Abstract
Nonalcoholic fatty liver disease has emerged as the most prevalent chronic liver disease. Nonalcoholic steatohepatitis (NASH); the more severe form of NAFLD has an increased risk for the progression to cirrhosis. Recently NAFLD has also been linked to the increased risk of cardiovascular morbidity and extrahepatic malignancy, which further predisposes these individuals to increased mortality from non-liver related deaths. The available data suggest a more benign course for individuals with steatosis alone, but increased morbidity and mortality among those with advanced histological severity such as NASH and fibrosis. However, the natural course of NAFLD has yet to be precisely defined in large part due to the lack of universally accepted histologic definition of NAFLD, sampling error intrinsic to liver biopsy, and inconsistency among pathologists to diagnose the histologic findings that are essential to the diagnosis of NASH. Despite these limitations, a few studies have identified specific histologic findings (particularly fibrosis regardless of stage) that are able to predict NAFLD related mortality being most important.
Keywords: Non alcoholic steatohepatitis, fibrosis, steatosis, liver related mortality, Cardiovascular Morbidity
(I) Introduction
Non-alcoholic fatty liver disease (NAFLD) has emerged as the most prevalent chronic liver disease. Population based studies in the United States estimate that the prevalence of NAFLD is between 17% to 30% in the general population and as high as 80% in obese individuals undergoing weight loss surgery [1, 2]. NAFLD represents a histopathologic spectrum ranging from steatosis alone, to necroinflammation - an entity described as nonalcoholic steatohepatitis (NASH); the latter having an increased risk for the progression to advanced fibrosis and cirrhosis. The prevalence of NASH is between 12%–17% with the highest rates observed in Hispanics (19%) and diabetics (22%) [3, 4]. Approximately 15% to 25% of individuals with NASH can progress to cirrhosis [5]. NASH cirrhosis is now the third most common cause of liver transplantation in the United States [6]. It is associated with an increased risk for hepatocellular carcinoma and mortality in patients awaiting orthotopic liver transplant [7] and it can recur post transplant [8]. Recently NAFLD has also been linked to an increased risk of cardiovascular morbidity, which further predisposes these individuals to increased mortality from non-liver related deaths [9]. A number of biomarkers and algorithms continue to be evaluated as noninvasive markers for the diagnosis and progression of NASH [10]. Although there is ongoing extensive research in the development of biomarkers, liver biopsy remains the gold standard in diagnosing and predicting severity of NAFLD [10, 11]. Despite the limitations of liver biopsy [12, 13], a few studies have identified specific histologic findings that are able to predict NAFLD related mortality. These data suggest a more benign course for individuals with steatosis alone, but increased morbidity and mortality among those with advanced histologic findings of NASH and fibrosis [14–17]. Most of these studies have been limited to tertiary centers and information from population based studies on long term outcomes in NAFLD are clearly lacking. Despite many studies and much discussion, the natural history of this disease remains ill defined. This chapter will review the data that have evaluated the natural history of NAFLD and discuss the relevance of histology to predict meaningful outcomes in NASH. Although NAFLD in children is also associated with increased morbidity and mortality [18], only adult NAFLD will be reviewed here.
(II) Histological Progression of NAFLD
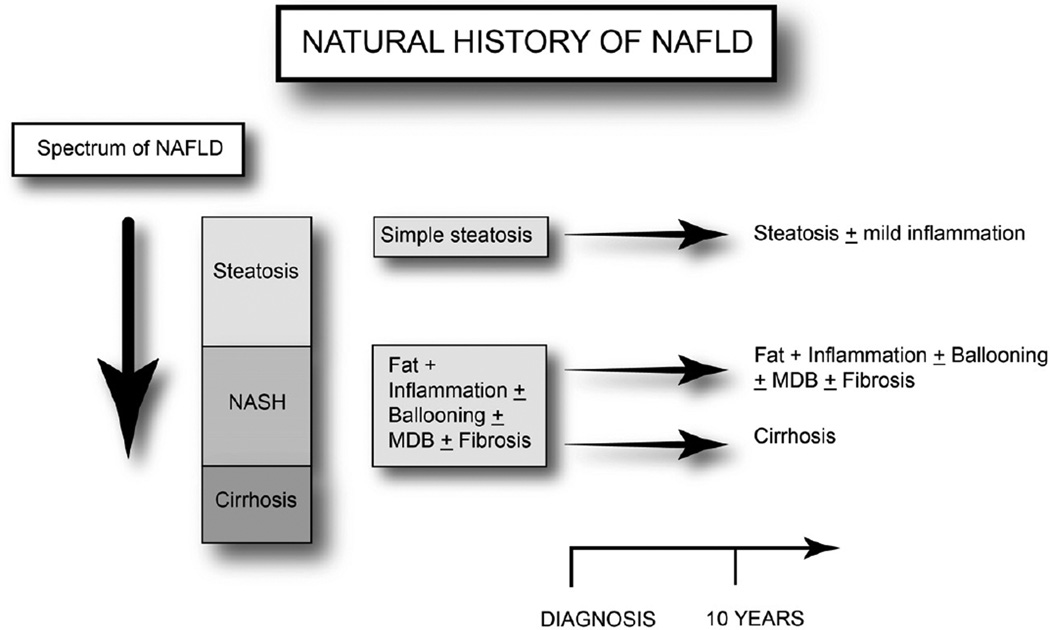
The spectrum of NAFLD (Figure 1) begins initially with simple fatty liver (steatosis) but can progress to necroinflammation (NASH) in a subgroup of patients. Progressive fibrosis as a result of the necroinflammation can lead to cirrhosis, the last and most severe form of the NAFLD spectrum. Patients with NASH develop progressive fibrosis in 25%–50% over a period of 4 to 6 years [14, 15, 19–22]. Less then 1% – 4% of patients with simple steatosis progress to a more advanced fibrosis [15, 23]. In contrast, 15% to 25% of individuals with NASH can progress to cirrhosis [5]. In a cohort of biopsy proven NAFLD patients, 37% of patients had progression of fibrosis, while 34% remained stable and 29% had some regression of fibrosis over 3–6 years [24]. In another study with a mean follow up of 13 years, 13.3% of NASH patient with mild to moderate fibrosis (F1–F2) and 50% of patients with stage 3 fibrosis developed cirrhosis [14].
Figure. 1.
