At the forefront of translational research to combat anxiety disorders is the idea of developing neuroscience-based adjuncts to traditional exposure-based treatment such as cognitive/behavioral therapy. The core concept of exposure therapy is Pavlovian extinction, where an anxiety-triggering stimulus is repeatedly presented so that that the patient learns that the stimulus predicts no negative consequences. This new association acts to inhibit the anxiety normally provoked by the stimulus. On its own, such exposure is quite effective, but it has some limitations. After exposure therapy, fear of the extinguished stimulus may return because of a stressful experience, a long passage of time since encountering the stimulus, or confronting the fear-provoking stimulus in a novel environment (1). Additionally in some anxiety disorders, notably post-traumatic stress disorder, extinction learning itself is compromised (2). The idea of neuroscience-based adjuncts given during exposure treatment is to overcome this limitation either by strengthening the extinction learning itself or by changing its nature. An example of the strengthening strategy is the use of d-cycloserine (DCS) to facilitate the NMDAmediated plasticity that normally mediates memory formation (2). To change the nature of extinction, researchers have administered extinction training during periods when the original fear memory lacks stability because it was very recently encoded or reactivated (2,3).
Pena et al. (4) present a novel and promising example of the strengthening extinction approach in this issue of Biological Psychiatry. Their strategy was based on the large literature indicating that emotional arousal leads to enhanced memory consolidation. This is the common experience that we better remember events that occur in the context of emotional arousal. For example, many of us know what we were doing at 9:03 am eastern time on September 11, 2001, but we cannot recall a similar episode from a less eventful day. This enhancement of memory consolidation is at least in part mediated by feedback from the autonomic nervous system to the amygdala. Beta-adrenergic receptors on the afferent arm of the vagus nerve detect release of epinephrine from the adrenal gland. This acts as a “something important just happened” signal that activates noradrenergic projection neurons in the locus coeruleus via the nucleus of the solitary tract (see Figure 4 reference 4). The resultant release of NE in several cortical and subcortical regions leads to enhanced memory consolidation and therefore we experience stronger memories for events that were accompanied by peripheral sympathetic arousal. Importantly, norepinephrine has a memory-enhancing action on the amygdala (4).
While the amygdala is most closely associated with the acquisition of fear, plasticity within the amygdala is also critical for extinction learning (2). Infusions of norepinephrine into the amygdala enhance extinction learning (5) suggesting that manipulations that mimic peripheral adrenergic activation will be beneficial to extinction via the vagal pathway described above. This prediction is supported by the findings that pharmacological activation or inhibition of adrenergic activity during extinction can enhance or decrease the rate of fear extinction, respectively (6).
A potential limitation of using autonomic arousal as an adjunct to exposure therapy is that most of these manipulations are anxiogenic. For example yohimbine facilitates adrenergic transmission and enhances extinction learning, but is anxiety provoking (6). An ideal treatment would be one that eliminates the anxiety-inducing aspects of autonomic arousal but retains the memory consolidating components. Pena et al (4) present a solution to this puzzle: bypass epinephrine and stimulate the vagus nerve directly. Prior work indicated that (VBS) could mimic epinephrine’s effects on memory consolidation but stimulation also reduced anxiety and enhanced mood. Rats were implanted with vagal stimulation electrodes prior to auditory fear conditioning with tone-shock pairings. Later the rats received presentation of the tone without shock to extinguish fear responding. Animals that received VBS during extinction extinguished more rapidly than controls that received similar stimulation at other times or were never stimulated. The benefit was still present at a 2-week follow-up test of fear. Since VBS is a well-tolerated treatment for epilepsy this is an approach that should be readily translatable to the clinic.
Typically patients do not seek treatment for anxiety disorders until symptoms have been present for a prolonged period and fear memories are known to change over time. Therefore, Pena et al (4) demonstrated that VBS had a similar benefit when extinction occurred 2 weeks after fear conditioning. This is critical as some approaches to enhancing the benefit of extinction have limited windows of efficacy and need treatment to begin not long after fear acquisition (2,7).
It has been suggested that fear must be experienced in order for extinction to occur. For example, administration of pharmacological agents that reduce anxiety concurrently with treatment typically reduce the benefits of exposure (1). While Pena et al (4) did not assess the direct effects of nerve stimulation on fear expression, others have reported a reduction in anxiety in patients undergoing stimulation to treat seizures. This raises the possibility that anxiolysis is not necessarily incompatible with treatment and that typical anxiolytics reduce the efficacy of treatment by a direct antagonist action on memory consolidation mechanisms or by causing a state-dependent learning that is only expressed when the drug is present (1). By enhancing fear extinction while quelling anxiety, vagal stimulation delivers a double hit against maladaptive fear. This may make vagal stimulation particularly useful in cases where severe anxiety prevents effective exposure therapy.
