Abstract
Purpose
To compare the potential application of 99mTc-3P-Arg-Gly-Asp (99mTc-3P4-RGD2) scintimammography (SMM) and 99mTc-methoxyisobutylisonitrile (99mTc-MIBI) SMM for the differentiation of malignant from benign breast lesions.
Method
Thirty-six patients with breast masses on physical examination and/or suspicious mammography results that required fine needle aspiration cytology biopsy (FNAB) were included in the study. 99mTc-3P4-RGD2 and 99mTc-MIBI SMM were performed with single photon emission computed tomography (SPECT) at 60 min and 20 min respectively after intravenous injection of 738±86 MBq radiotracers on a separate day. Images were evaluated by the tumor to non-tumor localization ratios (T/NT). Receiver operating characteristic (ROC) curve analysis was performed on each radiotracer to calculate the cut-off values of quantitative indices and to compare the diagnostic performance for the ability to differentiate malignant from benign diseases.
Results
The mean T/NT ratio of 99mTc-3P4-RGD2 in malignant lesions was significantly higher than that in benign lesions (3.54±1.51 vs. 1.83±0.98, p<0.001). The sensitivity, specificity, and accuracy of 99mTc-3P4-RGD2 SMM were 89.3%, 90.9% and 89.7%, respectively, with a T/NT cut-off value of 2.40. The mean T/NT ratio of 99mTc-MIBI in malignant lesions was also significantly higher than that in benign lesions (2.86±0.99 vs. 1.51±0.61, p<0.001). The sensitivity, specificity and accuracy of 99mTc-MIBI SMM were 87.5%, 72.7% and 82.1%, respectively, with a T/NT cut-off value of 1.45. According to the ROC analysis, the area under the curve for 99mTc-3P4-RGD2 SMM (area = 0.851) was higher than that for 99mTc-MIBI SMM (area = 0.781), but the statistical difference was not significant.
Conclusion
99mTc-3P4-RGD2 SMM does not provide any significant advantage over the established 99mTc-MIBI SMM for the detection of primary breast cancer. The T/NT ratio of 99mTc-3P4-RGD2 SMM was significantly higher than that of 99mTc-MIBI SMM. Both tracers could offer an alternative method for elucidating non-diagnostic mammograms.
Introduction
Breast cancer continues to be a major public health problem all over the world. The American Cancer Society estimates that there will be about 296,980 new cases of breast cancer in 2013, which is expected to account for 14% of female cancer deaths.
A realistic strategy for the reduction of breast cancer mortality rates and timely treatment is to detect the disease while it is still in an early stage.[1], [2]. The most common screening method for early breast cancer is mammography, which is very sensitive in the detection of malignant breast disease. However in several groups of breast cancer patients, including those with fibroadenoma breasts, post implants, mastectomy or severe dysplasia, mammography has a low predictive value (20%–30%) and is not accurate, requiring patients to undergo histopathological examinations for a definitive diagnosis [3], [4]. To improve diagnostic accuracy, new methods are being studied as alternatives to mammography. Over the last twenty years, Scintimammography (SMM) has been introduced as an adjunct modality to present imaging modalities for breast cancer imaging [5]. In addition to the imaging modality, several radiopharmaceuticals have also been investigated for diagnostic imaging procedures in patients with suspected breast cancer [6]. 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) [7] is proven to be the most effective in detection of breast cancer for diagnosis, staging and restaging, but its use is limited by the high cost of equipment and lack of general availability, especially in developing countries. Alternatively, single photon emission computed tomography (SPECT) is more widely used with a much lower cost worldwide.
99mTc-methoxyisobutylisonitrile (99mTc-MIBI) is an important tracer for oncological applications and has been widely used in breast tumor imaging. However, this tracer originated from nuclear medicine for cardiac imaging and was not specifically designed for tumor imaging. The exact mechanism of uptake in breast cancer cells is still not entirely clear. It is reported that 99mTc-MIBI is concentrated in cancer cells by an energy-requiring transport mechanism, specifically by transmembrane electrical potentials, as well as by non-specific mechanisms, and the tracer is stored within the mitochondria [8].
It is well documented that integrin αvβ3 plays a critical role in the regulation of tumor angiogenesis and metastasis [9], [10]. The integrin is upregulated on activated endothelial cells and is highly expressed in tumor cells of various tumor types, including breast cancer [11], [12]. Over the past decade, radiolabeled Arg-Gly-Asp (RGD) peptides and analogs that specifically target integrin αvβ3 have been intensively investigated for noninvasive imaging of tumors in pre-clinical and clinical studies [13]–[19]. We previously developed the αvβ3-specific tracer 99mTc-3P-Arg-Gly-Asp (99mTc-3P4-RGD2) for SPECT and already demonstrated that 99mTc-3P4-RGD2 SPECT allows specific imaging of αvβ3 expression with high accuracy in detecting malignant solitary pulmonary nodules (SPNs), esophageal cancer, and malignant gliomas [20]–[22].