The spectrum and the natural progression of nonalcoholic fatty liver disease
(III) Histologic Scoring Systems
Accumulation of greater than 5% of fat, particularly in the form of triglycerides, is generally considered to be the minimal requirement in the histologic diagnosis of NAFLD [25]. Since the original description of Ludwig [26], the histologic criteria for diagnosing NAFLD have evolved and several grading systems have been proposed to assess histological severity [15, 25, 27, 28]. Based on previously validated scoring systems [29], Matteoni et al [15] in 1999 characterized histologic subtypes that correlated with clinical outcomes (Table 1). In 2005, the NAFLD activity score was developed by the NIDDK supported NASH clinical research network (CRN) [25]. It consists of weighted sums of each of the following: steatosis, lobular inflammation and the presence of hepatocytes ballooning (Table 2) that comprises the NASH activity score (NAS), There is a separate classification for fibrosis used by NASH CRN (Table 3). Recently Younossi et al proposed a new classification to define NASH which included steatosis with the presence of centrolobular ballooning and/or Mallory Denk bodies or fibrosis [17]. According to these criteria (Table 4), a diagnosis of NASH was made if there was (1) any degree of steatosis along with centrilobular ballooning and/or Mallory-Denk bodies or if there was (2) any degree of steatosis along with centrilobular, pericellular/perisinusoidal fibrosis or bridging fibrosis in absence of other identifiable causes [17].
Table 1.
Classification of Non-alcoholic Fatty Liver Disease by Subtypes- Matteoni et al [15]
| NAFLD-subtype | Pathology | Clinicopathological correlation |
|---|---|---|
| Type 1 | Simple Steatosis alone | No NASH |
| Type 2 | Steatosis + lobular inflammation only | No NASH |
| Type 3 | Steatosis + hepatocellular ballooning | NASH without fibrosis |
| Type 4 | Steatosis, ballooning, Mallory bodies or fibrosis | NASH with fibrosis |
Table 2.
Classification of Non-alcoholic Fatty Liver Disease by NAFLD Activity Score (NASH CRN) [25]
| Histologic Finding | Grade |
|---|---|
| • Steatosis | 0 – 3 |
| • Lobular inflammation | 0 – 3 |
| • Hepatocellular ballooning | 0– 2 |
| Maximum Score | 8 |
| NASH requires a score of ≥ 4 with at least 1 point from ballooning injury† | |
Used in some clinical studies to diagnose NASH. However, it was developed as a scoring system to be used in clinical trials not to diagnose NASH based on a number. [33]
Table 3.
| Fibrosis Type* | Score |
|---|---|
| • None | 0 |
| Perisinusoidal Zone 3 | |
| -Mild | 1A |
| - Moderate | 1B |
| • Portal/Periportal | 1C |
| • Perisinusoidal and Portal/Periportal | 2 |
| • Bridging | 3 |
| • Cirrhosis | 4 |
- Not a prerequisite for diagnosis of NASH in this scoring system
Table 4.
Classification of Non-alcoholic Fatty Liver Disease by Subtypes- Younossi et al[17]
| Pathology | Clinicopathological correlation |
|---|---|
| Simple Steatosis alone | No NASH |
| Steatosis + lobular inflammation only | No NASH |
| Steatosis with centrilobular ballooning and/or Mallory-Denk bodies | NASH |
| Any Steatosis with centrilobular pericellular/perisinusoidal or bridging fibrosis | NASH |
The major differences between the NAFLD Scoring System [25] and the two scoring systems that used NAFLD subtype categories [15, 17] are that the latter two incorporate fibrosis and non fibrotic lesions into each subtype. More importantly, they provide a better prediction of long term liver related mortality in these patients [17]. Therefore, it is important to carefully consider the similarities and differences among the published scoring systems, as well as, the importance of individual histological lesions when assessing disease severity, the accuracy of biomarkers and the different imaging modalities to predict the histologic diagnosis, and most importantly the prognosis of NAFLD patients [30].
(IV) Role of Specific Histological Features in Prognostication
There are a number of individual histologic features that are both essential in the diagnosis of NASH as well as prediction of clinical outcomes.
(i) Ballooning
Hepatocellular ballooning is a key feature of NASH and typically denotes hepatocyte injury from steaohepatitis and is considered a marker for apoptosis [31]. The characteristic ballooned hepatocytes are typically large round cells with a reticulated cytoplasm [32]. More than 99% of patients with definitive NASH have some degree of ballooning which is considered the most import histological feature to predict steatohepatitis [33]. Therefore it is not surprising that ballooning degeneration (≥ 2) was associated with an increased liver related mortality (HR-5.32; P=0.0015) [17]. However, the findings of ballooning may be subtle and difficult to diagnose consistently even by a trained pathologist as noted in a study that reported a poor to fair reliability to diagnose inflammation, balloon degeneration and Mallory-Denk Bodies (MDBs) among pathologists [34]. Additionally the irregularity in distribution of ballooning degeneration and MDBs could lead to underreporting of NASH. A study of 51 NAFLD patients with paired liver biopsies taken simultaneously from the right lobe reported a low-moderate inter-biopsy agreement for various liver lesion: lobular inflammation- 0.13 (poor), ballooning degeneration-0.45 (moderate), Mallory bodies- 0.27 (poor) and acidophilic bodies- 0.07 (poor) [35].
(ii) Mallory-Denk bodies
Mallory-Denk Bodies (MDBs) also know as Mallory bodies are cytoplasmic inclusion bodies in the hepatocyte of patients with chronic liver disease containing an abnormal keratin protein that has been ubiquinated [36]. Both ballooning degeneration and the presence of MDBs can trigger the development of apoptosis. MDB’s are not specific to NASH and can be found in a number of other diseases including chronic hepatitis C [36–39]. The presence of MDBs is a pathological lesion that has been incorporated into various definitions to describe the severity of steatohepatitis[15, 17, 25], and has been associated with endoplasmic reticulum stress and oxidative stress [40, 41]. However, the clinical significance and prognostic value of MDBs in disease progression and outcomes in NAFLD is not completely understood. A number of studies in the past have alluded to presence of MDBs as being associated with disease severity and unfavorable outcomes in both alcoholic liver disease as well as NASH [15, 42, 43]. While the presence of MDBs is associated with the presence of ballooned hepatocytes, it can also form in their absence making them less interchangeable. Matteoni and colleagues were the first to report the importance of Mallory bodies in disease progression and suggest a possible prognostic role in steatohepatitis [15]. In a subsequent study comparing long term survival between patients with NASH and alcoholic steatohepatitis, those with nonalcoholic steatohepatitis had a better survival. However a subgroup of NASH patients with moderate to severe Mallory bodies and fibrosis had a significantly worse survival [43]. In a recent study Younossi et al observed that in patients with NASH who had a related Liver Related Mortality (LRM) (28%), the presence of MDBs were noted in 89% of the biopsy samples with 56% of them having at least moderate to severe Mallory bodies (grade ≥ 2). The presence of Mallory-Denk bodies (grade ≥ 2; HR= 4.21, P=0.002) was significantly associated with liver related mortality [17].
