Abstract
BACKGROUND & AIMS
ADAM10 is a cell surface sheddase that regulates physiological processes including Notch signaling. ADAM10 is expressed in all intestinal epithelial cell types but the requirement for ADAM10 signaling in crypt homeostasis is not well defined.
METHODS
We analyzed intestinal tissues from mice with constitutive (Vil-Cre;Adam10f/f mice) and conditional (Vil-CreER;Adam10f/f and Lgr5-CreER;Adam10f/f mice) deletion of ADAM10. We performed cell lineage tracing experiments in mice that expressed a gain-of-function allele of Notch in the intestine (Rosa26NICD) or mice with intestine-specific disruption of Notch (Rosa26DN-MAML), to examine the effects of ADAM10 deletion on cell fate specification and intestinal stem cell maintenance.
RESULTS
Loss of ADAM10 from developing and adult intestine caused lethality associated with altered intestinal morphology, reduced progenitor cell proliferation, and increased secretory cell differentiation. ADAM10 deletion led to the replacement of intestinal cell progenitors with 2 distinct, post-mitotic, secretory cell lineages: intermediate-like (Paneth/goblet) and enteroendocrine cells. Based on analysis of Rosa26NICD and Rosa26DN-MAML mice, we determined that ADAM10 controls these cell fate decisions by regulating Notch signaling. Cell lineage tracing experiments showed that ADAM10 is required for survival of Lgr5+ crypt-based columnar cells. Our findings indicate that Notch-activated stem cells have a competitive advantage for occupation of the stem cell niche.
CONCLUSIONS
ADAM10 acts in a cell autonomous manner within the intestinal crypt compartment to regulate Notch signaling. This process is required for progenitor cell lineage specification and crypt-based columnar cell maintenance.
Keywords: CBC, intestinal epithelium, development, differentiation
INTRODUCTION
The intestinal epithelium undergoes continuous renewal to preserve tissue integrity. Wnt and Notch signaling have distinct roles in controlling proliferation and cell lineage specification within the crypt compartment that must be precisely integrated to maintain intestinal homeostasis1. Cell lineage tracing studies have shown that Notch signaling is active in multipotent intestinal stem cells (ISCs)2–4 and that Notch activation during intestinal development leads to increased proliferation and amplification of intestinal progenitors5. Notch stimulation of this mitogenic response requires functional Wnt signaling6. Our previous studies demonstrated that Notch directly targets the Lgr5+ crypt base columnar cell (CBC) and is required for stem cell proliferation and survival7. However, the mechanism of Notch regulation and the extent to which Notch contributes to ISC replenishment within the crypt stem cell niche has not been clearly defined.
Notch controls cell fate decisions of short-lived, bipotent progenitors by regulating the key transcription factor Atoh16. Notch1 and Notch2 receptors and delta-like (Dll) 1 and Dll4 ligands control these events8,9. Upon Notch activation, Atoh1 expression is repressed in progenitors which drives differentiation into the enterocyte lineage10. Conversely, in the absence of Notch signaling, progenitors express Atoh1 and are fated into the secretory lineage. Atoh1 target genes such as Gfi1, Spdef and Neurog3 are responsible for later specification events in the secretory lineage11–14. Some evidence suggests that goblet and Paneth cells have a shared lineage7 but it is unclear how multipotent secretory progenitors are allocated and give rise to the major secretory cell types.
Canonical Notch receptor signaling is controlled by sequential processing, which requires extracellular (S2) cleavage by an α-secretase followed by intramembrane (S3) cleavage by a presenilin-dependent γ-secretase to release the Notch intracellular domain (NICD)1. The disintegrin-metalloproteinase ADAM10 was proposed to be a candidate Notch α-secretase because ADAM10−/− mice show an embryonic lethal phenotype that resembles Notch-deficient mice15. More recent analysis of conditional ADAM10-deficient mice and studies using transformed ADAM10−/− mouse embryonic fibroblasts have shown that ADAM10 is required for ligand-induced Notch activation during development16. However, the absolute dependency of Notch signaling on ADAM10 in vivo remains controversial, as other ADAMs (e.g. ADAM17) and metalloproteinases (e.g. MMP7) have been implicated in Notch activation within other contexts16–18. Here, using loss-of-function studies, we show that ADAM10 is required for Notch activation in the intestine and reveal that cell-autonomous ADAM10 signaling is essential for cell lineage specification and intestinal stem cell survival. Our findings also suggest a competitive advantage for Notch-activated stem cells to replenish the stem cell compartment.
EXPERIMENTAL PROCEDURES
Mice
All animal procedures were approved by the UCUCA at University of Michigan. The following mouse strains were used: Adam10f 19, Villin-Cre20, Villin-CreERT220, Lgr5-EGFP-ires-CreERT221, Rosa26YFP22, Atoh1LacZ23, Neurog3EYFP24, Ro-sa26NICD-ires-nEGFP25 and Rosa26DNMAML-GFP26.
Protocols for all procedures are provided in Supplementary Materials
RESULTS
ADAM10 inactivation leads to reduced viability, altered intestinal morphology and loss of progenitor cell proliferation
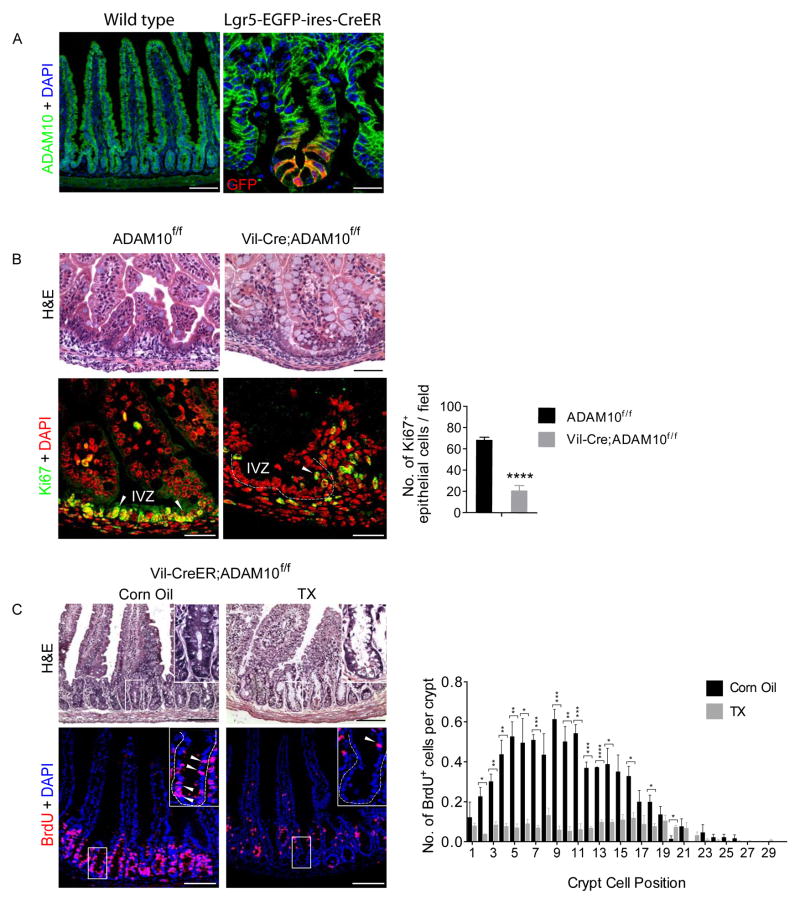
ADAM10 is abundantly expressed on the basolateral surface of all epithelial cell types in the intestine, including Lgr5+ CBCs (Figure 1A and Supplementary Figure 1A). To determine the role of ADAM10 in developing and adult intestine, we analyzed constitutive Villin-Cre;Adam10f/f (termed Vil-Cre;Adam10f/f) and tamoxifen (TX)-inducible Villin-CreERT2;Adam10f/f (termed Vil-CreER;Adam10f/f) mice. Vil-Cre;Adam10f/f pups were born at the correct Mendelian frequency, had normal body weights and intestinal length, however, no ADAM10-deficient pups survived beyond post-natal day 1 (Supplementary Figure 1B–D, Supplementary Table 1, data not shown). Because of this perinatal lethality, we used TX-inducible Vil-CreER;Adam10f/f mice to examine the effect of ADAM10 loss in the adult intestine. Adult Vil-CreER;Adam10f/f mice treated with TX (100 mg/kg i.p.) for 5 consecutive days achieved near complete ADAM10 deletion from intestinal epithelium (Supplementary Figure 1E–F). These mice rapidly lost weight, became moribund and did not survive beyond 7–9 days from the initial TX treatment.
