Abstract
Objectives
To determine the prospective risk of IUFD ≥ 34 weeks’ gestation for monochorionic (MC) and dichorionic (DC) twins receiving intensive antenatal fetal surveillance. The secondary objective is to calculate the incidence of prematurity-related neonatal morbidity/mortality, stratified by gestational week and chorionicity.
Study Design
A retrospective cohort study of all twins ≥ 34 weeks delivered at MUSC (1987–2010) was performed. Twins were cared for in a longstanding Twin Clinic with standardized management and surveillance protocols; supervised by a consistent Maternal-Fetal Medicine specialist. Gestational age specific fetal/neonatal mortality and composite neonatal morbidity rates were compared by chorionicity. A generalized linear mixed model was used to identify variables associated with increased composite neonatal morbidity.
Results
Among 768 twin gestations (601 DC and 167 MC), only one dichorionic IUFD occurred. The prospective risk of IUFD ≥34 weeks was 0.17% for DC twins and 0% for MC twins. Composite neonatal morbidity decreased with each gestational week (p<0.0001). Morbidity was increased by white race, gestational diabetes and elective indication for delivery. The nadir of composite neonatal morbidity occurred at 36/0-36/6 weeks for MC twins and 37/0-37/6 weeks for DC twins.
Conclusions
Our data do not support concern for an increased risk of stillbirth in uncomplicated intensively managed MC twins ≥34 weeks’ gestation. However, our data do show significantly increased rates of neonatal morbidity in late preterm MC twins that cannot be justified by a corresponding reduction in the risk of stillbirth. We feel that our data support delivery of uncomplicated MC twins at 37 weeks’ gestation.
Keywords: Delivery timing, Dichorionic twins, Monochorionic twins, Stillbirth
Introduction
The frequency of twin gestations has risen dramatically over the past 30 years, increasing by more than 60% between 1980 and 2006, and now represents almost 4% of all live births.1
Monochorionic twin gestations experience rates of stillbirth that are higher than either singleton or dichorionic twin gestations.2,3,4 This increased stillbirth rate is attributed primarily to placental vascular complications such as twin-twin transfusion syndrome.3,4 Other contributors include increased risks of congenital malformations, selective growth restriction, maternal obstetric complications, and co-twin demise due to intravascular shunting following a single intrauterine death.4
Recently, concerns have been raised that even “apparently uncomplicated” monochorionic twins are at increased risk for fetal demise. Third trimester stillbirth rates as high as 4.3% have been reported.5 Subsequent cohort studies, however, have reported a lower stillbirth risk in late pregnancy.6,7,8,9 A recent meta-analysis described an almost four-fold increased risk of stillbirth for “apparently uncomplicated” monochorionic twins.10
In February 2011, the NIH and Society for Maternal-Fetal Medicine collaborated to publish expert consensus-based recommendations on the optimal timing of delivery for high risk pregnancies.11 For uncomplicated dichorionic twins, delivery at 38 weeks’ gestation was recommended. Uncomplicated monochorionic twins, however, had less specific recommendations for delivery between 34–37 weeks’ gestation. This broad interval reflects the uncertainty over the possible greater risk of late stillbirth even for “apparently uncomplicated” monochorionic twins.
The purpose of this investigation was to review a large cohort of twin gestations cared for at a single institution in a specialized, antenatal Twin Clinic since 1987. The primary objective was to determine the prospective risk for stillbirth among continuing monochorionic and dichorionic twin gestations in the late preterm and early term gestational age time periods. Secondarily, we determined the gestational age-specific neonatal morbidity and mortality for monochorionic and dichorionic twins. This data will help better define the optimal balance between the risk of stillbirth and the neonatal morbidity associated with elective preterm delivery of monochorionic and dichorionic twins.
Material and Methods
This retrospective cohort study examined all dichorionic (DC) and monochorionic, diamniotic (MC) twins ≥34 weeks’ gestation delivered at the Medical University of South Carolina (MUSC) between 1987–2010. Following IRB approval (IRB #00012449; 9/13/2011), subjects were identified and maternal/neonatal variables were obtained using the Perinatal Information Network System (PINS) database. PINS is an institutional research quality database with multiple edits and audits to ensure accuracy. PINS was complemented by a second database created specifically for the Twin Clinic and maintained by a certified nurse-midwife involved in the care of those patients. Specific maternal variables including gestational age, indication for delivery, and chorionicity were confirmed by individual review of each maternal medical record.
Exclusion criteria included gestational age <34 weeks, monoamnionicity, aneuploidy, fetal anomalies requiring prolonged hospitalization or immediate surgery, co-twin demise <34 weeks or unknown chorionicity. Chorionicity was assigned prenatally by ultrasound assessment of the placental number, thickness of the dividing membrane, the presence or absence of a “twin-peak” sign, as well as fetal gender in all patients. Following delivery, the assignment of chorionicity was confirmed in all cases by inspection of the placentas and membranes. Chorionicity was further confirmed by individual review of placental pathology, which was available in 70% of patients. Gestational age was determined by the patients’ last menstrual period or date of fertilization in cases of assisted reproduction. When the menstrual dating was unknown or discordant with first or early second-trimester ultrasound measurements, the ultrasound-based dating criteria were used.
