Abstract
Excess dietary sodium has been linked to the development of hypertension and other cardiovascular diseases. In humans, the effects of sodium consumption on endothelial function have not been separated from the effects on blood pressure. The present study was designed to determine if dietary sodium intake affected endothelium-dependent dilation (EDD) independently of changes in blood pressure. Fourteen healthy salt resistant adults were studied (9M, 5F; age 33 ± 2.4 years) in a controlled feeding study. After a baseline run-in diet, participants were randomized to a 7 day high sodium (HS) (300-350 mmol/day) and 7 day low sodium (LS) (20 mmol/day) diet. Salt resistance, defined as a ≤ 5 mm Hg change in a 24-hour mean arterial pressure, was individually assessed while on the low sodium and high sodium diets and confirmed in the subjects undergoing study (LS: 85±1 mm Hg; HS: 85±2 mmHg). EDD was determined in each subject via brachial artery flow-mediated dilation on the last day of each diet. Sodium excretion increased during the high sodium diet (p < 0.01). EDD was reduced on the high sodium diet (Low: 10.3±0.9%, High: 7.3±0.7%, p < 0.05). The HS diet significantly suppressed plasma renin activity (PRA), plasma angiotensin II, and aldosterone (p < 0.05). These data demonstrate that excess salt intake in humans impairs endothelium-dependent dilation independently of changes in blood pressure.
Keywords: dietary sodium, endothelium-dependent dilation, blood pressure, salt-resistance
Introduction
Endothelial dysfunction, thought to be a primary causative event in the development of atherosclerosis, precedes clinical evidence of cardiovascular disease (CVD) [1], the leading global cause of death [2]. Excess dietary salt intake is an independent risk factor for the development of CVD [3]. Given the high levels of dietary salt habitually consumed in modern society [4], understanding the detrimental effects of salt on the cardiovascular system has important clinical and public health implications.
The deleterious effects of excess salt consumption in humans are typically attributed to increases in blood pressure (BP) [5-7]. However, recent experimental evidence in animals suggests that salt loading produces end-organ damage [7-11]. Dietary salt loading impaired cardiac, renal, and vascular function independent of arterial pressure in spontaneously hypertensive rats and also in normotensive Wistar-Kyoto rats [9-11]. Additional rodent studies have demonstrated vascular endothelial dysfunction during salt loading; these effects were independent of changes in BP [12-16]. In normotensive rats [12, 13] and mice [14] fed a high salt diet, skeletal muscle arteriolar responsiveness to endothelium-dependent stimulation, via exogenous acetylcholine and shear stress, was reduced in the absence of a change in BP.
Endothelium-dependent dilation (EDD), as assessed by brachial artery flow-mediated dilation (FMD), improves in overweight and obese normotensive adults, when switched from a typical salt diet (150 mmol sodium/day) to a low salt diet (50 mmol sodium/day) [17]. Decreases in BP during the low salt diet were also observed, and as such, the beneficial effects of dietary salt reduction on endothelial function could not be separated from the effects of decreases in BP. Additionally, a recent cross-sectional study suggests that habitual low salt intake is associated with increased EDD in middle-aged and older adults with elevated systolic BP [18]. Furthermore, impaired forearm blood flow responses to acetylcholine in response to dietary salt loading have been observed in healthy young men [19]; however, this study did not directly assess salt sensitivity or resistance. We have previously shown that an acute local infusion of hypertonic saline impairs cutaneous microvascular function in healthy normotensive individuals [20]. Importantly, these effects were observed without a change in resting BP suggesting a potential direct deleterious effect of salt on the vasculature.
Therefore, the purpose of the present study was to determine whether dietary sodium loading adversely affects endothelial function independently of changes in BP. We hypothesized that EDD, assessed via brachial artery FMD, would be impaired during a high sodium diet, even in the absence of a change in BP. To test this hypothesis, we individually assessed salt sensitivity of BP and EDD during a controlled feeding study in which subjects consumed both a low and high sodium diet for 7 days. Dietary sodium-induced declines in EDD in the absence of a change in BP (i.e., salt-resistance) would suggest that high dietary salt has deleterious vascular effects that are independent of BP.
Methods
Subjects
Fourteen healthy salt-resistant individuals participated in this study. Informed consent was obtained from all subjects, and the study protocol and procedures were approved by the Institutional Review Board of the University of Delaware and conform to the provisions of the Declaration of Helsinki.
Participants reported to the laboratory after a 12-hour fast. All participants provided a complete medical history. A resting 12-lead electrocardiogram (ECG), resting blood pressure (BP), and height and weight were determined and a venous blood sample was collected. The focus of this study was healthy adults; therefore exclusion criteria included a history of hypertension, cardiovascular disease, malignancy, diabetes mellitus, and renal impairment. Participants who were obese [body mass index (BMI) > 30 kg/m2] or used tobacco products were also excluded. Participant age ranged from 22-46 years. Menopausal women were excluded because salt sensitivity of BP increases with menopause [21].
