Abstract
The plasma level of the tumor necrosis factor-alpha receptor 2 (TNFR2) is associated with obesity phenotypes. However, the genetic polymorphisms for such an association have rarely been explored and are generally unknown. In this study, by employing a large sample of 1,873 subjects from 405 Caucasian nuclear families, we explored the association of 12 SNPs of the TNFR2 gene and obesity-related phenotypes, including body mass index (BMI), fat mass, and percentage fat mass (PFM). The within-family quantitative transmission disequilibrium test, which is robust to sample stratification, was implemented to evaluate the association of TNFR2 gene with obesity phenotypes. Evidence of association was obtained at SNP9 (rs5746059) with fat mass (P = 0.0002), BMI (P = 0.002), and PFM (P = 0.0006). The contribution of this polymorphism to the variation of fat mass and PFM was 6.24 and 7.82%, respectively. Individuals carrying allele A at the SNP9 site had a 4.6% higher fat mass and a 2.5% increased PFM compared to noncarriers. The results remained significant even after correction for multiple testing. Evidence of association between the TNFR2 gene and obesity phenotypes are also found in 700 independent Chinese Han and 1,000 random Caucasians samples. The results suggest that the TNFR2 gene polymorphisms contribute to the variation of obesity phenotypes.
Introduction
Obesity has become a common metabolic disorder associated with higher mortality, as it may lead to serious metabolic syndromes and diseases with lethal risks such as stroke, cancer, and sleep-breathing disorders (Kopelman 2000). Genetic factors contribute significantly to the etiology of obesity, with the heritability of body mass index (BMI), fat mass, and percentage fat mass (PFM) ranging from 0.5 to 0.9, 0.2 to 0.7, and 0.6 to 0.8, respectively (Liu et al. 2003).
Tumor necrosis factor-alpha (TNFα) regulates obesity through two genetically distinct receptors, TNFα receptor 1 (TNFR1) and TNFR2. This study focuses on TNFR2. Soluble TNFα plays an important role in energy regulation. In adipocytes, it regulates the secretion of leptin (Kirchgessner et al. 1997), which is important for systemic energy regulation. Polymorphisms of the TNFα gene are linked and associated with obesity (Herrmann et al. 1998; Norman et al. 1995). Moreover, the TNFα gene and protein expression level in adipose tissue is elevated in obesity rodent models and human beings, and decreases with weight loss (Good et al.2006; Hotamisligil et al. 1993, 1995; Kern et al. 1995). The TNFR2 gene expression correlated tightly with that of TNFα and it is also elevated in obesity (Good et al. 2006; Hotamisligil et al. 1997). Compared to the lean controls, the TNFR2 expression level increases twofold in fat tissue and sixfold in plasma in obese women (Hotamisligil et al.1997). The plasma level of TNFR2 is positively correlated with BMI, fat mass, and waist-to-hip ratio (Fernandez-Real et al. 1998). Consistently, a study in identical twin pairs who had an average 18-kg intrapair difference in body weight showed that the serum-soluble TNFR2 level was higher in the obese individual (Ronnemaa et al. 2000). All this evidence supports that TNFR2 is potentially important for obesity.
The TNFR2 gene is composed of 10 exons and is situated in chromosomal 1p32, a region that has been replicated to be linked with obesity-related phenotypes by several independent studies (Chagnon et al. 1997; Saar et al. 2003). Although the importance of TNFR2 for obesity has been revealed, few genetic association studies have comprehensively investigated the polymorphisms in the gene accounting for obesity phenotypes (Fernandez-Real et al. 2000; Huang et al. 2006). The earlier association studies on TNFR2 and obesity have two limitations: first, they examined only two common markers in the TNFR2 gene (Fernandez-Real et al. 2000; Huang et al. 2006); and second, potential population stratification/admixture in the unrelated samples (Chagnon et al. 1997) may yield false-positive results (Deng 2001). These limitations impeded testing a candidate gene exhaustively and definitively. In this study, we tested associations of the TNFR2 gene with the obesity phenotypes using high-density SNPs in a large sample of Caucasian nuclear families. The TDT method was employed to resolve the hidden population substructure, with the aim of providing robust findings for follow-up studies.
