edited, formatted or proofed. Consequently, Bioscientifica accepts no responsibility for any errors or omissions it may contain. The definitive version is now freely available at 10.1677/JME-08-0112 2009
References
- Adler GK, Chen R, Menachery AI, Braley LM, Williams GH. Sodium restriction increases aldosterone biosynthesis by increasing late pathway, but not early pathway, messenger ribonucleic acid levels and enzyme activity in normotensive rats. Endocrinology. 1993;133:2235–2240. doi: 10.1210/endo.133.5.8404675. [DOI] [PubMed] [Google Scholar]
- Bassett MH, White PC, Rainey WE. A role for the NGFI-B family in adrenal zonation and adrenocortical disease. Endocr Res. 2004a;30:567–574. doi: 10.1081/erc-200043715. [DOI] [PubMed] [Google Scholar]
- Bassett MH, White PC, Rainey WE. The regulation of aldosterone synthase expression. Mol Cell Endocrinol. 2004b;217:67–74. doi: 10.1016/j.mce.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Bassett MH, Zhang Y, White PC, Rainey WE. Regulation of human CYP11B2 and CYP11B1: comparing the role of the common CRE/Ad1 element. Endocr Res. 2000;26:941–951. doi: 10.3109/07435800009048620. [DOI] [PubMed] [Google Scholar]
- Bassett MH, Zhang Y, Clyne C, White PC, Rainey WE. Differential regulation of aldosterone synthase and 11beta-hydroxylase transcription by steroidogenic factor-1. J Mol Endocrinol. 2002;28:125–135. doi: 10.1677/jme.0.0280125. [DOI] [PubMed] [Google Scholar]
- Bassett MH, Suzuki T, Sasano H, White PC, Rainey WE. The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol. 2004c;18:279–290. doi: 10.1210/me.2003-0005. [DOI] [PubMed] [Google Scholar]
- Betancourt-Calle S, Calle RA, Isales CM, White S, Rasmussen H, Bollag WB. Differential effects of agonists of aldosterone secretion on steroidogenic acute regulatory phosphorylation. Mol Cell Endocrinol. 2001;173:87–94. doi: 10.1016/s0303-7207(00)00411-1. [DOI] [PubMed] [Google Scholar]
- Bird IM, Mason JI, Rainey WE. Protein kinase A, protein kinase C, and Ca(2+)-regulated expression of 21-hydroxylase cytochrome P450 in H295R human adrenocortical cells. J Clin Endocrinol Metab. 1998;83:1592–1597. doi: 10.1210/jcem.83.5.4825. [DOI] [PubMed] [Google Scholar]
- Bird IM, Pasquarette MM, Rainey WE, Mason JI. Differential control of 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase expression in human adrenocortical H295R cells. J Clin Endocrinol Metab. 1996;81:2171–2178. doi: 10.1210/jcem.81.6.8964847. [DOI] [PubMed] [Google Scholar]
- Cao XM, Koski RA, Gashler A, McKiernan M, Morris CF, Gaffney R, Hay RV, Sukhatme VP. Identification and characterization of the Egr-1 gene product, a DNA-binding zinc finger protein induced by differentiation and growth signals. Mol Cell Biol. 1990;10:1931–1939. doi: 10.1128/mcb.10.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabre O, Cornillon F, Bottari SP, Chambaz EM, Vilgrain I. Hormonal regulation of mitogen-activated protein kinase activity in bovine adrenocortical cells: cross-talk between phosphoinositides, adenosine 3′,5′-monophosphate, and tyrosine kinase receptor pathways. Endocrinology. 1995;136:956–964. doi: 10.1210/endo.136.3.7867605. [DOI] [PubMed] [Google Scholar]
- Chang SF, Chung BC. Difference in transcriptional activity of two homologous CYP21A genes. Mol Endocrinol. 1995;9:1330–1336. doi: 10.1210/mend.9.10.8544841. [DOI] [PubMed] [Google Scholar]