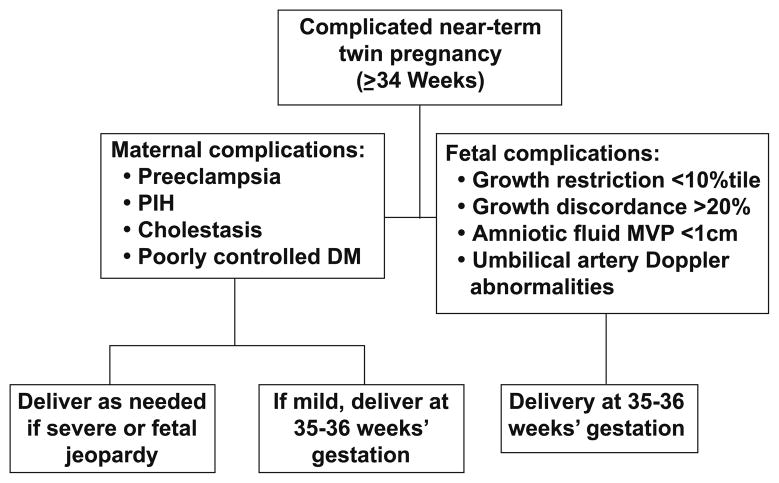
Twin gestations were cared for through a specialized Twin Clinic staffed by OB-GYN residents and a consistent, dedicated certified nurse-midwife. The Twin Clinic was directed by a single Maternal-Fetal Medicine physician. Twin gestations have been managed in a standardized fashion following protocols established by the supervising Maternal-Fetal Medicine specialist. Since inception of the Twin Clinic, third trimester fetal surveillance has included ultrasonographic surveillance of fetal growth, growth discordance and amniotic fluid volumes at least every four weeks. Since 2005, MC twins have undergone ultrasound surveillance at least every three weeks. Umbilical artery Doppler assessment was not routinely used for ultrasonographic fetal assessment. It was used selectively to further evaluate fetuses suspected of having an estimated fetal weight < 10th percentile, an abdominal circumference < 5th percentile, growth discordance > 20 percent, a deepest vertical pocket <2 cm, or suspected twin-twin transfusion syndrome. Weekly non-stress testing has been routinely initiated at 32 weeks’ gestation for MC twins and 34 weeks’ gestation for DC twins, unless earlier surveillance was indicated. All twins were seen on a weekly basis after 34 weeks’ gestation. Timing of delivery for uncomplicated DC and MC twins was recommended at 38 and at 37 weeks’ gestation, respectively (Figure 1). For twins with maternal or fetal complications, there was a liberal policy of delivery at any time after 35 weeks’ gestation (Figure 2).
Figure 1.
Recommended timing of delivery for uncomplicated near-term (≥ 34 weeks’ gestation) twin pregnancies at the MUSC Twin Clinic
Figure 2.
Recommended timing of delivery for complicated near-term (≥34 weeks’ gestation) twin pregnancies at the MUSC Twin Clinic
The primary outcomes in this study were stillbirth and composite neonatal morbidity/mortality, stratified by gestational age and chorionicity. Stillbirths were defined as an intrauterine fetal death between 34 weeks’ gestation and delivery. Composite neonatal morbidity was composed of: transient tachypnea of the newborn, respiratory distress syndrome, continuous positive air pressure ventilation or oxygen requirement outside of the delivery room, sepsis or sepsis work-up, necrotizing enterocolitis, any grade intra-ventricular hemorrhage, phototherapy, or neonatal death. Neonatal death was the death of a liveborn infant by 28 days of life. These diagnoses were extracted from PINS and represented the final discharge diagnoses assigned by the Neonatology attending staff.
Statistical analysis was performed with SAS software (version 9.2; SAS Institute, Cary NC) and SPSS software (version 12.0; SPSS, Inc, Chicago IL). Subjects were initially segregated by chorionicity (DC vs. MC). A univariate analysis was performed comparing continuous and categorical demographic and outcome variables. Continuous variables were compared using Mann-Whitney U, while Chi-square or Fisher’s exact test were used for categorical variables. To determine which variables were associated with a higher risk of stillbirth or composite neonatal morbidity/mortality, a generalized linear mixed model analysis was used. This model was used in preference to a standard logistic regression model because the outcomes of each twin were not independent of the co-twin. The prospective risks of IUFD for both MC and DC twins were calculated as the number of stillbirths during or after a given week-long gestational period divided by the total number of ongoing pregnancies at the start of the time period. 5,6 Gestational age-specific prospective risks of perinatal mortality were calculated using the concept of “fetuses at risk”.9,12–15 This calculation is performed by dividing the number of stillbirths and neonatal deaths during any given week by the number of fetuses remaining in utero, and thus “at risk”, at the beginning of that week. These risks were calculated weekly from 34 to 39+ weeks’ gestation and the MC and DC twin outcomes were compared.