Dietary Salt Perturbation
This experiment was a controlled feeding study. A registered dietitian prepared all the food. Habitual salt intake varies widely between individuals [22]; therefore, in order to normalize baseline dietary sodium intake, participants completed a 3-7 day run-in diet (100 mmol sodium/day). This was followed by 7 days of a low sodium (LS) diet (20 mmol sodium/day) and 7 days of a high sodium (HS) diet (300-350 mmol sodium/day), in random order. The specific sodium intakes were chosen in order to accurately classify adults with salt-resistant BP and are in agreement with previously published studies [23-25]. Dietary potassium intake averaged 70.9 ± 3.7 mmol/day, and the percentage of carbohydrates were 50%, fat 30%, and protein 20% across all conditions. Fluid intake was monitored and recorded daily. Participants were instructed to maintain their normal activity levels during the study.
Twenty-four hour urine and BP
Urine was collected during the last 24-hour period of the LS and HS conditions and kept in a cool dark container. The total volume, urinary electrolytes (EasyElectrolyte Analyzer, Medica, Bedford, MA), and urine osmolality (Advanced 3D3 Osmometer, Advanced Instruments, Norwood, MA) were assessed from an aliquot of the 24-hour collection period. Free water clearance, creatinine clearance (Christiana Care Health Systems), and fractional excretion of sodium and chloride were calculated using standard equations. An ambulatory BP monitor (Spacelabs Medical, Issaquah, WA) was worn on the non-dominant arm for the same 24-hour period. The monitor measured BP every 20 minutes while the participant was awake and every 30 minutes during sleep. Laboratory BP was also measured by an automated oscillometric sphygmomanometer (Dinamap Dash 2000, GE medical Systems) during the LS and HS testing visits to the laboratory.
Salt Resistance Classification
Salt resistance was defined as a ≤ 5 mm Hg change in 24-hour mean arterial pressure (MAP) determined while on the LS and the HS diets [26]. This assessment is reproducible within subjects [27]. The classification of salt resistance was determined after the participants completed the full protocol. Participants classified as salt-sensitive (a change in MAP of > 5 mmHg from the LS to the HS diet) were excluded from analysis, because they could not be used to test the a priori study hypothesis regarding the BP-independent effects of dietary salt.
Assessment of endothelium-dependent and independent dilation
Brachial artery FMD was used to assess EDD according to established guidelines [28]. Each participant was assessed at the end of the LS and HS phases of the dietary sodium perturbation. Participants were supine, with the right arm supported at heart level. A blood pressure cuff was placed on the proximal forearm ∼3 cm below the antecubital crease. Longitudinal images of the brachial artery and continuous Doppler blood velocity were obtained using a 12-MHz linear phased array ultrasound transducer (Logiq e, GE Healthcare, Waukesha, WI). Following 15 minutes of rest, baseline images were recorded. After baseline images and blood velocity were obtained, the cuff was rapidly inflated (AG101 rapid cuff inflator, Hokanson, Bellevue, WA) to 200 mm Hg for 5 minutes. Images and blood velocity were recorded during the last 15 seconds of occlusion and continued for 2 minutes following cuff release for the determination of peak diameter change and calculation of shear rate.
To assure that any observed changes in EDD are attributable to altered endothelial function and not altered smooth muscle responsiveness, we also assessed endothelium-independent dilation (EID) in five participants using sublingual nitroglycerin (NTG; 0.4 mg). Ultrasound images were recorded at baseline and for 10 minutes following NTG. The five participants underwent EID measurements due to the minimum blood pressure requirements for the administration of NTG at our institution.
Ultrasound images were transmitted to a National Instruments IMAQ PCI-1411 image acquisition board by way of an S-Video connection at a frequency of 20 frames/second. Brachial artery diameter was determined using custom designed automated edge detection software in National Instruments LabVIEW 8.0. Peak diameter was determined after applying a 3-second-wide median filter to each data point. Reproducibility in our lab for this technique is 1.3±1.1% and 1.9±1.6% (coefficient of variation) for baseline and peak brachial diameters, respectively. FMD was expressed as percent change from baseline and Doppler blood velocity data were used to calculate shear rate. The shear rate area under the curve (AUC) from cuff release to peak diameter best represents the reactive hyperemia shear stimulus for FMD [29]. Recent data suggest that the shear-FMD relationship varies between individuals [30] and since it is possible that dietary sodium intake may alter this relationship, we also report shear rate AUC.
Blood Analysis
A venous blood sample was used to measure hemoglobin (Hb 201+ model, HemoCue, Lake Forest, CA), hematocrit (Clay Adams Brand, Readacrit® Centrifuge, Becton Dickinson, Sparks, MD), serum electrolytes (EasyElectrolyte Analyzer, Medica, Bedford, MA), and plasma osmolality (Advanced 3D3 Osmometer, Advanced Instruments, Norwood, MA). Plasma renin activity (PRA), serum aldosterone, and angiotensin II were measured via RIA at the Hypertension Core Laboratory at Wake Forest University Baptist Medical Center. The inter-assay and intra-assay coefficients of variations (CV) were as follows: PRA, inter-assay, 14.8% CV for a mean of 5.8 ng/ml/hr; intra-assay, 10.0% CV for a mean of 1.6 ng/ml/hr; aldosterone, inter-assay precision of 6.6% CV and intra-assay precision of 5% CV, both at a mean of 25 ng/dl; angiotensin II, intra-assay CV of 12% with a minimal detectable level of 0.9 fmol (0.8 pg)/tube.
