Summary
Background and objective
Pancreatic cancer is a leading cause of cancer-related deaths in men and women. Early clinical studies suggest that photodynamic therapy (PDT) might be a useful modality in the management of this deadly disease. In this study, the photocytotoxicity of Photofrin-mediated PDT on different human pancreatic cancer cells (BxPc-3, HPAF-II, Mia PaCa-2, MPanc-96, PANC-1 and PL-45) was examined.
Materials and methods
After co-incubating cancer cells with Photofrin (0—10 [H9262]g/ml) for 4 h, the cells were irradiated with 0—6 J/cm2 of 630 nm light. The effect of Photofrin PDT on the survival of cells were examined using tetrazolium-based colorimetric assay and clonogenic assay. PDT-induced apoptosis was analyzed by flow cytometry. Expressions of apoptosis-related proteins were determined by western blot analysis.
Results
Photofrin PDT strongly inhibited the survival of pancreatic cancer cells. A small portion of cells (<15%) underwent apoptosis 24 h after PDT at LD50. Cleavage of caspase-3, caspase-8, caspase-9 and PARP after PDT were also confirmed. BxPc-3, Mia PaCa-2, MPanc-96, and PANC-1 cells were more sensitive and HPAF-II and PL-45 cells less sensitive.
Conclusion
Photofrin PDT can induce apoptosis and inhibit survival of human pancreatic cancer cells.
Keywords: Photofrin, Photodynamic therapy, Pancreatic cancer cells, Apoptosis
Introduction
Pancreatic cancer is a leading cause of cancer-related deaths in men and women. The prognosis for the pancreatic cancer, especially its common form, pancreatic ductal adenocarcinoma, is extremely poor and 5-year survival rate is less than 4% [1]. To date, it is still one of the most challenging solid tumors to treat. Although surgery and multimodal therapy (e.g. chemotherapy, radiotherapy, immunotherapy, and/or targeted therapy) might improve overall survival, an effective local treatment with minimal complications is urgently needed [2,3].
Photodynamic therapy (PDT) is a disease site-specific treatment modality and its potential for the treatment of pancreatic cancer has been proposed since the early 1980s [4,5]. PDT involves a local or systemic administration of a photosensitizer followed by the light irradiation of the targeted lesion site with non-thermal visible light of appropriate wavelength(s) [6]. In the presence of molecular oxygen (O2), the light irradiation of photosensitizer leads to a series of photochemical reactions and consequently generation of various cytotoxic species which can induce tumor ablation [7]. Light delivery in pancreatic PDT can be performed in minimally invasive fashion [4,5,8]. Early in vitro and in vivo studies have showed that PDT can inhibit the growth of pancreatic cancer cells [9,10]. A clinical study demonstrates that Foscan-mediated PDT can improve the survival of patients with cancers (2.5—6 cm in diameter) localized in the pancreatic head that could not be resected because of the advanced nature of the disease or the general condition of the patients [11]. PDT could become a potential adjuvant therapy in the management of pancreatic cancer. Nevertheless, more preclinical and clinical studies are needed to further improve and optimize PDT for the treatment of pancreatic cancer [12].
Photofrin® (Porfimer Sodium) is a commercially available photosensitizer. It is a proprietary combination of monomers, dimers, and oligomers derived from the chemical manipulation of hematoporphyrin [13]. Early in vitro and in vivo studies have demonstrated that pancreatic cancer cells are sensitive to Photofrin PDT [5,14—16]. Although the usefulness of Photofrin PDT has not been fully tested in patients with pancreatic cancer, the drug is approved worldwide for a number of other indications. This study will examine the photocytotoxic effect of Photofrin PDT on a panel of human pancreatic cancer cells.
The understanding of the regulation of apoptosis and necrosis, the two principal cell death pathways, is becoming exceedingly important in investigations of the pathogenesis and treatment of pancreatic cancer [17]. It is well-known that the apoptosis can rapidly occur during PDT-induced cancer cell death [18,19]. However, the rapid initiation of apoptosis by PDT depends on many factors such as the cell line and photosensitizer employed [20—22]. This study will also focus on the effect of Photofrin PDT on the initiation of apoptosis in human pancreatic cancer cells.
Materials and methods
Cell lines and culture conditions
The moderately well to poorly differentiated human pancreatic cancer cell lines (BxPc-3, HPAF-II, Mia PaCa-2, MPanc-96, PANC-1 and PL-45) were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and maintained in RPMI 1640 medium (HyClone Laboratories, Inc., Logan, UT) supplemented with 10% fetal bovine serum (FBS, HyClone Laboratories, Inc.) and antibiotics. All cell lines were cultured at 37 °C and 5% CO2 in a humidified incubator.
Photosensitizer and general PDT procedure
The photosensitizer stock solution was prepared as described previously [23]. Briefly, Photofrin powder (Axcan, Montreal, Canada) was dissolved in 5% Dextrose (Baxter Healthcare, Deerfield, IL) to give a final concentration of 1 mg/ml and kept frozen in the dark until use.