Results
A total of 1,779 twin gestations cared for at MUSC between 1987–2010 were assessed for eligibility. Following exclusions, 768 twin gestations were subsequently included in the analysis (Figure 3). Of the 1,011 twin gestations excluded, the majority were due to gestational age <34 weeks at delivery (N=846). Chorionicity could not be confirmed in 109 cases (6%). Other exclusions included fetal anomalies (23), co-twin demise <34 weeks (18), monoamnionicity (10), and outborn (5). Of the 768 twin gestations that met inclusion criteria, 601 (78%) were dichorionic (DC), delivering 1,202 neonates. There were 167 (22%) monochorionic (MC) twin gestations, delivering 334 neonates.
Figure 3.
Study population and exclusions from the MUSC Twin Clinic cohort
Maternal characteristics were compared between DC and MC twin sets (Table 1). Maternal age was the only statistically significant difference between the groups, with mothers of DC twins being a median of 3 years older than mothers of MC twins (p=0.0009). Maternal parity, race, pre-pregnancy BMI, tobacco use, and insurance status did not significantly differ.
Table 1.
Maternal Demographic Characteristicsa
| Characteristics | Dichorionic (n=601) | Monochorionic (n=167) | P |
|---|---|---|---|
| Maternal age (years) | 28 (15–47) | 25 (16–44) | 0.0009 |
|
| |||
| Parity | 1 (0–9) | 1 (0–7) | 0.14 |
|
| |||
| Maternal race/ethnicity | |||
| Black | 266 (44.3%) | 66 (39.5%) | 0.29 |
|
| |||
| Caucasian | 299 (49.8%) | 85 (50.9%) | 0.86 |
|
| |||
| Hispanic | 35 (5.8%) | 13 (7.8%) | 0.37 |
|
| |||
| Pre-pregnancy BMI | 24.3 (15.7–57.4) | 24.0 (14.2–50.6) | 0.98 |
|
| |||
| Smoker (%) | 46 (7.6%) | 14 (8.4%) | 0.74 |
|
| |||
| Insurance | |||
| Self-Pay | 44 (7.3%) | 17 (10.2%) | 0.26 |
|
| |||
| Medicaid | 275 (45.8%) | 83 (49.7%) | 0.38 |
|
| |||
| Private | 272 (45.3%) | 62 (37.1%) | 0.06 |
Data are median (range) or n (%)
Abbreviations: BMI, Body Mass Index
Neonatal outcomes were also compared (Table 2). Birth weight, incidence of low birth weight (<2500 gms), and Cesarean rates differed significantly between the two groups. Dichorionic twins had a slightly heavier median birthweight compared to monochorionic twins (2438 vs. 2325 gm; p=0.0015) as well as a lower rate of low birth weight (56.2% vs. 64.4%; p=0.007). Dichorionic twins also experienced a higher Cesarean rate (50.6% vs. 43.1%, p=0.02). The median gestational age at delivery was almost identical between the DC and MC twin sets at 36.4 and 36.3 weeks’ gestation, respectively.
Table 2.
Neonatal Characteristics and Outcomesa
| Characteristics/Outcomes | Dichorionic (n=1202) | Monochorionic (n=334) | P |
|---|---|---|---|
| Gestational age at delivery (weeks) | 36.4 (34–40) | 36.3 (34.0–39.8) | 0.27 |
| Term (37+ weeks) | 480 (39.9%) | 116 (34.7%) | 0.08 |
| Birth weight (grams) | 2438 (590–3815) | 2325 (1335–3690) | 0.0015 |
| LBW (<2500 g) | 675 (56.2%) | 215 (64.4%) | 0.007 |
| VLBW (<1500 g) | 20 (1.7%) | 5 (1.5%) | 1.0 |
| Cesarean (%) | 608 (50.6%) | 144 (43.1%) | 0.02 |
| NICU admit | 135 (11.2%) | 38 (11.4%) | 0.94 |
| Level 2 admit | 266 (22.1%) | 66 (19.8%) | 0.35 |
| Neonatal LOS (days) | 3 (1–245) | 3 (1–91) | 0.45 |
| ≥20% discordance | 188 (15.6%) | 49 (14.7%) | 0.66 |
| Antenatal death | 1 (0.08%) | 0 (0%) | 1.0 |
| Neonatal death | 4 (0.33%) | 0 (0%) | 0.58 |
Data are median (range) or n (%)
Abbreviations: LBW, low birth weight; VLBW, very low birth weight; LOS, length of stay.