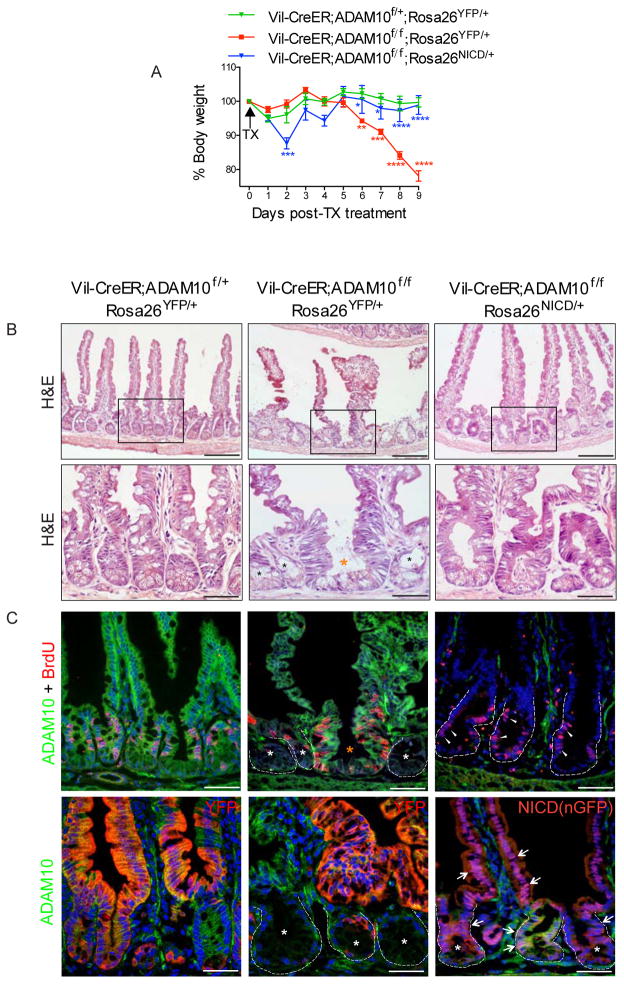
Figure 1. Reduced progenitor cell proliferation upon ADAM10 deletion.
(A), ADAM10 staining in adult small intestine of wild-type and Lgr5-EGFP-ires-CreER mice. Right, Co-staining with GFP (red). (B), H&E (upper) and Ki67+ staining (lower, arrowheads) of small intestine from newborn Adam10f/f and Vil-Cre;Adam10f/f mice. Quantification of Ki67+ cells per field (n=4). (C), H&E (upper) and BrdU+ staining (lower, arrowheads) of adult small intestine from Vil-CreER;Adam10f/f mice treated with TX (100 mg/kg i.p.) or vehicle for 5 consecutive days and analyzed the next day. Quantification of BrdU+ cells in crypts (n=3). *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001. Scale bars (μm) = A, left, 200 and right, 35; B, upper, 100 and lower, 50; and C, 200 (inset, 65).
Histological analysis of the small intestine from newborn Vil-Cre;Adam10f/f mice revealed that the epithelium was less cellular and villi were blunted with more goblet cells (Figure 1B). Importantly, the intervillus zone (IVZ) showed a marked reduction in the number of proliferating cells with only a few Ki67+ cells located at the villus boundary (Figure 1B). Similar morphological and proliferative changes were observed in TX-treated adult Vil-CreER;Adam10f/f mice with a significant reduction in BrdU+ cells throughout the crypt (Figure 1C). This was associated with a marked increase in active caspase-3 staining (Supplementary Figure 1F), indicating that apoptosis accompanied the loss of cell proliferation. These results demonstrate that loss of ADAM10 in either the immature or adult intestinal epithelium leads to diminished viability associated with altered intestinal morphology and reduced proliferation.
ADAM10 deficiency leads to increased secretory cell differentiation
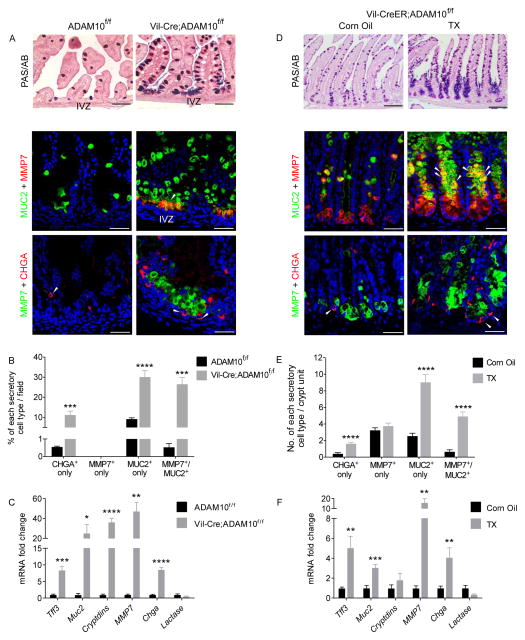
Further investigation into the differentiation status of newborn small intestine from Vil-Cre;Adam10f/f mice revealed dramatic increases in secretory cell marker expression for goblet, (PAS/AB+, Muc2+), Paneth (MMP7+, lysozyme+) and enteroendocrine (chromogranin A, CHGA+) cells (Figure 2A, data not shown). Analogous increases in secretory cell differentiation were found in TX-treated adult Vil-CreER;Adam10f/f mice, but here, an expanded crypt compartment was observed in which the mid/upper crypt regions were completely filled with differentiated secretory cells (Figure 2D). Conversely, the enterocyte marker, alkaline phosphatase, was markedly reduced in both ADAM10-deficient models (data not shown). Morphometric and qPCR analyses confirmed the dramatic increase in secretory cell differentiation observed in both ADAM10-deficient models (Figure 2B,C,E,F). Together, these results indicate that ADAM10 plays an important role in cell fate specification of the small intestine and that ADAM10 loss leads to increased secretory cell differentiation.
