Abstract
Background
Cognitive inflexibility is a core symptom of several mental disorders including schizophrenia. Brain imaging studies in schizophrenia patients performing cognitive tasks have reported decreased activation of the mediodorsal thalamus (MD). Using a pharmacogenetic approach to model MD hypofunction we recently showed that decreasing MD activity impairs reversal learning in mice. While this demonstrates causality between MD hypofunction and cognitive inflexibility, questions remain about the elementary cognitive processes that account for the deficit.
Methods
Using the ‘Designer Receptors Exclusively Activated by Designer Drugs’ (DREADD) system we reversibly decreased MD activity during behavioral tasks assessing elementary cognitive processes inherent to flexible goal-directed behaviors including extinction, contingency degradation, outcome devaluation and Pavlovian-to-instrumental transfer (n=134 mice).
Results
While MD hypofunction impaired reversal learning, it did not affect the ability to learn about non-rewarded cues nor the ability to modulate action selection based on the outcome value. In contrast, decreasing MD activity delayed the ability to adapt to changes in the contingency between actions and their outcomes. In addition, while Pavlovian learning was not affected by MD hypofunction, decreasing MD activity during Pavlovian learning impaired the ability of conditioned stimuli to modulate instrumental behavior.
Conclusion
MD hypofunction causes cognitive inflexibility reflected by an inability to adapt actions when their consequences change. Furthermore, it alters the encoding of environmental stimuli so that they cannot be properly utilized to guide behavior. Modulating MD activity could be a potential therapeutic strategy for promoting adaptive behavior in human subjects with cognitive inflexibility.
Keywords: Mediodorsal thalamus, DREADD system, schizophrenia, behavioral flexibility, goal-directed behavior, Pavlovian-to-instrumental transfer
Introduction
Behavioral flexibility reflects the ability of an individual to respond and adjust to important changes in the environment. Deficits in behavioral flexibility are core cognitive deficits in several psychiatric disorders including schizophrenia, obsessive compulsive disorder (OCD) and drug addiction (1–3) and have been associated with to poor decision-making (4, 5). Studies in healthy subjects and in patients suffering psychiatric diseases or local brain lesions have repeatedly involved frontal lobe dysfunction as a potential neural substrate underlying deficits in flexible and goal-directed behaviors (6–10). However, recent findings from human and animal studies further indicate that the neuronal circuitry mediating flexible behavior also includes subcortical structures connected with the prefrontal cortex (PFC) such as the ventral and dorsomedial striatum and the midline thalamic nuclei (11).
The mediodorsal thalamus (MD) is a higher order thalamic nucleus that shares dense reciprocal connections with the PFC, including the orbitofrontal (OFC) and prelimbic (PrL) cortices. The MD also receives inputs from the basolateral amygdala (BLA) and the basal ganglia, thus placing it in a position to integrate and relay information from these structures to the PFC (12, 13). Functional imaging studies in schizophrenia patients have consistently found decreased activation in the MD during executive function tasks (14). These findings suggest that MD hypofunction may also participate in the behavioral flexibility deficits observed in patients. Indeed, lesion studies performed in rodents and monkeys have supported a role for the MD in a number of cognitive functions (15–17), including flexible and goal-directed behaviors (18–23). However, the main limitation of lesion studies is that they permanently ablate the whole structure whereas the imaging studies performed in humans rather suggest an impaired functional activation of the MD under pathological conditions.
To circumvent this limitation we recently used the “Designer Receptor Exclusively Activated by a Designer Drug” (DREADD) system to test for a potential causal relationship between decreased MD activity and cognitive deficits. We expressed the modified muscarinic receptor hM4D selectively in the MD of mice by viral-mediated gene transfer. The hM4D is activated by a pharmacologically inert compound, clozapine-N-oxide (CNO) but not by endogenous acetylcholine. Upon CNO activation, hM4D hyperpolarizes neurons through a G-protein mediated activation of inward-rectifying potassium channels (24). Administration of CNO in awake mice induces an average decrease of 40% of the firing rate in about a third of MD neurons (25). This mild MD hypofunction was sufficient to induce a deficit in reversal learning along with an increase in perseverative behavior in an operant reversal learning task (25). Though these findings provide evidence that the MD performs a crucial function in flexible behavior, our previous study was not designed to identify basic cognitive processes by which the MD regulates flexible behavior.
Goal directed flexible behavior requires the encoding of the relationship between actions and their outcomes (or A-O contingencies) as well as a representation of the outcome value. The sensitivity to changes in A-O contingencies is tested in a contingency degradation task; the sensitivity to a change in the outcome value in an outcome devaluation task (26–29). In addition to the A-O association, reversal learning tasks often include environmental stimuli that become associated with the outcome (S-O association). The ability of those Pavlovian cues to guide responding for a specific outcome is tested in a Pavlovian-to-instrumental transfer (PIT) paradigm (30).
In the present study, we used the DREADD system in the mouse to decrease neuronal activity in the MD and investigated the effect of this manipulation on flexible and goal-directed behavior. The DREADD approach offers the possibility to transiently decrease MD activity during distinct phases of behavioral testing. Using this approach, we identified two cognitive processes sensitive to MD hypofunction. Decrease in MD activity impaired the ability to adapt to changes in A-O contingencies. In addition, inhibiting MD function during Pavlovian conditioning prevented the conditioned stimuli to later modulate instrumental behavior in a PIT test. In contrast, MD hypofunction neither affected the ability to decrease responding to a cue which is no longer rewarded nor the ability to represent the outcome value.