Even though there appears to be a role for MDBs in disease progression and outcomes, their presence is not considered a prerequisite to diagnose NASH [33].The presence of MDB’s are descriptively mentioned in the NAS scoring system but does not carry any weight in terms of the overall score [25], and additional data will be required to determine their prognostic importance. Apoptosis has been validated as an accurate marker for diagnosis of NASH based on immunochemistry in liver tissue [44]. This is of particular interest since serum levels of CK-18, a biomarker of apoptosis has been validated to differentiate NASH from no NASH [45].
(iii) Fibrosis
Although fibrosis predicts clinical outcomes in NASH, it is not included in all the currently published scoring systems. While evaluating 103 sequential liver biopsies in NAFLD subjects, Adams et al noted an interesting observation linking fibrosis progression and pathologic lesions in NASH [24]. A significant overall reduction in steatosis, inflammation, ballooning, Mallory hyaline and progression of fibrosis was noted between interval liver biopsies over a period of three years. It should be emphasized that reduction in steatosis but not the other NASH lesions correlated with fibrosis progression. It has been reported in previous studies that upto 47% of NAFLD patients without fibrosis at baseline developed fibrosis on subsequent follow up liver biopsy [14]. Approximately 37% to 41% of the NAFLD subjects have fibrosis progression over 3–10 years [14, 24]. A higher BMI, the presence of diabetes and a low initial fibrosis stage are associated with higher rates of fibrosis progression [24]. Based on this information, it appears that with disease progression overtime there is a decrease in the NAS score simultaneously with the worsening fibrosis, thereby decreasing the accuracy for diagnosing NASH (based on NAS CRN score). This issue is illustrated in a recent study done by Soderberg et al, who reported no differences in survival between NASH and no NASH, based on the NASH activity score [46]. An increased presence of fibrosis (72%) was noted even in the group classified as no NASH/borderline and additionally the presence of fibrosis (stage 1–4) was more common in NAFLD patients who died compared to those who survived (90% vs. 75%, respectively). In the same study, if one reclassified those patients who had any type of fibrosis as NASH and those with steatosis and inflammation without fibrosis as no-NASH, both the over all and the liver related mortality in the NASH group would be significantly higher than in the no NASH group. Absence of periportal fibrosis has a high negative predictive value (100%) to predict liver related outcomes [14]. The lack of difference in survival among the NASH and no NASH in previous studies lacked the prognostic utility for key pathologic lesions in NASH, as they were not adjusted for fibrosis [16, 46]. However in a subsequent study, it was clearly shown that the presence of advanced fibrosis is the only histological lesion shown to be associated with liver related mortality. Neither steatosis, inflammation, ballooning, nor Mallory hyaline were associated with liver related mortality after adjusting for the presence of fibrosis [17]. The inclusion of fibrosis could explain, in part, the reason, why the classifications for NASH used by Younossi and Matteoni and not the NAS score, independently correlated with liver related mortality [17]. Furthermore, fibrosis is the histological feature in NASH with the best inter and intra agreements among pathologist [34]. Using this observation one can make an argument that NAFLD in the presence of fibrosis, especially those who have NASH, are at a higher risk for death.
(V) NAFLD Related Mortality
(A) All Cause Mortality
There are several hospital and community based studies comparing overall and liver related outcomes in NAFLD patients to that of the general population (Table 5) with some studies lacking a histologic diagnosis of NAFLD [19, 47–50]. A community based study that used ultrasound to compare outcomes between NAFLD patients and the general population had higher all-cause mortality in the former group (Standardized mortality ratio=1.34; P=0.03) [47]. Similar findings were reported in females with NAFLD and no differences were observed between males and females (Hazard ratio: 1.2 vs. 0.92) [48].More recently in a study with a 15 year follow that used fatty liver index (FLI) as a surrogate marker for fatty liver, the FLI was independently associated with increased all-cause mortality compared to the general population [49]. Similar observations were also noted in studies that used histologic criteria for diagnosing NAFLD [14, 16, 46]. In a small Swedish cohort, there was a significantly decreased overall survival among biopsy proven NAFLD patients (78% vs.84%) compared to the reference population [14]. Another recent study by Soderberg et al validated these observation with higher all- cause mortality in NALFD patients followed for at least 21 years compared to the general population (SMR=1.7; 95% C.I: 1.24–2.25) [46]. In these studies, there is an incremental increase in overall mortality rates among subjects with biopsy proven NAFLD from 50% in the first decade of follow-up, to as high as 70% by the second decade [14, 46, 47]. NAFLD patients with metabolic syndrome; particularly with diabetes mellitus and insulin resistance have more advanced fibrosis and are prone to a more rapid progression of their fibrosis [24, 51]. NAFLD patients with type 2 diabetes mellitus can double the risk of overall mortality compared to the general population having diabetes without NAFLD (Hazard Ratio (HR)= 2.2) [52]. Compared to patients with alcoholic liver disease or viral hepatitis, NAFLD patients have lower risk of death but a higher risk than patients with autoimmune and genetic disorders [46].
Table 5.