In this study, we compare the diagnostic value of 99mTc-3P4-RGD2 SMM with 99mTc-MIBI SMM for the detection of breast cancer by receiver operating characteristic (ROC) curve analysis.
Materials and Methods
Patients
Thirty-six patients with breast masses on physical examination and/or suspicious mammographic findings that required fine needle aspiration cytology biopsy (FNAB) were included in this study. The patient mean age was 41.9±12.2 years (age range 22–65 years. All patients were referred for 99mTc-MIBI and 99mTc-3P4-RGD2 SMM on an individual basis. The time interval between the two imaging procedures was 3.2±1.4 days. Finally, 99mTc-3P4-RGD2 and 99mTc-MIBI SMM results were compared with each other and with the final histopathological diagnosis. Inclusion and exclusion criteria for entry into the study are summarized in Table 1. This study was approved by the Ethics Committee of China-Japan Union Hospital of Jilin University. Informed written consent to participate in the SMM studies was obtained from all patients.
Table 1. Inclusion and exclusion criteria of study.
| Inclusion Criteria | Exclusion Criteria |
| Female | Pregnancy |
| Not pregnant | Recurrent disease |
| Suspicious lesion of the breast | Pervious mastectomy |
| Recommendation for excision biopsy after mammography | Fine needle aspiration within 1 week prior to scintimammography |
| Informed consent from the patient | Previous chemotherapy |
| Medically unstable patient (severe arrhythmia, heart failure or recent surgery) |
Scintimammography protocol
Radiolabeling and quality control procedures for 3P4-RGD2 were performed as described previously [20]. Both 3P4-RGD2 and MIBI (ShiHong Drug Development Center, Beijing, China) were radiolabelled with 738±86 MBq 99mtechnetium and thereafter administered via a single intravenous bolus injection in the contralateral arm to the affected breast, followed by a 10 mL saline flush. The effective radiation dose to the body of 99mTc-3P4-RGD2 and 99mTc-MIBI were 2.89±0.34 mSv and 5.83±0.67 mSv, respectively [23], [24]. 99mTc-3P4-RGD2 and 99mTc-MIBI SMM were performed at 60 min and 20 min after intravenous injection, respectively. Patients were in supine position with raised arms during imaging.
SPECT was performed using a double-head γ camera (Precedence, Philips Healthcare), equipped with low-energy parallel hole collimators. The matrix was 128×128 pixels, and the photopeak was centered at 140 keV with a symmetrical 20% window. Imaging with both radiotracers was performed using 6° angular steps in a 20 s time frame. Distance between the breast and detector was minimized.
Data analysis
Both 99mTc-3P4-RGD2 and 99mTc-MIBI SMM uptake were evaluated by semiquantitative analysis. Regions of interest (ROIs) were drawn around the tumor and an area of normal breast tissue in the same breast on lateral images and used to determine the tumor to non-tumor ratios (T/NT) of 99mTc-3P4-RGD2 and 99mTc-MIBI.
All numerical results are reported as mean values with standard deviations (SDs). Student's t test was used for statistical comparison of quantitative indices between the malignant and benign breast disease groups. The IBM SPSS Statistics19 software was used to determine cut-off values of quantitative indices in the detection of primary breast cancer. The incremental diagnostic value of quantitative indices analysis was performed using calculated areas under the curve (AUCs) in ROC analysis. Statistical significance was defined as p<0.05.
Results
Samples for histological examination were obtained by surgery in 28 patients and by core needle biopsy in eight patients. Breast cancer was confirmed in 26 patients and resulted in a total of 28 cancer lesions with diameters ranging from 0.3 cm to 7.9 cm (mean ± SD: 2.86±1.73 cm). Benign breast disease was found in 10 patients with a total of 11 benign lesions ranging in diameter from 0.4 cm to 6.5 cm (mean ± SD: 2.83±1.91 cm). In this study, the yielding breast cancer prevalence was 71.8% (Table 2).