To determine the optimal photosensitizer-cell incubation time, the cellular uptake of Photofrin was examined by an epifluorescence microscope. Cells (~105) were seeded in 8-well plates (Greiner Bio-One Inc., Monroe, NC) containing a poly-L-lysine (0.1%, Sigma—Aldrich, St. Louis, MO) pre-treated glass slide in each well in 2 ml of complete medium containing 10% FBS. After 24 h of incubation, the medium was removed and replaced with fresh medium containing Photofrin (2 μg/ml). The cells were incubated with Photofrin for 0.5—4 h. At predetermined time points, cellular uptake of Photofrin was examined under the epifluorescence microscope after formalin fixation. Since 3—4 h of incubation produced intense fluorescence, the 4 h of incubation was used in following experiments.
Drug-cell incubation time, drug and light dose, and PDT experimental setup were similar to a previous report except that a diode laser was used in this study [24]. Briefly, after co-incubating cells with Photofrin (0—10 μg/ml) in culture plates or dishes for 4 h, the medium was removed and cells washed twice with PBS. The cells were then irradiated with light of 0—6 J/cm2, which was generated by a 630 nm diode laser (MicroMed, Shenzhen, China) and delivered though a microlens fiber (FD1, Biolitec AG, Jena, German). The average light intensity at the level of the cell monolayer was 10 mW/cm2.
Cell viability assay
Tetrazolium-based colorimetric assay was used to determine the effect of PDT on the cell viability. Briefly, exponential growth cells were suspended in the complete medium (HyClone Laboratories, Inc.), seeded into black-walled 96-well microculture plates (1 × 104 cells/well), and incubated for 24 h as described before. The medium was removed and replaced with fresh phenol-free medium containing 0—10 μg/ml Photofrin. Attached cells were incubated with Photofrin for 4 h and washed twice in PBS before receiving 0—6 J/cm2 of light irradiation. Control and PDT-treated cells were then washed twice and further incubated in the complete medium for 24 h. Then, 100 μl of fresh RPMI 1640 medium containing 0.5% FBS were added to each well and followed by addition of a mixture of 20 μl of phenazine methosulate (PMS, Sigma—Aldrich) and a tetrazolium compound MTS (Sigma—Aldrich). After incubation for 2—4 h, optical density at 490 nm was recorded with a microplate reader (Bio Tek Instruments Inc., Winooski, VT) and the percentages of surviving cells from each group relative to controls, defined as 100% survival, were determined by the reduction of MTS. Each drug and light dose were repeated at least five times.
Clonogenic cell survival assay
The effect of Photofrin PDT on cell survival was examined by clonogenic assay. Three poorly differentiated cell lines (i.e. Mia PaCa-2, MPanc-96 and PL-45) were grown in RPMI 1640 medium as described before. Exponential growth cells were resuspended in 2 ml of complete medium containing 10% FBS and seeded into 6-well plates (Greiner Bio-One) in triplicates at a density of approximately 2 × 102 cells/well. After 24 h incubation, the medium was removed and replaced with fresh medium containing Photofrin (5 or 10 μg/ml). The cells were incubated for 4 h and then irradiated at 3 or 6 J/cm2 as described before. Immediately after PDT, the cells were washed twice in PBS and incubated in the complete medium for up to 14 days. Medium was changed every 3 days during colony formation. The cell clones were stained with 0.5% of crystal violet (in 25% methanol) for 15 min followed by rinsing with tap water to remove excess dye. The numbers of colonies were counted by an imaging system (EAGLE EYETM II, Stratagene, La Jolla, CA).
Flow cytometric analysis of apoptosis
Flow cytometric analysis was performed to detect PDT-induced apoptosis [25]. Briefly, fresh cells were resus-pended in 8 ml of complete medium containing 10% FBS (~105 cells/ml) and transferred into 10 cm dishes (Greiner Bio-One). After 24 h incubation, the medium was removed and replaced with fresh medium with or without Photofrin (2 or 5 μg/ml). The cells were incubated for 4 h and then irradiated at dose levels of 3 or 6 J/cm2 as described before. After PDT the cells were washed twice in PBS and incubated with the complete medium for another 24 h. Then the cells were detached by addition of 0.25% trypsin (HyClone Laboratories, Inc.) and fixed with 70% ethanol. Cells were stained for total DNA content with a solution containing 50 μg/ml propidium iodide (PI, Sigma—Aldrich) and 100 μg/ml RNase (Sigma—Aldrich) at 37 °C for 30 min. DNA content was detected by a FACScan flow cytometer (Becton Dickinson, San Jose, CA) and cells with reduced DNA content were considered as apoptotic cells.
