Abstract
Background
Withdrawal, a diagnostic indicator of cannabis use disorder, is often minimized or ignored as a consequence of cannabis use, particularly among adolescents. This study aims to characterize cannabis withdrawal among adolescents in outpatient treatment for substance use disorder and evaluate the clinical significance of withdrawal as a predictor of substance-related outcomes.
Methods
Adolescent outpatients (N=127) reporting cannabis as their drug of choice (n=90) were stratified by presence of withdrawal and compared on demographic and clinical variables at treatment intake. Hierarchical linear models compared the effect of withdrawal on percent days abstinent (PDA) and related outcomes over a 1-year follow-up period.
Results
Adolescents reporting withdrawal (40%) were more likely to meet criteria for cannabis dependence, have higher levels of substance use severity, report more substance-related consequences, and have a mood disorder. Withdrawal was not associated with PDA over the follow-up period; however, this relationship was moderated by problem recognition such that adolescents reporting withdrawal and a drug problem improved at a greater rate with respect to PDA than those that didn’t recognize a problem with drugs and didn’t report withdrawal.
Discussion
Withdrawal is common among adolescent outpatients and is associated with a more clinically severe profile. In this sample, all adolescents reporting withdrawal met criteria for cannabis dependence, suggesting that withdrawal is a highly specific indicator of cannabis use disorder. While withdrawal doesn’t appear to be independently associated with substance use outcomes post-treatment, moderating factors such as drug problem recognition should be taken into account when formulating treatment and continuing care plans.
Keywords: withdrawal, adolescent, cannabis, marijuana, substance use disorder, addiction, cannabis
Introduction
The number of pathways to obtaining cannabis in the United States has increased during the past decade due to changes in state-level laws that have decriminalized or legalized both medical and, in some states, non-medical use and possession of cannabis. Along with these policies have come changes in the general public attitude towards cannabis. For the first time in history, U.S. opinion polls show that over fifty percent of the general population and sixty-five percent of the millennial generation supports the legalization of cannabis (Pew Research Center, 2013; Roffman, 2013). Similarly, attitudes towards cannabis and its perceived consequences are shifting such that an increasing number of adolescents perceive cannabis to be minimally harmful and not addictive (Hurd, Michaelides, Miller, & Jutras-Aswad, 2014). This is of particular concern given the vulnerability of the adolescent brain to neurotoxic exposures such as alcohol and drugs (Hurd et al., 2014).
While the perception of the addictiveness and risk is low, there is clear evidence to support a relationship between cannabis use and poor medical, neurocognitive, functional and psychosocial problems (Crean et al., 2011). Cannabis use during adolescence has been associated with an elevated risk of later problematic use of illicit drugs, impaired mental health and neurocognitive functioning, lower IQ, risky behavior, and criminal offenses (Copeland & Swift, 2009; Ehlers et al., 2010; Meier et al., 2012; NIDA, 2012). With approximately 10% of cannabis users becoming dependent (Copeland & Swift, 2009), the lower addictive potential of cannabis compared to other illicit drugs (Anthony, Warner, & Kessler, 1994) may be contributing to this perception of cannabis use as being low-risk. Low perception of cannabis risk is associated with intentions to use cannabis among adolescents (Lopez-Quintero & Neumark, 2010), suggesting that the changing attitudes towards cannabis risk in the United States may lead to increased use in the future. The high prevalence of cannabis use, particularly among adolescents, may in fact offset the relatively low risk of dependence with respect to the public health burden of cannabis. Currently, there are more people in the U.S. dependent on cannabis than any other illicit substance (Substance Abuse and Mental Health Services Administration, 2013) and, other than alcohol, it continues to be the most commonly misused substance by adolescents (Hurd et al., 2014).
Epidemiology of cannabis withdrawal
Cannabis use disorder can manifest through a combination of symptoms or criteria as outlined by the American Psychiatric Association(American Psychiatric Association, 2013). One of the hallmark characteristics of a substance use disorder (SUD) is the presence of withdrawal. Previous research has identified cannabis withdrawal as the most commonly reported criterion among adolescents with cannabis dependence (Cornelius, Chung, Martin, Wood, & Clark, 2008; Nocon, Wittchen, Pfister, Zimmermann, & Lieb, 2006). In previous studies, between 35–75% of treatment-seeking adolescents reported experiencing withdrawal symptoms when cutting down or abstaining from cannabis (Chung, Martin, Cornelius, & Clark, 2008; Crowley, Macdonald, Whitmore, & Mikulich, 1998; Preuss, Watzke, Zimmermann, Wong, & Schmidt, 2010; Vandrey, Budney, Hughes, & Liguori, 2008). Previous research suggests the prevalence of withdrawal does not differ by gender, lifetime history of drug and tobacco use, or psychopathology (Agrawal, Pergadia, & Lynskey, 2008; Allsop et al., 2012; Piontek, Kraus, Legleye, & Buhringer, 2011). Conversely, ethnicity, polysubstance use, concurrent tobacco cessation, family history of substance use, and certain genetic polymorphisms were shown to moderate cannabis withdrawal (Agrawal et al., 2008; Ehlers et al., 2010; Gizer et al., 2013; Haughey, Marshall, Schacht, Louis, & Hutchison, 2008; Preuss et al., 2010; Vandrey et al., 2008). The most common symptoms of withdrawal can be clustered into symptoms of weakness or symptoms of anxiety and depression (Hasin et al., 2008), with restlessness, appetite change, irritability, sleep problems and craving being most severe (Milin, Manion, Dare, & Walker, 2008; Vandrey et al., 2008; Allsop, Norberg, Copeland, Fu & Budney, 2011).