Table 2. Scintimammography results versus final histopathological diagnosis of 36 patients.
| Patient | Age (years) | Diameter (cm) | RGD (T/NT) | MIBI (T/NT) | Histopathological Diagnosis |
| 1 | 58 | 2.8 | 4.55 | 2.70 | Invasive ductal |
| 2 | 43 | 4.2 | 2.81 | 1.90 | Invasive lobular |
| 3 | 26 | 0.6 | 1.31 | 1.02 | Invasive ductal |
| 4 | 52 | 3.2 | 3.51 | 1.70 | DCIS |
| 5 | 53 | 2.5 | 5.70 | 3.10 | Invasive ductal |
| 6 | 65 | 1.8 | 3.33 | 1.90 | Invasive ductal |
| 7 | 45 | 0.9 | 2.71 | 1.54 | Invasive ductal |
| 8 | 59 | 7.9/3.0 | 4.24/3.32 | 3.51/1.85 | Invasive ductal/Invasive ductal |
| 9 | 49 | 0.3 | 1.29 | 1.14 | Invasive ductal |
| 10 | 49 | 1.8 | 2.91 | 1.68 | Invasive ductal |
| 11 | 33 | 3.7 | 2.48 | 2.33 | Invasive ductal |
| 12 | 23 | 2.5 | 5.04 | 3.65 | Invasive ductal |
| 13 | 36 | 0.4 | 1.02 | 1.23 | Invasive ductal |
| 14 | 29 | 6.0 | 8.27 | 4.87 | Invasive lobular |
| 15 | 31 | 2.2 | 2.96 | 1.31 | DCIS |
| 16 | 56 | 4.2 | 3.82 | 2.08 | Invasive ductal |
| 17 | 41 | 3.5 | 5.62 | 4.21 | Invasive mucinous |
| 18 | 37 | 3.8 | 4.20 | 2.10 | Invasive ductal |
| 19 | 22 | 4.5 | 2.52 | 1.62 | Invasive ductal |
| 20 | 31 | 1.7/0.8 | 4.12/2.49 | 1.85/1.91 | Invasive ductal/DCIS |
| 21 | 39 | 4.1 | 3.34 | 2.17 | Invasive ductal |
| 22 | 61 | 1.2 | 3.40 | 3.74 | Invasive ductal |
| 23 | 46 | 3.3 | 2.99 | 1.61 | Invasive mucinous |
| 24 | 58 | 2.9 | 5.23 | 3.21 | Invasive ductal |
| 25 | 27 | 2.0 | 3.01 | 1.60 | Invasive lobular |
| 26 | 44 | 4.2 | 2.79 | 1.82 | Invasive ductal |
| 27 | 41 | 4.3 | 1.11 | 1.34 | Fibroadenoma |
| 28 | 28 | 2.1 | 4.47 | 2.58 | Fibroadenoma with mastitis |
| 29 | 31 | 6.5/0.7 | 1.92/1.32 | 1.26/1.10 | Fibroadenoma/ductal ectasia |
| 30 | 47 | 3.2 | 1.34 | 2.81 | Fibroadenoma |
| 31 | 29 | 1.8 | 1.43 | 1.37 | Fibrocystic disease |
| 32 | 54 | 5.2 | 2.31 | 1.22 | Fibroadenoma |
| 33 | 49 | 3.3 | 1.1 | 1.09 | Fibroadenoma |
| 34 | 37 | 1.2 | 1.85 | 1.69 | Fibroadenoma |
| 35 | 25 | 2.4 | 2.17 | 1.02 | Fibrocystic disease |
| 36 | 55 | 0.4 | 1.08 | 1.17 | Ductal ectasia |
DCIS: ductal carcinoma in situ.
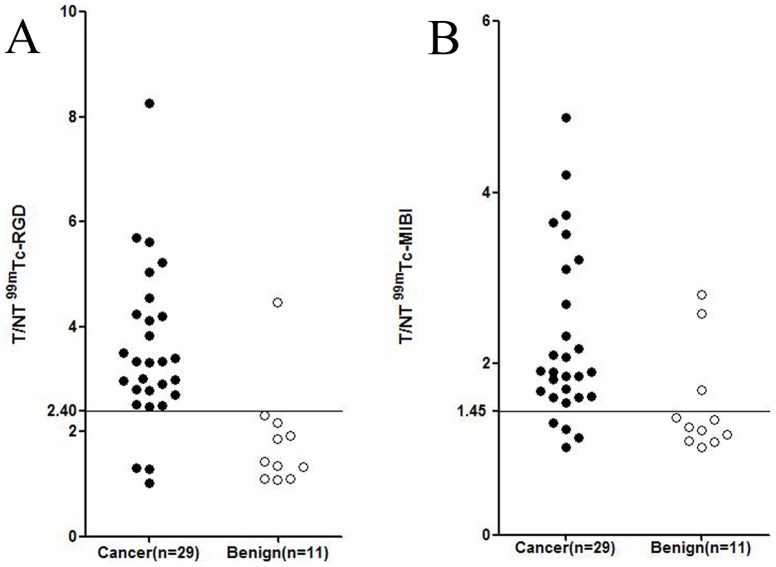
We observed high 99mTc-3P4-RGD2 uptake in breast cancer and low 99mTc-3P4-RGD2 uptake in benign lesions (Fig. 1A). In 99mTc-3P4-RGD2 SMM, the T/NT of breast cancer was 3.54±1.51 and that of benign lesions was 1.83±0.98. The difference was statistically significant (p<0.001). Similarly in 99mTc-MIBI SMM, high MIBI uptake was observed in breast cancer while low MIBI uptake was detected in benign lesions (Fig. 1B). The T/NT of breast cancer was 2.86±0.99 and that of benign lesions was 1.51±0.61. The difference was statistically significant (p<0.001).