Figure 2. ADAM10 deletion converts intestinal crypt progenitors to a secretory cell fate.
(A–C), Newborn small intestine from Adam10f/f and Vil-Cre;Adam10f/f mice (n=4). (D–E), Adult small intestine from Vil-CreER;Adam10f/f mice treated with TX as described in Figure 1 (n=5–7). (A,D), PAS/Alcian Blue staining (upper) and immunofluorescence analysis of goblet (MUC2), Paneth (MMP7) and enteroendocrine (CHGA) cell markers (lower). Intermediate (Paneth/goblet) and CHGA+ cells indicated by arrowheads. (B,E), Morphmometric analysis of secretory cell types. (C,F), qPCR analysis of cell lineage markers. *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001. Scale bars (μm) = All 100 except C, upper, 200.
ADAM10 deficiency produces distinct intermediate-like (Paneth/goblet) and endocrine cell populations
In γ-secretase inhibitor (GSI)-treated mice, we previously showed that increased intestinal secretory cell differentiation is associated with the appearance of distinct intermediate-like cells that co-express Paneth and goblet cell markers7. Similarly, in both ADAM10-deficient models, co-staining for MMP7 and MUC2 detected intermediate-like cells throughout the intestine, including the colon (Figure 2 and Supplementary Figure 2A). In newborn Vil-Cre;Adam10f/f mice, the intermediate-like cells were restricted to the IVZ (Figure 2A) whereas in TX-treated adult Vil-CreER;Adam10f/f mice, the intermediate-like cells were located primarily in the mid/upper crypt region. Ultrastructural analysis confirmed that mature Paneth cells were present at the crypt base. By contrast, the intermediate-like cells located in the mid-crypt region of ADAM10-deficient mice showed ultrastructural features of both Paneth and goblet cells, including mucus granules and variably-sized electron-dense granules characteristic of Paneth cells (Supplementary Figure 2B). Co-staining of the endocrine-specific marker CHGA with MMP7 showed that the intermediate-like cells did not overlap with endocrine cells in either ADAM10-deficient model (Figure 2A,D). Together, these data demonstrate that crypt progenitor cells are fated towards distinct intermediate-like (Paneth/goblet) or endocrine cell lineages in the absence of ADAM10 signaling.
ADAM10 controls Notch signaling associated with cell fate decisions
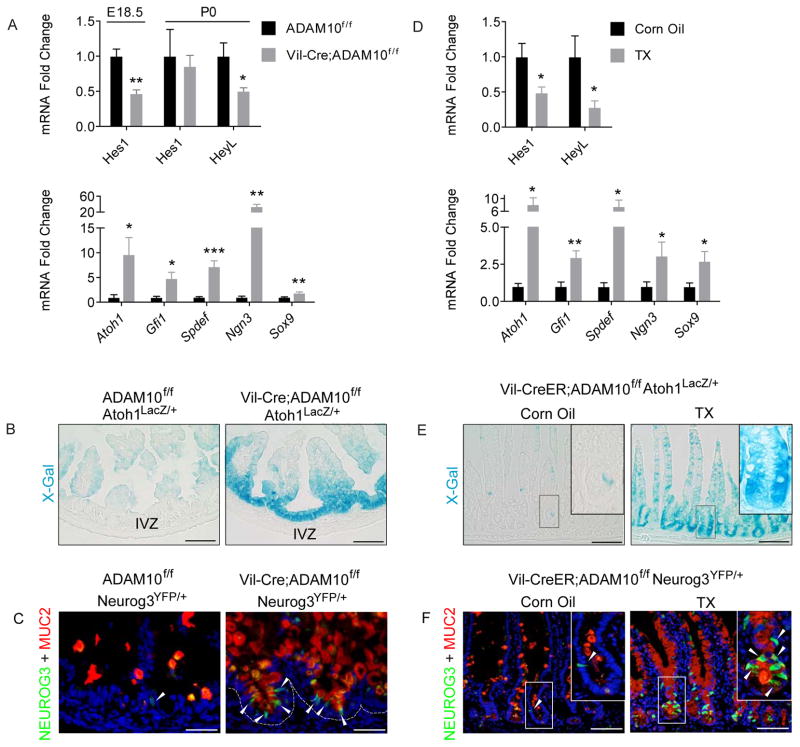
Notch signaling controls cell lineage specification in the intestine. In the absence of Notch signaling, the default programming of transit-amplifying (TA) progenitors is towards secretory cell differentiation1. The similarity of this intestinal phenotype with that observed in ADAM10-deficient mice led us to examine whether ADAM10 directly regulates Notch signaling. In both ADAM10-deficient models, gene expression analysis showed no significant changes in Notch1, Notch2, Dll-1 and Dll-4 mRNA levels suggesting that expression of Notch signaling components was not compromised (data not shown). However, qPCR analysis revealed a 2-fold reduction in expression of Notch target genes Hes1 and HeyL (Figures 3A,D and Supplementary Figure 3A). Consistent with a loss of Notch signaling, IHC analysis confirmed that nuclear Hes1 staining was completely absent from the IVZ of newborn Vil-Cre;Adam10f/f mice (Supplementary Figure 3B). Moreover, examination of Notch downstream effectors revealed a dramatic increase in the expression of key transcription factors required for secretory lineage specification, including Atoh1, Gfi1, Spdef, Neurog3 and Sox9. (Figure 3A,D). To examine Atoh1 and Neurog3 expression at a cellular level, Atoh1lacZ and Neurog3YFP reporter mice23,24, respectively, were bred to both ADAM10-deficient models. Consistent with the expansion of secretory cell lineages, abundant X-gal staining representing Atoh1+ cells was observed throughout the IVZ and crypt compartment (Figure 3B,E). Similarly, more Neurog3+ cells representing early endocrine progenitors were found in newborn and adult intestine that partially co-localized with the endocrine marker, CHGA. Moreover, the expanded Neurog3+ cell population was distinct from MUC2+-expressing intermediate cells (Figure 3C,F and Supplementary Figure 3B,C). Together, these results demonstrate that Notch and its downstream signaling events are compromised after ADAM10 inactivation in both developing and adult intestine.