Methods
Subjects
The Creighton University Institutional Review Board approved this study. All the study subjects signed informed-consent documents before entering the project. The study subjects came from an expanding database created for ongoing studies in the Osteoporosis Research Center (ORC) of Creighton University to search for genes underlying common human complex traits, including obesity and osteoporosis. The families were recruited without consideration of body weight/fat mass. The sampling scheme and exclusion criteria have been detailed elsewhere (Deng et al. 2002). Briefly, patients with chronic diseases and conditions that may potentially affect the development of human obesity as well as other studied traits were excluded from the study. All the study subjects were Caucasians of European origin and were recruited from Omaha, NE, Midwestern US.
In this study, we selected a total of 405 Caucasian nuclear families with 1,873 individuals. The sample includes 740 parents (ages with a mean ± SD of 62.62 ± 10.52), 389 male children (ages with a mean ± SD of 36.00 ± 10.92), and 744 female children (ages with a mean ± SD of 37.73 ± 10.33). The nuclear families were selected based on the following criteria: (1) families with two parents and at least two children have priority in selection; (2) if there is only one parent available, families with at least three children have priority in selection; and (3) age preference for offsprings of less than 50 years or premenopausal for females. Among them, 341 families comprised both parents and at least one offspring. The remaining 64 families, with one or no parent, contained two or more children. There were 27.2, 22.7, 22.7, and 27.4% nuclear families with one, two, three, and more than three children, respectively, yielding 1,512 sibling pairs in our sample. The descriptive characteristics of the study subjects have been detailed in our previous studies (Guo et al. 2006; Liu et al. 2004)
Phenotype measurement
We calculated BMI as body weight (in kilograms) divided by the square of height (in meters). Weight was measured in light indoor clothing without shoes, using a calibrated balance beam scale, and height was measured using a calibrated stadiometer. The PFM is the ratio of fat mass divided by body weight (i.e., the sum of fat mass, lean mass, and bone mass). Fat mass and lean mass were measured by dual-energy X-ray absorptiometry using a Hologic 2000+ or 4500 scanner (Hologic, Bedford, MA, USA). Both machines were calibrated daily. The measurement precision of BMI as reflected by the coefficient of variation was 0.2%. The coefficients of variation for fat mass, PFM, and lean mass were 2.2, 2.2 and 1.0%, respectively, for measurements obtained on the Hologic 2000+, and 1.2, 1.1 and 0.7%, respectively, for measurements obtained on the Hologic 4500. Generally, members of the same family were measured on the same type of machine, ensuring minimal or no effect on our analyses due to measurements by different scanners.
Genotyping
For each subject, DNA was extracted from peripheral blood using a Puregene DNA Isolation Kit (Gentra Systems, Minneapolis, MN, USA). DNA concentration was assessed by a DU530 UV/VIS Spectrophotometer (Beckman Coulter, Inc., Fullerton, CA, USA).
We selected SNPs on the basis of the following criteria: (1) validation status (validated experimentally in human populations), especially in Caucasians; (2) degree of heterozygosity, i.e., minor allele frequency (MAF) > 0.05; (3) reported to dbSNP by various sources; and (4) a high average density of one SNP per 5.4 kb. A total of 13 SNPs within and around the TNFR2 gene were selected and successfully genotyped using the high-throughput BeadArray SNP genotyping technology of Illumina Inc. (San Diego, CA, USA), with subjects’ phenotype information blinded. For the 13 SNPs, one SNP (rs625847) has MAF < 0.05 and was removed for subsequent data analyses according to common practice (Newton-Cheh and Hirschhorn 2005). PedCheck (O’Connell and Weeks 1998) was performed to ensure that the genotype data conform to Mendelian inheritance pattern at all the marker loci. Five of the overall 22,476 genotypes (0.02%) were omitted because of the violation of the Mendelian inheritance rule. Hardy–Weinberg equilibrium (HWE) was tested and SNPs that significantly departed from HWE at the P < 0.01 level among parents were discarded. For SNP genotyping, the reproducibility rate as revealed through blind duplicating was 100%.
Statistical analyses
In total, 12 SNPs with MAF > 0.05 were used for the data analyses. LD block structure of the TNFR2 gene was examined using the Haploview program (Barrett et al. 2005). The D′ values for all pairs of SNPs were calculated and the haplotype blocks were estimated using the confidence-interval method (Gabriel et al. 2002). SNPs with low MAF may inflate estimates of D′ and the use of confidence-bound estimates for D′ reduces this bias. The default settings were used in these analyses invoking a one-side upper 95% confidence bound of D′ > 0.98 and a lower bound of >0.7 to define SNP pairs in strong LD. A block is identified when at least 95% of SNP pairs in a region meet these criteria for strong LD. Haplotypes were reconstructed and their frequencies estimated using an accelerated expectation-maximization (EM) algorithm similar to the partition/ligation method (Qin et al. 2002) implemented in Haploview.