Only five perinatal deaths occurred; one IUFD and four neonatal deaths, all of which were in the DC group. The single IUFD in the DC group occurred in a woman without prenatal care who presented at 34 weeks gestation with undiagnosed twins. Gestational age was estimated based on the ultrasound assessment of the surviving twin. Due to the circumstances of her presentation she had not undergone any prior fetal surveillance. The four neonatal deaths in the DC group were a consequence of a variety of clinical scenarios, one of which was clearly amenable to antepartum fetal surveillance. One neonatal death occurred in a 34 week DC twin delivered with hypoxic ischemic encephalopathy and multi-organ failure. That infant was delivered by emergency Cesarean due to a severe placental abruption after a normal vaginal delivery of twin A. A second demise occurred in a 35 week DC twin with severe IUGR and absent end diastolic flow. That twin weighed 509 grams and the newborn had severe RDS. The third demise was another 35 week DC twin that died from complications of neonatal sepsis. The final demise was a 36 week DC twin that died as a consequence of RDS and other complications of prematurity. The prospective risk of IUFD was calculated for dichorionic twins, who had a 0.17% (95% CI 0.00–0.92%) prospective risk of fetal death at 34 weeks, reflecting the single IUFD within this group during that week. No monochorionic stillbirths occurred ≥34 weeks’ gestation, thus, the prospective risk of fetal death was 0% (95% CI 0.00–1.80%). The overall perinatal mortality rate ≥ 34 weeks’ gestation was 0% (0/334) for MC twins and 0.4% (5/1202) for DC twins. The prospective risk of perinatal mortality at each week of gestation is also provided (Table 3).
Table 3.
Prospective Risk of IUFD and Perinatal Mortality by Chorionicity and Week of Gestation
| Week of Gestation | Ongoing Pregnancies | IUFD (N) | Neonatal Demise (N) | Prospective Risk of IUFDa | Prospective Risk of Perinatal Mortalityb |
|---|---|---|---|---|---|
| Dichorionic Twins | |||||
| 340–346 | 601 | 1 | 1 | 0.17% | 0.17% |
| 350–356 | 491 | 0 | 2 | 0% | 0.2% |
| 360–366 | 358 | 0 | 1 | 0% | 0.14% |
| 370–376 | 240 | 0 | 0 | 0% | 0% |
| 380–386 | 99 | 0 | 0 | 0% | 0% |
| 39+ | 22 | 0 | 0 | 0% | 0% |
| Total | 601 | 1 | 4 | ||
| Monochorionic Twins | |||||
| 340–346 | 167 | 0 | 0 | 0% | 0% |
| 350–356 | 136 | 0 | 0 | 0% | 0% |
| 360–366 | 94 | 0 | 0 | 0% | 0% |
| 370–376 | 58 | 0 | 0 | 0% | 0% |
| 380–386 | 20 | 0 | 0 | 0% | 0% |
| 39+ | 3 | 0 | 0 | 0% | 0% |
| Total | 167 | 0 | 0 | ||
Abbreviations: IUFD, intrauterine fetal death.
Prospective risk of IUFD was calculated as the number of stillbirths during or after a given week-long gestational period divided by the total number of ongoing pregnancies at the start of the time period.5,6
Prospective risk of perinatal mortality was calculated by dividing the number of stillbirths and neonatal deaths during any given week by the number of fetuses remaining in utero at the beginning of that week.9
Composite neonatal morbidity/mortality decreased significantly with each completed week of gestation (Figure 4). The nadir of composite neonatal morbidity was observed during the 37th week for DC twins and the 36th week for MC twins. Greater gestational age was the only variable that significantly decreased the risk of composite neonatal morbidity (OR 0.39, 95% CI 0.31–0.47). After adjusting for confounding variables, being white as opposed to black (OR 1.72, 95% CI 1.16–2.57), having gestational diabetes (OR 2.71, 95% CI 1.32–5.59), and being delivered for elective indications (OR 1.81, 95% CI 1.09–3.01) all increased the risk of adverse neonatal outcome. Year of birth, mode of delivery, birth weight, discordance, birth order, gender, chronic hypertension, and pregestational diabetes did not affect neonatal risk. The analysis was also run separately excluding phototherapy from the composite outcome and the results did not change.
Figure 4.
Composite neonatal morbidity/mortality stratified by chorionicity and gestational week
To better illustrate our composite neonatal morbidity we have listed the frequency of individual morbidities by chorionicity and gestational week at delivery in Table 4. As expected, the frequencies of all morbidities were higher between 340 and 366 weeks’ gestation compared to 370 weeks or greater. The only significant differences between the MC and DC twins were higher rates of sepsis or sepsis work-up (55.9% vs. 37.1%; p=0.009) at 340–346 weeks and respiratory morbidity (15.7% vs. 5.6%; p=0.03) at 360–366 weeks experienced by the DC twin neonates. When the model was re-run using respiratory morbidity, sepsis/sepsis-work-up, or phototherapy as individual primary outcomes, the effect of gestational age at delivery remained statistically significant. Other components of our composite morbidity (neonatal death, intraventricular hemorrhage and necrotizing enterocolitis) were too infrequent to analyze individually.
Table 4.