Material and Methods
Subjects and drugs
All protocols used in this study were approved by the Institutional Animal Care and Use Committee (IACUC) at Columbia University and New York State Psychiatric Institute. C57/Bl6 male mice were purchased from Jackson Laboratory and housed under a 12-h, light-dark cycle in a temperature-controlled environment with food and water available ad libitum. For the behavioral experiments, mice were food restricted and maintained at 85% of their initial weight. CNO was dissolved in PBS to a final concentration of 0.2 mg/ml.
Experimental design
Mice were stereotactically injected with AAV2-hM4D virus (referred as MDhM4D mice) or AAV2-hrGFP control virus (referred as MDGFP mice) within the MD (see supplemental methods), and treated with either saline or CNO (2 mg/kg) 30 min before testing. A first cohort has been used for discrimination, reversal learning and contingency degradation tasks. A second cohort has been used for the outcome devaluation task. A third cohort has been used for the outcome specific Pavlovian-to-instrumental transfer.
Discrimination, reversal learning and contingency degradation
MDhM4D and MDGFP mice went through instrumental training and the discrimination task in CNO free conditions. Then the mice were subdivided into saline- and CNO-treated groups during extinction, reversal and degradation using a crossover design. Two-way ANOVA analyses were systematically performed to ensure that CNO treatment during previous task did not affect performance in the following task.
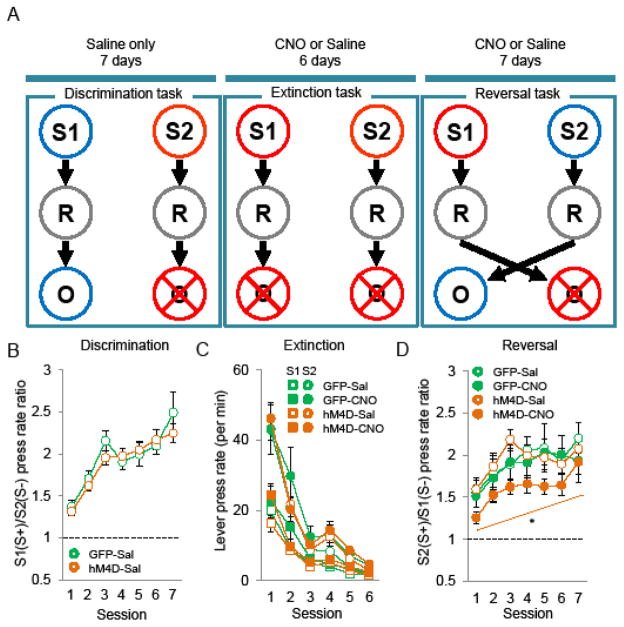
Discrimination, extinction and reversal task
Discrimination task has been previously described (25). Briefly, in this task, mice learn that a response (lever press) during the presentation of one visual cue (S+) is rewarded (condensed milk). The same response during another visual cue (S-) is not (see supplemental methods). The extinction task was identical to the discrimination task except that lever presses during both the prior S+ and S− were not rewarded. During the reversal phase the contingencies between the stimuli and the outcome were reversed.
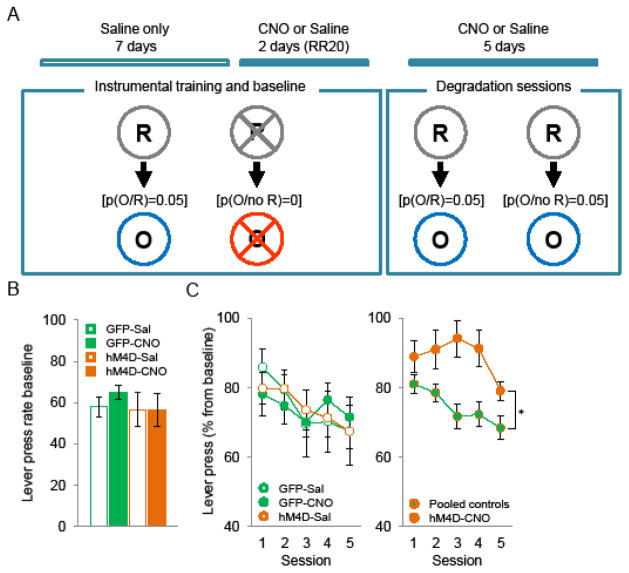
Instrumental contingency degradation
After the reversal task, mice were trained to press the lever on a random ratio (RR) schedule (see supplemental methods). Mice were then trained for two additional 20 min sessions of RR20 (5% chance to be rewarded for a lever press) under saline or CNO treatment for baseline level and then were exposed to 5 consecutive sessions during which the response-outcome contingency was degraded. In these sessions reward was still delivered for lever pressing on a RR20 schedule however the reward was also delivered non-contingently with the same probability in each second without a response.