Under a flexible variance-component framework, tests of population stratification, total association, and within-family associations were implemented for both the single SNP marker and haplotype blocks in the program package QTDT (quantitative transmission disequilibrium test, http://www.sph.umich.edu/csg/abecasis/QTDT/) (Abecasis et al. 2000a, b). The data analyses were performed under the assumption of an additive model. The total association evaluates the evidence for association at the whole population level using all information, including within-family and between-family components and is susceptible to spurious results due to population stratification. The within-family association test is a TDT method and therefore robust to population stratification. Initially, population stratification was tested for single SNPs. If population stratification existed, then only within-family association was tested. The permutation test built into the QTDT was performed to set up the threshold of the significance levels. When significant association is observed, the approximate phenotypic variation, due to the detected marker, is calculated as 2p(1 – p)a2, where p is the allele frequency of the marker, and a is the estimate of additive effect, i.e., E(βw) = a (Abecasis et al. 2000a). In all the statistical analyses, age and sex have been adopted as covariates (if they have significant effects in our sample) to adjust for BMI, fat mass, and PFM.
Results
The information about the 12 SNPs of the TNFR2 gene used for data analyses is listed in Table 1. The SNPs are within or around the TNFR2 gene, with average marker distance of ~5.4 kb. The SNP markers are presented according to their physical location, with an average MAF of 26%, ranging from 6 to 47%.
Table 1.
General information of the analyzed TNFR2 SNPs in this study
| SNP | Namea | Positionb | Intermarker interval (bp) |
Polymorphismcc | MAF | Role |
|---|---|---|---|---|---|---|
| 1 | rs1148459 | 12,140,439 | - | C/A | 0.467 | Promoter |
| 2 | rs590368 | 12,146,038 | 5,599 | G/A | 0.378 | Promoter |
| 3 | rs652625 | 12,147,938 | 1,900 | T/A | 0.065 | Promoter |
| 4 | rs496888 | 12,155,393 | 7,455 | A/G | 0.300 | Intron 1 |
| 5 | rs499646 | 12,161,676 | 6,283 | G/A | 0.057 | Intron 1 |
| 6 | rs17037696 | 12,165,861 | 4,185 | G/A | 0.232 | Intron 1 |
| 7 | rs945439 | 12,171,529 | 5,668 | A/G | 0.226 | Exon 2 |
| 8 | rs2275416 | 12,176,788 | 5,259 | G/A | 0.187 | Intron 7 |
| 9 | rs5746059 | 12,185,379 | 8,591 | A/G | 0.209 | Intron 9 |
| 10 | rs1061628 | 12,190,586 | 5,207 | G/A | 0.396 | Exon 10 |
| 11 | rs235214 | 12,194,090 | 3,504 | G/A | 0.151 | 3′ UTR |
| 12 | rs4846100 | 12,200,251 | 6,161 | C/G | 0.442 | 3′ UTR |
SNP ID in the dbSNP (http://www.ncbi.nlm.nih.gov/SNP)
Chromosome position is based on the dbSNP (http://www.ncbi.nlm.nih.gov/SNP)
The second allele is the minor allele
In our sample, both age and sex have significant effect on BMI (P < 1E-10), fat mass (P < 3E-16), and PFM (P < 3E-54). Age and sex together account for 9.40, 7.78, and 44.16% of fat mass, BMI, and PFM variation, respectively. Obesity phenotypes are adjusted for these two covariates of the data analyses, and all the presented results take into account the effects of age and sex. Table 2 presents the results of the QTDT population stratification test for single-locus analyses. Because population stratification was detected at SNP9 and SNP11 for all obesity phenotypes, only the within-family association test was adopted and the results are presented in Table 3.
Table 2.