Figure 1. T/NT for 99mTc-RGD and 99mTc-MIBI in malignant and benign tumors.
(A) The T/NT for 99mTc-RGD in breast cancer was significantly higher than that in benign lesions (p<0.001). (B) The T/NT for 99mTc-MIBI in breast cancer was significantly higher than that in benign lesions (p<0.001).
99mTc-3P4-RGD2 SMM was false negative in 3 breast cancer of invasive ductal which was the same as 99mTc-MIBI SMM. The tumor size was 0.6 cm or smaller in the long axis diameter. One patient with ductal carcinoma in situ (DCIS) in the long axis diameter of 2.2 cm was clear detected by 99mTc-3P4-RGD2 SMM, but not with 99mTc-MIBI SMM (Fig. 2). 99mTc-MIBI SMM was false positive in 3 benign lesions. Of the false positive cases, two were fibroadenoma and one was fibroadenoma with mastitis. The fibroadenoma with mastitis was also false positive in 99mTc-3P4-RGD2 SMM (Fig. 3).
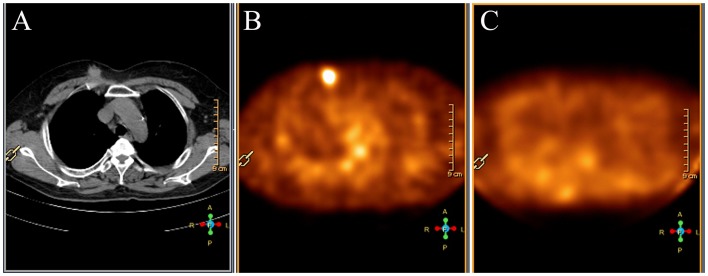
Figure 2. A 2.2 cm ductal carcinoma in situ of the right breast in a 31-year-old woman (Patient 15).
(A) CT scan demonstrates a mass in the right breast. (B) 99mTc-3P4-RGD2 SMM demonstrates focal uptake of 99mTc-3P4-RGD2 in the tumor (T/NT = 2.96). (C) 99mTc-MIBI SMM demonstrates low uptake of 99mTc-MIBI in the tumor (T/NT = 1.31).
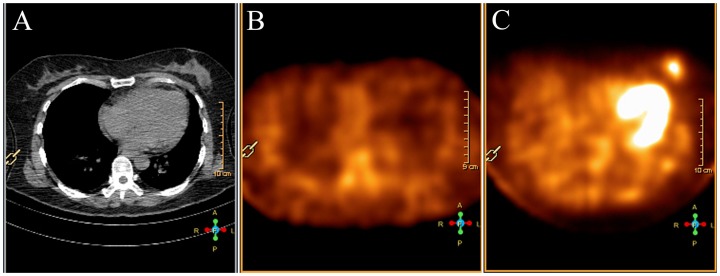
Figure 3. A 3.2 cm fibroadenoma of the left breast in a 47-year-old woman (Patient 30).
(A) CT scan demonstrates a mass in the left breast. (B) 99mTc-3P4-RGD2 SMM demonstrates low uptake of 99mTc-3P4-RGD2 in the tumor (T/NT = 1.34). (C) 99mTc-MIBI SMM demonstrates focal uptake of 99mTc-MIBI in the tumor (T/NT = 2.81).
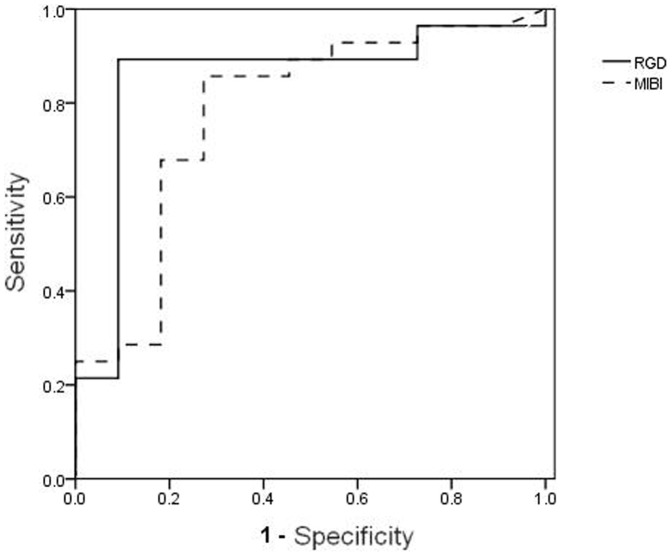
ROC analyses were performed to determine the optimal cut-off values of both 99mTc-3P4-RGD2 and 99mTc-MIBI SMM T/NT for the detection of malignant breast cancer. When a cut-off value was used based on the ROC analysis, the sensitivity, specificity and accuracy of 99mTc-3P4-RGD2 SMM were 89.3%, 90.9% and 89.7%, respectively (cutoff = 2.40 of T/NT), and those of 99mTc-MIBI SMM were 87.5%, 72.7% and 82.1%, respectively (cutoff = 1.46 of T/NT). The empirical ROC areas, which estimate the overall diagnostic performance, did not differ significantly among the two diagnostic analyses (Fig. 4). The value was 0.851 for 99mTc-3P4-RGD2 SMM and 0.781 for 99mTc-MIBI SMM.