- Cheng LE, Chan FK, Cado D, Winoto A. Functional redundancy of the Nur77 and Nor-1 orphan steroid receptors in T-cell apoptosis. Embo J. 1997;16:1865–1875. doi: 10.1093/emboj/16.8.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherradi N, Rossier MF, Vallotton MB, Timberg R, Friedberg I, Orly J, Wang XJ, Stocco DM, Capponi AM. Submitochondrial distribution of three key steroidogenic proteins (steroidogenic acute regulatory protein and cytochrome p450scc and 3beta-hydroxysteroid dehydrogenase isomerase enzymes) upon stimulation by intracellular calcium in adrenal glomerulosa cells. J Biol Chem. 1997;272:7899–7907. doi: 10.1074/jbc.272.12.7899. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Pezzi V, Stocco DM, Rainey WE. The steroidogenic acute regulatory protein is induced by angiotensin II and K+ in H295R adrenocortical cells. Mol Cell Endocrinol. 1995;115:215–219. doi: 10.1016/0303-7207(95)03683-0. [DOI] [PubMed] [Google Scholar]
- Clyne CD, Zhang Y, Slutsker L, Mathis JM, White PC, Rainey WE. Angiotensin II and potassium regulate human CYP11B2 transcription through common cis-elements. Mol Endocrinol. 1997;11:638–649. doi: 10.1210/mend.11.5.9920. [DOI] [PubMed] [Google Scholar]
- Condon JC, Pezzi V, Drummond BM, Yin S, Rainey WE. Calmodulin-dependent kinase I regulates adrenal cell expression of aldosterone synthase. Endocrinology. 2002;143:3651–3657. doi: 10.1210/en.2001-211359. [DOI] [PubMed] [Google Scholar]
- Cote M, Muyldermans J, Chouinard L, Gallo-Payet N. Involvement of tyrosine phosphorylation and MAPK activation in the mechanism of action of ACTH, angiotensin II and vasopressin. Endocr Res. 1998;24:415–419. doi: 10.3109/07435809809032625. [DOI] [PubMed] [Google Scholar]
- Crawford PA, Sadovsky Y, Woodson K, Lee SL, Milbrandt J. Adrenocortical function and regulation of the steroid 21-hydroxylase gene in NGFI-B-deficient mice. Mol Cell Biol. 1995;15:4331–4316. doi: 10.1128/mcb.15.8.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, Cavalli LR, Virolle V, Barbry P, Zambetti GP, Figueiredo BC, et al. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol. 2007;21:2968–2987. doi: 10.1210/me.2007-0120. [DOI] [PubMed] [Google Scholar]
- Dorn C, Ou Q, Svaren J, Crawford PA, Sadovsky Y. Activation of luteinizing hormone beta gene by gonadotropin-releasing hormone requires the synergy of early growth response-1 and steroidogenic factor-1. J Biol Chem. 1999;274:13870–13876. doi: 10.1074/jbc.274.20.13870. [DOI] [PubMed] [Google Scholar]
- Fernandez PM, Brunel F, Jimenez MA, Saez JM, Cereghini S, Zakin MM. Nuclear receptors Nor1 and NGFI-B/Nur77 play similar, albeit distinct, roles in the hypothalamo-pituitary-adrenal axis. Endocrinology. 2000;141:2392–2400. doi: 10.1210/endo.141.7.7562. [DOI] [PubMed] [Google Scholar]
- Guo IC, Huang CY, Wang CK, Chung BC. Activating protein-1 cooperates with steroidogenic factor-1 to regulate 3′,5′-cyclic adenosine 5′-monophosphate-dependent human CYP11A1 transcription in vitro and in vivo. Endocrinology. 2007;148:1804–1812. doi: 10.1210/en.2006-0938. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Hattori S, Akimoto K, Nishikimi T, Suzuki K, Matsuoka H, Kasai K. Globular adiponectin activates nuclear factor-kappaB and activating protein-1 and enhances angiotensin II-induced proliferation in cardiac fibroblasts. Diabetes. 2007;56:804–808. doi: 10.2337/db06-1405. [DOI] [PubMed] [Google Scholar]
- Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- Kelly SN, McKenna TJ, Young LS. Coregulatory protein-orphan nuclear receptor interactions in the human adrenal cortex. J Endocrinol. 2005;186:33–42. doi: 10.1677/joe.1.06005. [DOI] [PubMed] [Google Scholar]
- LeHoux JG, Lefebvre A. Transcriptional activity of the hamster CYP11B2 promoter in NCI-H295 cells stimulated by angiotensin II, potassium, forskolin and bisindolylmaleimide. J Mol Endocrinol. 1998;20:183–191. doi: 10.1677/jme.0.0200183. [DOI] [PubMed] [Google Scholar]
- LeHoux JG, Lefebvre A. Novel protein kinase C-epsilon inhibits human CYP11B2 gene expression through ERK1/2 signalling pathway and JunB. J Mol Endocrinol. 2006;36:51–64. doi: 10.1677/jme.1.01908. [DOI] [PubMed] [Google Scholar]