Studies on Long Term Mortality in NAFLD Patients in Comparison to the General Population
| Study | Year | Population (n) |
Follow- up (Years) |
Comparison Group |
Diagnosis | All-Cause Mortality |
Liver-Related Mortality |
Cardiovascular Mortality |
|---|---|---|---|---|---|---|---|---|
| Jespen [19] | 2003 | 1804 | 6.4 | Age and sex matched general population | Medical Records | SMR=2.26(2.4–2.9)† | SMR=19.7 (15.3–25.5)† | SMR=2.1(1.8–2.5)† |
| Dam-Larsen [20] | 2004 | 109 | 16.7 | General population | Biopsy | No differences between both groups | NR | NR |
| Adam [47] | 2005 | 420 | 7.6 | Age and sex matched general population | Imaging/Biopsy | SMR= 1.3(1.00–1.76)† | NR | NR |
| Ekstedt [14] | 2006 | 129 | 13.7 | Age and sex matched general population | Biopsy | 22% versus 16%† 30% versus 20%†• |
2.8% versus 0.2% (NASH)†• | 15.5% versus 7.5% (NASH)†• |
| Ong [60] | 2008 | 8.7 | General population | Ultrasound | HR=1.038 (1.036–1.041)† | HR=9.32(9.21–9.43)† | 25% versus 35% | |
| Haring [48] | 2009 | 4160 | 7.3 | General population | Ultrasound | Men: HR=0.92(0.7–1.2) Women: HR=1.2(0.8–1.8) |
NR | Men:HR 6.22 (1.22–31.62)¥ |
| Soderberg [46] | 2010 | 118 | 21 | Age and sex matched general population | Biopsy | SMR= 1.7(1.2–2.2)† SMR=1.86 (1.19–2.76)†• SMR= 1.55(0.98–2.32)‡ |
NR | NR |
| Adam [52] | 2010 | 337 | 10.9 | Type-2 DM without NAFLD | Imaging/Biopsy | HR=2.2(1.1–4.2)† | NR | HR=0.9(0.3–2.4) |
| Calori [49] | 2011 | 2011 | 15 | General population | Fatty Liver Index | HR=1.004(1.004–1.007)† | HR=1.04(1.02–1.05)† | HR= 1.006(1.00–1.01)# |
| Lazo [50] | 2011 | 11371 | NR | General population | Ultrasound/LFT |
Normal LFT-HR=0.92(0.78–0–1.09) Abnormal LFT – HR=0.80(0.52–1.22) |
Normal LFT-HR=0.64(0.12–3.59) Abnormal LFT –HR=1.17(0.15–8.93) |
Normal LFT-HR=0.86(0.67–1.12) Abnormal LFT –HR=0.59(0.29–1.2) |
SMR- Standardized Mortality Ratio and HR-Hazard Ratio are provided as ratio (95% Confidence Interval)
NR- Not Reported
- P<0.05
- Comparisons made between NASH patients and the general population
- Comparisons made between no-NASH and the general population
- Not significant in the presence of insulin resistance
- In the presence of elevated GGT
Within the NAFLD spectrum, patients with histologic evidence of steatosis alone in the absence of NASH appear to generally follow a more benign course. In a Danish cohort of 109 subjects with bland steatosis there was no difference in survival compared to the general population [20]. These findings have been corroborated by many other histology based studies in NAFLD [14, 16, 46, 53]. In contrast, the presence of histologic NASH is associated with worse outcomes compared to the general population [14, 46]. In a national registry based cohort of 129 with elevated liver enzymes and biopsy proven NAFLD followed for 13 years [14], Ekstedt et al found a significant increase in all-cause mortality in patients with NASH compared to the reference population. The overall survival of patients with NASH was significantly lower (70% vs.80%, respectively, P=0.01). The poor outcomes in this cohort were attributed more to both the presence of increased cardiovascular morbidity and to a lesser degree liver related mortality [14]. Similarly, in another study with a 28 year follow up, the overall mortality was higher in NASH compared to the general population (SMR 1.9; 95%CI, 1.19–2.76, P=0.007) [46]. In the same study all cause mortality in NASH patient remained significantly higher even after exclusion of those patients with stage 3 and stage 4 fibrosis.
There are only a few studies that have directly compared outcomes among those with NASH and no NASH (Table 6). Matteoni et al were the first group to analyze the long term outcomes of NAFLD patient in relation to their histologic subtypes [15]. A series of NAFLD patient were divided based on their histology into four distinct subtypes: type 1&−2 (steatosis ±lobular inflammation) and type 3&4 (steatosis + ballooning degeneration ± Mallory-Denk ± fibrosis). In a mean follow up of 18 years, those with type 3&4 histology (NASH) had higher rates of cirrhosis and liver related mortalities (30.8% vs. 5.6%) compared to type 1&2 (No NASH) but the overall survival was not different between the two groups [15]. Findings from this study pioneered the need to study liver histology for prognostic information on long-term survival among patients with steatohepatitis. The same group reported that the presence of diabetes mellitus decreased survival in these patients [15, 16]. In the study by Ekstedt and his colleagues, although NASH and no NASH were not directly compared, those patients with no NASH had a better better survival than patients with NASH compared to the general population [14]. However there were no differences in overall mortality between the two groups in other studies [16, 17, 46]. In one study, the presence of any fibrosis in NAFLD was associated with an increased all-cause mortality compared to those patients without any fibrosis [46]. NAFLD related advanced fibrosis is associated with an over all death rate of 13%–28% (Table 7) but is lower than other chronic liver diseases [54–57].
Table 6.
Studies comparing the overall and cause-specific mortality in no NASH compared to NASH
| Study | Year | Population (n) |
Follow up (Years) |
Comparison Groups |
Histology Classification |
All-Cause Mortality |
Liver- Related Mortality |
Cardiovascular Mortality |
Cancer related Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Matteoni [15] | 1999 | 132 | 18 | NASH versus steatosis± inflammation | Matteoni | 39.7% versus 32.2%● | 10.9% versus 1.69%* | NR | NR |
| Ekstedt [14] | 2006 | 129 | 13.7 | NASH versus steatosis± non-specific inflammation | Brunt | 26.7% versus 12%● | 2.8% versus none● | 15.5% versus 8.6%● | 5.6% versus 1.7%● |
| Rafiq [16] | 2009 | 173 | 18.5 | NASH versus steatosis± non -specific inflammation | Matteoni | No differences between both groups (P=0.30) | 17.5% versus 2.5% † | NR | NR |
| Soderberg [46] | 2010 | 118 | 45 | NASH versus simple steatosis | Kleiner | 47 % versus 34.3 %● | 5.8% versus 8.9%● | 13.7% versus 10.4%● | 15.6% versus 7.4%● |
| Younossi[17] | 2011 | 209 | 12 | NASH versus No NASH | Younossi | 30.5% versus 30.8%* | 13% versus 1.3% † | NR | NR |
NR-Not Reported,
- P<0.05,
-Significance not reported,
-Not significant
Table 7.