Figure 3. ADAM10 deletion leads to a loss of Notch signaling.
(A–C), Perinatal small intestine from Adam10f/f and Vil-Cre;Adam10f/f mice. (D–F), Adult small intestine from Vil-CreER;Adam10f/f mice treated with TX as described in Figure 1. (A,D), qPCR analysis of Notch gene targets (upper) and cell lineage-specific transcription factors (n=3–7). (B,E), X-Gal staining of Atoh1LacZ/+ reporter mice. (C,F), Immunostaining of Neurog3YFP/+ reporter mice. Arrowheads mark Neurog3+ cells. *p<0.05, **p<0.01 and ***p<0.001. Scale bars (μm) = B, 100; C, 65; E, 200; F, 135 and (insets 65).
Notch is the dominant signaling pathway regulated by ADAM10
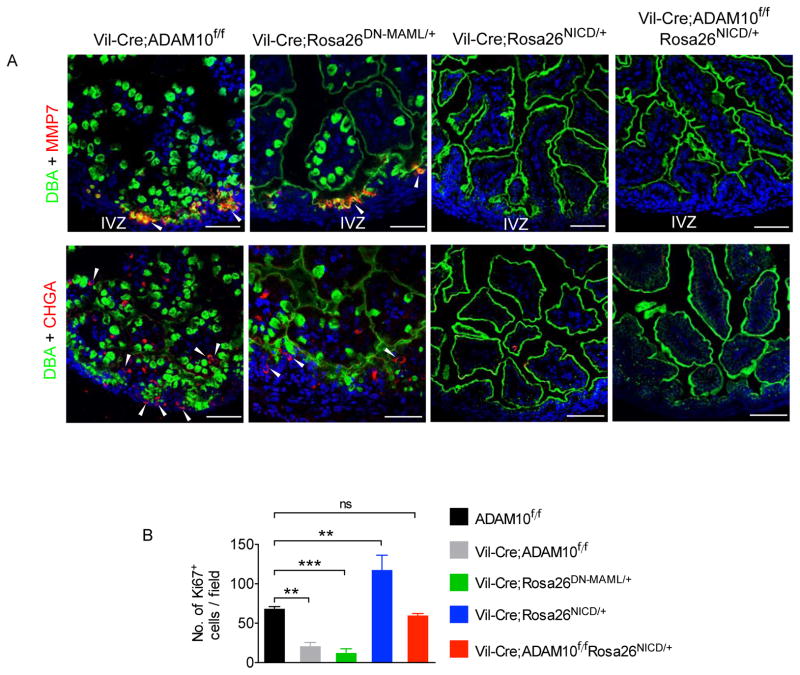
To verify that Notch signaling is the principal pathway controlled by ADAM10 in the intestine, we tested whether over-expression of constitutively active Notch could override ADAM10 deficiency. For this experiment, we used the Notch gain-of-function allele Rosa26NICD-ires-nGFP (termed Rosa26NICD), in which cDNAs encoding the mouse Notch1-ICD and nuclear GFP have been targeted to the Rosa26 locus25. Consistent with previous reports, Vil-Cre;Rosa26NICD/+ mice displayed a loss of secretory cell differentiation5. Importantly, the compound Vil-Cre;Adam10f/f;Rosa26NICD/+ mice, despite the loss of ADAM10 (data not shown), had an intestinal phenotype that was indistinguishable from Vil-Cre;Rosa26NICD/+ mice, indicating that activated Notch could override the loss of ADAM10 (Figure 4A). Next, we examined the Notch loss-of-function allele, Rosa26DNMAML/+, in which a cDNA encoding a GFP-tagged dominant-negative Mastermind-like construct is targeted to the Rosa26 locus26. In newborn Vil-Cre;Rosa26DNMAML/+ mice, co-staining of cell lineage markers revealed the appearance of intermediate-like cells within the IVZ and increased goblet and endocrine cell populations that closely resembled the phenotype found in Vil-Cre;Adam10f/f mice (Figure 4A and Supplementary Figure 4A). Furthermore, progenitor cell proliferation and nuclear Hes1 immunostaining mirrored the changes in cellular differentiation observed in the different Notch mutants (Figure 4B and Supplementary Figure 4B,C). Similar results were obtained when the same Notch mutants were examined in the adult intestine using the TX-inducible Vil-CreER line (Supplementary Fig 5). Together, these results demonstrate that Notch signaling is the dominant pathway regulated by ADAM10 in the developing and adult intestine.
Figure 4. ADAM10 mediated Notch signaling is required for cell lineage specification and progenitor cell proliferation.
(A), Immunofluorescence analysis of cell lineage markers in the newborn small intestine from Vil-Cre;Adam10f/f and in different Notch mutants. (B), Quantification of Ki67+ cells per field (n=3–4). p values of group comparisons between Adam10f/f and other genotypes are shown. *p<0.05, **p<0.01, ****p<0.0001. Scale bars (μm) = 100.
Mosaic ADAM10 deletion produces crypt degeneration that can be rescued by activated Notch
The pronounced changes in intestinal differentiation and rapid morbidity observed upon ADAM10 deletion prevented long-term fate analysis of adult ADAM10-deficient ISCs. To circumvent this problem, the TX treatment was modified to produce a mosaic pattern of ADAM10 recombination within crypts (Supplementary Figure 6). Vil-CreER;Adam10f/f mice given a single TX dose (100 mg/kg, i.p.) displayed reduced ADAM10 deletion but animals still lost weight, became moribund and had to be sacrificed at day 9 (Figure 5A). Histological evaluation of the small intestine revealed striking changes, with villus atrophy and crypt degeneration (Figure 5B). All degenerating crypts were AD-AM10-deficient, had reduced proliferation and numerous apoptotic cells were detected within the gut lumen and in degenerating crypts (Figure 5C and data not shown). By contrast, adjacent wild-type crypts contained tall, pseudostratified epithelial cells that were ADAM10+ and highly proliferative (BrdU+) suggesting that these crypts were involved in an intestinal repair response (Figure 5B,C).
Figure 5. ADAM10 loss leads to crypt degeneration that can be overridden by activated Notch.