Individual Neonatal Morbidities by Chorionicity and Gestational Week at Delivery
| Morbidity | Week Gestation | Dichorionic | Monochorionic | p-value | ||
|---|---|---|---|---|---|---|
| Neonates Delivered (N) | Number with Outcome (%) | Neonates Delivered (N) | Number with Outcome (%) | |||
| Respiratory* | 34º–346 | 220 | 71 (32.3%) | 62 | 13 (21.0%) | 0.12 |
| 35º–356 | 266 | 47 (17.7%) | 84 | 13 (15.5%) | 0.74 | |
| 36º–366 | 236 | 37 (15.7%) | 72 | 4 (5.6%) | 0.03 | |
| 37º–376 | 282 | 21 (7.5%) | 76 | 5 (6.6%) | 0.79 | |
| 38º–386 | 154 | 11 (7.1%) | 34 | 2 (5.9%) | 0.79 | |
| 39+ | 44 | 3 (6.8%) | 6 | 0 | 0.5 | |
| Sepsis or Sepsis Work-Up | 34º–346 | 220 | 123 (55.9%) | 62 | 23 (37.1%) | 0.009 |
| 35º–356 | 266 | 71 (26.7%) | 84 | 25 (29.8%) | 0.58 | |
| 36º–366 | 236 | 38 (16.1%) | 72 | 7 (9.7%) | 0.18 | |
| 37º–376 | 282 | 19 (6.7%) | 76 | 5 (6.6%) | 0.96 | |
| 38º–386 | 154 | 12 (7.8%) | 34 | 4 (11.8%) | 0.5 | |
| 39+ | 44 | 7 (15.9%) | 6 | 1 (16.7%) | 0.92 | |
| Necrotizing enterocolitis | 34º–346 | 220 | 2 (0.91%) | 62 | 1 (1.6%) | 0.53 |
| 35º–356 | 266 | 0 | 84 | 0 | n/a | |
| 36º–366 | 236 | 0 | 72 | 0 | n/a | |
| 37º–376 | 282 | 0 | 76 | 0 | n/a | |
| 38º–386 | 154 | 0 | 34 | 0 | n/a | |
| 39+ | 44 | 0 | 6 | 0 | n/a | |
| Intraventricular hemorrhage‡ | 34º–346 | 220 | 3 (1.36%) | 62 | 1 (1.6%) | 0.8 |
| 35º–356 | 266 | 2 (0.75%) | 84 | 0 | 0.6 | |
| 36º–366 | 236 | 3 (1.3%) | 72 | 0 | 0.5 | |
| 37º–376 | 282 | 1 (0.35%) | 76 | 0 | 0.8 | |
| 38º–386 | 154 | 0 | 34 | 0 | n/a | |
| 39+ | 44 | 0 | 6 | 0 | n/a | |
| Phototherapy | 34º–346 | 220 | 53 (24.1%) | 62 | 22 (35.5%) | 0.07 |
| 35º–356 | 266 | 42 (15.8%) | 84 | 7 (8.3%) | 0.09 | |
| 36º–366 | 236 | 17 (7.2%) | 72 | 4 (5.6%) | 0.8 | |
| 37º–376 | 282 | 15 (5.3%) | 76 | 5 (6.6%) | 0.8 | |
| 38º–386 | 154 | 5 (3.3%) | 34 | 1 (2.9%) | 0.7 | |
| 39+ | 44 | 0 | 6 | 0 | n/a | |
Includes use of any respiratory support device or supplemental oxygen outside the delivery room, as well as those babies with a stated diagnosis of transient tachypnea of the newborn or respiratory distress syndrome
Any grade intraventricular hemorrhage
Comment
Optimal delivery timing for MC twins has become an obstetrical controversy due to recent studies reporting an increased risk of late stillbirth in “apparently uncomplicated” monochorionic twins. Because of these concerns, a 2011 NIH and Society for Maternal-Fetal Medicine-sponsored workshop offered expert consensus-based recommendations that uncomplicated dichorionic twins be delivered at 38 weeks’ gestation and uncomplicated monochorionic twins be delivered between 34–37 weeks.11 However, antenatal surveillance was inconsistently reported across these studies, contributing to the possibility of unexpected adverse outcomes. The current investigation re-evaluates this question in a large, single-center experience where twin gestations were cared for in a specialized antenatal Twin Clinic with standardized protocols for fetal surveillance and pregnancy management.
Our data revealed no stillbirths during the late preterm/early term gestational period among 334 MC fetuses receiving intensive third trimester surveillance. There was only one intrauterine fetal demise occurring at 34 weeks’ gestation among 1,202 DC fetuses. Nor did the monochorionic twins experience any neonatal mortality. Late preterm delivery was associated with significantly increased neonatal morbidity. Prematurity-related neonatal morbidity decreased with advancing gestational age with the lowest composite rates occurring during the 36th week for MC twins and during the 37th week for DC twins. Delivery for preterm PROM, preterm labor, and other maternal/fetal indications resulted in less gestational age specific neonatal mortality compared to those twins delivered electively. It is important to remember that these results were specific to MC and DC twins at ≥34 weeks’ gestation. Twins delivered at earlier gestational ages were excluded from analysis.