Western blot analysis
Apoptosis-related protein expression levels were determined by western blot analysis. Cells (~105 in 2 ml) were seeded into 10 cm dishes (Greiner Bio-One) in 8 ml of complete medium containing 10% FBS. After 24 h incubation, the medium was removed and replaced with fresh medium with or without Photofrin at a final concentration of 0—5 μg/ml. The cells were incubated for 4 h and then irradiated at dose levels of 0—6 J/cm2 as described before. After PDT the cells were washed twice in PBS and incubated with complete medium for another 24 h. Then the cells were detached by addition of 0.25% trypsin and lysed in a buffer (pH 7.4) containing 50 mM Tris (Fisher Scientific, Pittsburgh, PA), 50 mM NaCl (Fisher Scientific), 0.5% NP-40, 50 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 25 g/ml leupeptin (Sigma—Aldrich), and 25 g/ml aprotinin (Sigma—Aldrich). The whole cell lysates were centrifuged at 13,200 RPM in a microcentrifuge for 20 min and the supernatants were collected for protein concentration determination by the Coomassie Plus protein assay reagent (Pierce Chemical Co., Rockford, IL). Equal amounts of cell lysates were boiled in Laemmli SDS-sample buffer, resolved by SDS-polyacrylamide gel electrophoresis (PAGE), transferred to nitrocellulose (Bio-Rad Laboratories, Hercules, CA), and probed with different primary antibodies. Anti-PARP (Poly ADP-ribose polymerase) antibody was obtained from BIOMOL International Inc. (Plymouth Meeting, PA), anti-caspase-3, caspase-8, and caspase-9 antibodies were from Cell Signaling Technology, Inc. (Danvers, MA), and anti-β-actin antibody was from Sigma—Aldrich. After the blots were incubated for another 1 h at room temperature with horseradish peroxidase-labeled secondary antibody (goat anti-rabbit IgG or goat anti-mouse IgG (Perkin Elmer, Boston, MA)), the signals were detected using the Enhanced Chemiluminance (ECL) assay (Amersham Life Science Inc., Arlington Heights, IL) according to the manufacturer's instructions.
Results
PDT affects cell viability
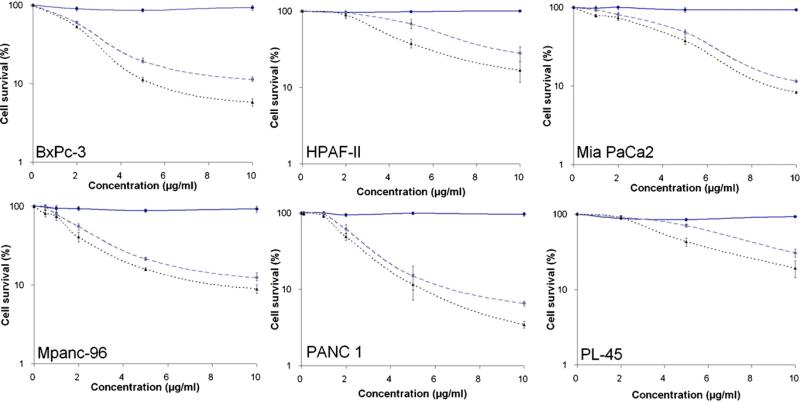
Conventional MTS colorimetric assay was used to determine the effect of Photofrin PDT on the cell viability. In order to establish the dose-effect relationship, pancreatic cancer cells were irradiated with a light dose of 0, 3 or 6 J/cm2 after incubating with various concentrations of Photofrin (0, 2, 5 and 10 μg/ml) for 4 h. MTS colorimetric assay was performed 24 h after PDT using drug only or light only as controls. Results showed a strong dose escalation effect on the cell viability of the six cell lines tested (Fig. 1).
Figure 1.
MTS assay. Dose escalation study of PDT effect on cell viability was performed at drug dose levels of 0—10 μg/ml and light dose levels of 0—6 J/cm2. Tetrazolium compound MTS assay was carried out 24 h after PDT. Solid line: light control; dash line: 3 J/cm2 and dot line: 6 J/cm2.
BxPc-3, Mia PaCa-2, MPanc-96, and PANC-1 cells were more sensitive to Photofrin-mediated photodynamic killing. At the drug and light dose of 10 μg/ml and 3 J/cm2, there were 88—94% of reduction in cell survival. At the drug and light dose of 10 μg/ml and 6 J/cm2, there were 92—96% of reduction. Comparing to above four cells lines, HPAF-II and PL-45 cells were less sensitive. At the drug and light dose of 10 μg/ml and 3 J/cm2, there were 70% of reduction in cell viability. At drug and light dose of 10 μg/ml and 6 J/cm2, there were 80% of reduction. These results suggested that Photofrin PDT could cause significant killing of pancreatic cancer cells in vitro.