Statistical Analysis
Demographic data, hemodynamic and renal measures and vascular function data were compared using two-tailed, paired t-tests. Statistical significance was set at P < 0.05 and results are reported as means ± SE.
Results
Participant characteristics are presented in Table 1. All participants were normotensive, with renal function, liver enzymes, and cholesterol within normal limits. Estimated creatinine clearance (Cockcoft-Gault equation) was normal in all subjects (>90 mL/min). All participants completed the standardized run-in diet and the subsequent two-week dietary sodium perturbation.
Table 1.
Baseline participant characteristics.
| Baseline Characteristic | Value |
|---|---|
| Demographic Data | |
| N (M/F) | 14 (9/5) |
| Age (yr) | 33 ± 2 |
| Height (cm) | 174 ± 3 |
| Mass (kg) | 76 ± 3 |
| BMI (kg/m2) | 24.6 ± 1.1 |
| Systolic BP (mmHg) | 114 ± 3 |
| Diastolic BP (mmHg) | 69 ± 2 |
| Heart rate (bpm) | 58 ± 3 |
| Biochemical Parameters | |
| Hemoglobin (g/dl) | 14.4 ± 0.5 |
| Hematocrit (%) | 42.2 ± 1.2 |
| Serum sodium (mmol/L) | 135.6 ± 2.8 |
| Serum potassium (mmol/L) | 4.1 ± 0.1 |
| Serum chloride (mmol/L) | 104.9 ± 1.1 |
| Plasma osmolality (mOsm/kg H2O) | 286.2 ± 1.1 |
| Serum creatinine (mg/dl) | 0.9 ± 0.04 |
| Blood urea nitrogen (mg/dl) | 13.2 ± 0.7 |
| Fasting glucose (mg/dl) | 82.7 ± 2.2 |
| Fasting total cholesterol (mg/dl) | 172.4 ± 5.9 |
| Fasting HDL (mg/dl) | 53.8 ± 5.3 |
| Fasting LDL (mg/dl) | 102.5 ± 5.9 |
| Fasting triglycerides (mg/dl) | 80.3 ± 10.6 |
Values are mean ± SE. BMI, body mass index; BP, blood pressure.
Dietary Salt Perturbation
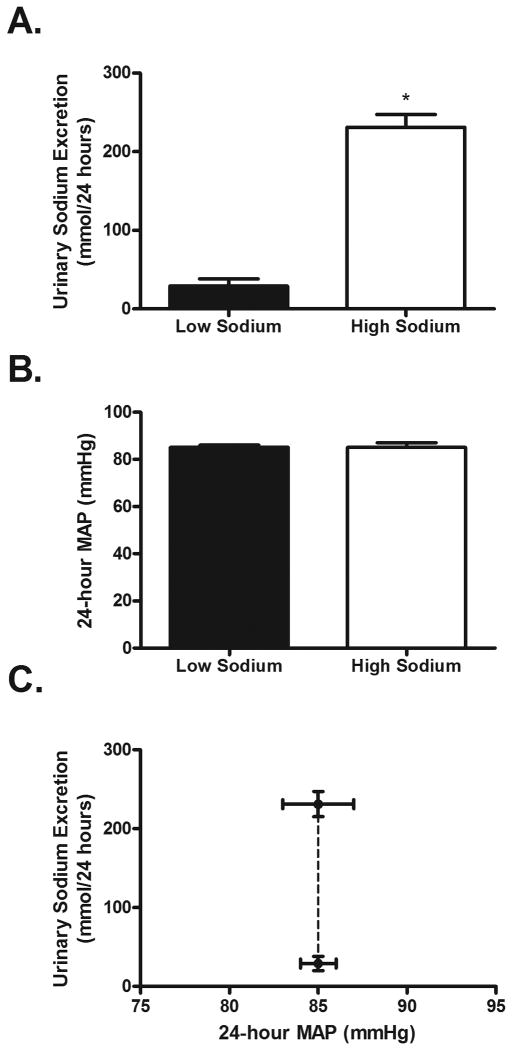
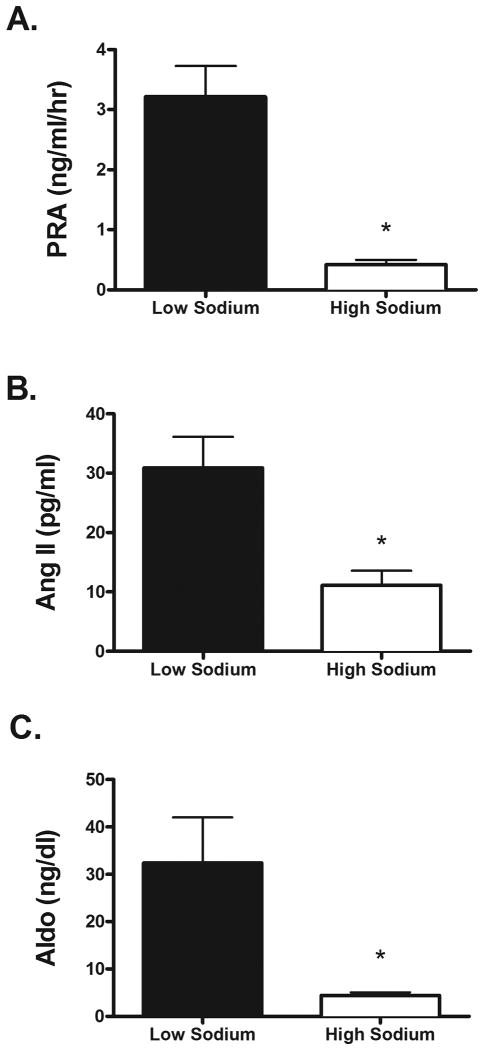
The HS diet elicited increases in serum sodium and plasma osmolality (Table 2). As expected, 24-hour urinary sodium excretion increased during the HS diet (Figure 1A). 24-hour urinary potassium excretion was not different between the HS and LS diets (HS: 36.2 ± 4.6 vs. LS: 32 ± 3.9 mmol/24 hours; P > 0.05). By design, 24-hour MAP did not differ while on the LS and HS diets (Table 2; Figure 1B). Also, systolic BP, diastolic BP and laboratory pressures did not differ while on the LS and HS diets (Table 