Although the severity of withdrawal among adolescents generally appears to be mild to moderate in clinical samples, there are cognitive and functional issues that often arise including functional impairment, structural and functional brain changes, using drugs to relieve withdrawal, exacerbation of mental health problems, and an increased risk of relapse (Agrawal et al., 2008; Allsop et al., 2012; Batalla et al., 2013; Bonn-Miller, Zvolensky, Marshall, & Bernstein, 2007). In a sample of adolescent cannabis users, participants retrospectively reported that withdrawal was associated with inability to complete school work and arguing beginning within 24 hours after cessation and persisting for several days if remaining abstinent (Dawes, Liguori, & Dougherty, 2006).
While there are studies that support the association between adolescent cannabis withdrawal and substance use outcomes (Cornelius et al., 2008), there are currently few prospective studies (Chung et al., 2008) that examine this relationship and include a follow-up period greater than one month. Furthermore, these studies exclude important clinical (substance use severity, psychiatric symptoms) and functional outcomes (substance use consequences). To further explore the relationship between dependence, withdrawal and substance use-related outcomes in adolescents with cannabis use disorder over one-year, this study aims to (a) determine the prevalence of cannabis withdrawal in a sample of adolescents receiving outpatient treatment for SUD who reported cannabis as their drug of choice; (b) describe the demographic, clinical and functional differences in adolescent cannabis users with and without withdrawal symptoms; and (c) explore the relationship between withdrawal and substance use-related outcomes (e.g. rates of substance use, substance use severity, substance use consequences, and psychiatric symptoms) during and post-treatment.
Methods
Participants
Participants were 127 adolescents who presented for treatment at an outpatient SUD treatment facility in the U.S. between 2006 and 2009. Treatment referral mechanisms were similar to those of other adolescent outpatient programs such that participants went to treatment because their parents required/encouraged it (52.8%), the court required/recommended it (13.4%), the participant wanted treatment (11.0%) or another reason (22.8%). In order to be eligible, patients had to be within their first month of treatment at this facility, between the ages of 14 and 19, have a parent/guardian willing to consent to their child’s participation (for those under 18), give assent/consent to participate, and be fluent in English. Adolescents were excluded from the study if they were actively psychotic or had an organic brain/cognitive disorder affecting their ability to comprehend the study and its risks and benefits. Of the 178 patients who presented for treatment, 160 (90.0%) were eligible to participate, however 8 (5.0%) refused to be contacted by study staff. Of those who were approached by study staff, 25 chose not to participate due to scheduling and transportation difficulties (52.0%) or refused to provide consent/participate (48.0%). Of the 127 participants who did enroll in the study, we only included participants who endorsed cannabis as their drug of choice in these analyses (n=90, 70.9%).
Of the 90 adolescents included in these analyses, follow-up rates were 93.3%, 90.0% and 84.4% at 3-, 6-, and 12-months respectively. To assess attrition bias, we compared participants who were lost to follow up to those who completed the assessments on baseline demographic and clinical variables using t-tests and Chi-square analyses. This study was reviewed and approved by the Partners Healthcare System Internal Review Board at Massachusetts General Hospital.
Materials
Demographics
The Background Information Form (BIF; Brown, Vik, & Creamer, 1989) assesses demographics, family history of substance use, and the patients’ motivation to enter treatment.
Substance Use Problem Recognition
In the BIF participants were asked “Do you think you might have a problem with drugs (other than alcohol)?” For the analyses, participants’ responses were dichotomized: yes vs. no. Some participants reported previously having a problem with drugs, but not at the present time. These responses were classified as not having a problem with drugs because we wanted to capture current problem recognition at baseline.
Substance Use and Withdrawal
We used a modified version of the CDDR (Brown et al., 1998), a structured interview that assesses substance involvement, past 90-day withdrawal symptoms, and DSM-IV lifetime cannabis abuse/dependence. The CDDR has been shown to have good internal consistency, reliability, and validity with adolescents (Brown et al., 1998). The Timeline Follow-Back (TLFB; Sobell & Sobell, 1992) and Form-90 (Miller & Del Boca, 1994) were used in conjunction to examine substance use in the past 90 days (or 180 days at 12-month follow-up).
Substance Use Severity
The PIS, a subscale of the Personal Experience Inventory (PEI; Henly & Winters, 1988), is a 29-item self-report measure of substance use severity. It assesses an individual’s degree of use across multiple settings, use for self-medicating purposes, and rearranging activities to facilitate substance use. It has been found to contribute substantial unique and reliable information, possess high internal consistency, as well as good convergent and discriminant validity (Winters, Stinchfield, & Henly, 1993).
Substance Use Consequences
To assess consequences we used the Inventory of Drug Use Consequences (InDUC-2R) (Tonigan & Miller, 2002), a self-report measure designed to assess physical, interpersonal, intrapersonal, impulse control and social responsibility consequences of alcohol and/or drug use over the past 90 days. The InDUC-2R has been shown to be sensitive to changes in drug-related consequences over a 3-month time period (Tonigan & Miller, 2002).
Psychiatric Symptoms
The Brief Symptom Inventory (BSI; Derogatis & Melisaratos, 1983) measures the degree of distress caused by 53 psychological symptoms in the past week. It has been shown to be reliable and have good convergent validity for use with adolescents (Sahin, Durak Batigun, & Ugurtas, 2002).
Psychiatric Diagnoses
The Computerized Diagnostic Interview Schedule for Children, version IV (C-DISC-IV; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000) is a computerized structured interview that assesses past-year mental health diagnoses using DSM-IV criteria. The Axis-I modules used in the present study were further classified as mood, anxiety or externalizing disorders. This instrument has been shown to have good reliability and validity (Schwab-Stone et al., 1996).
Biological Verification of Self-Report
Biological verification of self-reported abstinence was conducted using Intercept Oral Fluid Drug Test kits. There were no inconsistencies detected between self-reported abstinence and saliva test results.