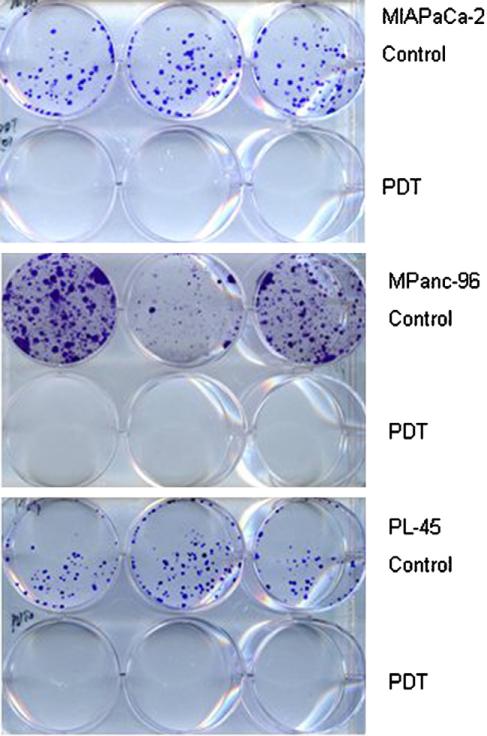
To further confirm PDT-induced cell killing, three poorly differentiated cell lines (Mia PaCa-2, MPanc-96 and PL-45) were subjected to the clonogenic assay immediately after PDT. At the seeding density of 200 cells/well, no colony formation was detected for any of those three cell lines (Fig. 2). PDT-induced reproductive death was estimated to be great than 99% under tested dose levels of 5 μg/ml and 3—6 J/cm2. For MPanc-96, Mia PaCa-2 and PL-45 cells, although there was 25—70% survival under the indicated drug/light doses (see Fig. 1), survival cells were not able to proliferate under the culture condition in vitro.
Figure 2.
Clonogenic assay. Poorly differentiated human pancreatic cancer cell lines were subjected to Photofrin PDT at the following drug and light doses — Mia PaCa-2: 5 μg/ml and 3 J/cm2; MPanc-96: 5 μg/ml and 3 J/cm2 and PL-45: 5 μg/ml and 6 J/cm2. Control (drug only) and treated cells were grown in a fresh medium immediately after PDT. Clonogenic assay was examined after two weeks. There was no colony formation in Photofrin PDT groups.
PDT induces apoptosis
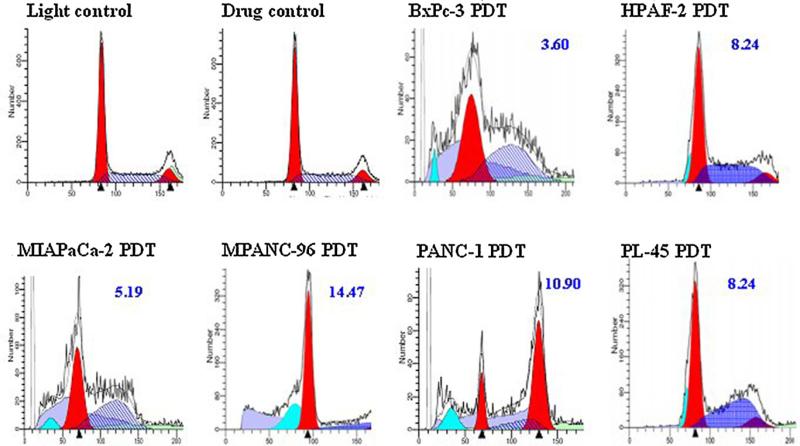
Microscopic examination of PDT-treated cells revealed apoptotic morphological alternations (e.g. cell shrinkage, nuclear breakdown, membrane blebbing and DNA fragmentation) (data not shown). When apoptotic cells were stained with fluorogenic PI, they displayed a broad hypodiploid (sub-G1) peak, whereas cells with normal diploid DNA displayed a narrow peak. The apoptotic rate was derived from their ratio. At around dose levels of LD50 determined by the MTS assay for each cell line, the flow cytometric analysis showed that there were 3.6—14.5% cells undergoing apoptosis at 24 h after PDT (Fig. 3). A heterogeneous pattern of ploidy content among the tested cell lines was also noted.
Figure 3.
Flow cytometric assay. PDT-induced apoptosis was detected by PI staining and flow cytometric analysis at 24 h after PDT. Pancreatic cancer cell lines were subjected to Photofrin PDT at the following drug and light doses respectively — BxPc-3: 2 μg/ml and 3 J/cm2; HPAF-II: 5 μg/ml and 3 J/cm2; Mia PaCa-2: 5 μg/ml and 3 J/cm2; MPanc-96: 2 μg/ml and 3 J/cm2; Panc-1: 2 μg/ml and 3 J/cm2 and PL-45: 5 μg/ml and 6 J/cm2. Representative light and drug controls are shown on the top panel. The percentage of apoptotic cells is indicated.