2). Based on MAP all subjects were characterized as having salt resistance (see Methods section), as indicated by a large increase in urinary sodium excretion during the HS diet without a concomitant change in MAP (Figure 1C). As expected, the HS diet significantly suppressed plasma renin activity (PRA), plasma angiotensin II, and aldosterone (Figure 2).
Table 2.
Hemodynamic and renal responses to dietary sodium perturbation.
| Low Sodium | High Sodium | |
|---|---|---|
| Mass (kg) | 75.2 ± 3.3 | 77.1 ± 3.5 |
| Hemoglobin (g/dl) | 15.1 ± 0.5 | 14.4 ± 0.4 * |
| Hematocrit (%) | 41.6 ± 1.1 | 39.9 ± 1.0 * |
| Serum sodium (mmol/L) | 136.6 ± 0.5 | 138.6 ± 0.6 * |
| Serum potassium (mmol/L) | 4.2 ± 0.1 | 4.0 ± 0.1 |
| Serum chloride (mmol/L) | 102.0 ± 0.5 | 104.7 ± 0.8 * |
| Plasma osmolality (mOsm/kg H2O) | 284.2 ± 1.0 | 286.3 ± 0.9 * |
| Urine osmolality (mOsm/kg H2O) | 455.1 ± 63.5 | 465.0 ± 40.7 |
| Urine flow rate (ml/min) | 1.08 ± 0.13 | 1.43 ± 0.09 * |
| Urinary Na+ Excretion (mmol/24hr) | 29 ± 9 | 231 ± 16 * |
| Urinary K+ Excretion (mmol/24hr) | 46.9 ± 5.7 | 52.2 ± 6.6 |
| FENa (%) | 0.12 ± 0.05 | 0.93 ± 0.09 * |
| FECl (%) | 0.36 ± 0.09 | 1.27 ± 0.11 * |
| Creatinine clearance (ml/min/1.73 cm2) | 140.2 ± 11.0 | 140.7 ± 9.1 |
| Free water clearance (ml/min) | -0.32 ± 0.16 | 0.62 ± 0.04 * |
| 24 hr systolic BP (mm Hg) | 115 ± 2 | 117 ± 3 |
| 24 hr diastolic BP (mm Hg) | 70 ± 1 | 70 ± 2 |
| 24 hr MAP (mm Hg) | 85 ± 1 | 85 ± 2 |
| 24 hr heart rate (bpm) | 68 ± 3 | 64 ± 3 * |
| Laboratory systolic BP (mm Hg) | 116 ± 3 | 120 ± 3 |
| Laboratory diastolic BP (mm Hg) | 64 ± 2 | 66 ±2 |
| Laboratory MAP (mmHg) | 80 ± 2 | 83 ± 2 |
Values are mean ± SE. FENa, fractional excretion of sodium; FECl, fractional excretion of chloride; BP, blood pressure.
P<0.05 v. low sodium.
Figure 1.
Urinary sodium excretion (Panel A; P < 0.01) and mean arterial pressure (MAP, Panel B; P = 0.87) during the LS (filled bars) and HS (open bars) diets. Renal function curve (Panel C) demonstrating that despite a significant increase in sodium intake/excretion during the HS diet, 24-hour MAP did not change, affirming the salt-resistant phenotype. * P<0.05. LS; low sodium. HS; high sodium.