Procedure
Eligible patients were informed about the study by the clinical program director(s) with which they completed their treatment intake assessment. If interested, study staff briefly screened for eligibility, gave the patient/parent a brief overview of the study, answered any questions that patients or parents had by phone and scheduled the baseline assessment. Study staff encouraged participants to complete a packet of self-report questionnaires, which was given to them by the clinical director(s), prior to the first meeting.
Participants completed the baseline assessment as close as possible to their treatment start date, followed by a 3-, 6-, and 12-month follow-up assessment 90, 180, and 360 days after their treatment start date, respectively. The baseline assessment took approximately two hours to complete, not including the time participants spent completing the self-report questionnaires prior to their appointment, whereas the 3-, 6-, and 12-month follow-ups took approximately 45–90 minutes to complete. Participants were paid by check at the end of each assessment: $50 for the baseline and 12-month assessments and $40 for the 3- and 6-month assessments. Assessments usually took place at the outpatient treatment facility (86.3%), but in cases where participants were unable to meet at the treatment facility, interviews were completed over the phone or in person at another location.
Data analysis Plan
We divided the sample into two groups, participants reporting at least one withdrawal symptom or using drugs to relieve withdrawal versus those reporting no withdrawal from cannabis. We compared baseline differences between these two groups using independent samples t-tests and chi-square analyses. Lifetime cannabis use days was positively skewed and required a square root transformation. All past 90-day substance use variables (excluding cannabis) were transformed into dichotomous variables due to their positively skewed distributions. Among patients who reported experiencing withdrawal, we calculated the proportion that endorsed each type of symptom. We also calculated the total number of withdrawal symptoms experienced by each participant in this subsample.
To examine the association between withdrawal symptoms and substance use outcomes over time we constructed hierarchical linear models (HLM), controlling for predictors of attrition (age, employment) and baseline levels of the outcome. The effects of interest were the main effect of withdrawal group and the interaction between withdrawal and time on substance use outcomes over 1-year. The outcomes were percent days abstinent, psychiatric symptoms, substance use severity and substance use consequences. Analyses were conducted using SAS Version 9.2, ©SAS Institute, Inc., Cary, NC, USA.
Results
Prevalence of cannabis dependence and withdrawal
In this sample of 90 adolescents initiating outpatient treatment for SUD that reported cannabis as their drug of choice, 84.44% (n=76) met DSM-IV criteria for cannabis dependence. Furthermore, 40% (n=36) reported experiencing cannabis withdrawal. All participants reporting withdrawal met criteria for dependence and endorsed an average of 5.47±1.13 dependence criteria (Table 1).
Table 1.
Baseline differences between patients reporting withdrawal and those not reporting withdrawal
| No Withdrawal (n=54) | Withdrawal (n=36) | t /χ2 | p | |
|---|---|---|---|---|
|
|
||||
| Demographics | ||||
| Age | 16.48±1.26 | 16.72±1.23 | −0.90 | 0.372 |
| Male | 44 (81.48) | 30 (83.33) | 0.05 | 0.822 |
| Race | 46 (85.19) | 31 (86.11) | 0.02 | 0.903 |
| Education (Yrs) | 9.94±1.68 | 10.17±1.25 | −0.68 | 0.499 |
| Employed | 24 (44.44) | 17 (47.22) | 0.07 | 0.796 |
| Clinical Characteristics | ||||
| Family history of alcohol use disorder | 35 (67.31) | 20 (62.50) | 0.20 | 0.653 |
| Family history of drug use disorder | 26 (50.00) | 20 (62.50) | 1.25 | 0.264 |
| Substance use severity | 1.60±0.58 | 2.06±0.55 | −3.70 | 0.000 |
| Drug use consequences | 45.25±18.42 | 33.17±23.41 | −2.54 | 0.013 |
| Self-motivated treatment entry | 13 (24.07) | 14 (38.89) | 2.26 | 0.133 |
| Previous SUD treatment | 26 (48.15) | 18 (50.00) | 0.03 | 0.863 |
| Problem Recognition | 17 (31.48) | 17 (47.22) | 2.28 | 0.131 |
| Substance Use | ||||
| Percent days abstinent | 42.77±34.56 | 40.49±31.78 | 0.32 | 0.752 |
| Percent days cannabis use | 52.39±36.70 | 54.10±33.54 | −0.22 | 0.823 |
| Lifetime cannabis use days | 908.9±1487.7 | 1134.8±1073.8 | −1.73 | 0.087 |
| Age at first use of cannabis | 13.81±1.49 | 13.65±1.12 | 0.52 | 0.601 |
| Age at first regular use of cannabis | 14.64±1.50 | 14.44±1.46 | 0.61 | 0.540 |
| Past 90 day substance use | ||||
| Alcohol | 49 (90.74) | 35 (97.22) | 1.46 | 0.227 |
| Nicotine | 48 (88.89) | 32 (88.89) | 0.00 | 1.000 |
| Hallucinogens | 16 (29.63) | 8 (22.22) | 0.61 | 0.436 |
| Cocaine/Crack | 7 (12.96) | 10 (27.78) | 3.09 | 0.079 |
| Amphetamines | 5 (9.26) | 8 (22.22) | 2.94 | 0.087 |
| Barbiturates | 0 (0.00) | 2 (5.56) | 3.07 | 0.080 |
| Sedatives/Tranquilizers | 4 (7.41) | 6 (16.67) | 1.88 | 0.171 |
| Heroin | 1 (1.85) | 1 (2.78) | 0.09 | 0.770 |
| Narcotics (other than heroin) | 8 (14.81) | 8 (22.22) | 0.81 | 0.368 |
| Inhalants | 2 (3.70) | 4 (11.11) | 1.90 | 0.168 |
| Other | 0 (0.00) | 2 (5.56) | 3.07 | 0.080 |
| Dependence | ||||
| Dependence diagnosis | 40 (74.07) | 36 (100.00) | 11.05 | 0.001 |
| # Dependence Criteria | 3.48±1.50 | 5.47±1.13 | −6.77 | 0.000 |
| # Dependence Criteria (exc. withdrawal) | 3.55±1.44 | 4.47±1.13 | −3.24 | 0.002 |
| Preoccupation | 44 (83.02) | 36 (100.00) | 6.80 | 0.009 |
| Reduced control | 34 (64.15) | 26 (72.22) | 0.64 | 0.425 |
| Tolerance | 34 (64.15) | 32 (88.89) | 6.85 | 0.009 |
| Attempts/difficulty cutting down | 45 (84.91) | 32 (88.89) | 0.29 | 0.589 |
| Given up activities | 19 (35.85) | 21 (58.33) | 4.38 | 0.036 |
| Withdrawal and/or withdrawal relief | 0 (0.00) | 36 (100.00) | 90.00 | 0.000 |
| Use despite medical/psychological problems | 12 (25.53) | 14 (48.28) | 4.12 | 0.042 |
Continuous variables were compared using an independent samples t-test. Descriptive statistics for continuous variables are reported as M±SD. Dichotomous variables were compared using a chi-square test. Descriptive statistics for dichotomous variables are reported as N(%).