(A–C), Adult mice were given a single TX (100 mg/kg, i.p.) dose and analyzed at day 9 post-treatment (n=3). (A), Body weight analysis. (B), H&E analysis. Black boxes are shown at high magnification (lower). In Vil-CreER;Adam10f/f;Rosa26YFP/+ mice, crypt degeneration (black asterisks) is observed adjacent to crypts with increased cellularity (orange asterisk). (C), Immunofluorescence analysis of ADAM10 with BrdU (upper) and YFP or NICD(nGFP) (lower). In Vil-CreER;Adam10f/f;Rosa26YFP/+ mice, degenerating crypts are ADAM10-deficient and lack cell proliferation (white asterisks) whereas adjacent regenerating crypts express ADAM10 and are highly proliferative (orange asterisk). In Vil-CreER;Adam10f/f;Rosa26NICD/+ mice, NICD(nGFP)+ ADAM10-deficient crypts (white asterisks) are filled with NICD(nGFP)+ cells (arrows) and maintain cell proliferation (arrowheads). p values from group comparisons of genotypes Vil-CreER;Adam10f/+;Rosa26YFP/+ vs Vil-CreER;Adam10f/f;Rosa26YFP/+ (red) and Vil-CreER;Adam10f/f;Rosa26YFP/+ vs Vil-CreER;Adam10f/f;Rosa26NICD/+ (blue) are shown in A. *p<0.05; **p<0.01, ***p<0.001, ****p<0.0001.
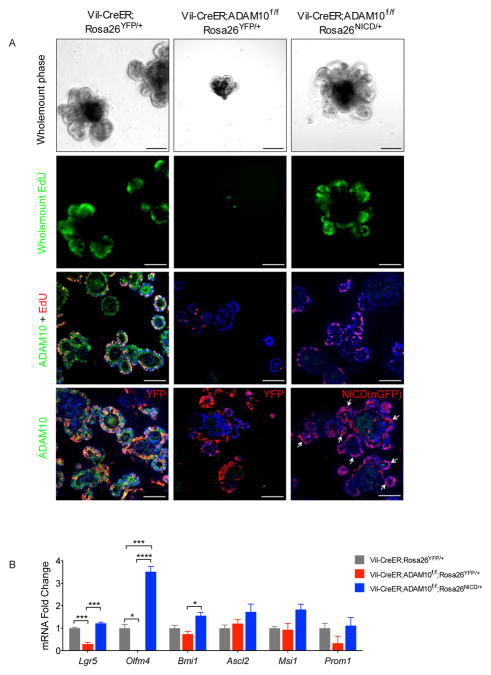
Next, we tested whether Notch activation could prevent crypt degeneration by examining Vil-CreER;Adam10f/f;Rosa26NICD/+ and genotype control mice under the same experimental conditions. Strikingly, TX-treated Vil-CreER;Adam10f/f;Rosa26NICD/+ mice were healthy, maintained weight and had normal intestinal histology (Figure 5A,B). Co-staining for nGFP (a surrogate marker of NICD) and ADAM10 showed that NICD(nGFP)+ ADAM10-deficient crypts were readily detected and had comparable proliferation to wild-type controls (Figure 5C). Similar results were obtained when recombination was induced with 4-hydroxytamoxifen (4OH-TX) in crypt organoid cultures derived from the same mice. 4OH-TX-treated organoids from Vil-CreER;Adam10f/f mice showed reduced cell proliferation and growth failure whereas 4OH-TX-treated organoids from Vil-CreER;Adam10f/f;Rosa26NICD/+ mice displayed robust cell proliferation and organoid growth similar to wild-type organoids (Figure 6A). Together, these results demonstrate that Notch activation could protect ADAM10-deficient crypts from degeneration and restore organoid growth suggesting that ADAM10 is required for maintenance of ISCs. Consistent with this interpretation was the observed loss of the Notch transcriptional target and stem cell marker Olfm4 from ADAM10-deficient organoids (Figure 6B) and from ADAM10-deficient immature and adult intestine (Supplementary Figure 7).
Figure 6. Activated Notch restores proliferation and Olfm4 expression in AD-AM10-deficient organoids.
(A), Organoid cultures from adult mice treated with 4-OH-TX (5μM) for 12 h and then analyzed at day 5 post-treatment. Whole mount analysis of morphology (upper) and EdU staining (mid-upper). Co-staining of ADAM10, EdU and YFP or NICD(nGFP) (lower panels). Arrows indicate NICD(nGFP)+ cells. Scale bars (μm) = B, 200 (inset, 65); C, 65; D, all 200. (B), qPCR analysis of ISC markers in 4-OH-TX treated organoid cultures (n=3). *p<0.05, ***p<0.001, ****p<0.0001.
ADAM10-mediated Notch signaling is required for long-term maintenance of Lgr5+ CBCs
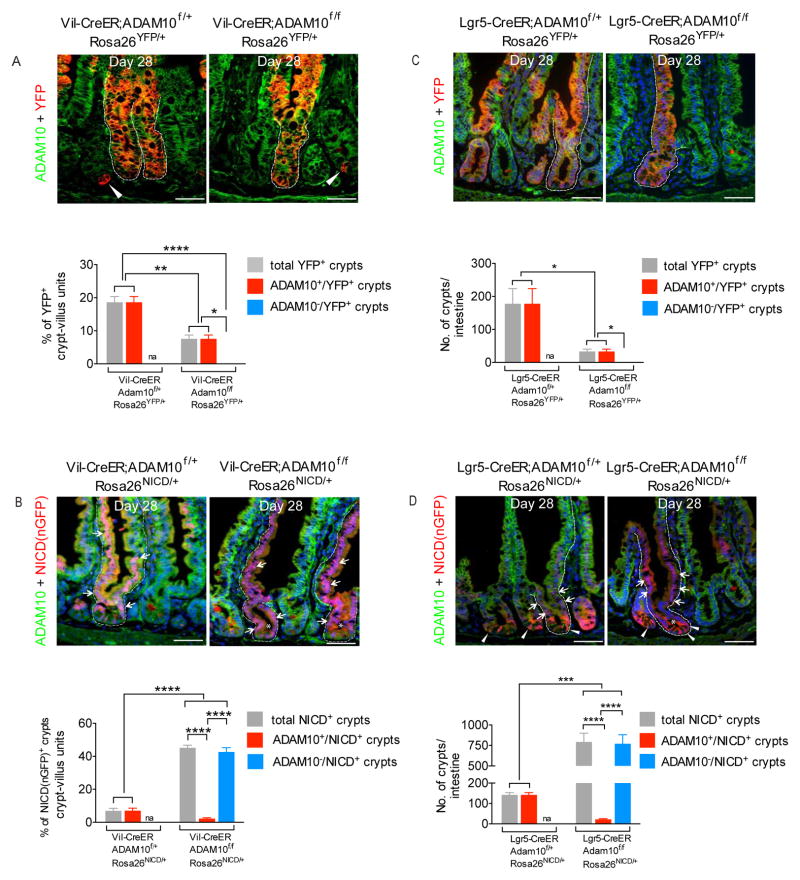
To formally test the fate of ADAM10-deficient ISCs, lineage tracing was performed using Vil-CreER;Adam10f/f;Rosa26YFP and genotype control mice given a single TX dose (50 mg/kg, i.p.) and then chased over time. At day 5 of chase, an equivalent % of YFP+ crypts were found in both genotypes. In TX-treated Vil-CreER;Adam10f/f;Rosa26YFP crypts, both YFP+/ADAM10− and YFP−/ADAM10− cells were detected due to the stochastic nature of recombination (Supplementary Figure 8A,B). However, by day 28 of chase, selection of monoclonal lineage tracing events had occurred. In TX-treated genotype control mice, ADAM10+/YFP+ crypt-villus units were detected. By contrast, in TX-treated ADAM10-deficient mice, there was a marked reduction in the number of YFP+ lineage tracing events and all of these had “escaped” ADAM10 deletion, indicating that ADAM10-deficient cells observed at day 5 had not survived (Figure 7A). Similarly, no YFP+ ADAM10-deficient crypt-villus units were detected when lineage tracing was performed using Lgr5-CreER;Adam10f/f;Rosa26YFP mice (Figure 7C). The failure to detect any YFP+ ADAM10-deficient crypt-villus units in either genetic model, together with the inability to detect DN-MAML+ crypt-villus units when the same experiments were performed with Rosa26DNMAML allele (data not shown) indicate that ADAM10-deficient and Notch-deficient Lgr5+ CBCs were not capable of maintaining crypt homeostasis.