Figure 2.
Plasma renin activity (A), plasma angiotensin II (B), and plasma aldosterone (C). Compared to corresponding values obtained during the LS diet, all were suppressed (P<0.05) during the HS diet. LS; low sodium. HS; high sodium.
Vascular Function
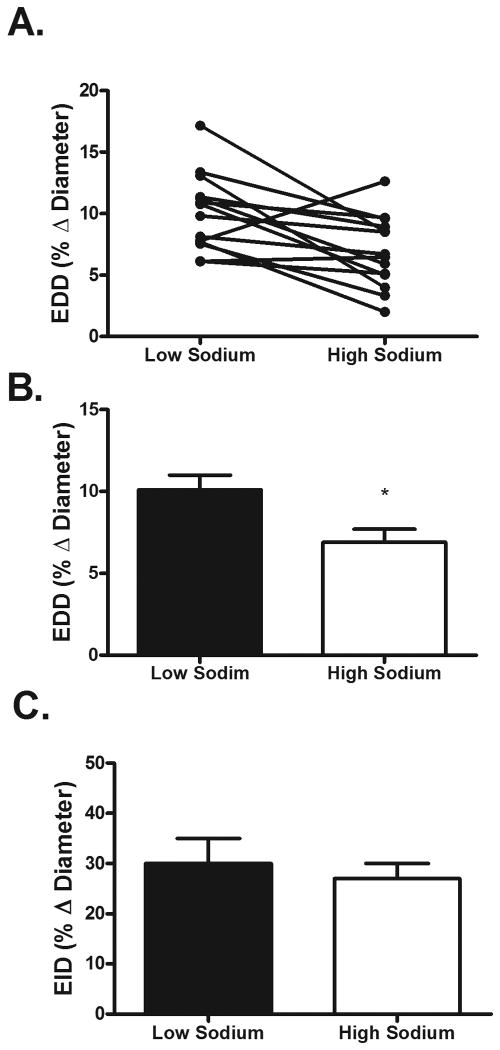
Baseline brachial artery diameters did not differ between diets (LS: 3.74 ± 0.21 vs. HS: 3.80 ± 0.20 mm; P > 0.05). EDD was reduced by approximately 32% during the HS diet (Figure 3). Shear rate AUC, an estimate for the shear stimulus for dilation, did not differ between LS and HS diets (LS: 18280 ± 5455 vs. HS: 18409 ± 6216; P > 0.05). In contrast to EDD, EID did not differ between diets (Figure 3); (LS: 30 ± 5% vs. HS: 27 ± 3%; P > 0.05).
Figure 3.
Individual (Panel A) and group (Panel B) endothelium-dependent dilation (EDD) responses during the LS and HS diets. EDD was significantly decreased during the HS diet. Endothelium-independent dilation EID (Panel C) did not differ between the LS and HS diets. *P < 0.05. LS; low sodium. HS; high sodium.
Discussion
Previous clinical studies have not been able to separate the effects of dietary salt intake and blood pressure on endothelial function. The present study examined healthy, normotensive adults who manifested salt resistance in order to characterize the effects of chronic dietary salt intake on the vasculature in the absence of changes in blood pressure. The primary finding of this study is that a high-sodium diet impaired endothelium-dependent dilation (EDD), but not endothelium-independent dilation (EID), in salt-resistant participants. Thus, our findings suggest that high dietary sodium intake directly impacts endothelial function.
Endothelial dysfunction is thought to precede the development of cardiovascular disease [1], which remains the leading cause of death in the United States [2]. Excess dietary salt has been linked to hypertension and other cardiovascular diseases [3]. However, the detrimental effects of salt have consistently been linked with changes in BP in humans [17-19]. The trials of hypertension prevention phase 1 and 2 (TOHP) in which individuals with pre-hypertensive BP underwent a chronic sodium restricted diet, resulted in a ∼25% reduction in cardiovascular event rate [31]. These individuals also experienced a reduction in BP during sodium restriction [31]. Additional evidence has demonstrated that improved EDD was associated with a decline in BP in overweight/obese patients that were switched from a usual salt diet to a low salt diet [17]. Similarly, Jablonski et al. reported that an increase in EDD was associated with a low salt diet in middle-aged and older adults with elevated systolic BP [18]. A high salt diet has also been shown to impair forearm blood flow responses to acetylcholine and increase 24 hour MAP in young normotensive adults [19]. In the present study, we classified our participants as salt-sensitive or salt-resistant, because we wanted to examine changes in vascular function in response to dietary salt independent of alterations in BP. Focusing on normotensive salt-resistant individuals is important because the majority of young to middle aged normotensive adults have salt-resistant BP [32]. To our knowledge, these are the first data to demonstrate that the effects of dietary sodium on vascular function are independent of BP in normotensive humans.