Baseline characteristics
Adolescents reporting past 90-day cannabis withdrawal were not significantly different with respect to demographic characteristics compared to cannabis users who did not report withdrawal at baseline. Regarding clinical characteristics, participants reporting withdrawal had more substance-related consequences (t=2.54, p=0.013) and reported higher substance use severity (t=−3.70, p=0.000) than did participants who did not report withdrawal. There were no significant between-group differences with respect to cannabis, alcohol or other illicit drug use. However, participants reporting withdrawal were more likely to meet criteria for cannabis dependence (χ2=11.05, p=0.001) and endorsed significantly more dependence criteria even when the withdrawal criterion was excluded from the analysis (t=−3.24, p=0.002). More specifically, they were significantly more likely to report preoccupation, tolerance, giving up activities and using cannabis despite medical or psychological problems that were caused or made worse by their use (p<0.05; Table 1). The psychiatric profiles were similar between groups with the exception of mood disorders. Participants reporting withdrawal were more likely to have a mood disorder (27.78%) compared to participants without withdrawal (11.11%, χ2=4.10, p=0.043). The presence of a cannabis dependence diagnosis was not associated with having additional psychiatric diagnoses (Table 2).
Table 2.
Prevalence of psychiatric diagnoses stratified by dependence and withdrawal
| No Withdrawal (n=54) | Withdrawal (n=36) | t /χ2 | p | |
|---|---|---|---|---|
| Number of psychiatric disorders1 | 0.85±1.20 | 1.39±1.40 | −1.94 | 0.055 |
| Anxiety disorder | 3 (5.56) | 6 (16.67) | 2.96 | 0.085 |
| Mood disorder | 6 (11.11) | 10 (27.78) | 4.10 | 0.043 |
| Externalizing disorder | 22 (40.74) | 16 (44.44) | 0.12 | 0.728 |
| Psychiatric symptoms | 0.91±0.70 | 1.08±0.66 | −1.15 | 0.254 |
| No Dependence (n=14) | Dependence (n=76) | t /χ2 | p | |
|---|---|---|---|---|
| Number of psychiatric disorders1 | 0.71±1.44 | 1.13±1.28 | −1.10 | 0.274 |
| Anxiety disorder | 2 (14.29) | 7 (9.21) | 0.34 | 0.561 |
| Mood disorder | 1 (7.14) | 15 (19.74) | 1.28 | 0.257 |
| Externalizing disorder | 3 (21.43) | 35 (46.05) | 2.94 | 0.087 |
| Psychiatric symptoms | 1.01±0.87 | 0.97±0.65 | 0.18 | 0.857 |
Anxiety disorders: panic disorder, agoraphobia, or social phobia; Mood disorders: major depressive disorder, dysthymic disorder, or manic/hypomanic episode; Externalizing disorders: attention deficit hyperactivity disorder, oppositional defiant disorder, or conduct disorder
Cannabis withdrawal and substance use outcomes
Participants reporting withdrawal had an average of 2.03±1.25 withdrawal symptoms. The maximum number of symptoms reported was six. Twenty-four (66.67%) of these participants reported using drugs to relieve or prevent withdrawal symptoms. The most common withdrawal symptoms included difficulty sleeping (30.56%), headaches (13.89%) and feeling irritable (13.89%) (Table 3).
Table 3.
Prevalence of withdrawal symptoms among patients who report withdrawal (n=36)
| Symptom | % (n) |
|---|---|
| Using drugs to relieve withdrawal | 66.67 (24) |
| Difficulty sleeping | 30.56 (11) |
| Headaches | 13.89 (5) |
| Feeling irritable | 13.89 (5) |
| Stomach upset, nausea, vomiting | 11.11 (4) |
| Fatigue, excessive yawning | 11.11 (4) |
| Feeling angry, hostile, or acting aggressive | 11.11 (4) |
| Loss of appetite | 11.11 (4) |
| Feeling depressed | 8.33 (3) |
| Feeling anxious or nervous | 8.33 (3) |
| Increased dreaming | 5.56 (2) |
| Trouble concentrating | 5.56 (2) |
| Moodiness | 2.78 (1) |
| Impulsivity | 2.78 (1) |
| Loss of consciousness | 2.78 (1) |
| Restless | 2.78 (1) |
| Chest pains | 2.78 (1) |
| Feeling weak/faint | 2.78 (1) |
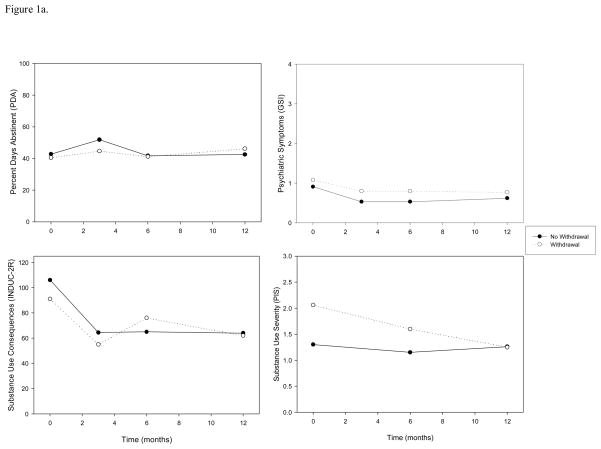
Longitudinal mixed-effects models controlling for predictors of attrition found that there was neither a significant main effect of withdrawal, nor an interaction between withdrawal and time, on PDA over the 12-month follow-up period. Similarly, there was no longitudinal relationship between withdrawal and psychiatric symptoms. There was a significant interaction between withdrawal and time on substance use-related consequences (F=4.97, p=0.027) and substance use severity (F=7.56, p=0.008) over 12-months such that consequences and severity decreased at a greater rate for participants with withdrawal relative to participants who did not report withdrawal symptoms at baseline (Table 4; Figure 1a).