Figure 7. ADAM10 is required for maintenance of adult Lgr5+ CBCs.
(A–B), Lineage tracing of Vil-CreER;Adam10f/f;Rosa26YFP/+, Vil-CreER;Adam10f/f;Rosa26NICD/+ and genotype control mice given a single TX (50 mg/kg i.p.) dose and then chased for 28 days (n=3). Co-staining of ADAM10 and YFP or NICD(nGFP). (A), In Vil-CreER;Adam10f/f;Rosa26YFP/+ mice, no ADAM10-deficient cells were detected. Arrowheads indicate individual YFP+ Paneth cells (Supplementary Figure 8A). (B), In both genotypes, NICD(nGFP)+ cells are detected (arrowheads). Asterisks mark NICD(nGFP)+ ADAM10-deficient crypt-villus units. (C–D), Lineage tracing of Lgr5-CreER;Adam10f/f;Rosa26YFP/+, Lgr5-CreER;Adam10f/f;Rosa26NICD/+ and genotype control mice given a single TX (400 mg/kg o.g.) dose and then chased for 28 days (n=3). Immunofluorescence analysis of ADAM10 and YFP or NICD(nGFP). Note that EGFP+-Lgr5 cells can co-express YFP or NICD(nGFP). (C), In Lgr5-CreER;Adam10f/f;Rosa26YFP/+ mice, no ADAM10-deficient cells were detected. Strong YFP expression from the Rosa26YFP allele masks the weaker EGFP+- Lgr5 cells. (D), NICD(nGFP)+ cells (arrows), EGFP+-Lgr5 cells displaying stronger cytoplasmic staining (arrowheads) and NICD(nGFP)+ ADAM10-deficient crypt-villus unit (asterisk) are shown. (AD), Lower panels, Quantification of each lineage tracing event. *p<0.05; **p<0.01;***p<0.001, ****p<0.0001; na, not applicable. Scale bars (μm) = 100.
Notch-activated ADAM10-deficient Lgr5+ CBCs have a competitive advantage to occupy the stem cell niche
To test whether activated Notch could rescue ADAM10-deficient ISCs, lineage tracing was performed using Vil-CreER;Adam10f/f;Rosa26NICD/+ and genotype control mice. At day 5 of chase, NICD(nGFP)+ cells were found in both genotypes. However, the Vil-CreER;Adam10f/f;Rosa26NICD/+ mice had a significant increase in the % of NICD(nGFP)+ crypts and these crypts primarily expressed NICD(nGFP)+/ADAM10− cells (Supplementary Figure 8C). By day 28 of chase, NICD(nGFP)+ ADAM10-deficient crypt-villus units were readily detected indicating that activated Notch had rescued the ability of ADAM10-deficient ISCs to populate the crypt compartment (Figure 7B). Moreover, there was a six-fold increase in the percentage of NICD(nGFP)+ lineage tracing events observed in Vil-CreER;Adam10f/f; Rosa26NICD/+ mice compared to genotype controls suggesting that there was a selective advantage in the ADAM10-deficient state for NICD(nGFP)+ ADAM10-deficient ISCs to occupy the stem cell niche (Figure 7B). When this same experiment was repeated using the Lgr5-CreER line, a five-fold increase in the number of NICD(nGFP)+ ADAM10-deficient lineage tracing events was observed in Lgr5-CreER;Adam10f/f;Rosa26NICD/+ mice compared to genotype controls (Figure 7D). Notch signaling was clearly active in NICD(nGFP)+ ADAM10-deficient crypts as demonstrated by the loss of secretory differentiation and the restoration of crypt cell proliferation (Supplementary Figures 8D and 9). Interestingly, although there was no significant difference in progenitor cell proliferation between NICD(nGFP)+/ADAM10− and NICD(nGFP)+/ADAM10+ crypts, NICD(nGFP)+ ADAM10-deficient crypt villus units represented the majority (>97%) of lineage tracing events in ADAM10-deficient state. This enhanced NICD(nGFP)+ lineage tracing was not due to an expansion of EGFP+-expressing Lgr5+ crypts in the ADAM10-deficient state as the total number of these crypts remained constant in both genotypes (see Supplementary Methods). These results demonstrate that activated Notch can rescue ADAM10-deficient Lgr5+ CBCs. While the precise mechanism(s) for how ADAM10-deficiency leads to enhanced NICD(nGFP)+ lineage tracing is still unclear, the results clearly demonstrate that Notch-activated ADAM10-deficient stem cells have a competitive advantage to occupy the stem cell niche. We conclude that cell-autonomous AD-AM10-mediated Notch signaling plays an important role in the maintenance of Lgr5+ CBCs and crypt homeostasis.
DISCUSSION
The progenitor cell compartment of the small intestine is comprised of slowly and actively cycling intestinal stem cells (ISCs) located within the crypt base27. While the interplay between these different ISC populations and transit-amplifying progenitors is crucial for crypt homeostasis, the signaling pathways required for ISC function within the stem cell niche are not well defined. In this study, we demonstrate that cell-autonomous ADAM10 activity functions within the crypt compartment to regulate Notch signaling essential for maintenance of ISCs and progenitor cell lineage specification. We also demonstrate that Notch is the dominant substrate regulated by ADAM10 within the crypt compartment, consistent with ADAM10 deficiency in other organ systems in vivo19,28. In addition, we show that activated Notch can rescue ADAM10-deficient Lgr5+ CBCs and enhance their ability to re-populate the crypt compartment, indicating that Notch-activated stem cells have a competitive advantage to occupy the stem cell niche and re-establish ISC homeostasis.