In contrast to clinical studies, there is preclinical evidence that endothelial dysfunction in response to high dietary salt intake can occur independently of changes in BP [10, 13, 14, 16, 33]. Impaired arteriolar responses to acetylcholine were observed in mice and rats fed a high salt diet, but significant differences in MAP were not observed during the high salt diet in these rodents [13, 14]. We extend these findings by reporting, for the first time, that conduit vessel function declines in response to dietary salt loading independent of changes in BP in humans.
Endothelial dysfunction is the primary event in the development of cardiovascular disease and is characterized by a decreased bioavailability of nitric oxide (NO), which inhibits platelet aggregation, leukocyte adhesion, and smooth muscle cell proliferation. Endothelial dysfunction has also been linked to acute cardiovascular events and has been shown to be a predictor of future cardiovascular events in patients with coronary artery disease [34, 35]. Therefore, the presence of impaired endothelial dependent dilation (EDD) in response to a high salt diet suggests an increased risk of cardiovascular disease. While we did not investigate the mechanism of altered NO production/bioavailability, several animal studies have identified increases in reactive oxygen species (ROS), specifically superoxide, as a mechanism of impaired EDD in response to a high salt diet [13-15]. Additionally, we have recently reported a role for oxidative stress in dietary salt-induced impairments in microvascular function in normotensive salt-resistant adults [36]. These recent findings, in conjunction with the findings of the present study, suggest that the deleterious effects of high dietary salt may occur in multiple vascular beds. ROS scavengers TEMPOL and catalase have also been shown to reverse the suppressed arteriolar dilation response to acetylcholine in mice and rats fed a high salt diet [13, 14]. Additionally, mesenteric resistance arteries from rats fed a high salt diet exhibited significant relaxation with exposure to TEMPOL [15]. Recent evidence has demonstrated that decreases in acetylcholine-induced arteriolar dilation in mice fed a high salt diet is due to eNOS uncoupling via a low level of tetrahydrobiopterin availability, which leads to superoxide generation [37]. Animal studies have identified the specific sources of excess superoxide to be NAD(P)H oxidase [13, 15], xanthine oxidase [13, 15], and un-coupled eNOS [14, 37]. Oxidative stress has also been implicated as a mechanism of endothelial dysfunction in several human models, including aging [38], hypertension [39], chronic kidney disease [40], and diabetes[41].
As seen in figure 2, we observed an expected suppression of plasma angiotensin II (ANG II) levels in response to the high sodium diet. The reduction in ANG II may play a role in reducing vascular function. Normalization of plasma ANG II via intravenous infusion restored vascular function in rats fed a high salt diet [42-44]. ANG II infusion has also been shown to prevent a reduction in the expression of Cu/Zn SOD in rats fed a high salt diet [45] and increase Cu/Zn SOD activity and improve aortic relaxation in ecSOD-deficient mice [46]. Taken together, these results suggest that physiological levels of ANG II are required to maintain normal SOD expression/activity. Thus, the suppression of ANG II as a result of high dietary sodium consumption observed in our study may have played a role in reducing vascular function via oxidative stress by disrupting endogenous antioxidant defenses. Alternatively, it is possible that the renin-angiotensin-aldosterone system plays little to no role in the high salt induced impairments in EDD that we observed.
Physiological increases in sodium directly increase vascular endothelial cell stiffness and decrease endothelial NO release [47]. We observed a significant increase of 2mmol/L in serum sodium from the low to high salt diet in our participants. However, when exposed to increases in extracellular potassium concentrations, endothelial cell stiffness is reversed and NO release is improved [47]. Cook et al. have reported that a high sodium-to-potassium ratio is associated with an increased CVD risk [48]. These findings suggest an interesting interaction between dietary potassium and excess salt intake. For this reason, similar dietary potassium intake was maintained during changes in dietary sodium intake in this study. Serum potassium concentration remained within the normal range and did not differ between the two dietary conditions. Additionally, urinary potassium excretion did not differ between diets, suggesting that potassium consumption was consistent between the low and high sodium diets. Future studies are warranted in order to determine whether alterations in dietary potassium may protect against the deleterious effects of dietary salt on vascular function in humans. Further, it is possible that different effects on vascular function and/or BP may be observed during longer and greater degrees of dietary salt loading [49].
In summary, by studying salt-resistant humans, our data demonstrated that a high-sodium diet impaired EDD independent of BP. Although current public health guidelines recommend a low salt diet, habitual sodium intake remains high [22]. Our data, along with others [12-14, 16-19, 33, 37] suggest that it may be beneficial to associate dietary salt with overall vascular health in addition to BP. Future studies are needed to elucidate specific mechanisms of dietary salt-induced vascular impairments.
Acknowledgments
The authors would like to thank Gabrielle Snyder-Marlow, R.D. for all food preparation, Allen Prettyman, Ph.D, MSN, FNP-BC for assistance with medical oversight, and Carol Catanese, MS for assistance in the conduction of the studies. We thank the subjects for their participation.
Sources of Funding: This work was supported by grants 2 P20 RR016472-11 and R01 HL104106. Dr. Sanders is supported by NIH grants R01 DK046199 and P30 DK079337 and by the Medical Research Service of the Department of Veterans Affairs.