Initial concern for “apparently uncomplicated” MC twins came from Barigye et al. who evaluated the prospective risk of fetal death in 151 uncomplicated MC twins. Barigye reported a 4.3% risk of fetal death after 32 weeks’ gestation. However, this work has been criticized as the frequency of fetal surveillance was every other week and there was no dichorionic control group.5 Lee et al. also calculated a prospective risk of fetal death in an “apparently normal” cohort of 130 MC twins.6 The prospective risk of fetal death was 1.7% between 30–33 weeks, increased to 2% at 34 weeks and then remained relatively constant until >38 weeks’ gestation. A systematic review and meta-analysis by Danon et al. found a significantly increased risk of stillbirth in uncomplicated MC twins with a 3.67-fold higher odds ratio at 34–35 weeks’ gestation compared with uncomplicated DC twins.10 Of the four studies included in the meta-analysis, there were five stillbirths during the 34th and 35th week among 391 MC twins (1.2%) compared to a rate of 0.3% for the DC twins (p=0.03).6,16–18
More reassuring data, however, has been reported by other investigators. Lewi et al. prospectively followed 149 MC twins at two centers.7 They reported a prospective risk of stillbirth of 1.1% at 30–32 weeks’ gestation, which fell to 0.7% at 36 weeks. There were no fetal deaths among the remaining MC twins after 36 weeks. Simoes et al. reported on an unselected cohort of 193 MC twins and found a prospective risk of fetal death of 1.1% at 30–32 weeks’ gestation which decreased to 0.4% at 36 weeks.8 There were no stillbirths among the 171 remaining fetuses undelivered after 36 weeks. Sullivan et al. reviewed the outcomes of 5,894 DC and 1,704 MC twin pregnancies delivered between 2000–2009 at 18 hospitals in Utah.9 Overall, the perinatal mortality rate was higher (p<0.0001) for the MC twins (5.6%; 96 out of 1,704) compared to the DC twins (1.9%; 110 out of 5,894). However, the prospective risk of fetal death in MC twins was only 0.14% at 32 weeks’ gestation with a non-significant upward trend to 0.46% at 37 weeks. Among the subgroup of MC and DC twins that did not have medically indicated deliveries, there was no difference in the risk of serious adverse perinatal outcome after 31 weeks’ gestation. Severe neonatal morbidity was significantly more likely and neonatal care charges greater among MC twins delivered at every gestational week until 36 completed weeks compared to MC twins delivered subsequently.
The explanation for this seemingly conflicted data may lie in the intensity and consistency of fetal surveillance. In many of the prior studies, data regarding antenatal surveillance was either not provided, varied among multiple centers, or over-relied on intermittent ultrasound examination. Our hypothesis is that consistent and intensive fetal surveillance of both MC and DC twins will be associated with a low risk of unexpected fetal demise. This hypothesis is supported by older studies. Knuppel and colleagues instituted weekly non-stress testing after 32 weeks of gestation, regardless of chorionicity, and had no stillbirths among the 90 twin pregnancies cared for after the protocol was established.19 Sherman and colleagues reported on 665 twin gestations at the busy Los Angeles County-University of Southern California Women’s Hospital. Weekly non-stress testing was performed on 230 twin gestations with only a single fetal demise. Among the 435 twin pregnancies without non-stress testing, there were 10 gestations in which one or both fetuses died. The risk of stillbirth was reduced more than five-fold (p=0.062) among twins receiving weekly non-stress tests.20 The major strength of the current study is that it represents a 23 year, single center experience involving 1,779 twin gestations, of which 601 DC and 167 MC pregnancies were managed to ≥34 weeks’ gestation. Each of these twin gestations received consistent and intensive fetal surveillance in the third trimester. The Medical University of South Carolina initiated a specialized Twin Clinic in 1987, which has been continuously directed by a single Maternal-Fetal Medicine physician with expertise in multiple gestations. The success of specialized antenatal care for twins in this clinic has been reported previously.21,22 Maternal and fetal surveillance of twin gestations at MUSC in the third trimester has been directed by standardized protocols. Established antenatal surveillance protocols include: 1) weekly office visits ≥34 weeks’ gestation; 2) weekly nonstress testing or biophysical profile routinely initiated at 32 weeks’ gestation for MC twins and at 34 weeks for DC twins; 3) comprehensive ultrasound evaluation every 3–4 weeks; and 4) planned delivery of uncomplicated MC and DC twins at 37 and 38 weeks’ gestation, respectively.
While the tragedy of a late preterm stillbirth cannot be underestimated, it has also become clear that there are significant adverse neonatal outcomes associated with late preterm delivery. Neonates born between 34–37 weeks’ gestation experience a significantly increased risk of neonatal morbidity compared with either twins or singletons delivered at ≥ 37 weeks.9,23–26 Our findings are consistent with these reports. The nadir of composite neonatal morbidity occurred during the 37th week for DC twins and during the 36th week for MC twins. Since we were unable to express our outcomes in smaller than weekly intervals, our recommendations are to deliver uncomplicated DC twins with the achievement of 38 weeks’ gestation and to deliver uncomplicated MC twins with the achievement of 37 weeks’ gestation.