Comparison of Outcomes in NAFLD cirrhosis in comparison to Hepatitis C Related Cirrhosis
| Study | Year | Population Cirrhosis with NASH (n) |
Follow up (years) |
Comparison Groups |
All- Cause Mortality |
Liver- Related Mortality |
Cardiovascular Mortality |
|---|---|---|---|---|---|---|---|
| Hui [56] | 2003 | 23 | 7 | NASH versus HCV (total) | 26 % versus 39.1%● | 21.7% versus 30.4%● | 0% versus 2.2%● |
| Sanyal [54] | 2006 | 152 | 10 | NASH versus HCV | 19.1% versus 29.3† | 14.5% versus 28%‡ | 5.2% versus 0.6%† |
| Yatsuji [55] | 2009 | 68 | 5 | NASH versus HCV | 27.9% versus 40.6%●- | 22% versus 37.6%● | None in both groups |
| Bhala [57] | 2011 | 247 | 7 | NASH versus HCV | 13.4% versus 9.4%* | 5.7% versus 7.9%* | No differences |
-P< 0.05,
- P< 0.05 for Child A but no B&C,
-Not significant,
-Significance not reported
(B) Cardiovascular Related Mortality
NAFLD patients have a higher risk for cardiovascular morbidity and mortality compared to a general population controlled for age and gender [58]. Both hospital and community based studies have established cardiovascular complications as one of the leading causes of death in the NAFLD cohort [14–16, 19, 47, 59].The death from cardiovascular disease in NAFLD ranges from 13% to 30% and is the most common cause of mortality followed by malignancy (6%–28%) and liver related deaths (2.8% to 19%) respectively [14, 16, 46, 47, 60]. The prevalence of cardiovascular disease is higher in NASH compared to other chronic liver diseases other than alcoholic liver disease [46]. Furthermore, cardiovascular deaths remain significantly higher in patients with NASH cirrhosis (the second highest cause of mortality) compared to patients with HCV- related cirrhosis (sixth leading cause) [54]. These findings are not surprising given that 41% to 58% of subjects who undergo coronary angiogram have NAFLD on imaging and furthermore the presence of NAFLD was also an independent predictor of coronary artery calcification [61, 62]. The risk of death from cardiovascular events among the histological subtypes has shown variable results [14, 15, 46]. Ekstedt et al reported higher deaths from cardiovascular disease in patients with NASH compared to the general population (15.5% vs. 7.5%, P= 0.04) as well as those with no NASH (15.5% vs. 8.6%) [14], a similar but not significant trend was noted in a Swedish cohort [46].However more recent data suggest patients with NASH do not have an increased risk of cardivascular disease compared to no NASH [63]. Several large population based studies that have used non-histologic modalities of diagnosis have reported no difference in cardiovascular mortality among patients with NAFLD compared to the general population [49, 50, 52]. In a large population based cohort of patients with diabetes mellitus, NAFLD with diabetes was associated with increased overall mortality including deaths from malignancy but not with cardiovascular disease compared to those without NAFLD [52]. Moreover the presence of NAFLD is also an independent predictor for cardiovascular mortality. Therefore, whether cardiovascular mortality is related to NASH independent from the co-morbidities in the metabolic syndrome remains to be determined [59, 64]. While the rates of cardiovascular disease is higher in both NAFLD and NASH compared to the general population, the differences in mortality between NASH and no NASH has yet to be clearly ascertained.
(C) Liver Related Mortality
NASH is associated with progression to advanced fibrosis and cirrhosis leading to the development of liver related complications and deaths [65, 66]. Additionally cirrhosis can also predispose to the development of hepatocellular cancer and need for transplantation. While liver disease is the third leading cause of death in NAFLD patients, it is the 11th and 13th cause of death in obese patients without fatty liver and in the general population: respectively [16, 60]. Liver related deaths are significantly higher in patients with NASH (13%–17.5%) compared to simple steatosis and no NASH (1.3% – 2.5%) [16, 17]. Among the causes of liver related death hepatocellular carcinoma is the most common occurrence [14, 16, 46]. Besides NASH, the presence of NASH related fibrosis including both mild fibrosis (F1–F2) and advanced fibrosis (F3–F4) have significantly higher liver related mortality rates then simple steatosis [21]. The ability of the NASH subtype classification to predict liver related mortality outcomes (LRM) in NASH was tested in a cohort of 209 biopsy proven NAFLD patients followed for a median of 146 months [17]. The liver related mortality was significantly higher in NASH (13%) compared to no NASH (1.3%). After adjusting for confounding variables, the proposed scoring of NASH had a Cox adjusted hazard ratio (aHR) of 4.43 (95% CI=0.97–20.20; P=0.05) as a predictor of LRM. Assessment of individual components of pathological features found that advanced fibrosis ≥ 3 (stage3 fibrosis and cirrhosis) but not grades of steatosis, lobular inflammation or ballooning degeneration predicted increased risk of LRM in NASH. The over all liver related deaths in patients with NASH and advanced fibrosis is as high as 22% (Table 7) but is lower than other chronic liver diseases [54–56]. More recently a large study with mean follow up of 85.6 months comparing outcomes between NAFLD and hepatitis C related cirrhosis showed a fewer liver related complications, death and transplant rates in the NAFLD group [57]. However, most studies data comparing NAFLD cirrhosis with other chronic liver disease are hampered by small size and variability in length of follow up.
(D) NASH Related Post-Transplant Outcomes
Liver transplantation is an effective therapy for chronic end-stage liver disease with over 90% and 70% survival at 1 year and 5 years respectively [67]. The percentage of patients undergoing a liver transplant for NASH in the last decade has increased from 1.2% to 9.7%. NASH is now the third most common indication for liver transplantation in the United States behind hepatitis C and alcoholic liver disease and is the only liver related indication that continues to increase [6]. Patients after transplantation can develop a number of well-recognized complications, including infection, graft rejection, cardiovascular disorders, renal insufficiency, diabetes, hypertension, chronic rejection, dyslipidemia, and certain types of malignancies [8]. A common link in the development of cardiovascular, renal disorders and malignancies is the presence of posttransplant metabolic syndrome (PTMS). The prevalence of PTMS after transplant is approximately 50% and is most often associated with development of insulin resistance in the setting of immunosupression [68]. It is not surprising that the development of metabolic disorders after OLT can result in denovo steatosis and NASH in up to 70% and 30 % patients respectively, with a minority of patients developing cirrhosis [69, 70]. Recurrent nonalcoholic steatohepatitis patients did not develop allograft failure or require retransplantation at 3 years [69]. The over-all posttransplant survival rates of patients with NASH at 1 and 3 years are as high as 84% and 78%, respectively, compared with 87% and 78% for other indications (P=0.67) [6]. More recently the 5 year survival was reported as 76.4% with cardiovascular mortality (26%) being the second highest cause of death after sepsis [70, 71]. Among those transplanted for NASH cirrhosis, a combination of older age, a higher BMI, diabetes and hypertension have an associated mortality rate of at least 50% [72].
(VI) Histologic Features and Clinical outcomes
Data from several large population and hospital-based studies have confirmed that the presence of NAFLD by using either histology or abnormal liver imaging is associated with an increased all- cause and liver related mortality compared to age and sex matched general population [14, 19, 46, 47, 52, 60]. In these studies, the all-cause mortality from NAFLD increased by 50% in the first 10 years and peaked to 70% in the second decade [14, 46, 47, 52]. The presence of diabetes in NAFLD patients doubles the risk of disease progression and is prognostic for higher all-cause mortality in NAFLD patients [16, 52]. However, NAFLD includes the presence of simple steatosis and steatohepatitis and one must use caution while extrapolating these results to the entire spectrum of disease. The long term outcomes vary across the disease stage and are based on histological severity among NAFLD subtypes. While NAFLD by itself has significantly poorer outcomes than age and sex matched general populations, studies reported to date have uniformly concluded that the presence of simple steatosis, either alone or with minimal inflammation, in the absence of ballooning degeneration or fibrosis are associated with similar outcomes to age and gender matched general population without NAFLD [14, 20, 46]. However, one aspect that has clearly stood out is the lack of difference within the spectrum of NASH with no differences in all-cause mortality between NASH compared to no NASH [16, 17, 46]. Given that NASH is a more histologically severe form of NAFLD, with an increased predisposition to cirrhosis, one should have expected findings to the contrary. A number of different possibilities may explain this discrepancy including: (1) difficulty in establishing a true definition for NASH [73], (2) determining the exact role of pathologic features including fibrosis in NASH outcomes and (3) limitations in the role of liver biopsy in NAFLD related outcome studies [29].