- LeHoux JG, Dupuis G, Lefebvre A. Control of CYP11B2 gene expression through differential regulation of its promoter by atypical and conventional protein kinase C isoforms. J Biol Chem. 2001;276:8021–8028. doi: 10.1074/jbc.M009495200. [DOI] [PubMed] [Google Scholar]
- Lehoux JG, Hales DB, Fleury A, Briere N, Martel D, Ducharme L. The in vivo effects of adrenocorticotropin and sodium restriction on the formation of the different species of steroidogenic acute regulatory protein in rat adrenal. Endocrinology. 1999;140:5154–5164. doi: 10.1210/endo.140.11.7101. [DOI] [PubMed] [Google Scholar]
- Li LA, Chang YC, Wang CJ, Tsai FY, Jong SB, Chung BC. Steroidogenic factor 1 differentially regulates basal and inducible steroidogenic gene expression and steroid synthesis in human adrenocortical H295R cells. J Steroid Biochem Mol Biol. 2004;91:11–20. doi: 10.1016/j.jsbmb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Miura S, Zhang J, Matsuo Y, Saku K, Karnik SS. Activation of extracellular signal-activated kinase by angiotensin II-induced Gq-independent epidermal growth factor receptor transactivation. Hypertens Res. 2004;27:765–770. doi: 10.1291/hypres.27.765. [DOI] [PubMed] [Google Scholar]
- Mukai K, Mitani F, Shimada H, Ishimura Y. Involvement of an AP-1 complex in zone-specific expression of the CYP11B1 gene in the rat adrenal cortex. Mol Cell Biol. 1995;15:6003–6012. doi: 10.1128/mcb.15.11.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai K, Mitani F, Agake R, Ishimura Y. Adrenocorticotropic hormone stimulates CYP11B1 gene transcription through a mechanism involving AP-1 factors. Eur J Biochem. 1998;256:190–200. doi: 10.1046/j.1432-1327.1998.2560190.x. [DOI] [PubMed] [Google Scholar]
- Muller J. Aldosterone: the minority hormone of the adrenal cortex. Steroids. 1995;60:2–9. doi: 10.1016/0039-128x(94)00021-4. [DOI] [PubMed] [Google Scholar]
- Naville D, Bordet E, Berthelon MC, Durand P, Begeot M. Activator protein-1 is necessary for angiotensin-II stimulation of human adrenocorticotropin receptor gene transcription. Eur J Biochem. 2001;268:1802–1810. [PubMed] [Google Scholar]
- Nilsson M, Ford J, Bohm S, Toftgard R. Characterization of a nuclear factor that binds juxtaposed with ATF3/Jun on a composite response element specifically mediating induced transcription in response to an epidermal growth factor/Ras/Raf signaling pathway. Cell Growth Differ. 1997;8:913–920. [PubMed] [Google Scholar]
- Nogueira EF, Vargas CA, Otis M, Gallo-Payet N, Bollag WB, Rainey WE. Angiotensin-II acute regulation of rapid response genes in human, bovine, and rat adrenocortical cells. J Mol Endocrinol. 2007;39:365–374. doi: 10.1677/JME-07-0094. [DOI] [PubMed] [Google Scholar]
- Peters B, Clausmeyer S, Obermuller N, Woyth A, Kranzlin B, Gretz N, Peters J. Specific regulation of StAR expression in the rat adrenal zona glomerulosa. An in situ hybridization study. J Histochem Cytochem. 1998;46:1215–1221. doi: 10.1177/002215549804601101. [DOI] [PubMed] [Google Scholar]
- Pezzi V, Clyne CD, Ando S, Mathis JM, Rainey WE. Ca(2+)-regulated expression of aldosterone synthase is mediated by calmodulin and calmodulin-dependent protein kinases. Endocrinology. 1997;138:835–838. doi: 10.1210/endo.138.2.5032. [DOI] [PubMed] [Google Scholar]
- Quinn SJ, Williams GH. Regulation of aldosterone secretion. Annu Rev Physiol. 1988;50:409–426. doi: 10.1146/annurev.ph.50.030188.002205. [DOI] [PubMed] [Google Scholar]
- Rainey WE. Adrenal zonation: clues from 11beta-hydroxylase and aldosterone synthase. Mol Cell Endocrinol. 1999;151:151–160. doi: 10.1016/s0303-7207(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Naville D, Mason JI. Regulation of 3 beta-hydroxysteroid dehydrogenase in adrenocortical cells: effects of angiotensin-II and transforming growth factor beta. Endocr Res. 1991;17:281–296. doi: 10.1080/07435809109027202. [DOI] [PubMed] [Google Scholar]