Results of the population stratification test
| SNP | Name | Fat mass | BMI | PFM |
|---|---|---|---|---|
| 1 | rs1148459 | 0.085 | 0.135 | 0.172 |
| 2 | rs590368 | 0.496 | 0.996 | 0.349 |
| 3 | rs652625 | 0.805 | 0.928 | 0.559 |
| 4 | rs496888 | 0.880 | 0.816 | 0.689 |
| 5 | rs499646 | 0.649 | 0.612 | 0.848 |
| 6 | rs17037696 | 0.262 | 0.203 | 0.214 |
| 7 | rs945439 | 0.652 | 0.705 | 0.617 |
| 8 | rs2275416 | 0.179 | 0.208 | 0.111 |
| 9 | rs5746059 | 0.002 | 0.038 | 0.002 |
| 10 | rs1061628 | 0.156 | 0.841 | 0.129 |
| 11 | rs235214 | 0.034 | 0.058 | 0.035 |
| 12 | rs4846100 | 0.520 | 0.862 | 0.561 |
Empirical P values <0.05 are in bold
Table 3.
P values of within-family association test (TDT) and permutation results
| SNP | Name | Fat mass | BMI | PFM |
|---|---|---|---|---|
| 1 | rs1148459 | 0.005 (0.015) | 0.016 (0.032) | 0.022 (0.057) |
| 2 | rs590368 | 0.161 | 0.331 | 0.149 |
| 3 | rs652625 | 0.961 | 0.650 | 0.540 |
| 4 | rs496888 | 0.584 | 0.616 | 0.641 |
| 5 | rs499646 | 0.745 | 0.570 | 0.955 |
| 6 | rs17037696 | 0.124 | 0.060 (0.109) | 0.186 |
| 7 | rs945439 | 1.000 | 0.710 | 0.830 |
| 8 | rs2275416 | 0.078 (0.056) | 0.049 (0.045) | 0.088 (0.071) |
| 9 | rs5746059 | 0.0002 (< 0.0001) * | 0.002 (0.001) * | 0.0006 (<0.0001) * |
| 10 | rs1061628 | 0.176 | 0.884 | 0.121 |
| 11 | rs235214 | 0.024 (0.012) | 0.014 (0.003) | 0.039 (0.033) |
| 12 | rs4846100 | 0.671 | 0.896 | 0.624 |
|
| ||||
| LD block | SNPs in the Block | Fat mass | BMI | PFM |
|
| ||||
| Block 1 | SNP1, SNP2 | 0.043 (0.070) | 0.113 | 0.096 (0.157) |
| Block 2 | SNP3-SNP5 | 0.025 (0.051) | 0.027 (0.054) | 0.071 (0.146) |
| Block 3 | SNP6-SNP8 | 0.195 | 0.109 | 0.242 |
The permutation test was performed for those TDT results with P < 0.05 and listed in the parenthesis. Bold values indicate significant P values (P < 0.05)
The results remain significant even after the correction for multiple testing
Three SNPs (SNP1, SNP9, and SNP11) showed evidence of association with fat mass, BMI, and PFM (P < 0.05). Evidence of association was obtained at SNP1 (P = 0.005), SNP9 (P = 0.0002), and SNP11 (P = 0.024) with fat mass; SNP1 (P = 0.016), SNP8 (P = 0.049), SNP9 (P = 0.002), and SNP11 (P = 0.014) with BMI; SNP1 (P = 0.022), SNP9 (P = 0.0006), and SNP11 (P = 0.039) with PFM. Among the 12 SNPs, SNP9 showed the most significant consistent associations across all phenotypes (P < 0.01).
It should be pointed out that the above significant associations were only at the nominally significant level (P < 0.05) before the multiple testing corrections. Because of the dense markers and three correlated phenotypes used for data analysis, the Monte-Carlo permutation was performed for 1,000 repetitions to adjust for the multiple tests (Table 3). After 1,000 permutations, it was established that a P < 0.002 was required for an individual test to achieve a global significance level of 0.05. Thus, SNP9 retained significant association with all three obesity phenotypes (P ≤ 0.001) even after correction for multiple testing. The contribution of SNP9 to the phenotypic variation of fat mass, BMI, and PFM was 6.24, 0.57, and 7.82%, respectively. Individuals carrying allele A had 4.6% higher fat mass and 2.5% increased PFM compared to noncarriers.