Figure 4. Comparison the sensitivity and specificity of 99mTc-3P4-RGD2 SMM and 99mTc-MIBI SMM.
Comparison between 99mTc-3P4-RGD2 SMM and 99mTc-MIBI SMM in the differential diagnosis of breast cancer and benign lesions using ROC analysis (solid line: 99mTc-3P4-RGD2 SMM, dashed line: 99mTc-MIBI SMM). The area under the curve of both 99mTc-3P4-RGD2 SMM and 99mTc-MIBI SMM are 0.851 and 0.781, respectively. The difference was not significant.
Discussion
Over the last twenty years, SMM has been proposed to be a complementary tool to mammography in the diagnosis of primary breast cancer [5]. An already widely used radiopharmaceutical, 99mTc-MIBI appears to be a suitable SMM scanning agent. Many publications have reported favorable sensitivity and specificity results, 84%–96% and 72%–94%, respectively, for 99mTc-MIBI scintigraphy in the diagnosis of breast cancer [25]–[32]. 99mTc-3P4-RGD2 is a new agent with a high affinity for the αvβ3 integrin, a receptor associated with angiogenesis. In our previous study, we found that 99mTc-3P4-RGD2 could accumulate in a variety of malignant lesions [20]–[22]. However, a comparative study between 99mTc-3P4-RGD2 and 99mTc-MIBI SMM has not been previously reported.
In this present study, to differentiate benign from malignant lesions, ROC analyses were performed to determine the optimal cut-off values of T/NT of 99mTc-3P4-RGD2 and 99mTc-MIBI SMM. When T/NT of 2.40 was used as a cut-off point, the sensitivity, specificity and accuracy of 99mTc-3P4-RGD2 SMM were 89.3%, 90.9% and 89.7%, respectively. With a T/NT of 1.45 as a cut-off value, the same findings were 87.5%, 72.7% and 82.1% in 99mTc-MIBI SMM, respectively. The sensitivities reported in this study for 99mTc-3P4-RGD2 SMM are comparable with our previous reports; however the specificity is slightly higher than previous studies, which may be due to the low total number of benign breast lesions [20]–[22]. For 99mTc-MIBI SMM, the results reported here are comparable with those in previous studies [25]–[32]. Although the sensitivity, specificity and accuracy of 99mTc-3P4-RGD2 SMM was slightly superior to that of 99mTc-MIBI SMM in this study, the difference was not statistically significant. The area under the curve of 99mTc-3P4-RGD2 SMM was slightly larger than that of 99mTc-MIBI SMM, although this difference was also not significant.
It is generally accepted that the detection sensitivity of SMM is much lower for small breast cancer lesions with a diameter less than 1 cm over larger lesions [29]. Data from a multicentre European study showed a sensitivity of 26%–56% for lesions less than 1 cm [33]. Similarly in the present study, neither 99mTc-3P4-RGD2 nor 99mTc-MIBI was sufficient to visualize tumors in three patients having malignant lesions with diameters at 0.3 cm, 0.4 cm and 0.6 cm, respectively. The 99mTc-3P4-RGD2 and 99mTc-MIBI uptake in small lesions is considered to be underestimated due to partial volume effects from the relatively low spatial resolution of the SPECT device, though other factors such as the degree of radiopharmaceutical uptake by tumors and normal breast tissue may have contributed as well.