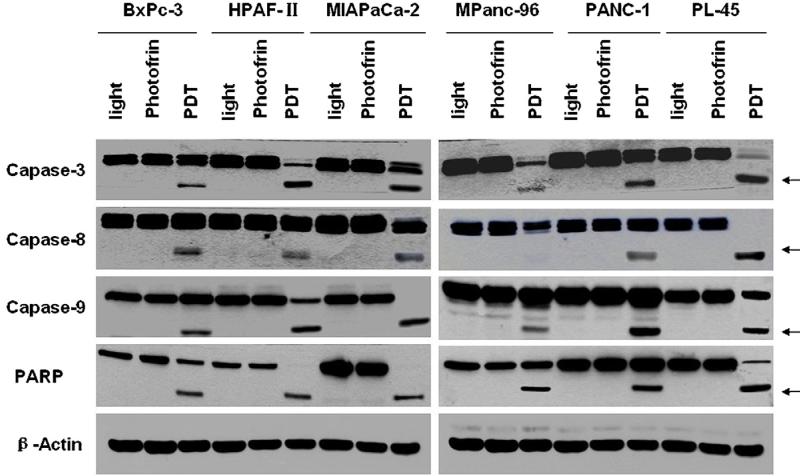
To further identify apoptotic pathways associated with Photofrin PDT and examine apoptosis-related protein expression, western blot analyses were performed using cell lysates with anti-caspase-3, anti-caspase-8, anti-caspase-9 and anti-PARP antibodies that could react with full length and cleaved fragments of these proteins. The cleavage of caspase-9, which occurred during mitochondrial damage and cytochrome c release, was detected in pancreatic cancer cells after PDT treatment (Fig. 4). Cleaved caspase-9 could further process other caspase members (e.g. caspase-3 and caspase-7) and initiate a caspase cascade and ultimately the apoptosis. By detecting the cleaved caspase-3 fragment and by the disappearance of the uncleaved precursor form of caspases-3, PDT-induced apoptosis was further confirmed. Caspase-3 is one of the key executioners of apoptosis and responsible for the proteolytic cleavage of many key proteins such as PARP. Western blot analyses also demonstrated that the cleavage of PARP from 116 to 85 kDa occurred in all six human pancreatic cancer cell lines, indicating that apoptotic cell death was indeed involved in PDT-induced photocytotoxicity. Meanwhile, cellular levels of the uncleaved precursor form of caspase-8 decreased and that of cleaved caspase-8 fragment increased, indicating that activation of caspase-8 was also involved in PDT-induced apoptosis.
Figure 4.
Western blot analysis. PDT-induced apoptosis was confirmed by detecting the cleaved caspase-3, caspase-8, caspase-9, and PARP fragments (arrows) and by the disappearance of the uncleaved precursors of these proteins in pancreatic cancer cells.
Discussion
Photofrin is a well-studied photosensitizer and has been used in the photodynamic treatment of various types and stages of cancers. A major drawback of Photofrin is its prolonged cutaneous phototoxicity. Optimizing its dose might offer reliable tumor ablation and meanwhile reduce normal tissue damage [26]. Although previous in vitro studies demonstrate that Photofrin PDT possesses a strong phototoxicity toward human pancreatic cancer cells [14—16], the mechanisms of action of Photofrin-mediated PDT on human pancreatic cells have not been fully explored. In this study, the mechanisms of Photofrin PDT-mediated photocytotoxicity were explored in a panel of human pancreatic cancer cell lines.
As expected, different cells lines showed different sensitivity toward Photofrin PDT in terms of the survival rate under the same drug/light dose. Roughly, six human pancreatic cancer cell lines tested in this study could be divided into two groups: BxPc-3, Mia PaCa-2, MPanc-96, and PANC-1 cells were more sensitive and HPAF-II and PL-45 cells less sensitive (see Fig. 1). It seemed that the sensitivity was unrelated to the degree of cell differentiation. For poorly differentiated cell lines, although some cells survived at the indicated drug/light doses (5 μg/ml and 3 or 6 J/cm2), the survival cells were unable to proliferate under the culture condition (see Fig. 2). Clearly, Photofrin PDT had a strong impact on cell viability and proliferation.
Other groups have demonstrated that Photofrin can be preferentially accumulated in the mitochondria of various cancer cells [27—30]. Chronological cellular events from the early apoptotic event to the downstream cascades were studied. Our data indicated that Photofrin-mediated photocytotoxicity might be mainly attributed to the cellular uptake of Photofrin by pancreatic cancer cells and severe direct damage to the mitochondria during Photofrin photosensitization.
There is considerable evidence strongly suggesting that the mitochondria serve as the critical mediators of apoptosis by rapid release of apoptotic inducing factor, cytochrome c, activation of caspase-9 and caspase-3 and possibly other protein factors, which promote caspase activation [30]. PDT induced apoptosis has attracted great attention since the last decade [19]. PDT is a potent modulator of apoptosis in many cell types and apoptosis is more effective than necrosis for cell inactivation at a lower dose. Two major apoptotic pathways have been characterized: the death receptor-mediated and the mitochondria-mediated apoptosis. PDT-induced cytotoxic species have a short lifetime and only act within a limited distance. Therefore, the uptake and subcellular localization of photosensitizer by cancer cells is critical in PDT-induced direct cytotoxicity [31,32]. The links between PDT effects and mitochondria-mediated apoptotic pathways have been identified in numerous photosensitizers that can be localized in the mitochondria [33].
Apoptosis, or programmed cell death, is a highly regulated cell death process and morphologically characterized by cell shrinkage, membrane blebbing and DNA fragmentation. All those typical apoptotic morphological changes (e.g. cell shrinkage, membrane blebbing) were observed in pancreatic cancer cells lines at the dose level of LD50 (data not shown). Further analysis using propidium iodide staining and flow cytometry confirmed that there was still a small portion of PDT-treated cells underwent apoptosis at the time point of 24 h after PDT treatment (see Fig. 3).