It is well-established that Notch signaling plays a crucial role in controlling cell lineage specification of intestinal progenitors1. Earlier Notch loss-of-function studies showed that Notch inhibition resulted in goblet cell hyperplasia8,9,29 while more recent reports have demonstrated robust differentiation of all secretory cell lineages7,30,31. Recently, VanDussen et al., showed that Notch inhibition with a γ-secretase inhibitor induces accumulation of a distinct “intermediate” cell population that expresses both Paneth and goblet cell markers throughout the intestine including the colon7. Consistent with a loss of Notch signaling, morphological and transcriptional analysis of the ADAM10-deficient intestine showed robust secretory cell differentiation in the immature and adult intestine. Moreover, analysis of Notch loss-of-function (Rosa26DN-MAML) and gain-of-function (Rosa26NICD) mutants provided direct genetic evidence that cell-autonomous AD-AM10 activity regulates Notch signaling in these cell fate decisions.
Currently, the mechanism(s) by which secretory progenitors give rise to the major secretory cell lineages remain controversial. In one model, it has been proposed that multipotent secretory progenitors must rapidly commit to a specific, secretory cell fate32 whereas recent analysis of different Atoh1-, Neurog3- and Gfi1-deficient mouse models suggests that multipotent Atoh1+ progenitors give rise to committed enteroendocrine progenitor and bipotent goblet/Paneth progenitor populations10–13. In the ADAM10-deficient crypts, the progenitor cell population was completely replaced by Atoh1+ cells, which is indicative of cells undergoing secretory differentiation. In addition, we confirmed using the Neurog3YFP reporter line that the “intermediate” (Paneth/goblet) cells were distinct from Neurog3+ progenitors required for enteroendocrine lineage specification. A similar accumulation of distinct intermediate and endocrine cell populations was observed upon expression of DN-MAML, demonstrating that cell-autonomous Notch inhibition was directly responsible for the appearance of these different secretory cell types.
Notch plays an essential role in crypt cell proliferation5,29 but it is only recently that lineage tracing and Notch inhibition studies have demonstrated that active Notch signaling is present2–4,33 and required for maintenance of ISCs7,34. Notch inhibition led to reduced proliferation, loss of the stem cell marker, Olfm4, and apoptosis of CBCs, indicating that active Notch signaling is required for survival of Lgr5+ CBCs. In the immature and adult intestine, intestine-specific ADAM10 inactivation closely recapitulated these findings, including reduced progenitor proliferation and loss of Olfm4. Further analysis of ADAM10-deficient crypts in vivo or cultured organoid in vitro revealed severe crypt degeneration and organoid failure associated with a loss of progenitor proliferation and increased apoptosis. In addition, our data from organoid experiments clearly demonstrates that ADAM10 acts in a cell autonomous manner and does not require any mesenchymal signaling to fulfill its functions. Importantly, the ability of activated Notch to completely restore progenitor proliferation and cell viability in both these settings demonstrates that ADAM10-mediated Notch signaling is essential for long-term crypt survival. Interestingly, a recent study using mice constitutively-expressing dominant-negative ADAM10 under the cryptdin-2 promoter reported mis-localized Paneth cells, a phenotype linked to a loss of Eph signaling35. By contrast, our results clearly demonstrate that ADAM10 deficiency in the stem/progenitor cell compartment produces a dominant Notch loss-of-function phenotype. While ADAM10 likely regulates other substrates in the crypt compartment, the loss of stem/progenitor cell proliferation and rapid crypt degeneration observed in our current ADAM10-deficient models has not permitted analysis of ADAM10 signaling in post-mitotic intestinal cells including Paneth cells and this will be an important area for further investigation.
Mosaic recombination epigenetically found in the Lgr5-EGFP-ires-CreERT2 line or produced by reduced tamoxifen dosing in the Villin-CreERT2 line allowed long-term lineage tracing of ADAM10-deficient ISCs. In both models, ADAM10-deficient ISCs failed to populate the crypt compartment and no dormant AD-AM10-deficient ISCs were detected, indicating that ADAM10-deficient ISCs did not survive long-term. Similar results were found when lineage tracing was performed with the Notch loss-of-function mutant DN-MAML. The demise of AD-AM10-deficient ISCs was clearly illustrated by the ability of activated Notch to rescue ADAM10-deficient ISCs and restore their capacity to populate the crypt-villus axis. Together, these results demonstrate that ADAM10-mediated Notch signaling is crucial for the maintenance of these ISC populations.
It has been proposed that neutral drift and symmetric cell divisions are critical elements that control ISC homeostasis. ISCs within the same crypt form an equi-potent population, in which the loss of one stem cell can be replaced stochastically by another stem cell36,37. In addition, lineage tracing after injury or ablation of Lgr5+ CBCs demonstrates that the Lgr5+ stem cell compartment can be repopulated through mobilization of quiescent stem cell populations38,39 or by de-differentiation of crypt progenitors40,41. Analogous to these Lgr5+ cell injury/ablation studies, our data suggest that ADAM10 deletion within Lgr5+ CBCs leads to stem cell loss and an imbalance within the stem cell niche that promotes permissive signals for wild-type ISCs and/or progenitor populations to reestablish ISC homeostasis. Unexpectedly, lineage tracing in the presence of activated Notch revealed a 5-6-fold increase in the total number of NICD+ lineage tracing events within the ADAM10-deficient state compared to genotype control mice. Our data suggest that loss of ADAM10-deficient CBCs creates permissive conditions in which Notch-activated stem cells have a competitive advantage to replenish the ISC compartment. A possible explanation based on the kinetics of ADAM10 deletion and functional NICD expression is that the immediate progeny of NICD+/ADAM10-deficient Lgr5+ CBCs efficiently re-populate the stem cell compartment. This result is consistent with the plasticity of secretory progenitors to revert to a stem-cell state40,41. It will be interesting to test whether this enhanced compensatory mechanism observed with active Notch signaling can occur in other regenerative processes in which imbalances of ISC homeostasis are found.
In summary, Notch is the dominant substrate regulated by ADAM10 in the crypt compartment. ADAM10-mediated Notch signaling is essential for cell lineage specification and maintenance of Lgr5+ CBCs. Importantly, loss of ADAM10-deficient Lgr5+ CBCs created a competitive advantage for Notch-activated stem cells to re-populate the stem cell niche and restore ISC homeostasis.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by grants from the UM Comprehensive Cancer Center (PJD), CCFA (PJD) and the NIH, R01-DK093697 (PJD), R01-AI091627 (IM), R01-DK096972 (LCS) and R01-CA159222 (HCC). This work utilized the Morphology and Image Analysis Core of the Michigan Diabetes Research Center funded by NIH NIDDK P60DK020572. KLV was supported by an AGA Foundation Graduate Student Fellowship and a Rackham Predoctoral Fellowship.