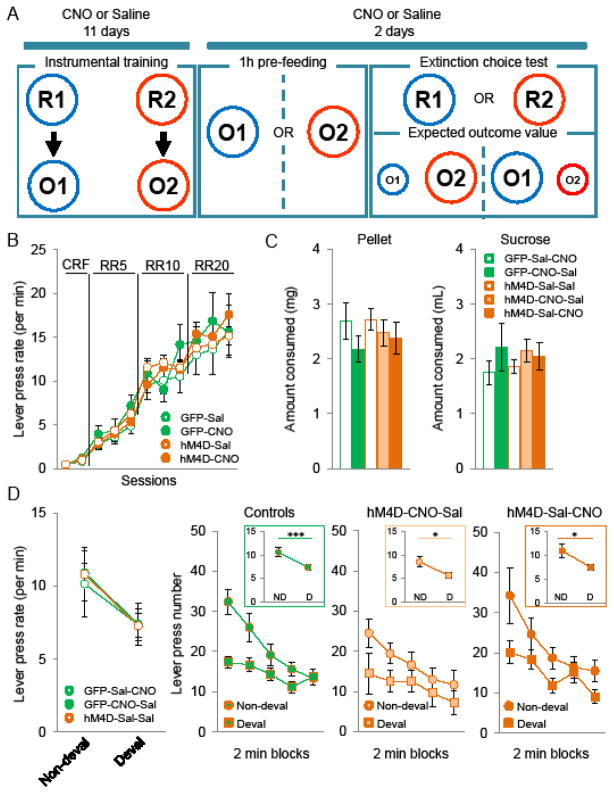
Instrumental outcome specific devaluation task
In this task, MDhM4D and MDGFP mice received saline or CNO either during instrumental training or during devaluation test, just before pre-feeding. Outcome specific devaluation task was adapted from Ostlund et al., (22) (see supplemental methods). Briefly, mice went through 11 days of instrumental training during which each lever (left and right) was associated with one outcome (pellet and 20% sucrose solution). Mice were then given two devaluation tests during which one of the two outcomes was devalued by pre-feeding the mice with it for 1h before a 10 min choice extinction test in which both levers were available but no outcomes were delivered. The number of lever presses for the devalued versus non-devalued outcome was recorded.
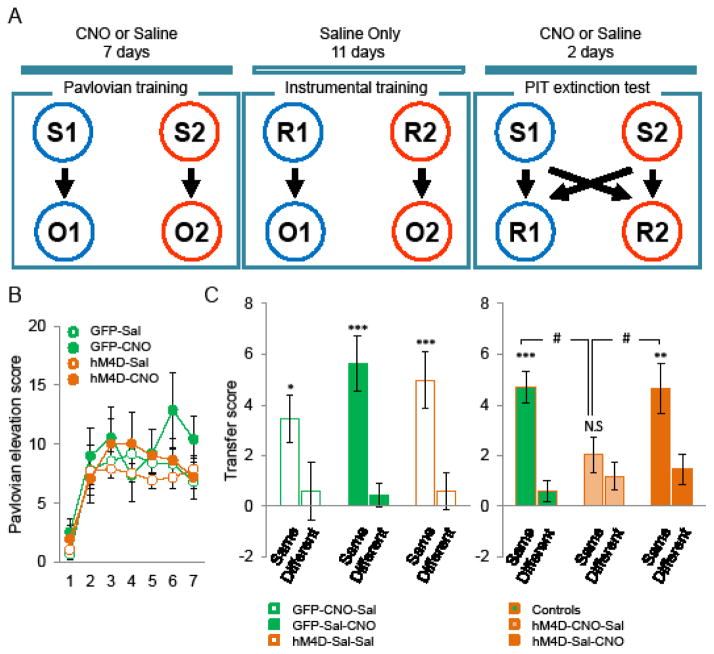
Outcome specific Pavlovian-to-instrumental transfer
In this task, MDhM4D and MDGFP mice received saline or CNO either during Pavlovian training or during PIT testing. Outcome specific PIT was adapted from Ostlund et al., (22) (see supplemental methods). Briefly, mice went through 7 days of Pavlovian training during which two conditioned stimuli (CSs) (tone or white noise) were paired with either sucrose or pellets. Performance in Pavlovian conditioning was assessed using a Pavlovian elevation score [(head entries rate during CS) – (head entries rate during the pre-CS period)]. After Pavlovian training, mice received 11 days of instrumental training as described for the outcome devaluation task. Mice went then through two PIT extinction test sessions during which both levers were inserted into the box, but no outcomes were delivered. After 8 min extinction, each CS was presented four times separated by 3 min fixed ITIs. A transfer score was calculated by measuring the difference in lever press responding in the presence versus absence of CS. The “Same” responses are lever presses on the lever that is paired with the same outcome as the CS. The “Different” responses are lever presses on the lever that is paired with the different outcome than the CS.
Results
Histology
We stereotactically injected into the MD an AAV enabling the co-expression of hM4D with GFP (AAV2-hM4D) (25). For all mice we analyzed the pattern of transgene expression after behavioral testing by visualizing GFP expression (Figure 1A and B). The virus spread almost entirely among the anterio-posterior axis of the MD whereas it stayed within the dorso-ventral and latero-medial axis of the MD (Figure 1B). Limited viral expression was present in the caudal portion of the thalamic paraventricular nucleus and a few infected cells were occasionally observed in centrolateral nucleus and lateral habenula.
Figure 1.
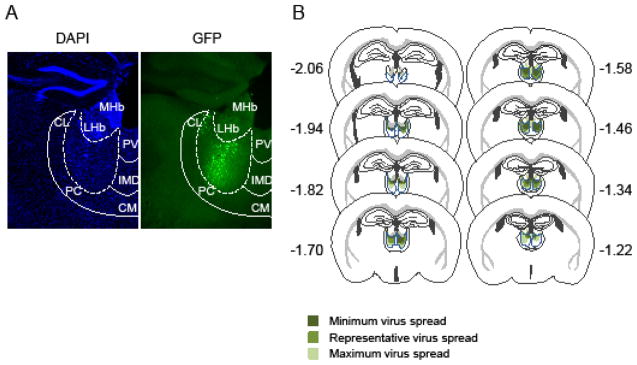
Viral approach and histological results. A, Representative example of viral infection as assayed by GFP autofluoresence. Coronal sections are co-labeled for nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI). Abbreviations: Centrolateral (CL), paracentral (PC), centromedial (CM), intermediodorsal (IMD) and paraventricular (PV) thalamic nuclei. Lateral (LHb) and medial habenula (MHb). B, Representation of the viral spread in the MD (outlined with dash blue lines) for all the mice used in this study. Coronal sections are drawn from Paxinos mouse brain atlas. Bregma −1.22 mm to −2.06 mm depicts anterior-posterior axis.