Table 4.
Substance use outcomes by presence of withdrawal over 12-months
| Independent Variable | Percent Days Abstinent
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Main Effects Only
|
Interaction
|
|||||||
| β | SE | F | p | β | SE | F | p | |
| Age | −4.168 | 2.400 | 3.02 | 0.086 | −4.145 | 2.400 | 2.98 | 0.088 |
| Employment | 5.884 | 5.773 | 1.04 | 0.311 | 6.019 | 5.776 | 1.09 | 0.301 |
| Baseline PDA | 0.326 | 0.085 | 14.82 | 0.000 | 0.324 | 0.085 | 14.66 | 0.000 |
| Time | −0.328 | 0.472 | 0.48 | 0.488 | 0.240 | 0.712 | 0.31 | 0.577 |
| Withdrawal Group | −0.032 | 5.723 | 0.00 | 0.996 | −7.142 | 8.798 | 0.66 | 0.419 |
| Withdrawal Group x Time | 1.012 | 0.951 | 1.13 | 0.289 | ||||
| Independent Variable | Psychiatric Symptoms
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Main Effects Only
|
Interaction
|
|||||||
| B | SE | F | p | β | SE | F | p | |
| Age | 0.031 | 0.034 | 0.82 | 0.368 | 0.031 | 0.034 | 0.81 | 0.371 |
| Employment | −0.020 | 0.079 | 0.06 | 0.805 | −0.022 | 0.079 | 0.08 | 0.781 |
| Baseline Psychiatric Symptoms | 0.345 | 0.059 | 33.87 | 0.000 | 0.345 | 0.059 | 33.88 | 0.000 |
| Time | 0.004 | 0.007 | 0.31 | 0.579 | −0.005 | 0.011 | 0.20 | 0.657 |
| Withdrawal Group | 0.136 | 0.080 | 2.93 | 0.091 | 0.251 | 0.133 | 3.58 | 0.062 |
| Withdrawal Group x Time | −0.016 | 0.015 | 1.17 | 0.281 | ||||
| Independent Variable | Substance Use Consequences
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Main Effects Only
|
Interaction
|
|||||||
| B | SE | F | p | β | SE | F | p | |
| Age | 0.440 | 1.150 | 0.15 | 0.703 | 0.419 | 1.149 | 0.13 | 0.716 |
| Employment | 0.677 | 2.780 | 0.06 | 0.808 | 0.520 | 2.780 | 0.03 | 0.852 |
| Baseline Consequences | 0.369 | 0.066 | 31.76 | 0.000 | 0.372 | 0.066 | 32.17 | 0.000 |
| Time | −0.131 | 0.214 | 0.37 | 0.543 | −0.661 | 0.318 | 0.77 | 0.382 |
| Withdrawal Group | 0.272 | 2.853 | 0.01 | 0.924 | 6.815 | 4.094 | 2.77 | 0.100 |
| Withdrawal Group x Time | −0.949 | 0.426 | 4.97 | 0.027 | ||||
| Independent Variable | Substance Use Severity
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Main Effects Only
|
Interaction
|
|||||||
| B | SE | F | p | β | SE | F | p | |
| Age | 0.053 | 0.054 | 0.99 | 0.324 | 0.057 | 0.054 | 1.11 | 0.296 |
| Employment | 0.022 | 0.128 | 0.03 | 0.862 | 0.015 | 0.128 | 0.01 | 0.907 |
| Baseline Severity | 0.204 | 0.116 | 3.07 | 0.084 | 0.202 | 0.116 | 3.00 | 0.088 |
| Time | −0.035 | 0.030 | 1.36 | 0.247 | −0.120 | 0.042 | 2.14 | 0.149 |
| Withdrawal Group | 0.112 | 0.139 | 0.65 | 0.422 | 0.826 | 0.295 | 7.86 | 0.006 |
| Withdrawal Group x Time | −0.157 | 0.057 | 7.56 | 0.008 | ||||
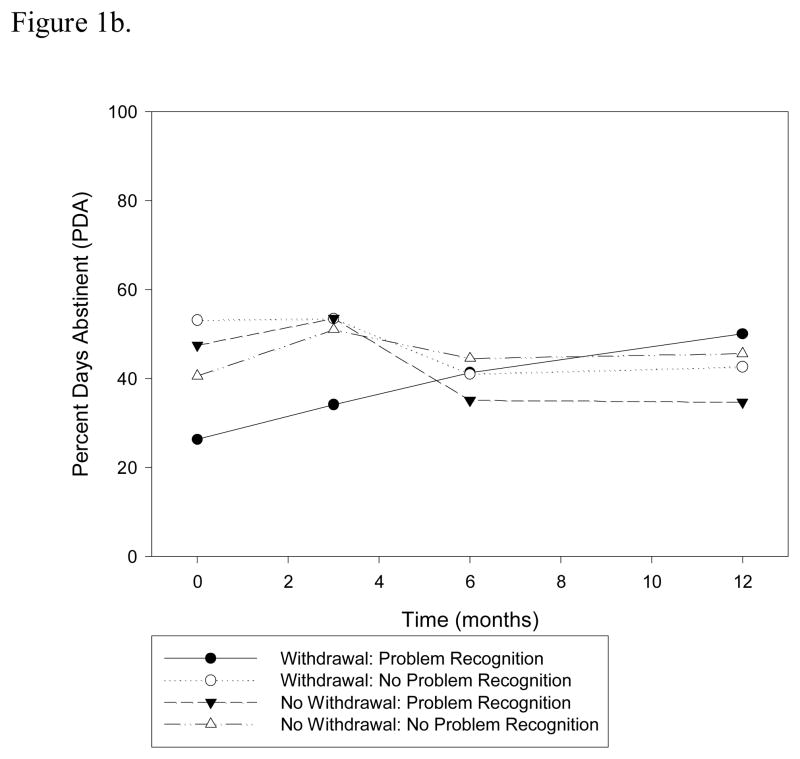
Figure 1.