In one case of DCIS with a long axis diameter of 2.2 cm was false negative on 99mTc-MIBI SMM but not 99mTc-3P4-RGD2 SMM. Some reports state that 99mTc-MIBI SMM always showed a low sensitivity for detecting DCIS. Vassilios Papantoniou et al. [34] studied the diagnostic accuracy of 99mTc-MIBI SMM in 13 cases of DCIS and achieved a low sensitivity of 46%. Reinhard Obwegeser et al. [35] reported that 99mTc-MIBI SMM could not detect all four DCIS in their study. They conceived it may be due to the histological type of DCIS, which is known to show a lower density of tumor cells per square unit than invasive ductal carcinomas. Conversely, experimental studies using in vivo assays have shown that breast carcinoma in situ may be antigenic [36]. Sections stained for endothelial markers have shown increased vascularity around DCIS [37]–[39]. A more detailed study demonstrated two patterns of increased vascularity: cuffs of vessels close to the involved ducts and vessels diffusely arranged in the interductal stroma [40]. The true positive result with 99mTc-3P4-RGD2 SMM in this common malignant tumor may be an advantage of RGD targeting. Further studies with a larger patient population is needed to determine this issue.
Three of the 11 patients with fibroadenoma showed focal 99mTc-MIBI uptake, and one patient from this group who was diagnosed with fibroadenoma with severe mammitis on histopathological examination showed high focal tracer accumulation of 99mTc-3P4-RGD2. The false positive results obtained with 99mTc-MIBI in three fibroadenoma may be due to the high cellular activity associated in fibroadenoma. Previous studies have demonstrated that integrin αvβ3 is preferentially expressed on several types of cancer cells including melanoma, glioma, and ovarian and breast cancer. However, because expression is very low in existing blood vessels and absent in normal tissue, the accumulation of 99mTc-3P4-RGD2 may be mainly due to its higher specificity [41]–[44]. As is known to all, inflammation was different from other benign lesions, always showed high cell density and vascularity, likely responsible for the increased uptake. Previous studies have also shown that the integrin αvβ3 can exist on neutrophils, monocytes, and vascular smooth muscle cells [45], which can be the main reason for the false positive result using 99mTc-3P4-RGD2 SMM.
In conclusion, 99mTc-3P4-RGD2 SMM does not provide any significant advantage over the established 99mTc-MIBI SMM for differentiating breast lesions. The uptake of 99mTc-3P4-RGD2 in breast cancer was higher than that of 99mTc-MIBI. 99mTc-3P4-RGD2 seems to be more accurate than 99mTc-MIBI in the detection of DCIS and fibroadenoma. But with only a few patients, there was no statistically significant difference between 99mTc-3P4-RGD2 and 99mTc-MIBI SMM. Future studies will involve higher sample numbers.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This research was supported by the National Natural Science Foundation of China (NSFC) projects (51373144, 81271606 and 81201129), and Research Fund of Science and Technology Department of Jilin Province (201015185 and 201201041). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Panel NIoHCD (2001) National Institutes of Health Consensus Development Conference statement: adjuvant therapy for breast cancer, November 1–3, 2000. Journal of the National Cancer Institute 93: 979–989. [DOI] [PubMed] [Google Scholar]
- 2. Buscombe JR, Cwikla JB, Holloway B, Hilson AJ (2001) Prediction of the usefulness of combined mammography and scintimammography in suspected primary breast cancer using ROC curves. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 42: 3–8. [PubMed] [Google Scholar]
- 3. Kopans DB (1992) The positive predictive value of mammography. AJR. American journal of roentgenology 158: 521–526. [DOI] [PubMed] [Google Scholar]
- 4. Murphy IG, Dillon MF, Doherty AO, McDermott EW, Kelly G, et al. (2007) Analysis of patients with false negative mammography and symptomatic breast carcinoma. Journal of surgical oncology 96: 457–463. [DOI] [PubMed] [Google Scholar]
- 5. Schillaci O, Danieli R, Romano P, Santoni R, Simonetti G (2005) Scintimammography for the detection of breast cancer. [DOI] [PubMed] [Google Scholar]
- 6. Liberman M, Sampalis F, Mulder DS, Sampalis JS (2003) Breast cancer diagnosis by scintimammography: a meta-analysis and review of the literature. Breast cancer research and treatment 80: 115–126. [DOI] [PubMed] [Google Scholar]