(a) Lack of Consensus for a True Definition to NASH
The definition of NASH is still evolving. A number of different histologic definitions have been reported, which makes it difficult to establish a universally accepted diagnosis of histologic NASH that can predict meaningful clinical outcomes [15, 17, 25, 33, 74]. For example, a recent study that included a cohort of 257 patients with NAFLD proposed a new histologic definition of NASH and compared their definition interprotocol agreement with three other existing classifications including Brunt’s classification, NAS ≥5 and the Matteoni subtypes, which has been discussed earlier in this article. The reported measure of interprotocol agreement of their definition (k statistic= 0.896) was in almost perfect agreement with Matteoni classification to distinguish NASH. The agreement between the proposed definition and the now widely used NAS> 5 and Brunt’s classification was moderate (k=0.511) and at best fair (k=0.365) respectively. Lowering NAS≥4 resulted in better agreement between the NAS and the new NAFLD subtype classification [17]. The results are not surprising as both the NASH definitions that had the best interprotocol agreement have some key similarity in their definitions [15, 17]. This study also compared the association of liver related mortality (LRM) with the existing NASH definitions in 209 NAFLD patients followed over a median period of 12 years. After adjusting for various confounders, the diagnosis of NASH using the Younossi and Matteoni definitions showed significant associations with LRM. However, NASH defined by the NAS≥ 5 and the original Brunt’s criteria did not reach statistical significance [17]. However as recently emphasized, the NAS CRN scoring system was developed as a tool for descriptive purposes to measure changes in NAFLD during therapeutic trials- not to diagnose NASH [33]. The diagnosis should not be made based on an NAS calculated number, but rather on the pathologist’s overall evaluation of histological patterns and individual lesions on the liver biopsy. Using 934 biopsies taken from the NASH CRN network database, the use of NAS cutoffs (≥ 5 vs. ≤ 4) for histological diagnosis of steatohepatitis versus borderline/no NASH did not provide sufficient sensitivity and specificity to support their use for NASH diagnosis [33]. These results do not in any way suggest a superiority of one definition over another but rather emphasizes the need for uniformity across studies especially concerning outcomes related studies in NAFLD.
(b) Variability in diagnosing individual pathologic lesions in NASH
Another possible reason for the lack of differences in mortality within the NAFLD spectrum could be explained in part by the heterogeneity in distribution of individual pathologic lesions in the liver and intraobserver variability using these lesions to define NASH on a biopsy. By far the highest variability in intra and inter observer agreement has been for lobular inflammation, ballooning degeneration and presence of certain intracytoplasmic inclusion [29, 34, 35] that are central to the diagnosis of histologic steatohepatitis [25] that were discussed above.
(c) Limitations of Liver Biopsy in Outcome Studies
NAFLD is essentially an asymptomatic disease, discovered incidentally by the presence of elevated liver enzymes during routine blood work and abnormal imaging done for a variety of reasons [75]. Liver biopsy continues to remain the gold standard to assess severity in NAFLD, making it logistically difficult to utilize this method in the general population due to the invasive nature of the procedure and related complications [76]. Furthermore, the chronicity of NAFLD would require many years of follow up to reach the hard end points of cardiovascular and liver related morbidity/mortality, resulting in the paucity of population based data to elucidate the natural history of NAFLD [77].In addition, regardless of the scoring system used, the presence of sampling error, adequacy of sample size for grading and staging (Table 8) and interobserver variability among pathologists to diagnose NAFLD remains an important limitation for both clinical care, investigative trials and outcome studies [13, 35, 78, 79].
Table 8.
Liver Biopsy in NAFLD with Minimum Criteria for Size of Biopsy
(VI) Conclusion
Unfortunately, the natural course of NAFLD has yet to be precisely defined due in large part to the difficulty in establishing the exact disease burden of NAFLD in the general population and identifying those NAFLD patients at risk for increased morbidity and mortality. Based on the data available and review of the current literature, particularly in general population studies, it appears that NAFLD, including NASH but not simple steatosis, is associated with worse outcomes compared to the general population. However, in spite of these observations, the differences in all cause mortality between no NASH and NASH are not significantly different. Recently, there also has been increasing interest in the association NAFLD and cardiovascular disease [9]. While histologic severity in NAFLD was associated with an increased LRM, cardiovascular mortality did not significantly differ between the NAFLD despite it being a leading cause of death in NAFLD. Potential explanations for this include (1) early deaths from liver disease and cancer, (2) overlapping co-morbid risk factor for heart disease, and (3) most studies had small sample sizes have investigated this issue. In addition, the relevance of histological severity to predict outcomes are also confounded by the presence of certain metabolic derangements especially diabetes mellitus and dyslipidemia. Therefore, additional large, prospective, randomized, multicenter trials are needed to determine the precise role of NAFLD histology on the morbidity and mortality for both liver related and cardiovascular outcomes. Given the recent evidence supporting the importance of fibrosis in disease progression, prospective studies are required to compare the outcomes among NASH patients with and without fibrosis to identify the 25% patients with NASH who will progress to advanced liver disease. Additional work is also required to (1) form a consensus of the histologic diagnosis of NASH, (2) increase the accuracy of among pathologists to identify the key pathologic lesions in NASH, (3) clarify the role of fibrosis across the spectrum of NAFLD, and (4) identify biomarkers that can accurately stage NAFLD and obviate the need for repeat liver biopsy in these patients. Until such data are available, it seems reasonable to consider that the stage of disease is a critical determinant in the overall prognosis of NAFLD. The presence of NASH on liver biopsy especially among those having any degree of fibrosis should necessitate enhanced surveillance for cardiovascular disease and malignancies, aggressive management of comorbidities and referral (if possible) to a medical center with expertise in NAFLD.
Unfortunately, the natural course of NAFLD has yet to be precisely defined due in large part to the difficulty in establishing the exact disease burden of NAFLD in the general population and identifying those NAFLD patients at risk for increased morbidity and mortality.
Based on the data available and review of the current literature, particularly in general population studies, it appears that NAFLD, including NASH but not simple steatosis, is associated with worse outcomes compared to the general population. However, in spite of these observations, the differences in all cause mortality between no NASH and NASH are not significantly different.
Given the recent evidence supporting the importance of fibrosis in disease progression, prospective studies are required to compare the outcomes among NASH patients with and without fibrosis to identify the 25% patients with NASH who will progress to advanced liver disease.