We thank the Michigan Gut Group for helpful discussions, Dr. Sudo for Hes1 antibody, Dr. Gradwohl for Neurog3YFP line, Dr. Robine for Villin-Cre and Villin-CreER lines and Rebecca Tucker and Connie Brindley for technical assistance.
Abbreviations
- CBCs
crypt based columnar cells
- DAPI
4′-6-diamidino-2-phenylindole
- Lgr5
Leucine-rich repeat-containing GPCR5
- qPCR
quantitative polymerase chain reaction
- MMP7
matrix metalloproteinase-7
- Neurog3
Neurogenin-3
- GSI
gamma-secretase inhibitor
Footnotes
Disclosures
The authors declare no competing financial interests.
Writing Assistance
None.
Author Contributions
Y-H.T, K. L.V, C.K, S.R, R.G.B and P.J.D performed experiments and prepared data, E.T.S, A.S, A.W.W, H.C.C and P.J.D performed characterization of Adam10f mice, I.M provided reagents, Y-H.T, K.L.V, I.M, L.C.S, H.C.C and P.J.D. analyzed data and edited manuscript, P.J.D wrote the manuscript and supervised the project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Noah TK, Shroyer NF. Notch in the intestine: regulation of homeostasis and pathogenesis. Annu Rev Physiol. 2013;75:263–88. doi: 10.1146/annurev-physiol-030212-183741. [DOI] [PubMed] [Google Scholar]
- 2.Vooijs M, Ong CT, Hadland B, et al. Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development. 2007;134:535–44. doi: 10.1242/dev.02733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopinke D, Brailsford M, Shea JE, et al. Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development. 2011;138:431–41. doi: 10.1242/dev.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fre S, Hannezo E, Sale S, et al. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PLoS One. 2011;6:e25785. doi: 10.1371/journal.pone.0025785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fre S, Huyghe M, Mourikis P, et al. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–8. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 6.Fre S, Pallavi SK, Huyghe M, et al. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci U S A. 2009;106:6309–14. doi: 10.1073/pnas.0900427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.VanDussen KL, Carulli AJ, Keeley TM, et al. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139:488–97. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riccio O, van Gijn ME, Bezdek AC, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377–83. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pellegrinet L, Rodilla V, Liu Z, et al. Dll1− and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140:1230–1240. e1–7. doi: 10.1053/j.gastro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanDussen KL, Samuelson LC. Mouse atonal homolog 1 directs intestinal progenitors to secretory cell rather than absorptive cell fate. Dev Biol. 2010;346:215–23. doi: 10.1016/j.ydbio.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjerknes M, Cheng H. Cell Lineage metastability in Gfi1-deficient mouse intestinal epithelium. Dev Biol. 2010;345:49–63. doi: 10.1016/j.ydbio.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Gregorieff A, Stange DE, Kujala P, et al. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–45. e1–3. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 13.Shroyer NF, Wallis D, Venken KJ, et al. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 2005;19:2412–7. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Diaz L, Jain RN, Keeley TM, et al. Intestinal Neurogenin 3 directs differentiation of a bipotential secretory progenitor to endocrine cell rather than goblet cell fate. Dev Biol. 2007;309:298–305. doi: 10.1016/j.ydbio.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmann D, de Strooper B, Serneels L, et al. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet. 2002;11:2615–24. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- 16.Weber S, Saftig P. Ectodomain shedding and ADAMs in development. Development. 2012;139:3693–709. doi: 10.1242/dev.076398. [DOI] [PubMed] [Google Scholar]
- 17.Brou C, Logeat F, Gupta N, et al. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–16. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 18.Sawey ET, Johnson JA, Crawford HC. Matrix metalloproteinase 7 controls pancreatic acinar cell transdifferentiation by activating the Notch signaling pathway. Proc Natl Acad Sci U S A. 2007;104:19327–32. doi: 10.1073/pnas.0705953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibb DR, El Shikh M, Kang DJ, et al. ADAM10 is essential for Notch2-dependent marginal zone B cell development and CD23 cleavage in vivo. J Exp Med. 2010;207:623–35. doi: 10.1084/jem.20091990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.el Marjou F, Janssen KP, Chang BH, et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–93. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 21.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 22.Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Q, Bermingham NA, Finegold MJ, et al. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–8. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 24.Mellitzer G, Martin M, Sidhoum-Jenny M, et al. Pancreatic islet progenitor cells in neurogenin 3-yellow fluorescent protein knock-add-on mice. Mol Endocrinol. 2004;18:2765–76. doi: 10.1210/me.2004-0243. [DOI] [PubMed] [Google Scholar]
- 25.Murtaugh LC, Stanger BZ, Kwan KM, et al. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–5. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu L, Fang TC, Artis D, et al. Notch signaling is an important regulator of type 2 immunity. J Exp Med. 2005;202:1037–42. doi: 10.1084/jem.20050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–84. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Weber S, Niessen MT, Prox J, et al. The disintegrin/metalloproteinase Adam10 is essential for epidermal integrity and Notch-mediated signaling. Development. 2011;138:495–505. doi: 10.1242/dev.055210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–63. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 30.Kazanjian A, Noah T, Brown D, et al. Atonal homolog 1 is required for growth and differentiation effects of notch/gamma-secretase inhibitors on normal and cancerous intestinal epithelial cells. Gastroenterology. 2010;139:918–28. 928 e1–6. doi: 10.1053/j.gastro.2010.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim TH, Shivdasani RA. Genetic evidence that intestinal Notch functions vary regionally and operate through a common mechanism of Math1 repression. J Biol Chem. 2011;286:11427–33. doi: 10.1074/jbc.M110.188797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116:7–14. doi: 10.1016/s0016-5085(99)70222-2. [DOI] [PubMed] [Google Scholar]
- 33.Stamataki D, Holder M, Hodgetts C, et al. Delta1 expression, cell cycle exit, and commitment to a specific secretory fate coincide within a few hours in the mouse intestinal stem cell system. PLoS One. 2011;6:e24484. doi: 10.1371/journal.pone.0024484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Es JH, de Geest N, van de Born M, et al. Intestinal stem cells lacking the Math1 tumour suppressor are refractory to Notch inhibitors. Nat Commun. 2010;1:18. doi: 10.1038/ncomms1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solanas G, Cortina C, Sevillano M, et al. Cleavage of E-cadherin by ADAM10 mediates epithelial cell sorting downstream of EphB signalling. Nat Cell Biol. 2011;13:1100–7. doi: 10.1038/ncb2298. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Garcia C, Klein AM, Simons BD, et al. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330:822–5. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- 37.Snippert HJ, van der Flier LG, Sato T, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–44. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery RK, Carlone DL, Richmond CA, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108:179–84. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian H, Biehs B, Warming S, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–9. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buczacki SJ, Zecchini HI, Nicholson AM, et al. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–9. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- 41.van Es JH, Sato T, van de Wetering M, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.