The mitochondria are one of the initiating targets concerned for apoptosis. The major apoptotic factor in the mitochondria-regulated pathway is cytochrome c. A disruption of mitochondria membrane function will cause a rapid loss of the mitochondrial inner transmembrane potential (Δψm) and promote leakage of cytochrome c from the mitochondria. Cytochrome c in turn triggers caspase activation by binding to the caspase-activating protein, Apaf-1. In the presence of deoxyadenosine triphosphate (dATP), Apaf-1 subsequently activates procaspase-9 resulting in the formation of functional apoptosome that will activate procaspase-3, one of the effector caspases. The downstream of these effector caspases (including 3, 6, 7 and 8) will provoke the degradation of genomic DNA into nucleosome-sized fragments [34]. The cleavage of caspase-9, which occurred during mitochondrial damage and cytochrome c release, was detected after PDT (see Fig. 4). Cleaved caspase-9 could further process other caspase members (e.g. caspase-3 and caspase-7) and initiate a caspase cascade. By detecting the cleaved caspase-3 fragment and by the disappearance of the uncleaved precursor form of caspases-3, Photofrin PDT-induced apoptosis was further confirmed. Caspase-3 is one of the key executioners of apoptosis and responsible for the proteolytic cleavage of many key proteins including PARP which was detected in all six pancreatic cancer cell lines, indicating that apoptotic cell death was indeed involved in Photofrin PDT-mediated photocytotoxi-city. Meanwhile, cellular levels of the uncleaved precursor form of caspase-8 decreased and that of cleaved caspase-8 fragment increased, indicating the activation of another downstream effector.
Pancreatic cancer is a challenging disease with a median survival of less than 6 months and has an overall 5-year survival rate of less than 4%. Effective therapies for locally advanced or metastatic tumors are very limited and curatively resected patients experience relapse in over 80% of cases. This bad prognosis reflects the aggressive biology of the disease that can lead to unrestrained proliferation, insensitivity to growth inhibitory signals as well as evasion of apoptosis [35,36]. Although in vitro study demonstrates that Photofrin PDT can induce apoptosis, the role of PDT-induced apoptosis in clinical setup need to be further examined.
Previous in vitro and in vivo studies suggest that pancreatic cancer cells are sensitive to PDT-mediated with other photosensitizers (e.g. ALA, AlPcS, hypericin, pheopharbide, Pterin and verteporfin) [37—42]. PDT might be effective for chemotherapy insensitive pancreatic cancer cells [43]. Although some of those photosensitizers are superior to Photofrin in terms of known chemical structure, longer absorption wavelength, potency and shorter skin photosensitization, in vitro study of Photofrin PDT on a panel of human pancreatic cancer cells can serve as a good reference or baseline for comparison purposes.
In summary, Photofrin PDT can effectively induce early apoptotic responses after mitochondrial damage in human pancreatic cancer cells and the efficiency of photodynamic killing is high. Further in vivo studies are worth investigating.
Acknowledgements
The authors are grateful to Dr. Ann Thor (Department of Pathology, University of Colorado Denver) for her constantly support in this work, Biolitec A.G. (Germany) and Shenzhen MicroMed Tech Co. Ltd. (China) for providing the diode laser and fibers, and the Clinical Educational Foundation of Changhai Hospital for the financial support to Dr. Luowei Wang. This work was supported in part by an NIH grant (CA 43892).