Extinction test
The deficit in reversal learning previously observed after MD hypofunction (25) may arise from an inability to decrease responding to a cue that is no longer rewarded. To test this, we performed an extinction task in mice that before had acquired an instrumental discrimination task (Figure 2A). Saline injected hM4D and GFP mice were first trained in a visual instrumental discrimination task for 7 days. Both groups learned the discrimination (two-way ANOVA, session F(6,252)=27.11; p<0.001, group F(1,42)=0.25; ns, interaction F(6,252)=1.03, ns) (Figure 2B). After acquisition, the mice were treated either with saline or CNO during the extinction task. A two-way mixed ANOVA resulted in a significant main effect of session (F(5,200)=142.84; p<0.001) but no effect of group (F(3,40)=0.61; ns) or interaction (F(15,200)=0.64; ns) indicating that both groups equally lowered responding to the former S+ (Figure 2C). Mice also decreased lever press rate during the S− presentations (repeated ANOVA, session F(5,200)=76.8; p<0.001, group F(3,40)=1.23, p>0.05; session x group F(15,200)=1.30, p>0.05), thereby keeping a constant ratio of responses between both S+ and S− trials.
Figure 2.
Effect of decreased MD activity on extinction and reversal learning task. A, Schematic drawing of the experiment design. Abbreviations: Visual stimulus 1 (S1), visual stimulus 2 (S2), lever-press response (R), outcome (milk) (O). B, Discrimination task, ratio between mean S+ and S− response rate (±SEM) (GFP-Sal n=15, hM4D-Sal n=29). C, Extinction task. mean lever press performed per minute (±SEM) during S+ and S− presentations (GFP-Sal n=8, GFP-CNO n=7, hM4D-Sal n=15, hM4D-CNO n=14). D, Reversal task, ratio between mean S+ and S− response rate (±SEM) (GFP-Sal n=8, GFP-CNO n=5, hM4D-Sal n=13, hM4D-CNO n=15).
Reversal learning test
We then tested whether a deficit in reversal learning would persist after an extinction phase. For this task, three mice (2 MDGFP and 1 MDhM4D) that did not reach learning criterion (S+/S− rate > 2 during all the sessions) during the acquisition task were excluded. We found that even after extinction, mice with decreased MD activity showed a deficit in reversal learning (Figure 2D). A two-way mixed ANOVA revealed a main effect of session (F(6,222)=8.71; p<0.001) and group (F(3,37)=3.97; p<0.05) but no interaction (F(18,222)=0.51; ns). Fischer’s post hoc analysis found significant differences between CNO-treated MDhM4D and each of the three control groups confirming that CNO-treated MDhM4D mice performance is impaired during reversal.
Contingency degradation test
We then tested the impact of MD inhibition on sensing or acting on changes in A-O contingencies in an instrumental contingency degradation task (Figure 3A). Mice were first trained on random ratio 20 (RR20) in which each lever press had a 0.05 probability of producing a rewarding outcome. Baseline lever press rate was not affected by decreasing MD activity (one-way ANOVA, group effect F(3,40)=0.9; ns) (Figure 3B). During contingency degradation, the reward is still delivered for lever pressing; however the reward is also delivered non-contingently for free with the same probability. The three control groups were equally sensitive to changes in contingency and decreased responding across sessions (two-way ANOVA, session F(4,108)=5.05; p<0.01, group F(2,27)=0.001; ns, interaction F(8,108)=0.67; ns) (Figure 3C left panel). We therefore pooled the control groups and compared them to CNO-treated MDhM4D mice. A two-way mixed ANOVA resulted in a significant main effect of session (F(4,168)=4.75; p<0.01) and group (F(1,42)=7.03; p<0.05) reflecting reduced sensitivity to contingency degradation when MD activity is decreased. We did not find a significant interaction between session and group (F(4,168)=1.76; p>0.05) meaning that decreased MD activity did not fully abolish sensitivity to contingency degradation (Figure 3C right panel). Finally, both controls and CNO-treated MDhM4D mice made a constant and similar number of head entries in the food magazine across the five degradation sessions ruling out any response competition between lever press and food magazine approach (Controls: 174.2 ±17.1 to 189.3 ±35.9; CNO-treated MDhM4D: 146.5 ±19.2 to 171.1 ±31.9).
Figure 3.
Effect of decreased MD activity on instrumental contingency degradation. A, Schematic drawing of the experiment design. Abbreviations: Lever-press response (R), outcome (milk) (O). [p(O/R)=0.05]: probability of obtaining outcome after lever press is 5%. [p(O/no R)=0]: probability of obtaining outcome meanwhile no pressing is 0% B, Mean lever press performed per minute (±SEM) during 20 minutes instrumental training session. C, Mean lever presses (in percentage from baseline) (±SEM) across the contingency degradation sessions. Left panel: control groups. Right panel: pooled controls vs CNO-treated MDhM4D mice (GFP-Sal n=8, GFP-CNO n=7, hM4D-Sal n=15, hM4D-CNO n=14).