Figure 1a. Substance use outcomes over the 12-month follow-up period by cannabis withdrawal group
Figure 1b. Interaction between withdrawal and problem recognition on PDA over time
Subsidiary analyses
There is mixed prior evidence on the relationship between withdrawal and substance use outcomes among adolescent cannabis users (Chung et al., 2008; Cornelius et al., 2008) and the recent macro-level changes toward more permissible cannabis use has been associated with perceived lower risk of harm. This perceived lower risk of harm may translate into a failure to identify a cannabis problem, because adolescents may assign any problems to causes outside of their drug use. We wanted to explore this further by examining the role of cannabis problem recognition and its relationship to withdrawal on substance use outcomes since this construct is an important construct in predicting intentions to use substances in adolescent samples (Hurd et al., 2014; Lopez-Quintero & Neumark, 2010). We were interested in investigating whether this relationship also existed among adolescents with current cannabis use problems. To explore this question, we added a higher order interaction between problem recognition, withdrawal, and time, to the longitudinal model predicting PDA. We also controlled for substance use consequences, severity and baseline PDA in order to isolate the effect of perceived problem recognition from the actual degree of problems that the participant has incurred. We found that problem recognition among participants reporting withdrawal was associated with a significantly greater rate of increase in PDA over the follow-up period relative to participants reporting withdrawal and no problem recognition as well as those not reporting withdrawal, (F=4.02, df=151, p=0.047; Figure 1b).
Discussion
Results of this investigation suggest that cannabis dependence is prevalent among treatment-seeking adolescent cannabis users (84.4%), despite a relatively low frequency of problem recognition (37.8%). On average, this sample had only been using cannabis for 2.8 years and regularly for 2.0 years, indicating a rapid progression from initial to regular use and dependence. The prevalence of withdrawal (40.0%) and average number of withdrawal symptoms (2.03±1.25) is similar to a previous study of adolescents with cannabis use problems in outpatient treatment (Chung et al., 2008), but lower than samples of adolescents with cannabis dependence in inpatient treatment or comorbid psychopathology (Cornelius et al., 2008; Crowley et al., 1998; Preuss et al., 2010). Contrary to previous literature, the presence of self-reported withdrawal did not differ by demographic variables or substance use at baseline (Agrawal et al., 2008; Gizer et al., 2013).
Identifying cannabis withdrawal has clinical implications for adolescents being treated for cannabis use disorder. All patients reporting withdrawal met criteria for dependence, suggesting that the presence of withdrawal is a highly specific indicator of cannabis use disorder among adolescents and has a high positive predictive value. However, given that the majority (74.1%) of patients without withdrawal also met criteria for dependence, we must acknowledge that absence of withdrawal doesn’t signify a lack of dependence. Furthermore, patients presenting with symptoms of withdrawal appear to have a more severe clinical profile with respect to substance use severity, consequences and psychiatric comorbidity (e.g. mood disorder), however these functional trajectories appear to become similar to patients without withdrawal over the course of the follow-up period. When considered alone, cannabis withdrawal appears to have no effect on substance use rates during and post-treatment in this sample. The exploratory results from our subsidiary analysis suggest that it is important to consider problem recognition among patients experiencing withdrawal such that problem recognition may motivate adolescents toward adaptive changes in use over time. This finding is consistent with previous research that found adolescents’ perceived risk and recognition of cannabis problems is negatively correlated with cannabis use (Lopez-Quintero & Neumark, 2010).
The importance of problem recognition has direct implications for secondary and tertiary prevention. Adolescents perceive cannabis use as low-risk and may therefore not attribute functional consequences and problems, such as withdrawal, to their cannabis use. This misattribution may be in part due to the increasingly relaxed laws on cannabis possession and the increasing social acceptability of cannabis use (Pew Research Center, 2013; Roffman, 2013). Furthermore, as supported by results from this study, there is a relatively rapid progression from first to regular cannabis use and dependence among adolescents, resulting in a shorter window for secondary and tertiary prevention of cannabis use disorder (Crowley et al., 1998). To prevent this progression from experimentation to dependence and the consequences that are often associated with an SUD, it is important to intervene early and to inform youth about the consequences associated with cannabis use. Being aware of the neuropsychological, physiological and social consequences may increase the likelihood of problem recognition and, consequently, a reduction in substance misuse, particularly among adolescents that are experiencing withdrawal symptoms. It is possible that educating adolescents about cannabis-related problems (e.g. withdrawal), such that they are better able to identify them, may increase the likelihood of problem recognition and appropriate attribution of related consequences, thereby improving substance use trajectories over time. Further research determining the population attributable risk (PAR) associated with improved knowledge about cannabis use among adolescents will allow us to predict the degree to which we can reduce the rates and burden of cannabis use through increased education and public awareness of cannabis-related harms.