The presence of NASH on liver biopsy especially among those having any degree of fibrosis should necessitate enhanced surveillance for cardiovascular disease and malignancies, aggressive management of co-morbidities and referral (if possible) to a medical center with expertise in NAFLD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Mangesh. R.Pagadala is supported by the grant number T32 DK061917.
Dr. Arthur J McCullough is supported in part by NIH grant 5 U01 DK 061732.
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Luyckx FH, Desaive C, Thiry A, et al. Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty. Int J Obes Relat Metab Disord. 1998;22(3):222–226. doi: 10.1038/sj.ijo.0800571. [DOI] [PubMed] [Google Scholar]
- 3.Bambha K, Belt P, Abraham M, et al. Ethnicity and nonalcoholic fatty liver disease. Hepatology. 2012;55(3):769–780. doi: 10.1002/hep.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 5.McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40(Suppl 1):S17–S29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 6.Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Growing evidence of an epidemic? Hepatol Res. 2012;42(1):1–14. doi: 10.1111/j.1872-034X.2011.00872.x. [DOI] [PubMed] [Google Scholar]
- 8.Pagadala M, Dasarathy S, Eghtesad B, et al. Posttransplant metabolic syndrome: an epidemic waiting to happen. Liver Transpl. 2009;15(12):1662–1670. doi: 10.1002/lt.21952. [DOI] [PubMed] [Google Scholar]
- 9.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 10.Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis. 2008;28(4):386–395. doi: 10.1055/s-0028-1091983. [DOI] [PubMed] [Google Scholar]
- 11.Adams LA, Angulo P. Role of liver biopsy and serum markers of liver fibrosis in non-alcoholic fatty liver disease. Clin Liver Dis. 2007;11(1):25, 35, viii. doi: 10.1016/j.cld.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Janiec DJ, Jacobson ER, Freeth A, et al. Histologic variation of grade and stage of non-alcoholic fatty liver disease in liver biopsies. Obes Surg. 2005;15(4):497–501. doi: 10.1381/0960892053723268. [DOI] [PubMed] [Google Scholar]
- 13.Merriman RB, Ferrell LD, Patti MG, et al. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44(4):874–880. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]
- 14.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 15.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 16.Rafiq N, Bai C, Fang Y, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7(2):234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Younossi ZM, Stepanova M, Rafiq N, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53(6):1874–1882. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- 18.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, et al. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58(11):1538–1544. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jepsen P, Vilstrup H, Mellemkjaer L, et al. Prognosis of patients with a diagnosis of fatty liver--a registry-based cohort study. Hepatogastroenterology. 2003;50(54):2101–2104. [PubMed] [Google Scholar]
- 20.Dam-Larsen S, Becker U, Franzmann MB, et al. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44(10):1236–1243. doi: 10.1080/00365520903171284. [DOI] [PubMed] [Google Scholar]
- 21.Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43(8):617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 22.Angulo P, Keach JC, Batts KP, et al. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30(6):1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 23.Dam-Larsen S, Becker U, Franzmann MB, et al. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44(10):1236–1243. doi: 10.1080/00365520903171284. [DOI] [PubMed] [Google Scholar]
- 24.Adams LA, Sanderson S, Lindor KD, et al. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42(1):132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig J, Viggiano TR, McGill DB, et al. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55(7):434–438. [PubMed] [Google Scholar]
- 27.Mendler MH, Kanel G, Govindarajan S. Proposal for a histological scoring and grading system for non-alcoholic fatty liver disease. Liver Int. 2005;25(2):294–304. doi: 10.1111/j.1478-3231.2005.01052.x. [DOI] [PubMed] [Google Scholar]
- 28.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 29.Younossi ZM, Gramlich T, Liu YC, et al. Nonalcoholic fatty liver disease: assessment of variability in pathologic interpretations. Mod Pathol. 1998;11(6):560–565. [PubMed] [Google Scholar]
- 30.Adams LA, Feldstein AE. Non-invasive diagnosis of nonalcoholic fatty liver and nonalcoholic steatohepatitis. J Dig Dis. 2011;12(1):10–16. doi: 10.1111/j.1751-2980.2010.00471.x. [DOI] [PubMed] [Google Scholar]
- 31.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43(2) Suppl 1:S31–S44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 32.Lackner C, Gogg-Kamerer M, Zatloukal K, et al. Ballooned hepatocytes in steatohepatitis: the value of keratin immunohistochemistry for diagnosis. J Hepatol. 2008;48(5):821–828. doi: 10.1016/j.jhep.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 33.Brunt EM, Kleiner DE, Wilson LA, et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53(3):810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukusato T, Fukushima J, Shiga J, et al. Interobserver variation in the histopathological assessment of nonalcoholic steatohepatitis. Hepatol Res. 2005;33(2):122–127. doi: 10.1016/j.hepres.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 36.Zatloukal K, French SW, Stumptner C, et al. From Mallory to Mallory-Denk bodies: what, how and why? Exp Cell Res. 2007;313(10):2033–2049. doi: 10.1016/j.yexcr.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 37.Zhong B, Strnad P, Selmi C, et al. Keratin variants are overrepresented in primary biliary cirrhosis and associate with disease severity. Hepatology. 2009;50(2):546–554. doi: 10.1002/hep.23041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rakoski MO, Brown MB, Fontana RJ, et al. Mallory-Denk bodies are associated with outcomes and histologic features in patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2011;9(10):902, 909.e1. doi: 10.1016/j.cgh.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omary MB, Ku NO, Strnad P, et al. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest. 2009;119(7):1794–1805. doi: 10.1172/JCI37762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanada S, Strnad P, Brunt EM, et al. The genetic background modulates susceptibility to mouse liver Mallory-Denk body formation and liver injury. Hepatology. 2008;48(3):943–952. doi: 10.1002/hep.22436. [DOI] [PubMed] [Google Scholar]
- 41.Hanada S, Snider NT, Brunt EM, et al. Gender dimorphic formation of mouse Mallory-Denk bodies and the role of xenobiotic metabolism and oxidative stress. Gastroenterology. 2010;138(4):1607–1617. doi: 10.1053/j.gastro.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gramlich T, Kleiner DE, McCullough AJ, et al. Pathologic features associated with fibrosis in nonalcoholic fatty liver disease. Hum Pathol. 2004;35(2):196–199. doi: 10.1016/j.humpath.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 43.Cortez-Pinto H, Baptista A, Camilo ME, et al. Nonalcoholic steatohepatitis--a long-term follow-up study: comparison with alcoholic hepatitis in ambulatory and hospitalized patients. Dig Dis Sci. 2003;48(10):1909–1913. doi: 10.1023/a:1026152415917. [DOI] [PubMed] [Google Scholar]