Outcome specific devaluation test
We then assessed whether MD function is essential to maintain a flexible representation of the outcome value in an instrumental outcome devaluation procedure. To assess whether MD activity is needed for encoding A-O associations or required for the later modulation of action-selection by changes in outcome value, MDhM4D mice and controls received saline or CNO either during instrumental training or during the devaluation test (Figure 4A). During the instrumental learning, decreasing MD activity did not alter the rate at which mice were pressing the lever to obtain a reward. This is confirmed by a two-way mix ANOVA showing a significant main effect of instrumental session (F(10,420)=141.8; p<0.001) but no group effect (F(3,42)=0.26; ns) or interaction (F(30, 420)=1.02; ns) (Figure 4B). During the pre-feeding of test day, all groups consumed the same amount of food pellets (one-way ANOVA, group F(4,41)=0.51; ns) (Figure 4C left panel) and sucrose solution (one-way ANOVA, group F(4,41)=0.52; ns) (Figure 4C right panel). During the test, the three control groups showed comparable suppression of responding for the devalued outcome and were therefore pooled for further analysis (two-way ANOVA, value F(1,18)=17.06; p<0.001, groups(F(2,18)=0.02; ns, interaction F(2,18)=0.09; ns) (Figure 4D). Figure 4E represents the test results in successive 2 min bins for the action that had earned the devalued outcome and the action that had earned the non-devalued outcome. MDhM4D mice treated with CNO during instrumental training (hM4D-CNO-Sal) or during the devaluation test (hM4D-Sal-CNO) showed the same ability than the controls to decrease their responses to the lever associated with the devalued outcome. This is confirmed by a three-way mixed ANOVA showing a significant main effect of value (F(1,43)=25.80; p<0.001 ) but no effect of group (F(1,43)=1.30; ns) and no group x value interaction (F(1,43)=0.07; ns).
Figure 4.
Effect of decreased MD activity on instrumental outcome devaluation task. A, Schematic drawing of the experiment design. Abbreviations: Response 1 (left lever press) (R1), response 2 (right lever) (R2), outcome 1 (pellet) (O1), outcome 2 (sucrose) (O2). B, Mean lever press performed per minute (±SEM) during instrumental training (GFP-Sal n=6, GFP-CNO n=5, hM4D-Sal n=23, hM4D-CNO n=12). C, Mean of consumed food (±SEM) (pellet on left panel, sucrose on right panel) during the pre-feeding phase. D, Mean lever press performed per minute (±SEM) on the non-devalued and devalued lever in the three control groups. E, Mean number of lever press on the non-devalued and devalued lever (±SEM) across successive two-minutes blocks of extinction choice test in controls (left panel) and CNO-treated MDhM4D mice. Insets: mean lever press performed per minute (±SEM) on the non-devalued and devalued lever (GFP-Sal-CNO n=6, GFP-CNO-Sal n=5, hM4D-Sal-Sal n=10, hM4D-CNO-Sal n=12, hM4D-Sal-CNO n=13).
Outcome specific Pavlovian-to-instrumental transfer task
MD hypofunction may also alter the acquisition of conditioned stimuli or their ability to guide action-selection. To test this hypothesis, we performed an outcome specific PIT test in which we measured the modulation of instrumental responding during the presentation of conditioned stimuli (30). In this experiment MDhM4D mice and controls received saline or CNO either during Pavlovian training (hM4D CNO-Sal) or during PIT testing (hM4D Sal-CNO) (Figure 5A). Decreased MD activity did not alter Pavlovian learning as CNO-treated MDhM4D mice showed the same approach behavior as controls when CSs were presented (two-way ANOVA, session F(6,240)=15.64; p<0.001, group F(3,40)=0.93; ns, interaction F(18,240)=0.67; ns) (Figure 5B). After instrumental training, the PIT test was performed. All three control groups were statistically indistinguishable (two-way ANOVA, group F(2,22)=0.59; group x transfer: F(2,22)=0.56), displayed outcome-specific PIT (transfer F(1,22)=24.09; p<0.001) (Figure 5C left panel) and were pooled for further analysis. Surprisingly, while decreasing MD activity during the test did not alter PIT expression, mice with inhibited MD activity during Pavlovian training showed a decreased and non-specific PIT (Figure 5C right panel). A two-way ANOVA showed an effect of transfer (F(1,41)=22.41; p<0.001) and a significant group x transfer interaction (F(2,41)=18.8; p<0.05) but no main effect of group (F(2,41)=1.88; p=0.16). Simple effects analyses revealed significant specific transfer in both control (F(1,24)=27.48; p<0.001), and hM4D Sal-CNO (F(1,8)=11.98; p<0.01) groups, but not in hM4D CNO-Sal group (F(1,9)=0.92; ns). Although diminished compared to other groups, hM4D CNO-Sal mice exhibited a significant PIT as revealed by one-sample t-test analysis (transfer score ≠ 0; same: t(9)=2.83, p<0.05; different: t(9)=2.19, p=0.056). Importantly, these effects reflect a deficit in transfer rather than a global decrease in lever press during the test. At no point during baseline periods (absence of CS) did performance on the levers differ between groups (one-way ANOVA F(2,41)=1.78; p>0.05). Also, impaired PIT in hM4D CNO-Sal group cannot be attributed to response competition as they did a similar number of food magazine head entries than the other groups during testing (data not shown).
Figure 5.