- 7. Soussan M, Orlhac F, Boubaya M, Zelek L, Ziol M, et al. (2014) Relationship between Tumor Heterogeneity Measured on FDG-PET/CT and Pathological Prognostic Factors in Invasive Breast Cancer. PloS one 9: e94017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tiling R, Tatsch K, Sommer H, Meyer G, Pechmann M, et al. (1998) Technetium-99m-sestamibi scintimammography for the detection of breast carcinoma: comparison between planar and SPECT imaging. Journal of nuclear medicine: official publication, Society of Nuclear Medicine 39: 849–856. [PubMed] [Google Scholar]
- 9. Hood JD, Cheresh DA (2002) Role of integrins in cell invasion and migration. Nature Reviews Cancer 2: 91–100. [DOI] [PubMed] [Google Scholar]
- 10. Ruoslahti E (2002) Specialization of tumour vasculature. Nature Reviews Cancer 2: 83–90. [DOI] [PubMed] [Google Scholar]
- 11. Desgrosellier JS, Cheresh DA (2010) Integrins in cancer: biological implications and therapeutic opportunities. Nature Reviews Cancer 10: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niu G, Chen X (2011) Why integrin as a primary target for imaging and therapy. Theranostics 1: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jia B, Liu Z, Zhu Z, Shi J, Jin X, et al. (2011) Blood Clearance Kinetics, Biodistribution, and Radiation Dosimetry of a Kit-Formulated Integrin αvβ3-Selective Radiotracer 99mTc-3PRGD2 in Non-Human Primates. Molecular Imaging and Biology 13: 730–736. [DOI] [PubMed] [Google Scholar]
- 14. Wang L, Shi J, Kim Y-S, Zhai S, Jia B, et al. (2008) Improving tumor-targeting capability and pharmacokinetics of 99mTc-labeled cyclic RGD dimers with PEG4 linkers. Molecular pharmaceutics 6: 231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beer AJ, Haubner R, Sarbia M, Goebel M, Luderschmidt S, et al. (2006) Positron emission tomography using [18F] Galacto-RGD identifies the level of integrin αvβ3 expression in man. Clinical Cancer Research 12: 3942–3949. [DOI] [PubMed] [Google Scholar]
- 16. Bach-Gansmo T, Danielsson R, Saracco A, Wilczek B, Bogsrud TV, et al. (2006) Integrin receptor imaging of breast cancer: a proof-of-concept study to evaluate 99mTc-NC100692. Journal of Nuclear Medicine 47: 1434–1439. [PubMed] [Google Scholar]
- 17. Kenny LM, Coombes RC, Oulie I, Contractor KB, Miller M, et al. (2008) Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. Journal of nuclear medicine 49: 879–886. [DOI] [PubMed] [Google Scholar]
- 18. Liu Z, Jia B, Shi J, Jin X, Zhao H, et al. (2010) Tumor uptake of the RGD dimeric probe 99mTc-G3-2P4-RGD2 is correlated with integrin αvβ3 expressed on both tumor cells and neovasculature. Bioconjugate chemistry 21: 548–555. [DOI] [PubMed] [Google Scholar]
- 19. Bhojani MS, Ranga R, Luker GD, Rehemtulla A, Ross BD, et al. (2011) Synthesis and investigation of a radioiodinated F3 peptide analog as a SPECT tumor imaging radioligand. PloS one 6: e22418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qingjie M, Bin J, Bing J, Shi G, Tiefeng J, et al. (2011) Differential diagnosis of solitary pulmonary nodules using 99mTc-3P4-RGD2 scintigraphy. Eur J Nucl Med Mol Imaging 38: 2145–2152. [DOI] [PubMed] [Google Scholar]
- 21. Gao S, Ma Q, Cui Q, Liu L, Zhou X, et al. (2013) A pilot study on yymTc-3PRGD2 scintigraphy in diagnosis of brain glioma. Nuclear Science and Techniques 020301. [Google Scholar]
- 22. Gao S, Ma Q, Wen Q, Jia B, Liu Z, et al. (2013) 99mTc-3P4-RGD2 radiotracers for SPECT/CT of esophageal tumor. Nuclear Science and Techniques 24: 040302. [Google Scholar]
- 23. Guanghui C, Shi G, Tiefeng J, Qingjie M, Bing J, et al. (2012) Pharmacokinetics and radiation dosimetry of∼(99m) Tc-3PRGD_2 in healthy individuals: A pilot study. Nuclear Science and Techniques 23: 349-349–354. [Google Scholar]
- 24. Mitchell D, Hruska CB, Boughey JC, Wahner-Roedler DL, Jones KN, et al. (2013) 99mTc-Sestamibi Using a Direct Conversion Molecular Breast Imaging System to Assess Tumor Response to Neoadjuvant Chemotherapy in Women With Locally Advanced Breast Cancer. Clinical nuclear medicine 38: 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burak Z, Argon M, Memis A, Erdem S, Balkan Z, et al. (1994) Evaluation of palpable breast masses with 99Tcm-MIBI: a comparative study with mammography and ultrasonography. Nuclear medicine communications 15: 604–612. [DOI] [PubMed] [Google Scholar]