References
- 1.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–20. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleeff J, Michalski C, Friess H, Büchler MW. Pancreatic cancer: from bench to 5-year survival. Pancreas. 2006;33:111–8. doi: 10.1097/01.mpa.0000229010.62538.f2. [DOI] [PubMed] [Google Scholar]
- 3.Costello E, Neoptolemos JP. Pancreatic cancer in 2010: new insights for early intervention and detection. Nature Reviews Gastroenterology and Hepatology. 2011;8:71–3. doi: 10.1038/nrgastro.2010.214. [DOI] [PubMed] [Google Scholar]
- 4.Moesta KT, Schlag P, Douglass HJ, Mang TS. Evaluating the role of photodynamic therapy in the management of pancreatic cancer. Lasers in Surgery and Medicine. 1995;16:84–92. doi: 10.1002/lsm.1900160112. [DOI] [PubMed] [Google Scholar]
- 5.Ayaru L, Bown SG, Pereira SP. Photodynamic therapy for pancreatic carcinoma: experimental and clinical studies. Photodiagnosis and Photodynamic Therapy. 2004;1:145–55. doi: 10.1016/S1572-1000(04)00038-9. [DOI] [PubMed] [Google Scholar]
- 6.Huang Z, Xu H, Meyers AD, et al. Photodynamic therapy for treatment of solid tumors — potential and technical challenges. Technology in Cancer Research & Treatment. 2008;7:309–20. doi: 10.1177/153303460800700405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castano AP, Tatiana N, Demidova Michael R. Hamblin mechanisms in photodynamic therapy: part one — photosensitizers, photochemistry and cellular localization. Photodiagnosis and Photodynamic Therapy. 2004;1:279–93. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Z. A review of progress in clinical photodynamic therapy. Technology in Cancer Research & Treatment. 2005;4:283–93. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayaru L, Bown SG, Pereira SP. Photodynamic therapy for pancreatic and biliary tract carcinoma. International Journal of Gastrointestinal Cancer. 2005;35:1–13. doi: 10.1385/IJGC:35:1:001. [DOI] [PubMed] [Google Scholar]
- 10.Fan BG, Andrén-Sandberg A. Photodynamic therapy for pancreatic cancer. Pancreas. 2007;34:385–9. doi: 10.1097/mpa.0b013e3180439c50. [DOI] [PubMed] [Google Scholar]
- 11.Bown SG, Rogowska AZ, Whitelaw DE, et al. Photodynamic therapy for cancer of the pancreas. Gut. 2002;50:549–57. doi: 10.1136/gut.50.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfsen HC. Photodynamic therapy for pancreatic cancer: let's get serious. Gastrointestinal Endoscopy. 2008;67:961–3. doi: 10.1016/j.gie.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Allison RR, Downie GH, Cuenca R, Hu XH, Childs CJH, Sibata CH. Photosensitizers in clinical PDT. Photodiagnosis and Photo-dynamic Therapy. 2004;1:27–42. doi: 10.1016/S1572-1000(04)00007-9. [DOI] [PubMed] [Google Scholar]
- 14.Mang TS, Wieman TJ. Photodynamic therapy in the treatment of pancreatic carcinoma: dihematoporphyrin ether uptake and photobleaching kinetics. Photochemistry and Photobiology. 1987;46:853–8. doi: 10.1111/j.1751-1097.1987.tb04859.x. [DOI] [PubMed] [Google Scholar]
- 15.Schroder T, Chen IW, Sperling M, Bell RH, Jr, Brackett K, Joffe SN. Hematoporphyrin derivative uptake and photodynamic therapy in pancreatic carcinoma. Journal of Surgical Oncology. 1988;38:4–9. doi: 10.1002/jso.2930380103. [DOI] [PubMed] [Google Scholar]
- 16.Moesta KT, Greco WR, Nurse-Finlay SO, Parsons JC, Mang TS. Lack of reciprocity in drug and light dose dependence of photodynamic therapy of pancreatic adenocarcinoma in vitro. Cancer Research. 1995;55:3078–84. [PubMed] [Google Scholar]
- 17.Gukovskaya AS, Pandol SJ. Cell death pathways in pancreatitis and pancreatic cancer. Pancreatology. 2004;4:567–86. doi: 10.1159/000082182. [DOI] [PubMed] [Google Scholar]
- 18.Zaidi SI, Oleinick NL, Zaim MT, Mukhtar H. Apoptosis during photodynamic therapy-induced ablation of RIF-1 tumors in C3H mice: electron microscopic, histopathologic and biochemical evidence. Photochemistry and Photobiology. 1993;58:771–6. doi: 10.1111/j.1751-1097.1993.tb04969.x. [DOI] [PubMed] [Google Scholar]
- 19.Castano AP, Tatiana N, Demidova Hamblin MR. Mechanisms in photodynamic therapy: part two — cellular signaling, cell metabolism and modes of cell death. Photodiagnosis and Photodynamic Therapy. 2005;2:1–23. doi: 10.1016/S1572-1000(05)00030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He XY, Sikes RA, Thomsen S, Chung LW, Jacques SL. Photo-dynamic therapy with photofrin II induces programmed cell death in carcinoma cell lines. Photochemistry and Photobiology. 1994;59:468–73. doi: 10.1111/j.1751-1097.1994.tb05066.x. [DOI] [PubMed] [Google Scholar]
- 21.Dellinger M. Apoptosis or necrosis following Photofrin photosensitization: influence of the incubation protocol. Photochemistry and Photobiology. 1996;64:182–7. doi: 10.1111/j.1751-1097.1996.tb02440.x. [DOI] [PubMed] [Google Scholar]