Effect of decreased MD activity on outcome-specific pavlovian to instrumental transfer task. A, Schematic drawing of the experiment design. Abbreviations: stimulus 1 (S1), stimulus 2 (S2), outcome 1 (pellet) (O1), outcome 2 (sucrose) (O2), response 1 (left lever) (R1), response 2 (right lever) (R2), PIT: pavlovian instrumental transfer B, Mean number of conditioned magazine entries performed per minute (±SEM) during pavlovian training, plotted as the difference in responding during CS and pre-CS periods (GFP-Sal n=8, GFP-CNO n=6, hM4D-Sal n=20, hM4D-CNO n=10). C, Mean number of lever press performed per minute (±SEM) during PIT test in control groups (left panel), plotted as the difference in responding during each CS (same and different) and pre-CS periods (transfer score). Right panel, same as left panel with pooled control groups, CNO-Sal MDhM4D and Sal-CNO MDhM4D groups (GFP-CNO-Sal n=6, GFP-Sal-CNO n=8, hM4D-Sal-Sal n=11, hM4D-CNO-Sal n=10, hM4D-Sal-CNO n=9).
Discussion
We used a pharmacogenetic approach to reversibly decrease neuronal activity within the MD of mice to address the impact of MD hypofunction on goal-directed and flexible behavior. We found that MD hypofunction decreased the sensitivity to changes in the contingency between a response and its outcome. In addition, transient MD hypofunction during Pavlovian conditioning prevented the mice to later use the acquired conditioned stimuli to modulate instrumental behavior in a PIT task. In contrast, MD hypofunction neither alters the ability to decrease responding to a cue which is no longer rewarded nor the ability to represent the outcome value.
Previous studies in rats showed a deficit in outcome devaluation when the MD was lesioned before instrumental training but not when it was ablated just before the devaluation test itself (20, 22). This differs from, a recent study that found no effect of MD lesions on devaluation independent of the time of the lesion (31). Our findings are in agreement with this later study as we did not found any deficits in outcome devaluation, whether inhibiting the MD during testing or throughout instrumental training. In contrast, we found that the MD is important in the ability to adapt to changes in the contingency between actions and outcomes. Such a deficit could arise from a difficulty in detecting or in learning the new contingency. Independent of the origin of the deficit it could underlie the impairment in cognitive flexibility observed in the reversal learning task.
Problems in reversal learning could also arise from a difficulty in remapping the relationship between stimuli and their associated outcomes and/or retrieving and using the new knowledge to guide action selection. Therefore, we investigated the potential role of the MD in encoding S-O relationships, in the retrieval of these associations, and their ability to guide instrumental actions in an outcome specific PIT task. One prior study reported a disruption of outcome selective transfer in rats that received post-training lesions of the MD (22). In contrast, we did not find an effect of decreasing MD activity during PIT testing on the instrumental transfer as CNO treated MDhM4D mice increased lever pressing to the same degree as the three control groups. This result indicates that a mild MD hypofunction during PIT test might not be sufficient to impair retrieval of S-O and A-O associations. Surprisingly, although mice with MD hypofunction during Pavlovian training learned the association between the stimulus and the outcome as shown by the increased head entries in the food magazine, they were not able to use this information later to modulate instrumental behavior. One hypothesis would be that decreasing MD activity during Pavlovian training prevents the CS from acquiring incentive properties of the outcome thereby preventing the flexible use and the transfer of the previously acquired S-O association to a new context such as an instrumental task. There is evidence that the BLA, which sends projections to the MD (13), plays a similar role. Lesions of the BLA do not affect the acquisition of simple Pavlovian training, but pre-training lesions of the BLA have been shown to impair PIT in a similar way to what we observed after MD inhibition during Pavlovian training (32). Thus, the BLA-MD circuit seems to be involved in encoding S-O associations in a manner that leaves them accessible in novel situations such as a PIT test, an instrumental task in which an unexpected Pavlovian stimulus is presented.
Our results demonstrate a role for the MD in both instrumental and Pavlovian processes. Evidence from lesion studies revealed that these two processes involve partially distinct neural substrates. The PrL has been essentially involved in instrumental processes (33) while the OFC has been shown to play a role in Pavlovian outcome expectancies (34). Cell body lesion in the PrL prior to instrumental training impairs outcome devaluation and contingency degradation without altering PIT (33). In addition, a recent study showed that pre-training dopamine depletion or post-training D1/D2 receptor antagonist infusion within the PrL led to similar deficits in instrumental contingency degradation as those observed after decreasing MD activity (35). Taken together, these results suggest that signaling from the MD and dopamine midbrain input to the PrL may be necessary for encoding instrumental A-O contingencies. In contrast, OFC lesions have been shown to alter outcome selective PIT without altering instrumental action selection based on current outcome value (36). Interestingly, both PrL and OFC also share reciprocal projections with the BLA (37, 38). Similar to our findings with the MD, the BLA is involved in both instrumental and Pavlovian learning (39). Thus, BLA-MD circuitry might encode for the flexible use of both A-O and SO associations. Further studies aiming at dissecting these circuits will be needed to understand the respective contribution of BLA-PFC and MD-PFC networks in both goal-directed and stimulus guided action selection.