Limitations
This study was conducted in a single, private, suburban outpatient clinic in the northeastern United States. The sample was fairly homogeneous with respect to race, gender and age and thereby limits the generalizability of the findings to other populations. Withdrawal was assessed using a symptom checklist instead of a validated withdrawal scale to determine whether the participants experienced cannabis withdrawal, which may result in some misclassification. However, in this sample we did identify several clinically meaningful differences between withdrawal groups suggesting that there may be relevance to classifying groups using this approach. Furthermore, the measure used to determine cannabis dependence assesses lifetime rather than current diagnosis. To minimize the likelihood of including a participant with a previous cannabis use disorder that is currently in remission, we only included participants that reported cannabis as their current drug of choice. A methodological limitation was that the oral toxicology test may not be maximally sensitive to THC, which may therefore produce some false negatives.
Another limitation is related to the possible confounding effects of psychiatric comorbidity. Participants reporting withdrawal were more likely to have a mood disorder. Whether some of the reported cannabis withdrawal symptoms may have been a product of a mood disorder cannot be determined. On the contrary, mood-related withdrawal symptoms may increase the likelihood of meeting criteria for a mood disorder. Future research needs to be conducted to disentangle mood-related symptoms and their etiology. In addition, problem recognition was measured as a dichotomy and it may be that the degree of perceived severity of that problem is a more sensitive and predictive measure.
Conclusions
Cannabis dependence and withdrawal is prevalent among adolescent outpatients reporting cannabis as their drug of choice. Typically adolescents presenting with cannabis withdrawal have a more severe clinical profile with respect to consequences, severity and psychiatric comorbidity (mood disorders). The relationship between cannabis withdrawal and future rates of substance use seems to be moderated by problem recognition. It appears that recognizing the risks and problems associated with ones’ cannabis use is directly associated with a greater rate of reduction in substance use among adolescents with cannabis withdrawal. While cannabis withdrawal appears to be a highly specific indicator of severity, problem recognition may be a better predictor of substance use outcomes during and post-treatment. Efforts should be made to increase cannabis related-problem attribution and recognition among adolescent cannabis users.
Acknowledgments
This research was funded by the National Institute of Alcohol Abuse and Alcoholism (NIAAA; R01AA015526).
Contributor Information
M. Claire Greene, Center for Addiction Medicine Departments of Psychiatry Massachusetts General Hospital and Harvard Medical School, Boston, MA.
John F. Kelly, Center for Addiction Medicine Departments of Psychiatry Massachusetts General Hospital and Harvard Medical School, Boston, MA.
References
- 1.Agrawal A, Pergadia ML, Lynskey MT. Is there evidence for symptoms of cannabis withdrawal in the national epidemiologic survey of alcohol and related conditions? [Research Support, N.I.H., Extramural] Am J Addict. 2008;17(3):199–208. doi: 10.1080/10550490802019519. [DOI] [PubMed] [Google Scholar]
- 2.Allsop DJ, Copeland J, Norberg MM, Fu S, Molnar A, Lewis J, Budney AJ. Quantifying the clinical significance of cannabis withdrawal. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] PLoS One. 2012;7(9):e44864. doi: 10.1371/journal.pone.0044864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119(1–2):123–129. doi: 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington: American Psychiatric Association; 2013. [Google Scholar]
- 5.Anthony JC, Warner LA, Kessler RC. Comparative epidemioloy of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the national comorbidity survey. Exp Clin Psychopharmacol. 1994;2(3):244–268. [Google Scholar]
- 6.Batalla A, Bhattacharyya S, Yucel M, Fusar-Poli P, Crippa JA, Nogue S, Martin-Santos R. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. [Research Support, Non-U.S. Gov’t Review] PLoS One. 2013;8(2):e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonn-Miller MO, Zvolensky MJ, Marshall EC, Bernstein A. Incremental validity of anxiety sensitivity in relation to marijuana withdrawal symptoms. [Research Support, N.I.H., Extramural] Addict Behav. 2007;32(9):1843–1851. doi: 10.1016/j.addbeh.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 9.Brown SA, Vik PW, Creamer VA. Characteristics of relapse following adolescent substance abuse treatment. Addict Behav. 1989;14(3):291–300. doi: 10.1016/0306-4603(89)90060-9. [DOI] [PubMed] [Google Scholar]
- 10.Chung T, Martin CS, Cornelius JR, Clark DB. Cannabis withdrawal predicts severity of cannabis involvement at 1-year follow-up among treated adolescents. [Research Support, N.I.H., Extramural Validation Studies] Addiction. 2008;103(5):787–799. doi: 10.1111/j.1360-0443.2008.02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copeland J, Swift W. Cannabis use disorder: epidemiology and management. [Review] Int Rev Psychiatry. 2009;21(2):96–103. doi: 10.1080/09540260902782745. [DOI] [PubMed] [Google Scholar]
- 12.Cornelius JR, Chung T, Martin C, Wood DS, Clark DB. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addict Behav. 2008;33(11):1500–1505. doi: 10.1016/j.addbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crean RD, Tapert SF, Minassian A, Macdonald K, Crane NA, Mason BJ. Effects of chronic, heavy cannabis use on executive functions. [Case Reports Research Support, N.I.H., Extramural] J Addict Med. 2011;5(1):9–15. doi: 10.1097/ADM.0b013e31820cdd57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowley TJ, Macdonald MJ, Whitmore EA, Mikulich SK. Cannabis dependence, withdrawal, and reinforcing effects among adolescents with conduct symptoms and substance use disorders. [Research Support, U.S. Gov’t, P.H.S.] Drug Alcohol Depend. 1998;50(1):27–37. doi: 10.1016/s0376-8716(98)00003-9. [DOI] [PubMed] [Google Scholar]