- 22.Kessel D, Luo Y, Deng Y, Chang CK. The role of subcellular localization in initiation of apoptosis by photodynamic therapy. Photochemistry and Photobiology. 1997;65:422–6. doi: 10.1111/j.1751-1097.1997.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Z, Chen Q, Shakil A, et al. Hyperoxygenation enhances the tumor cell killing of photofrin-mediated photodynamic therapy. Photochemistry and Photobiology. 2003;78:496–502. doi: 10.1562/0031-8655(2003)078<0496:hettck>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Saczko J, Chwiłkowska A, Kulbacka J, et al. Photooxidative action in cancer and normal cells induced by the use of photofrin in photodynamic therapy. Folia Biologica. 2008;54:24–9. [PubMed] [Google Scholar]
- 25.Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nature Protocols. 2006;1:1458–61. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- 26.Allison RR, Sibata CH. Photofrin® photodynamic therapy: 2.0 mg/kg or not 2.0 mg/kg that is the question. Photodiagnosis and Photodynamic Therapy. 2008;5:112–9. doi: 10.1016/j.pdpdt.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Musser DA, Oseroff AR. The use of tetrazolium salts to determine sites of damage to the mitochondrial electron transport chain in intact cells following in vitro photodynamic therapy with Photofrin II. Photochemistry and Photobiology. 1994;59:621–6. doi: 10.1111/j.1751-1097.1994.tb09666.x. [DOI] [PubMed] [Google Scholar]
- 28.Wilson BC, Olivo M, Singh G. Subcellular localization of Photofrin and aminolevulinic acid and photodynamic cross-resistance in vitro in radiation-induced fibrosarcoma cells sensitive or resistant to photofrin-mediated photodynamic therapy. Photochemistry and Photobiology. 1997;65:166–76. doi: 10.1111/j.1751-1097.1997.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 29.Saczko J, Mazurkiewicz M, Chwiłkowska A, et al. Intracellular distribution of Photofrin in malignant and normal endothelial cell lines. Folia Biologica. 2007;53:7–12. [PubMed] [Google Scholar]
- 30.Green DR, Amarante-Mendes GP. The point of no return: mitochondria, caspases, and the commitment to cell death. Results and Problems in Cell Differentiation. 1998;24:45–61. doi: 10.1007/978-3-540-69185-3_3. [DOI] [PubMed] [Google Scholar]
- 31.Gomer CJ. Preclinical examination of first and second generation photosensitizers used in photodynamic therapy. Photochemistry and Photobiology. 1991;54:1093–107. doi: 10.1111/j.1751-1097.1991.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 32.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: Part three — photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis and Photodynamic Therapy. 2005;2:91–106. doi: 10.1016/S1572-1000(05)00060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almeida RD, Manadas BJ, Carvalho AP, Duarte CB. Intracellular signaling mechanisms in photodynamic therapy. Biochimica et Biophysica Acta. 2004;1704:59–86. doi: 10.1016/j.bbcan.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–6. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 35.Shi X, Friess H, Kleeff J, Ozawa F, Büchler MW. Pancreatic cancer: factors regulating tumor development, maintenance and metastasis. Pancreatology. 2001;1:517–24. doi: 10.1159/000055854. [DOI] [PubMed] [Google Scholar]
- 36.Welsch T, Kleeff J, Friess H. Molecular pathogenesis of pancreatic cancer: advances and challenges. Current Molecular Medicine. 2007;7:504–21. doi: 10.2174/156652407781387082. [DOI] [PubMed] [Google Scholar]
- 37.Ward AJ, Matthews EK. Cytotoxic, nuclear, and growth inhibitory effects of photodynamic drugs on pancreatic carcinoma cells. Cancer Letters. 1996;102:39–47. doi: 10.1016/0304-3835(96)04152-3. [DOI] [PubMed] [Google Scholar]
- 38.Hajri A, Coffy S, Vallat F, Evrard S, Marescaux J, Aprahamian M. Human pancreatic carcinoma cells are sensitive to photo-dynamic therapy in vitro and in vivo. British Journal of Surgery. 1999;86:899–906. doi: 10.1046/j.1365-2168.1999.01132.x. [DOI] [PubMed] [Google Scholar]
- 39.Liu CD, Kwan D, Saxton RE, McFadden DW. Hypericin and photodynamic therapy decreases human pancreatic cancer in vitro and in vivo. Journal of Surgical Research. 2000;93:137–43. doi: 10.1006/jsre.2000.5949. [DOI] [PubMed] [Google Scholar]
- 40.Whitaker CJ, Battah SH, Forsyth MJ, Edwards C, Boyle RW, Matthews EK. Photosensitization of pancreatic tumour cells by delta-aminolaevulinic acid esters. Anti-Cancer Drug Design. 2000;15:161–70. [PubMed] [Google Scholar]
- 41.Ayaru L, Wittmann J, Macrobert AJ, Novelli M, Bown SG, Pereira SP. Photodynamic therapy using verteporfin photosensitization in the pancreas and surrounding tissues in the Syrian golden hamster. Pancreatology. 2007;7:20–7. doi: 10.1159/000101874. [DOI] [PubMed] [Google Scholar]
- 42.Miyoshi T, Arai T, Nonogawa M, et al. Anticancer photodynamic and non-photodynamic effects of pterin derivatives on a pancreatic cancer cell line. Journal of Pharmacological Science. 2008;107:221–5. doi: 10.1254/jphs.08002sc. [DOI] [PubMed] [Google Scholar]
- 43.Celli JP, Solban N, Liang A, Pereira SP, Hasan T. Verteporfin-based photodynamic therapy overcomes gemcitabine insensitivity in a panel of pancreatic cancer cell lines. Lasers in Surgery and Medicine. 2011;43:565–74. doi: 10.1002/lsm.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]