Impairment in behavioral flexibility has been observed in several psychiatric disorders including schizophrenia, OCD and drug addiction (1–3). Our study was designed in the context of schizophrenia where deficits in MD activation during executive function tasks have been reported (14). Impairment in different forms of behavioral flexibility, such as set-shifting and reversal learning are reliable cognitive deficits of schizophrenia and have been observed in chronic as well as in first-episode patients (2, 40, 41). Interestingly, set shifting deficits in schizophrenia have been found to relate to poor executive function (2). This is consistent with the well-established finding of dorsolateral PFC hypofunction in patients (42). In contrast, deficits in reversal learning have been observed regardless of the current intellectual function of patients (2) and may arise from OFC dysfunctions (43, 44). In a study by Heerey and Gold, patients with schizophrenia self-reported similar depths of emotion to positive, neutral and negative stimuli than control subjects but failed at coupling their behavior to the motivational properties of a stimulus (45). Thus, deficits in reversal learning in schizophrenia may arise from a disability to translate experience into goal-directed action. Although this finding seems to relate to our PIT findings, the stimuli used may be considered as secondary reinforcers rather than as conditioned stimuli such as the ones used in PIT. This distinction precludes any direct comparison between the two experiments. Interestingly, PIT has been recently studied in human subjects. Consistent with rodent literature, nucleus accumbens and amygdala have been found to be activated during PIT in healthy human subjects (46, 47). Whether PIT also involves thalamic activation in human and whether both PIT performance and potential thalamic activation are altered in patients with schizophrenia are still unanswered questions.
Finally, caution has to be used when extrapolating data from mouse models to human conditions. Indeed, while thalamo-frontal circuits share homologies across species, the parvocellular region of the MD is less prominent in rodents compared to humans or non-human primates (12). In addition, the main target of this parvocellular region in humans is the dorsolateral PFC for which the homologous region in rodents is still debated. Despite of this distinction, it is clear that similar to humans and primates, rodents do present a specific pattern of topographic and reciprocal innervation between the MD and the PFC. As such, the medial part of the MD shares connections with the medial-ventral PFC including the PrL, while the central part of the MD is interconnected with the lateral OFC (13). Because the MD in mice is relatively small, we cannot distinguish the specific role of each MD sub-region in our study. Independent of the thalamo-cortical sub-circuit involved, our findings along with the clinical observations point to modulation of MD activity as a potential strategy for improving flexible behaviors in the context of schizophrenia and other disorders with altered MD function.
Supplementary Material
Acknowledgments
We would like to thanks Mariya Shegda for excellent technical assistance and Matthew Bailey for help with data analysis. CNO was obtained from the NIH as part of the Rapid Access to Investigative Drug Program funded by the NINDS. This work was supported by NARSAD young investigator awards from the Brain and Behavior Research Foundation (to SP and CK), by US National of Health Grants MH068073 (to PDB) and by National Institute of Mental Health Grant F32MH090750-01 (to RDW). SP, PDB and CK designed research, SP, KT, SSB and RDW performed research, SP, KT, PDB and CK analyzed the data, and SP, PDB and CK wrote the paper. All the authors participated in interpreting the data and edited the manuscript.
Abbreviations
- AAV
adeno-associated virus
- A-O
action-outcome
- BLA
basolateral amygdala
- CNO
clozapine-N-oxide
- CS
conditioned stimulus
- DREADD
designer receptor exclusively activated by a designer drug
- MD
mediodorsal thalamus
- OCD
obsessive compulsive disorder
- OFC
orbitofrontal cortex
- PFC
prefrontal cortex
- PIT
Pavlovian-to-instrumental transfer
- PrL
prelimbic cortex
- S-O
stimulus-outcome
Footnotes
Financial disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cavedini P, Ferri S, Scarone S, Bellodi L. Frontal lobe dysfunction in obsessive-compulsive disorder and major depression: a clinical-neuropsychological study. Psychiatry research. 1998 Mar 20;78(1–2):21–28. doi: 10.1016/s0165-1781(97)00153-4. [DOI] [PubMed] [Google Scholar]
- 2.Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, et al. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biological psychiatry. 2009 Sep 15;66(6):586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-Serrano MJ, Perales JC, Moreno-Lopez L, Perez-Garcia M, Verdejo-Garcia A. Neuropsychological profiling of impulsivity and compulsivity in cocaine dependent individuals. Psychopharmacology. 2012 Jan;219(2):673–683. doi: 10.1007/s00213-011-2485-z. [DOI] [PubMed] [Google Scholar]
- 4.Crider A. Perseveration in schizophrenia. Schizophrenia bulletin. 1997;23(1):63–74. doi: 10.1093/schbul/23.1.63. [DOI] [PubMed] [Google Scholar]
- 5.Hutton SB, Murphy FC, Joyce EM, Rogers RD, Cuthbert I, Barnes TR, et al. Decision making deficits in patients with first-episode and chronic schizophrenia. Schizophrenia research. 2002 Jun 1;55(3):249–257. doi: 10.1016/s0920-9964(01)00216-x. [DOI] [PubMed] [Google Scholar]
- 6.Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. Journal of cognitive neuroscience. 2000 Jan;12(1):142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- 7.Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, et al. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of cognitive neuroscience. 2004 Apr;16(3):463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- 8.Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, et al. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Archives of general psychiatry. 2006 Nov;63(11):1225–1236. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- 9.O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003 Aug 27;23(21):7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, et al. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science (New York, NY. 2008 Jul 18;321(5887):421–422. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- 11.Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behavioural brain research. 2009 Dec 7;204(2):396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Jones EG. The thalamus. 2. Cambridge University Press; New York, N.Y: 2007. [Google Scholar]