As with any retrospective study, ours has several limitations. Given the relative rarity of stillbirth even among MC twin gestations, a significant concern is a sample size that is too small to provide an accurate estimate of mortality risk. The confidence intervals around our point estimates of stillbirth risk provide a measure of the strength of our findings. The confidence intervals for the MC and DC twins overlap and both include zero. While we controlled for multiple potential confounding variables in our regression analysis, it is impossible to rule out the influence of unidentified confounders. The accuracy of data collection is also a concern in retrospective cohort analysis. We used a research quality institutional perinatal database which has been in place since the 1970s and has proven accurate and reliable. This was complemented by a second database created specifically for the Twin Clinic. Because of its critical nature, one of the authors (JLB) reviewed each patient’s medical record to confirm gestational age at delivery, indication for delivery, and chorionicity. Despite using multiple sources, we cannot exclude the possibility of database deficiencies. For instance, despite our extraction of delivery indication, we were unable to directly relate those indications to components of our fetal surveillance protocol or determine which twins may have been delivered emergently due to concerning fetal testing, thereby avoiding a potential stillbirth. The absence of placental pathology in 30% of cases could also be criticized, however, we have previously published on the exceptional accuracy of early ultrasound examination in determining chorionicity at our own institution.27 The cohort could also be criticized because of the two decade interval of case accumulation. To control for this temporal influence, we included year of birth in our generalized linear mixed model analysis and found no effect on neonatal risk. There are also limitations associated with the use of a composite neonatal outcome. While we acknowledge this limitation, we felt that it was the most appropriate approach to measuring the multiple and overlapping neonatal morbidities associated with prematurity. Importantly, several of the individual morbidities were also noted to be significantly associated with gestational age at delivery. In our study, elective delivery was independently associated with an increased risk of composite neonatal morbidity. It would be illustrative to know the benefit versus risk of awaiting spontaneous labor for twins beyond the 37 or 38 week recommendations. It is unlikely, however, that any such study will be performed given the current guidelines.
In summary, the data presented here are consistent with the current NIH and Society for Maternal-Fetal Medicine consensus-based recommendations for delivery of uncomplicated DC twins at 38 weeks’ gestation. However, for uncomplicated MC twins, we would advise delivery at the upper end of the recommended delivery interval (37 weeks’ gestation). Elective late preterm delivery of MC twins is associated with significantly increased neonatal morbidity that does not seem justifiable by a corresponding reduction in the risk of stillbirth. Despite this large, single-center clinical experience, our sample size is still insufficient to make definitive conclusions on stillbirth risk in the late preterm period. We are certain that some risk of stillbirth remains despite the absence of this outcome among our monochorionic twins. However, with specialized antenatal care for twins and intensive fetal surveillance, delivery at 37 weeks’ gestation appears to be associated with the best outcomes for uncomplicated monochorionic twins.
Acknowledgments
The project described was supported by Award Number UL1TR000062 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Myla Ebeling, Medical University of South Carolina
Rebecca Nida, Medical University of South Carolina
Footnotes
The authors report no conflict of interest.
Presented as an oral presentation at the Annual Meeting of the South Atlantic Association of Obstetricians and Gynecologists. Lake Buena Vista, Florida, Jan 19–22, 2014.
Reprints will not be available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin JA, Hamilton BE, Sutton P, et al. Births: Final data for 2006. National Vital Statistics Report. 2009;57:1–102. [PubMed] [Google Scholar]
- 2.Minakami H, Honma Y, Matsubara S, Uchida A, Shiraishi H, Sato I. Effects of placental chorionicity on outcome in twin pregnancies: A cohort study. J Reprod Med. 1999;44:595–600. [PubMed] [Google Scholar]
- 3.Gratacos E, Ortiz JU, Martinez JM. A systematic approach to the differential diagnosis and management of complications of monochorionic twin pregnancies. Fetal Diagn Ther. 2012;32:145–55. doi: 10.1159/000342751. [DOI] [PubMed] [Google Scholar]
- 4.Lewi L, Gucciardo L, Van Mieghem T, et al. Monochorionic/diamniotic twin pregnancies: Natural history and risk stratification. Fet Diagn Ther. 2010;27:121–33. doi: 10.1159/000313300. [DOI] [PubMed] [Google Scholar]