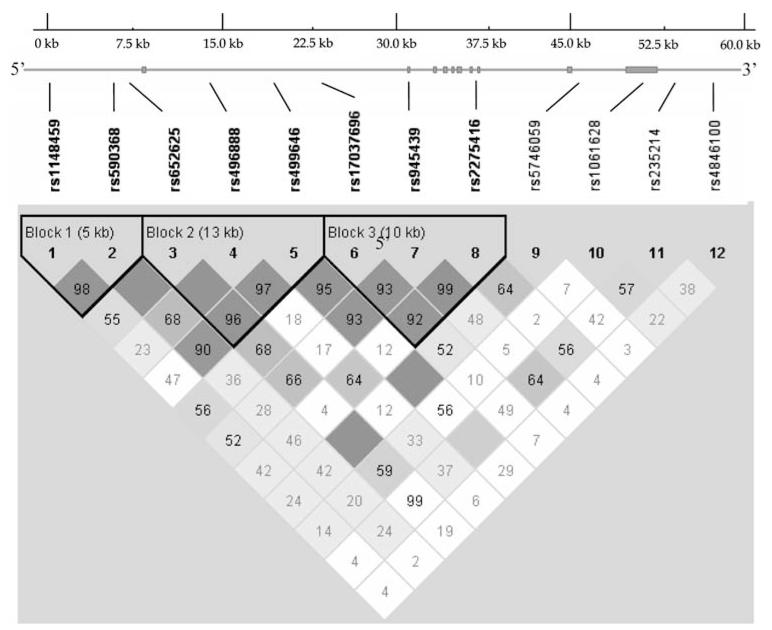
We identified three blocks with high linkage disequilibrium (LD), ranging in size from 5 to 10 kb (Fig. 1). Blocks 1–3 mainly spanned from promoter to intron 7. Four SNPs near/in the 3′-UTR had low LD with each other, and could not be assigned to any blocks. Based on the haplotypes constructed by SNPs, a multiallelic within-family association test was conducted for the identified haplotype blocks. The haplotype analyses, as an important complementary source in the study, further supported the association of the TNFR2 gene with obesity. The haplotype Block 1, representing SNP1 and SNP2, was found to be associated with fat mass (P = 0.043). Haplotype Block 2, containing SNP3, SNP4, and SNP5, was associated with fat mass (P = 0.025) and BMI (P = 0.027). However, these associations only attained marginal significance after multiple testing corrections (Table 3).
Fig. 1.
Gene structure and LD patterns for the TNRF2 gene. Exons are depicted as filled boxes. Positions of the 12 SNPs used in the association study are sketched. LD block structure, as depicted by Haploview, is shown in the bottom frame. The increasing degree of darkness from white to black represents the increasing strength of LD. Values for D′ = 1 are dark black boxes and D′ < 1 (indicated as original value multiple 100) are shown in the cells
Discussion
In our study, associations with various obesity phenotypes were detected for some TNFR2 common polymorphisms. Our results are in agreement with those previously reported by Fernandez-Real et al. (2000), in which the polymorphism at the 3′-UTR is associated with BMI in human subjects and diet-treated type 2 diabetic patients (Fernandez-Real et al.2000). Our preliminary study (Huang et al. 2006) also detected a marginally significant association (P = 0.042) between the 13-repeat allele in intron 4 with the PFM. Compared with the previous studies, our study has several notable strengths. First, SNPs distributed densely across the TNFR2 gene were genotyped and exhaustively studied. Second, LD analysis was conducted based on the haplotype information extracted from the multiple SNPs, and the inferred blocks were used in the association tests. Third, the SNPs with high heterozygosity were used to maximize the power to find associations. Fourth, our study highlights the importance of the large sample size and the hidden population substructures in the genetic association study. The TDT approach, which is robust for the potential population stratification was employed in current study, and the 1,873 individuals from 405 nuclear families ensure sufficient power for detection of important variants. Fifth, the permutation approach was applied to circumvent the multiple testing problems afflicting the association study, which highly minimized the number of false-positive results.
It is anticipated that positive association results were detected here, because TNFR2 is overexpressed in adipose tissue in obese subjects (Hotamisligil et al. 1995), and the gene expression and plasma levels of TNFR2 is different between the obese and lean (Fernandez-Real et al. 1998; Hotamisligil et al. 1997; Ronnemaa et al. 2000), as we briefly summarized in the “Introduction”. Additionally, mice lacking TNFR2 exhibit lower body weight and improved insulin sensitivity (Schreyer et al. 1998). More-over, different independent studies reported that TNFR2 is correlated with obesity-associated diseases, such as hypertension (Glenn et al. 2000), hyperlipidemia (Geurts et al.2000), coronary artery disease (Benjafield et al. 2001), and insulin resistance (Fernandez-Real et al. 2000).