- 13.Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988 Feb;24(2):379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- 14.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of general psychiatry. 2009 Aug;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baxter MG. Mediodorsal thalamus and cognition in non-human primates. Frontiers in systems neuroscience. 2013;7:38. doi: 10.3389/fnsys.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funahashi S. Thalamic mediodorsal nucleus and its participation in spatial working memory processes: comparison with the prefrontal cortex. Frontiers in systems neuroscience. 2013;7:36. doi: 10.3389/fnsys.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell AS, Chakraborty S. What does the mediodorsal thalamus do? Frontiers in systems neuroscience. 2013;7:37. doi: 10.3389/fnsys.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Floresco SB, Braaksma DN, Phillips AG. Thalamic-cortical-striatal circuitry subserves working memory during delayed responding on a radial arm maze. J Neurosci. 1999 Dec 15;19(24):11061–11071. doi: 10.1523/JNEUROSCI.19-24-11061.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chudasama Y, Bussey TJ, Muir JL. Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. The European journal of neuroscience. 2001 Sep;14(6):1009–1020. doi: 10.1046/j.0953-816x.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- 20.Corbit LH, Muir JL, Balleine BW. Lesions of mediodorsal thalamus and anterior thalamic nuclei produce dissociable effects on instrumental conditioning in rats. The European journal of neuroscience. 2003 Sep;18(5):1286–1294. doi: 10.1046/j.1460-9568.2003.02833.x. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell AS, Browning PG, Baxter MG. Neurotoxic lesions of the medial mediodorsal nucleus of the thalamus disrupt reinforcer devaluation effects in rhesus monkeys. J Neurosci. 2007 Oct 17;27(42):11289–11295. doi: 10.1523/JNEUROSCI.1914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostlund SB, Balleine BW. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J Neurosci. 2008 Apr 23;28(17):4398–4405. doi: 10.1523/JNEUROSCI.5472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradfield LA, Hart G, Balleine BW. The role of the anterior, mediodorsal, and parafascicular thalamus in instrumental conditioning. Frontiers in systems neuroscience. 2013;7:51. doi: 10.3389/fnsys.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proceedings of the National Academy of Sciences of the United States of America. 2007 Mar 20;104(12):5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parnaudeau S, O’Neill PK, Bolkan SS, Ward RD, Abbas AI, Roth BL, et al. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. 2013 Mar 20;77(6):1151–1162. doi: 10.1016/j.neuron.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams CD, Disckinson A. Instrumental responding following reinforcer devaluation. Q J Exp Psychol. 1981;33B:109–121. [Google Scholar]
- 27.Colwill RM, Rescorla RA. Postconditioning devaluation of a reinforcer affects instrumental responding. J Exp Psychol: Anim Behav Process. 1985;(11):120–132. [Google Scholar]
- 28.Colwill RM, Rescorla RA. Associative structures in instrumental learning. Psychol Learn Motiv. 1986;(20):55–104. [Google Scholar]
- 29.Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998 Apr-May;37(4–5):407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 30.Rescorla RA, Solomon RL. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychological review. 1967 May;74(3):151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- 31.Pickens CL. A limited role for mediodorsal thalamus in devaluation tasks. Behav Neurosci. 2008;122(3):659–676. doi: 10.1037/0735-7044.122.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci. 2005 Jan 26;25(4):962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behavioural brain research. 2003 Nov 30;146(1–2):145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature reviews. 2009 Dec;10(12):885–892. doi: 10.1038/nrn2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naneix F, Marchand AR, Di Scala G, Pape JR, Coutureau E. A role for medial prefrontal dopaminergic innervation in instrumental conditioning. J Neurosci. 2009 May 20;29(20):6599–6606. doi: 10.1523/JNEUROSCI.1234-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci. 2007 May 2;27(18):4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44(1):1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- 38.McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996 Mar;71(1):55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 39.Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends in neurosciences. 2006 May;29(5):272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophrenia research. 2007 Jul;93(1–3):296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray GK, Cheng F, Clark L, Barnett JH, Blackwell AD, Fletcher PC, et al. Reinforcement and reversal learning in first-episode psychosis. Schizophrenia bulletin. 2008 Sep;34(5):848–855. doi: 10.1093/schbul/sbn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weinberger DR, Berman KF. Prefrontal function in schizophrenia: confounds and controversies. Philosophical transactions of the Royal Society of London. 1996 Oct 29;351(1346):1495–1503. doi: 10.1098/rstb.1996.0135. [DOI] [PubMed] [Google Scholar]
- 43.Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Gaser C, et al. Reduced cortical thickness in first episode schizophrenia. Schizophrenia research. 2010 Feb;116(2–3):204–209. doi: 10.1016/j.schres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Takayanagi Y, Takahashi T, Orikabe L, Masuda N, Mozue Y, Nakamura K, et al. Volume reduction and altered sulco-gyral pattern of the orbitofrontal cortex in first-episode schizophrenia. Schizophrenia research. 2010 Aug;121(1–3):55–65. doi: 10.1016/j.schres.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. Journal of abnormal psychology. 2007 May;116(2):268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- 46.Talmi D, Seymour B, Dayan P, Dolan RJ. Human pavlovian-instrumental transfer. J Neurosci. 2008 Jan 9;28(2):360–368. doi: 10.1523/JNEUROSCI.4028-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prevost C, Liljeholm M, Tyszka JM, O’Doherty JP. Neural correlates of specific and general Pavlovian-to-Instrumental Transfer within human amygdalar subregions: a high-resolution fMRI study. J Neurosci. 2012 Jun 13;32(24):8383–8390. doi: 10.1523/JNEUROSCI.6237-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.