- Romero DG, Plonczynski M, Vergara GR, Gomez-Sanchez EP, Gomez-Sanchez CE. Angiotensin II early regulated genes in H295R human adrenocortical cells. Physiol Genomics. 2004;19:106–116. doi: 10.1152/physiolgenomics.00097.2004. [DOI] [PubMed] [Google Scholar]
- Romero DG, Rilli S, Plonczynski MW, Yanes LL, Zhou MY, Gomez-Sanchez EP, Gomez-Sanchez CE. Adrenal transcription regulatory genes modulated by angiotensin II and their role in steroidogenesis. Physiol Genomics. 2007;30:26–34. doi: 10.1152/physiolgenomics.00187.2006. [DOI] [PubMed] [Google Scholar]
- Seta K, Nanamori M, Modrall JG, Neubig RR, Sadoshima J. AT1 receptor mutant lacking heterotrimeric G protein coupling activates the Src-Ras-ERK pathway without nuclear translocation of ERKs. J Biol Chem. 2002;277:9268–9277. doi: 10.1074/jbc.M109221200. [DOI] [PubMed] [Google Scholar]
- Shibata H, Ogishima T, Mitani F, Suzuki H, Murakami M, Saruta T, Ishimura Y. Regulation of aldosterone synthase cytochrome P-450 in rat adrenals by angiotensin II and potassium. Endocrinology. 1991;128:2534–2539. doi: 10.1210/endo-128-5-2534. [DOI] [PubMed] [Google Scholar]
- Shibata H, Kobayashi S, Kurihara I, Suda N, Yokota K, Murai A, Ikeda Y, Saito I, Rainey WE, Saruta T. COUP-TF and transcriptional co-regulators in adrenal steroidogenesis. Endocr Res. 2004;30:795–801. doi: 10.1081/erc-200044042. [DOI] [PubMed] [Google Scholar]
- Sirianni R, Sirianni R, Carr BR, Pezzi V, Rainey WE. A role for src tyrosine kinase in regulating adrenal aldosterone production. J Mol Endocrinol. 2001;26:207–215. doi: 10.1677/jme.0.0260207. [DOI] [PubMed] [Google Scholar]
- Song KH, Park JI, Lee MO, Soh J, Lee K, Choi HS. LH induces orphan nuclear receptor Nur77 gene expression in testicular Leydig cells. Endocrinology. 2001;142:5116–5123. doi: 10.1210/endo.142.12.8525. [DOI] [PubMed] [Google Scholar]
- Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev. 2004;84:489–539. doi: 10.1152/physrev.00030.2003. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- Thomas WG, Qian H. Arresting angiotensin type 1 receptors. Trends Endocrinol Metab. 2003;14:130–136. doi: 10.1016/s1043-2760(03)00023-7. [DOI] [PubMed] [Google Scholar]
- Tremblay A, Parker KL, Lehoux JG. Dietary potassium supplementation and sodium restriction stimulate aldosterone synthase but not 11 beta-hydroxylase P-450 messenger ribonucleic acid accumulation in rat adrenals and require angiotensin II production. Endocrinology. 1992;130:3152–3158. doi: 10.1210/endo.130.6.1597135. [DOI] [PubMed] [Google Scholar]
- Wang XL, Bassett M, Zhang Y, Yin S, Clyne C, White PC, Rainey WE. Transcriptional regulation of human 11beta-hydroxylase (hCYP11B1) Endocrinology. 2000;141:3587–3594. doi: 10.1210/endo.141.10.7689. [DOI] [PubMed] [Google Scholar]
- Watanabe G, Lee RJ, Albanese C, Rainey WE, Batlle D, Pestell RG. Angiotensin II activation of cyclin D1-dependent kinase activity. J Biol Chem. 1996;271:22570–22577. doi: 10.1074/jbc.271.37.22570. [DOI] [PubMed] [Google Scholar]
- Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Mouw AR, Weaver CA, Milbrandt J, Parker KL. The orphan nuclear receptor NGFI-B regulates expression of the gene encoding steroid 21-hydroxylase. Mol Cell Biol. 1993;13:861–868. doi: 10.1128/mcb.13.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Nakamura Y, Lalli E, Rainey WE. Differential Effects of High and Low Steroidogenic Factor-1 Expression on CYP11B2 Expression and Aldosterone Production in Adrenocortical Cells. Endocrinology. 2008 doi: 10.1210/en.2008-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Lin Y, Bacanamwo M, Chang L, Chai R, Massud I, Zhang J, Garcia-Barrio MT, Thompson WE, Chen YE. Interleukin-1 beta-induced Id2 gene expression is mediated by Egr-1 in vascular smooth muscle cells. Cardiovasc Res. 2007;76:141–148. doi: 10.1016/j.cardiores.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]