- 5.Barigye O, Pasquini L, Galea P, Chambers H, Chappell L, Fisk NM. High risk of unexpected late fetal death in monochorionic twins despite intensive ultrasound surveillance: A cohort study. PLoS Med. 2005;2:e172. doi: 10.1371/journal.pmed.0020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YM, Wylie BJ, Simpson LL, D’Alton ME. Twin chorionicity and the risk of stillbirth. Obstet Gynecol. 2008;111:301–8. doi: 10.1097/AOG.0b013e318160d65d. [DOI] [PubMed] [Google Scholar]
- 7.Lewi L, Jani J, Blickstein I, et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: A prospective cohort study. Am J Obstet Gynecol. 2008;199:514, e1–8. doi: 10.1016/j.ajog.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 8.Simoes T, Amaral N, Lerman R, Ribeiro F, Dias E, Blickstein I. Prospective risk of intrauterine death of monochorionic/diamniotic twins. Am J Obstet Gynecol. 2006;195:134–9. doi: 10.1016/j.ajog.2006.01.099. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan AE, Hopkins PN, Weng H-I, et al. Delivery of monochorionic twins in the absence of complications: Analysis of neonatal outcomes and costs. Am J Obstet Gynecol. 2012;206:257, e1–7. doi: 10.1016/j.ajog.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Danon D, Sekar R, Hack KEA, Fisk NM. Increased stillbirth in uncomplicated monochorionic twin pregnancies: A systematic review and meta-analysis. Obstet Gynecol. 2013;121:1318–26. doi: 10.1097/AOG.0b013e318292766b. [DOI] [PubMed] [Google Scholar]
- 11.Spong CY, Mercer BM, D’Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118:323–33. doi: 10.1097/AOG.0b013e3182255999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman GB. Prospective risk of stillbirth. Obstet Gynecol. 1992;79:547–53. [PubMed] [Google Scholar]
- 13.Yudkin PL, Wood L, Redman CW. Risk of unexplained stillbirth at different gestational ages. Lancet. 1987;1:1192–4. doi: 10.1016/s0140-6736(87)92154-4. [DOI] [PubMed] [Google Scholar]
- 14.Hilder L, Costeloe K, Thilaganathan B. Prolonged pregnancy: Evaluating gestation-specific risks of fetal and infant mortality. Br J Obstet Gynaecol. 1998;105:169–73. doi: 10.1111/j.1471-0528.1998.tb10047.x. [DOI] [PubMed] [Google Scholar]
- 15.Sairam S, Costeloe K, Thilaganathan B. Prospective risk of stillbirth in multiple gestation pregnancies: A population-based analysis. Obstet Gynecol. 2002;100:638–41. doi: 10.1016/s0029-7844(02)02174-9. [DOI] [PubMed] [Google Scholar]
- 16.Domingues AP, Fonseca E, Vasco E, Moura P. Should apparently uncomplicated monochorionic twins be delivered electively at 32 weeks? J Matern Fetal Neonatal Med. 2009;22:1077–80. doi: 10.3109/14767050903042579. [DOI] [PubMed] [Google Scholar]
- 17.Hack KE, Derks JB, Elias SG, Van MFA, Koopman-Esseboom C, Mol BW, et al. Perinatal mortality and mode of delivery in monochorionic diamniotic twin pregnancies ≥ 32 weeks of gestation: A multicentre retrospective cohort study. BJOG. 2011;118:190–7. doi: 10.1111/j.1471-0528.2011.02955.x. [DOI] [PubMed] [Google Scholar]
- 18.Mahony R, Mulcahy C, McAuliffe F, Herlihe CO, Carroll S, Foley ME. Fetal death in twins. Acta Obstet Gynecol Scand. 2011;90:1274–80. doi: 10.1111/j.1600-0412.2011.01239.x. [DOI] [PubMed] [Google Scholar]
- 19.Knuppel RA, Rattom PK, Scerbo JC, O’Brien WF. Intrauterine fetal death in twins after 32 weeks of gestation. Obstet Gynecol. 1985;65:172–5. [PubMed] [Google Scholar]
- 20.Sherman SJ, Kovacs BW, Medearis AL, Bear MB, Paul RH. Non-stress test assessment of twins. J Reprod Med. 1992;37:804–8. [PubMed] [Google Scholar]
- 21.Ellings JM, Newman RB, Hulsey TC, et al. Reduction in very low birth weight deliveries and perinatal mortality in a specialized, multidisciplinary Twin Clinic. Obstet Gynecol. 1993;81:387–91. [PubMed] [Google Scholar]
- 22.Newman RB, Ellings JM. Antepartum management of the multiple gestation: A case for specialized care. Semin Perinatol. 1995;19:387–403. doi: 10.1016/s0146-0005(05)80016-3. [DOI] [PubMed] [Google Scholar]
- 23.Luke B, Brown MB, Alexandre PK, Kinoshi T, O’Sullivan MJ, Martin D, et al. The cost of twin pregnancy: Maternal and neonatal factors. Am J Obstet Gynecol. 2005;192:909–15. doi: 10.1016/j.ajog.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 24.Engle WA, Tomashek KM, Wallman C. “Late-preterm” infants: A population at risk. Pediatrics. 2007;120:1390–401. doi: 10.1542/peds.2007-2952. [DOI] [PubMed] [Google Scholar]
- 25.Khashu M, Narayanan M, Bhargava S, Osiovich H. Perinatal outcomes associated with preterm birth at 33–36 weeks gestation: A population-based cohort study. Pediatrics. 2009;123:109–113. doi: 10.1542/peds.2007-3743. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro-Mendoza CK, Tomashek KM, Kotelchuk M, Barfield W, Weiss J, Evans S. Risk factors for neonatal morbidity and mortality among “healthy”, late preterm newborns. Semin Perinatol. 2006;30:54–60. doi: 10.1053/j.semperi.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Scardo JA, Ellings JM, Newman RB. Prospective determination of chorionicity, amnionicity, and zygosity in twin gestations. Am J Obstet Gynecol. 1995;173:1376–80. doi: 10.1016/0002-9378(95)90619-3. [DOI] [PubMed] [Google Scholar]