Like DCS, vagal nerve stimulation enhanced the rate of acquisition of extinction learning. However, the rate of extinction is not the only issue. Even after very strong extinction training fear responding is prone to “renewal” if the previously feared stimulus is encountered in a context that is different from that of extinction (1). Even when extinction learning is enhanced by DCS fear is still prone to renewal (8). Pena et al., did not test whether or not VBS mitigates renewal so this remains an important concern
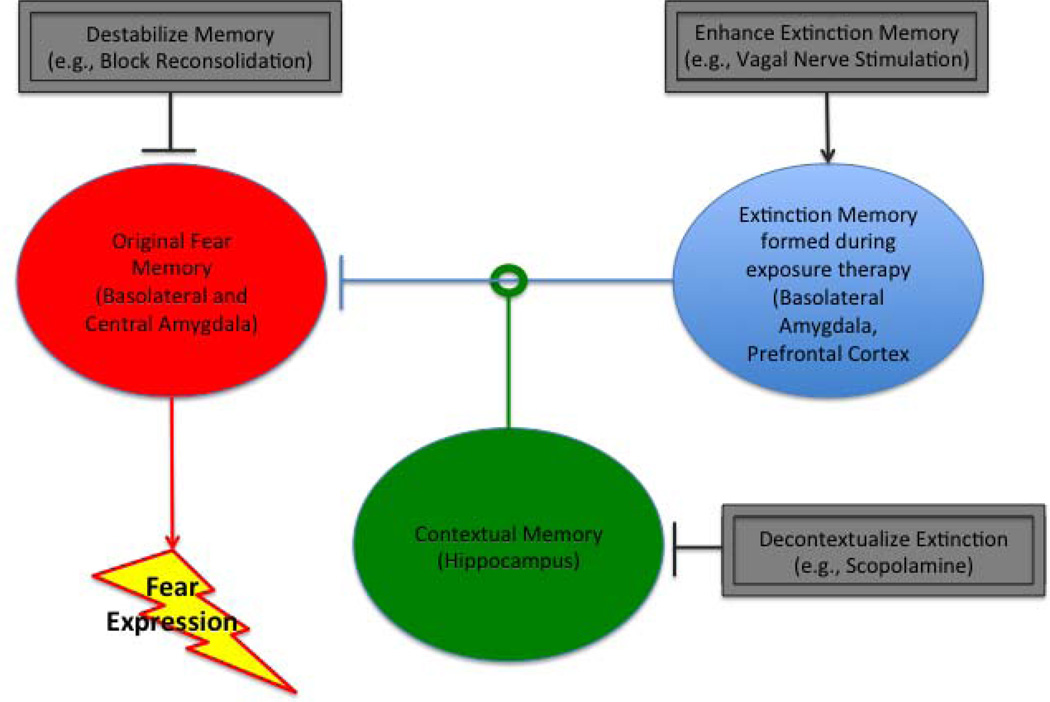
However, basic research also suggests a way to address the problem of renewal. The hippocampus provides contextual information that is used by the amygdala and prefrontal cortex to generate renewal; hippocampal ablation or inactivation eliminates renewal (9). Hippocampus-dependent learning is exquisitely sensitive to the blockade of cholinergic activity produced by scopolamine. Recently, my lab found that systemic administration of a low dose of scopolamine during extinction prevented later fear renewal (10). Scopolamine is used in the treatment of psychiatric and neurological disorders and therefore should also be translatable (10). An ideal set of adjuncts to exposure therapy would enhance extinction memory acquisition or consolidation processes in the amygdala and medial prefrontal cortex but simultaneously decontextualize that learning by inhibiting the hippocampus (see Figure 1).
Figure 1.
Fear expression depends on a circuit comprising the amygdala, prefrontal cortex and hippocampus that process fear memories, extinction memories and contextual memories. Hippocampus provides a contextual memory that can gate the effectiveness of the extinction memory in inhibiting fear expression. Neuroscience research suggests that exposure therapy can be enhanced by targeting these different memories. In the target paper, Pena et al (4) enhance extinction memory by pairing exposure with vagal nerve stimulation.
Manipulation of memory mechanisms as an adjunct to behavioral therapy is a new treatment frontier. Basic neuroscientific research is suggesting many new avenues to enhance the benefits of therapy. It remains to be determined which of these adjuncts is best matched with a particular anxiety disorder. Time elapsing between onset and treatment is also likely to turnout to be a factor in the choice of a particular adjunct. Some approaches, such as reconsolidation update, may be most effective with recently acquired fears (3, 7), while VBS is effective with a fear acquired two weeks earlier (4). The melding of neuroscientific and behavioral research is entering a very exciting period and optimism about potential treatment advances is well justified.
Acknowledgements
Supported by NIH RO1 MH62122.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The author has no biomedical financial interests and has no conflicts of interest relevant to this commentary.
References
- 1.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 2.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 3.Monfils M-H, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pena DF, Engineer ND, McIntyre Rapid Remission of Conditioned Fear Expression with Extinction Training Paired with Vagus Nerve Stimulation. Biol. Psychiatry. 2013;XX:YY–ZZ. doi: 10.1016/j.biopsych.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci. 2006;120:1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- 9.Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zelikowsky M, Hast TA, Bennett RZ, Merjanian M, Nocera NA, Ponnusamy R, Fanselow MS. Cholinergic Blockade Frees Fear Extinction from Its Contextual Dependency. Biol. Psychiatry. 2013;73:345–352. doi: 10.1016/j.biopsych.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]