- 15.Dawes MA, Liguori A, Dougherty DM. Cannabis withdrawal among adolescent cannabis users in an outpatient research setting. [Research Support, N.I.H., Extramural] Am J Addict. 2006;15(6):485–486. doi: 10.1080/10550490601000637. [DOI] [PubMed] [Google Scholar]
- 16.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13(3):595–605. [PubMed] [Google Scholar]
- 17.Ehlers CL, Gizer IR, Vieten C, Gilder DA, Stouffer GM, Lau P, Wilhelmsen KC. Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Addict Behav. 2010;35(2):102–110. doi: 10.1016/j.addbeh.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gizer IR, Gilder DA, Lau P, Wang T, Wilhelmsen KC, Ehlers CL. Contributions of ethnicity to differential item functioning of cannabis abuse and dependence symptoms. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] J Stud Alcohol Drugs. 2013;74(2):320–328. doi: 10.15288/jsad.2013.74.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasin DS, Keyes KM, Alderson D, Wang S, Aharonovich E, Grant BF. Cannabis withdrawal in the United States: results from NESARC. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t] J Clin Psychiatry. 2008;69(9):1354–1363. doi: 10.4088/jcp.v69n0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haughey HM, Marshall E, Schacht JP, Louis A, Hutchison KE. Marijuana withdrawal and craving: influence of the cannabinoid receptor 1 (CNR1) and fatty acid amide hydrolase (FAAH) genes. [Research Support, N.I.H., Extramural] Addiction. 2008;103(10):1678–1686. doi: 10.1111/j.1360-0443.2008.02292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henly GA, Winters KC. Personal Experience Inventory. Los Angeles CA: Western Psychological Services; 1988. [Google Scholar]
- 22.Hurd YL, Michaelides M, Miller ML, Jutras-Aswad D. Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology. 2014;76(Pt B):416–424. doi: 10.1016/j.neuropharm.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Quintero C, Neumark Y. Effects of risk perception of marijuana use on marijuana use and intentions to use among adolescents in Bogota, Colombia. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Drug Alcohol Depend. 2010;109(1–3):65–72. doi: 10.1016/j.drugalcdep.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Proc Natl Acad Sci U S A. 2012;109(40):E2657–2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milin R, Manion I, Dare G, Walker S. Prospective assessment of cannabis withdrawal in adolescents with cannabis dependence: a pilot study. J Am Acad Child Adolesc Psychiatry. 2008;47(2):174–178. doi: 10.1097/chi.0b013e31815cdd73. [DOI] [PubMed] [Google Scholar]
- 26.Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol Suppl. 1994;12:112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- 27.NIDA. Marijuana Abuse. Bethesda, MD: National Institute on Drug Abuse, National Institutes of Health; 2012. [Google Scholar]
- 28.Nocon A, Wittchen HU, Pfister H, Zimmermann P, Lieb R. Dependence symptoms in young cannabis users? A prospective epidemiological study. [Research Support, Non-U.S. Gov’t] J Psychiatr Res. 2006;40(5):394–403. doi: 10.1016/j.jpsychires.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Pew Research Center. Majority now supports legalizing marijuana. 2013 from http://www.people-press.org/2013/04/04/majority-now-supports-legalizing-marijuana/
- 30.Piontek D, Kraus L, Legleye S, Buhringer G. The validity of DSM-IV cannabis abuse and dependence criteria in adolescents and the value of additional cannabis use indicators. [Research Support, Non-U.S. Gov’t Validation Studies] Addiction. 2011;106(6):1137–1145. doi: 10.1111/j.1360-0443.2010.03359.x. [DOI] [PubMed] [Google Scholar]
- 31.Preuss UW, Watzke AB, Zimmermann J, Wong JW, Schmidt CO. Cannabis withdrawal severity and short-term course among cannabis-dependent adolescent and young adult inpatients. [Research Support, Non-U.S. Gov’t] Drug Alcohol Depend. 2010;106(2–3):133–141. doi: 10.1016/j.drugalcdep.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Rey JM, Martin A, Krabman P. Is the party over? Cannabis and juvenile psychiatric disorder: the past 10 years. [Research Support, U.S. Gov’t, P.H.S.Review] J Am Acad Child Adolesc Psychiatry. 2004;43(10):1194–1205. doi: 10.1097/01.chi.0000135623.12843.60. [DOI] [PubMed] [Google Scholar]
- 33.Roffman RA. Legalization of marijuana: unraveling quandaries for the addiction professional. Front Psychiatry. 2013;4:50. doi: 10.3389/fpsyt.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahin NH, Durak Batigun A, Ugurtas S. The validity, reliability and factor structure of the Brief Symptom Inventory (BSI) Turk Psikiyatri Derg. 2002;13(2):125–135. 49 [pii] [PubMed] [Google Scholar]
- 35.Schwab-Stone ME, Shaffer D, Dulcan MK, Jensen PS, Fisher P, Bird HR, Rae DS. Criterion validity of the NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3) J Am Acad Child Adolesc Psychiatry. 1996;35(7):878–888. doi: 10.1097/00004583-199607000-00013. S0890-8567(09)62466-7 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. S0890-8567(09)66098-6 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 38.Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: SAMHSA; 2013. [Google Scholar]
- 39.Tonigan JS, Miller WR. The inventory of drug use consequences (InDUC): test-retest stability and sensitivity to detect change. Psychol Addict Behav. 2002;16(2):165–168. [PubMed] [Google Scholar]
- 40.Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. [Comparative Study Research Support, N.I.H., Extramural] Drug Alcohol Depend. 2008;92(1–3):48–54. doi: 10.1016/j.drugalcdep.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winters KC, Stinchfield RD, Henly GA. Further validation of new scales measuring adolescent alcohol and other drug abuse. Journal of Studies on Alcohol. 1993;54(5):534–541. doi: 10.15288/jsa.1993.54.534. [DOI] [PubMed] [Google Scholar]