In our study, SNP11 (rs235214) resided in the 3′-UTR of the TNFR2 gene, and was found to be marginally associated with obesity phenotypes. The 3′-UTR region was associated with obesity (Fernandez-Real et al. 2000), hypertension (Glenn et al. 2000), and cardiovascular disease (Benjafield et al. 2001), and effected gene expression and mRNA stability (Puga et al. 2005). Independent support for the importance of 3′-UTR is provided by our genome-wide association study on obesity (Liu et al. 2008). SNP rs235237 in 3′-UTR of the TNFR2 gene was geno-typed in 1,000 Caucasian subjects, and it was significantly associated with PFM (P = 0.0156), fat mass (P = 0.0376), and BMI (P = 0.0082).
In this study, the most significant obesity-associated polymorphism is SNP9 (rs5746059). It remains significant even after the adjustment of multiple testing. Similar LD patterns around the SNP9 were observed when we compared the LD structure in our sample with the Caucasian samples’ LD structure in the HapMap project (http://www.hapmap.org/). Both the LD structure for HapMap and ours is in low LD, and the SNP9 (rs5746059) could not be assigned to any of the blocks with any SNPs measured in the 3′-UTR. We hypothesize that the strong association between SNP9 and obesity may be attributed to the strong LD of SNP9 with potential functional genetic variants.
Current and earlier association studies on the TNFR2 and obesity (Fernandez-Real et al. 2000; Huang et al. 2006) are all limited to Caucasian samples. To test the association between the TNFR2 gene and obesity in non-Caucasian ethnic groups, we analyzed the data in our genome-wide association study using Affymetrix 500K SNP arrays in a sample of 700 unrelated Han Chinese. For the TNFR2 gene, SNP rs496888 (SNP4 in Table 3) was significantly associated with fat mass (P = 0.0042) and PFM (P = 0.0025). The results suggest that our finding can be generalized to non-Caucasian ethnic groups.
In conclusion, this study further confirmed a significant association between the TNFR2 gene and obesity. Our efforts, combined together with the previous evidence of TNFR2 function in metabolism, supported its variants as important genetic factors for obesity.
Acknowledgments
Investigators of this work were partially supported by grants from NIH (R01 AR050496, R21 AG 027110, R01 AG026564, R21 AA015973, and P50 AR055081) and from the Cancer and Smoking Disease Research Bone Biology Program, and the Nebraska tobacco settlement biomedical research development award, both supported by the State of Nebraska. The study also benefited from grants from National Science Foundation of China, Huo Ying Dong Education Foundation, Hunan Province, Xi’an Jiaotong University, and the Ministry of Education of China. We thank Mr. Darin L. Jensen and Ms. Ann Goering for their constructive input during the preparation of this manuscript.
Contributor Information
Lan-Juan Zhao, Osteoporosis Research Center, Creighton University, Omaha, NE 68131, USAlanjuanzhao@creighton.edu; Department of Biomedical Sciences, Creighton University, Omaha, NE 68131, USA; Department of Orthopedic Surgery, University of Missouri-Kansas City, 2411 Holmes Street, Room M3-CO3, Kansas City, MO 64108-2792, USA; Department of Basic Medical Sciences, University of Missouri-Kansas City, 2411 Holmes Street, Room M3-CO3, Kansas City, MO 64108-2792, USA.
Dong-Hai Xiong, Osteoporosis Research Center, Creighton University, Omaha, NE 68131, USA; Department of Biomedical Sciences, Creighton University, Omaha, NE 68131, USA.
Feng Pan, Osteoporosis Research Center, Creighton University, Omaha, NE 68131, USA; Department of Biomedical Sciences, Creighton University, Omaha, NE 68131, USA.
Xiao-Gang Liu, The Key Laboratory of Biomedical Information Engineering of Ministry of Education and Institute of Molecular Genetics, School of Life Science and Technology, Xi’an Jiaotong University, Xi’an 710049, People’s Republic of China.