- 26. Khalkhali I, Mena I, Diggles L (1994) Review of imaging techniques for the diagnosis of breast cancer: a new role of prone scintimammography using technetium-99m sestamibi. European journal of nuclear medicine 21: 357–362. [DOI] [PubMed] [Google Scholar]
- 27. Khalkhali I, Cutrone JA, Mena IG, Diggles LE, Venegas RJ, et al. (1995) Scintimammography: the complementary role of Tc-99m sestamibi prone breast imaging for the diagnosis of breast carcinoma. Radiology 196: 421–426. [DOI] [PubMed] [Google Scholar]
- 28. Khalkhali I, Cutrone J, Mena I, Diggles L, Venegas R, et al. (1995) Technetium-99m-sestamibi scintimammography of breast lesions: clinical and pathological follow-up. Journal of nuclear medicine: official publication, Society of Nuclear Medicine 36: 1784–1789. [PubMed] [Google Scholar]
- 29. Palmedo H, Schomburg A, Grünwald F, Mallmann P, Krebs D, et al. (1996) Technetium-99m-MIBI scintimammography for suspicious breast lesions. Journal of nuclear medicine: official publication, Society of Nuclear Medicine 37: 626–630. [PubMed] [Google Scholar]
- 30. Taillefer R, Robidoux A, Lambert R, Turpin S, Laperrière J (1995) Technetium-99m-sestamibi prone scintimammography to detect primary breast cancer and axillary lymph node involvement. The Journal of nuclear medicine 36: 1758–1765. [PubMed] [Google Scholar]
- 31. Tiling R, Sommer H, Pechmann M, Moser R, Kress K, et al. (1997) Comparison of technetium-99m-sestamibi scintimammography with contrast-enhanced MRI for diagnosis of breast lesions. Journal of nuclear medicine: official publication, Society of Nuclear Medicine 38: 58–62. [PubMed] [Google Scholar]
- 32.Waxman A, Nagaraj N, Ashok G, Khan S, Yadegar J, et al. (1994) Sensitivity and specificity of TC-99M methoxy isonitrile (MIBI) in the evaluation of primary-carcinoma of the breast-comparison of palpable lesions with mammography. SOC NUCLEAR MEDICINE INC 1850 SAMUEL MORSE DR, RESTON, VA 20190-5316. pp. P22-22.
- 33. Scopinaro F, Schillaci O, Ussof W, Nordling K, Capoferro R, et al. (1996) A three center study on the diagnostic accuracy of 99mTc-MIBI scintimammography. Anticancer research 17: 1631–1634. [PubMed] [Google Scholar]
- 34. Papantoniou V, Tsiouris S, Mainta E, Valotassiou V, Souvatzoglou M, et al. (2004) Imaging in situ breast carcinoma (with or without an invasive component) with technetium-99m pentavalent dimercaptosuccinic acid and technetium-99m 2-methoxy isobutyl isonitrile scintimammography. Breast Cancer Research 7: R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brem SS, Jensen HM, Gullino PM (1978) Angiogenesis as a marker of preneoplastic lesions of the human breast. Cancer 41: 239–244. [DOI] [PubMed] [Google Scholar]
- 36. Obwegeser R, Berghammer P, Rodrigues M, Granegger S, Hohlagschwandtner M, et al. (1999) A head-to-head comparison between technetium-99m-tetrofosmin and technetium-99m-MIBI scintigraphy to evaluate suspicious breast lesions. European journal of nuclear medicine 26: 1553–1559. [DOI] [PubMed] [Google Scholar]
- 37. Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. New England Journal of Medicine 324: 1–8. [DOI] [PubMed] [Google Scholar]
- 38. Bosari S, Lee AK, DeLellis RA, Wiley BD, Heatley GJ, et al. (1992) Microvessel quantitation and prognosis in invasive breast carcinoma. Human pathology 23: 755–761. [DOI] [PubMed] [Google Scholar]
- 39. Schor A, Van Hoef M, Dhesi S, Howell A, Knox W (1993) Assessment of tumour vascularity as a prognostic factor in lymph node negative invasive breast cancer. European Journal of Cancer 29: 1141–1145. [DOI] [PubMed] [Google Scholar]
- 40. Guidi AJ, Fischer L, Harris JR, Schnitt SJ (1994) Microvessel density and distribution in ductal carcinoma in situ of the breast. Journal of the National Cancer Institute 86: 614–619. [DOI] [PubMed] [Google Scholar]
- 41. van der Flier A, Sonnenberg A (2001) Function and interactions of integrins. Cell and tissue research 305: 285–298. [DOI] [PubMed] [Google Scholar]
- 42. Eliceiri B, Cheresh D (2000) Role of alpha v integrins during angiogenesis. Cancer journal (Sudbury, Mass.) 6: S245–249. [PubMed] [Google Scholar]
- 43. Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nature medicine 6. [DOI] [PubMed] [Google Scholar]
- 44. Kuwano M, Fukushi J-i, Okamoto M, Nishie A, Goto H, et al. (2001) Angiogenesis factors. Internal medicine (Tokyo, Japan) 40: 565–572. [DOI] [PubMed] [Google Scholar]
- 45. Horton MA (1997) The αvβ3 integrin “vitronectin receptor”. The international journal of biochemistry & cell biology 29: 721–725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.