Funding sources: Grants 2 P20 RR016472-11 and R01 HL104106.
PWS is supported by NIH grants R01 DK046199 and P30 DK079337.
Footnotes
Disclaimers: NONE
References
- 1.Luscher TF, Tanner FC, Tschudi MR, Noll G. Endothelial dysfunction in coronary artery disease. Annu Rev Med. 1993;44:395–418. doi: 10.1146/annurev.me.44.020193.002143. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Jousilahti P, Rastenyte D, Moltchanov V, Tanskanen A, Pietinen P, et al. Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet. 2001;357:848–51. doi: 10.1016/S0140-6736(00)04199-4. [DOI] [PubMed] [Google Scholar]
- 4.Frassetto L, Morris RCJ, Sellmeyer DE, Todd K, Sebastian A. Diet, evolution and aging--the pathophysiologic effects of the post-agricultural inversion of the potassium-to-sodium and base-to-chloride ratios in the human diet. Eur J Nutr. 2001;40:200–13. doi: 10.1007/s394-001-8347-4. [DOI] [PubMed] [Google Scholar]
- 5.Elliott P, Stamler J, Nichols R, Dyer AR, Stamler R, Kesteloot H, et al. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. Intersalt Cooperative Research Group. BMJ. 1996;312:1249–53. doi: 10.1136/bmj.312.7041.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Law MR, Frost CD, Wald NJ. By how much does dietary salt reduction lower blood pressure? I--Analysis of observational data among populations. Bmj. 1991;302:811–5. doi: 10.1136/bmj.302.6780.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Midgley JP, Matthew AG, Greenwood CM, Logan AG. Effect of reduced dietary sodium on blood pressure: a meta-analysis of randomized controlled trials. JAMA. 1996;275:1590–97. doi: 10.1001/jama.1996.03530440070039. [DOI] [PubMed] [Google Scholar]
- 8.Frisbee JC, Lombard JH. Acute elevations in salt intake and reduced renal mass hypertension compromise arteriolar dilation in rat cremaster muscle. Microvasc Res. 1999;57:273–83. doi: 10.1006/mvre.1998.2138. [DOI] [PubMed] [Google Scholar]
- 9.Frohlich ED, Chien YSS, Pegram BL. Relationship between dietary sodium intake, hemodynamics, and cardiac mass in SHR and WKY rats. Am J Physiol. 1993;264:R30–4. doi: 10.1152/ajpregu.1993.264.1.R30. [DOI] [PubMed] [Google Scholar]
- 10.Matavelli LC, Zhou X, Varagic J, Susic D, Frohlich ED. Salt loading produces severe renal hemodynamic dysfunction independent of arterial pressure in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H814–9. doi: 10.1152/ajpheart.00671.2006. [DOI] [PubMed] [Google Scholar]
- 11.Yu HC, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, et al. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation. 1998;98:2621–8. doi: 10.1161/01.cir.98.23.2621. [DOI] [PubMed] [Google Scholar]
- 12.Lenda DM, Boegehold MA. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol. 2002;282:H395–402. doi: 10.1152/ajpheart.0354.2001. [DOI] [PubMed] [Google Scholar]
- 13.Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol. 2000;279:H7–H14. doi: 10.1152/ajpheart.2000.279.1.H7. [DOI] [PubMed] [Google Scholar]
- 14.Nurkiewicz TR, Boegehold MA. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1550–6. doi: 10.1152/ajpregu.00703.2006. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res. 2007;44:382–90. doi: 10.1159/000102955. [DOI] [PubMed] [Google Scholar]
- 16.Boegehold MA. Flow-dependent arteriolar dilation in normotensive rats fed low-or high-salt diets. Am J Physiol. 1995;269:H1407–14. doi: 10.1152/ajpheart.1995.269.4.H1407. [DOI] [PubMed] [Google Scholar]
- 17.Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr. 2009:485–90. doi: 10.3945/ajcn.2008.26856. [DOI] [PubMed] [Google Scholar]
- 18.Jablonski KL, Gates PE, Pierce GL, Seals DR. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Ther Adv Cardiovasc Dis. 2009;7(6):805–12. doi: 10.1177/1753944709345790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzesmos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension. 2008;51:1525–30. doi: 10.1161/HYPERTENSIONAHA.108.109868. [DOI] [PubMed] [Google Scholar]
- 20.DuPont JJ, Farquhar WB, Edwards DG. Intradermal microdialysis of hypertonic saline attenuates cutaneous vasodilation in response to local heating. Exp Physiol. 2011;96:674–80. doi: 10.1113/expphysiol.2011.058404. [DOI] [PubMed] [Google Scholar]
- 21.Schulman IH, Aranda P, Raij L, Veronesi M, Aranda FJ, Martin R. Surgical menopause increases salt sensitivity of blood pressure. Hypertension. 2006;47:1168–74. doi: 10.1161/01.HYP.0000218857.67880.75. [DOI] [PubMed] [Google Scholar]
- 22.CDC. Sodium intake among adults - United States, 2005-2006. MMWR Morb Mortal Wkly Rep. 2010;59:746–9. [PubMed] [Google Scholar]
- 23.He FJ, Markandu ND, MacGregor GA. Importance of the renin system for determining blood pressure fall with acute salt restriction in hypertensive and normotensive whites. Hypertension. 2001;38(3):321–5. doi: 10.1161/01.hyp.38.3.321. [DOI] [PubMed] [Google Scholar]