Robert R. Recker, Osteoporosis Research Center, Creighton University, Omaha, NE 68131, USA; Department of Biomedical Sciences, Creighton University, Omaha, NE 68131, USA
Hong-Wen Deng, Laboratory of Biomedical Information Engineering of Ministry of Education and Institute of Molecular Genetics, School of Life Science and Technology, Xi’an Jiaotong University, Xi’an 710049, People’s Republic of China; The Key Laboratory of Biomedical Information Engineering of Ministry of Education and Institute of Molecular Genetics, School of Life Science and Technology, Xi’an Jiaotong University, Xi’an 710049, People’s Republic of China; Department of Orthopedic Surgery, University of Missouri-Kansas City, 2411 Holmes Street, Room M3-CO3, Kansas City, MO 64108-2792, USA; Department of Basic Medical Sciences, University of Missouri-Kansas City, 2411 Holmes Street, Room M3-CO3, Kansas City, MO 64108-2792, USA.
References
- Abecasis GR, Cardon LR, Cookson WO. A general test of association for quantitative traits in nuclear families. Am J Hum Genet. 2000a;66:279–292. doi: 10.1086/302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abecasis GR, Cookson WO, Cardon LR. Pedigree tests of transmission disequilibrium. Eur J Hum Genet. 2000b;8:545–551. doi: 10.1038/sj.ejhg.5200494. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Benjafield AV, Wang XL, Morris BJ. Tumor necrosis factor receptor 2 gene (TNFRSF1B) in genetic basis of coronary artery disease. J Mol Med. 2001;79:109–115. doi: 10.1007/s001090000168. [DOI] [PubMed] [Google Scholar]
- Chagnon YC, Perusse L, Lamothe M, Chagnon M, Nadeau A, Dionne FT, Gagnon J, Chung WK, Leibel RL, Bouchard C. Suggestive linkages between markers on human 1p32-p22 and body fat and insulin levels in the Quebec Family Study. Obes Res. 1997;5:115–121. doi: 10.1002/j.1550-8528.1997.tb00651.x. [DOI] [PubMed] [Google Scholar]
- Deng HW. Population admixture may appear to mask, change or reverse genetic effects of genes underlying complex traits. Genetics. 2001;159:1319–1323. doi: 10.1093/genetics/159.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng HW, Deng H, Liu YJ, Liu YZ, Xu FH, Shen H, Conway T, Li JL, Huang QY, Davies KM, Recker RR. A genomewide link-age scan for quantitative-trait loci for obesity phenotypes. Am J Hum Genet. 2002;70:1138–1151. doi: 10.1086/339934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Real JM, Broch M, Ricart W, Casamitjana R, Gutierrez C, Vendrell J, Richart C. Plasma levels of the soluble fraction of tumor necrosis factor receptor 2 and insulin resistance. Diabetes. 1998;47:1757–1762. doi: 10.2337/diabetes.47.11.1757. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Vendrell J, Ricart W, Broch M, Gutierrez C, Casamitjana R, Oriola J, Richart C. Polymorphism of the tumor necrosis factor-alpha receptor 2 gene is associated with obesity, leptin levels, and insulin resistance in young subjects and diet-treated type 2 diabetic patients. Diabetes Care. 2000;23:831–837. doi: 10.2337/diacare.23.6.831. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Geurts JM, Janssen RG, van Greevenbroek MM, van der Kallen CJ, Cantor RM, Bu X, Aouizerat BE, Allayee H, Rotter JI, de Bruin TW. Identification of TNFRSF1B as a novel modifier gene in familial combined hyperlipidemia. Hum Mol Genet. 2000;9:2067–2074. doi: 10.1093/hmg/9.14.2067. [DOI] [PubMed] [Google Scholar]
- Glenn CL, Wang WY, Benjafield AV, Morris BJ. Linkage and association of tumor necrosis factor receptor 2 locus with hypertension, hypercholesterolemia and plasma shed receptor. Hum Mol Genet. 2000;9:1943–1949. doi: 10.1093/hmg/9.13.1943. [DOI] [PubMed] [Google Scholar]
- Good M, Newell FM, Haupt LM, Whitehead JP, Hutley LJ, Prins JB. TNF and TNF receptor expression and insulin sensitivity in human omental and subcutaneous adipose tissue—influence of BMI and adipose distribution. Diab Vasc Dis Res. 2006;3:26–33. doi: 10.3132/dvdr.2006.003. [DOI] [PubMed] [Google Scholar]