- 44.Feldstein AE, Wieckowska A, Lopez AR, et al. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50(4):1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machado MV, Cortez-Pinto H. Cell death and nonalcoholic steatohepatitis: where is ballooning relevant? Expert Rev Gastroenterol Hepatol. 2011;5(2):213–222. doi: 10.1586/egh.11.16. [DOI] [PubMed] [Google Scholar]
- 46.Soderberg C, Stal P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51(2):595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 47.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Haring R, Wallaschofski H, Nauck M, et al. Ultrasonographic hepatic steatosis increases prediction of mortality risk from elevated serum gamma-glutamyl transpeptidase levels. Hepatology. 2009;50(5):1403–1411. doi: 10.1002/hep.23135. [DOI] [PubMed] [Google Scholar]
- 49.Calori G, Lattuada G, Ragogna F, et al. Fatty liver index and mortality: the Cremona study in the 15th year of follow-up. Hepatology. 2011;54(1):145–152. doi: 10.1002/hep.24356. [DOI] [PubMed] [Google Scholar]
- 50.Lazo M, Hernaez R, Bonekamp S, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ. 2011;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Angulo P, Alba LM, Petrovic LM, et al. Leptin, insulin resistance, and liver fibrosis in human nonalcoholic fatty liver disease. J Hepatol. 2004;41(6):943–949. doi: 10.1016/j.jhep.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 52.Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105(7):1567–1573. doi: 10.1038/ajg.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teli MR, James OF, Burt AD, et al. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22(6):1714–1719. [PubMed] [Google Scholar]
- 54.Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43(4):682–689. doi: 10.1002/hep.21103. [DOI] [PubMed] [Google Scholar]
- 55.Yatsuji S, Hashimoto E, Tobari M, et al. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24(2):248–254. doi: 10.1111/j.1440-1746.2008.05640.x. [DOI] [PubMed] [Google Scholar]
- 56.Hui JM, Kench JG, Chitturi S, et al. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003;38(2):420–427. doi: 10.1053/jhep.2003.50320. [DOI] [PubMed] [Google Scholar]
- 57.Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54(4):1208–1216. doi: 10.1002/hep.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Treeprasertsuk S, Leverage S, Adams LA, et al. The Framingham risk score and heart disease in nonalcoholic fatty liver disease. Liver Int. 2012 doi: 10.1111/j.1478-3231.2011.02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stepanova M, Younossi ZM. Independent Association Between Nonalcoholic Fatty Liver Disease and Cardiovascular Disease in the US Population. Clin Gastroenterol Hepatol. 2012 doi: 10.1016/j.cgh.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 60.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49(4):608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 61.Chen CH, Nien CK, Yang CC, et al. Association between nonalcoholic fatty liver disease and coronary artery calcification. Dig Dis Sci. 2010;55(6):1752–1760. doi: 10.1007/s10620-009-0935-9. [DOI] [PubMed] [Google Scholar]
- 62.Wong VW, Wong GL, Yip GW, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut. 2011;60(12):1721–1727. doi: 10.1136/gut.2011.242016. [DOI] [PubMed] [Google Scholar]
- 63.Domanski JP, Park SJ, Harrison SA. Cardiovascular disease and nonalcoholic Fatty liver disease: does histologic severity matter? J Clin Gastroenterol. 2012;46(5):427–430. doi: 10.1097/MCG.0b013e31822fb3f7. [DOI] [PubMed] [Google Scholar]
- 64.Targher G, Bertolini L, Padovani R, et al. Increased prevalence of cardiovascular disease in Type 2 diabetic patients with non-alcoholic fatty liver disease. Diabet Med. 2006;23(4):403–409. doi: 10.1111/j.1464-5491.2006.01817.x. [DOI] [PubMed] [Google Scholar]
- 65.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2) Suppl 1:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 66.Rinella ME. Will the increased prevalence of nonalcoholic steatohepatitis (NASH) in the age of better hepatitis C virus therapy make NASH the deadlier disease? Hepatology. 2011;54(4):1118–1120. doi: 10.1002/hep.24634. [DOI] [PubMed] [Google Scholar]
- 67.Roberts MS, Angus DC, Bryce CL, et al. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transpl. 2004;10(7):886–897. doi: 10.1002/lt.20137. [DOI] [PubMed] [Google Scholar]
- 68.Laish I, Braun M, Mor E, et al. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 2011;17(1):15–22. doi: 10.1002/lt.22198. [DOI] [PubMed] [Google Scholar]
- 69.Malik SM, Devera ME, Fontes P, et al. Recurrent disease following liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Transpl. 2009;15(12):1843–1851. doi: 10.1002/lt.21943. [DOI] [PubMed] [Google Scholar]
- 70.Bhagat V, Mindikoglu AL, Nudo CG, et al. Outcomes of liver transplantation in patients with cirrhosis due to nonalcoholic steatohepatitis versus patients with cirrhosis due to alcoholic liver disease. Liver Transpl. 2009;15(12):1814–1820. doi: 10.1002/lt.21927. [DOI] [PubMed] [Google Scholar]
- 71.Afzali A, Berry K, Ioannou GN. Excellent posttransplant survival for patients with nonalcoholic steatohepatitis in the United States. Liver Transpl. 2012;18(1):29–37. doi: 10.1002/lt.22435. [DOI] [PubMed] [Google Scholar]
- 72.Malik SM, deVera ME, Fontes P, et al. Outcome after liver transplantation for NASH cirrhosis. Am J Transplant. 2009;9(4):782–793. doi: 10.1111/j.1600-6143.2009.02590.x. [DOI] [PubMed] [Google Scholar]
- 73.Brunt EM, Kleiner DE, Behling C, et al. Misuse of scoring systems. Hepatology. 2011;54(1):369, 70. doi: 10.1002/hep.24347. author reply 370-1. [DOI] [PubMed] [Google Scholar]
- 74.Promrat K, Lutchman G, Uwaifo GI, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39(1):188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 75.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49(1):306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Piccinino F, Sagnelli E, Pasquale G, et al. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2(2):165–173. doi: 10.1016/s0168-8278(86)80075-7. [DOI] [PubMed] [Google Scholar]
- 77.Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54(1):344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goldstein NS, Hastah F, Galan MV, et al. Fibrosis heterogeneity in nonalcoholic steatohepatitis and hepatitis C virus needle core biopsy specimens. Am J Clin Pathol. 2005;123(3):382–387. doi: 10.1309/EY72-F1EN-9XCB-1KXX. [DOI] [PubMed] [Google Scholar]
- 79.Arun J, Jhala N, Lazenby AJ, et al. Influence of liver biopsy heterogeneity and diagnosis of nonalcoholic steatohepatitis in subjects undergoing gastric bypass. Obes Surg. 2007;17(2):155–161. doi: 10.1007/s11695-007-9041-2. [DOI] [PubMed] [Google Scholar]