- 24.Eisenach JH, Gullixson LR, Kost SL, Joyner MJ, Turner ST, Nicholson WT. Sex differences in salt sensitivity to nitric oxide dependent vasodilation in healthy young adults. J Appl Physiol. 2012;112(6):1049–53. doi: 10.1152/japplphysiol.01197.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenner MM, Edwards DG, Ray CA, Rose WC, Gardner TJ, Stillabower M, et al. Celecoxib does not alter cardiovascular and renal function during dietary salt loading. Clin Exp Pharmacol Physiol. 2011;38(8):543–9. doi: 10.1111/j.1440-1681.2011.05546.x. [DOI] [PubMed] [Google Scholar]
- 26.Schmidlin O, Sebastian AF, Morris RCJ. What initiates the pressor effect of salt in saltsensitive humans? Observations in normotensive blacks. Hypertension. 2007;49:1032–39. doi: 10.1161/HYPERTENSIONAHA.106.084640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinberger MH. Is salt-sensitivity of blood pressure a reproducible phenomenon-commentary. J Hypertens. 1996;14:1461–62. doi: 10.1097/00004872-199612000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Coretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 29.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol. 2007;102:1510–9. doi: 10.1152/japplphysiol.01024.2006. [DOI] [PubMed] [Google Scholar]
- 30.Thijssen DHJ, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Heart Circ Physiol. 2011;300(1):H2–H12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP) Bmj. 2007;334:885. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–90. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 33.Boegehold MA. Effect of dietary salt on arteriolar nitric oxide in striated muscle of normotensive rats. Am J Physiol Heart Circ Physiol. 1993;264:H1810–H6. doi: 10.1152/ajpheart.1993.264.6.H1810. [DOI] [PubMed] [Google Scholar]
- 34.Schachinger V, Zeiher AM. Prognostic implications of endothelial dysfunction: does it mean anything? Coron Artery Dis. 2001;12:435–43. doi: 10.1097/00019501-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DRJ, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 36.Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol. 2012 doi: 10.1113/jphysiol.2012.236992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nurkiewicz TR, Wu G, Li P, Boegehold MA. Decreased arteriolar Tetrahydrobiopterin is linked to Superoxide Generation from Nitric Oxide Synthase in Mice Fed High Salt. Microcirculation. 2009;17:147–57. doi: 10.1111/j.1549-8719.2009.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–66. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 39.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–58. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Cross JM, Donald AE, Nuttall SL, Deanfield JE, Woolfson RG, MacAllister RJ. Vitamin C improves resistance but not conduit artery endothelial function in patients with chronic renal failure. Kidney Int. 2003;63:1433–42. doi: 10.1046/j.1523-1755.2003.00852.x. [DOI] [PubMed] [Google Scholar]
- 41.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 42.Weber DS, Lombard JH. Elevated salt intake impairs dilation of rat skeletal muscle resistance arteries via ANG II suppression. Am J Physiol Heart Circ Physiol. 2000;278(2):H500–6. doi: 10.1152/ajpheart.2000.278.2.H500. [DOI] [PubMed] [Google Scholar]
- 43.Weber DS, Lombard JH. Angiotensin II AT1 receptors preserve vasodilator reactivity in skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol. 2001;280(5):H2196–202. doi: 10.1152/ajpheart.2001.280.5.H2196. [DOI] [PubMed] [Google Scholar]
- 44.Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2003;284(4):H1124–33. doi: 10.1152/ajpheart.00835.2002. [DOI] [PubMed] [Google Scholar]
- 45.McEwen ST, Schmidt JR, Somberg L, Cruz Lde L, Lombard JH. Time-course and mechanisms of restored vascular relaxation by reduced salt intake and angiotensin II infusion in rats fed a high-salt diet. Microcirculation. 2009;16(3):220–34. doi: 10.1080/10739680802544177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, et al. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48(3):473–81. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 47.Oberleithner H, Callies C, Kusche-Vihrog K, Schillers H, Shahin V, Riethmuller C, et al. Potassium softens vascular endothelium and increases nitric oxide release. PNAS. 2009;106:2829–34. doi: 10.1073/pnas.0813069106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, et al. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch Intern Med. 2009;169:32–40. doi: 10.1001/archinternmed.2008.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon G, Jaeckel M, Illyes G. Development of structural vascular changes in salt-fed rats. Am J Hypertens. 2003;16(6):488–93. doi: 10.1016/s0895-7061(03)00568-5. [DOI] [PubMed] [Google Scholar]