- Guo YF, Xiong DH, Shen H, Zhao LJ, Xiao P, Guo Y, Wang W, Yang TL, Recker RR, Deng HW. Polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J Med Genet. 2006;43:798–803. doi: 10.1136/jmg.2006.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann SM, Ricard S, Nicaud V, Mallet C, Arveiler D, Evans A, Ruidavets JB, Luc G, Bara L, Parra HJ, Poirier O, Cambien F. Polymorphisms of the tumour necrosis factor-alpha gene, coronary heart disease and obesity. Eur J Clin Invest. 1998;28:59–66. doi: 10.1046/j.1365-2362.1998.00244.x. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, Atkinson RL, Spiegelman BM. Differential regulation of the p80 tumor necrosis factor receptor in human obesity and insulin resistance. Diabetes. 1997;46:451–455. doi: 10.2337/diab.46.3.451. [DOI] [PubMed] [Google Scholar]
- Huang QY, Shen H, Deng HY, Conway T, Elze L, Davies KM, Recker RR, Deng HW. Linkage and association between CA repeat polymorphism of the TNFR2 gene and obesity phenotypes in two independent Caucasian populations. Yi Chuan Xue Bao. 2006;33:775–781. doi: 10.1016/S0379-4172(06)60110-8. [DOI] [PubMed] [Google Scholar]
- Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchgessner TG, Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Tumor necrosis factor-alpha contributes to obesity-related hyperleptinemia by regulating leptin release from adipocytes. J Clin Invest. 1997;100:2777–2782. doi: 10.1172/JCI119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Araujo S, Recker RR, Deng HW. Molecular and genetic mechanisms of obesity: implications for future management. Curr Mol Med. 2003;3:325–340. doi: 10.2174/1566524033479735. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Liu XG, Wang L, Dina C, Yan H, Liu JF, Levy S, Papasian CJ, Drees BM, Hamilton JJ, Meyre D, Delplanque J, Pei YF, Zhang L, Recker RR, Froguel P, Deng HW. Genome-wide association scans identified CTNNBL1 as a novel gene for obesity. Hum Mol Genet. 2008;17:1803–1813. doi: 10.1093/hmg/ddn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Rocha-Sanchez SM, Liu PY, Long JR, Lu Y, Elze L, Recker RR, Deng HW. Tests of linkage and/or association of the LEPR gene polymorphisms with obesity phenotypes in Caucasian nuclear families. Physiol Genomics. 2004;17:101–106. doi: 10.1152/physiolgenomics.00213.2003. [DOI] [PubMed] [Google Scholar]
- Newton-Cheh C, Hirschhorn JN. Genetic association studies of complex traits: design and analysis issues. Mutat Res. 2005;573:54–69. doi: 10.1016/j.mrfmmm.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Norman RA, Bogardus C, Ravussin E. Linkage between obesity and a marker near the tumor necrosis factor-alpha locus in Pima Indians. J Clin Invest. 1995;96:158–162. doi: 10.1172/JCI118016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga I, Lainez B, Fernandez-Real JM, Buxade M, Broch M, Vendrell J, Espel E. A polymorphism in the 3′ untranslated region of the gene for tumor necrosis factor receptor 2 modulates reporter gene expression. Endocrinology. 2005;146:2210–2220. doi: 10.1210/en.2004-1366. [DOI] [PubMed] [Google Scholar]
- Qin ZS, Niu T, Liu JS. Partition–ligation–expectation–maximization algorithm for haplotype inference with single-nucleotide polymorphisms. Am J Hum Genet. 2002;71:1242–1247. doi: 10.1086/344207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnemaa T, Pulkki K, Kaprio J. Serum soluble tumor necrosis factor-alpha receptor 2 is elevated in obesity but is not related to insulin sensitivity: a study in identical twins discordant for obesity. J Clin Endocrinol Metab. 2000;85:2728–2732. doi: 10.1210/jcem.85.8.6720. [DOI] [PubMed] [Google Scholar]
- Saar K, Geller F, Ruschendorf F, Reis A, Friedel S, Schauble N, Nurnberg P, Siegfried W, Goldschmidt HP, Schafer H, Ziegler A, Remschmidt H, Hinney A, Hebebrand J. Genome scan for childhood and adolescent obesity in German families. Pediatrics. 2003;111:321–327. doi: 10.1542/peds.111.2.321. [DOI] [PubMed] [Google Scholar]
- Schreyer SA, Chua SC, Jr, LeBoeuf RC. Obesity and diabetes in TNF-alpha receptor-deficient mice. J Clin Invest. 1998;102:402–411. doi: 10.1172/JCI2849. [DOI] [PMC free article] [PubMed] [Google Scholar]