Abstract
Chronic intermittent ethanol (CIE) alters neural functions and behaviors mediated by the dorsolateral striatum (DLS) and prefrontal cortex. Here, we examined the effects of prolonged (16-bout) CIE on DLS plasticity and DLS-mediated behaviors. Ex vivo electrophysiological recordings revealed loss in efficacy of DLS synaptically induced activation and absent long-term depression after CIE. CIE increased two-bottle choice drinking and impaired Pavlovian-to-instrumental transfer but not discriminated approach. These data suggest prolonged CIE impaired DLS plasticity, to produce associated changes in drinking and cue-controlled reward-seeking. Given recent evidence that less-prolonged CIE can promote certain dorsal striatal-mediated behaviors, CIE may drive chronicity-dependent adaptations in corticostriatal systems regulating behavior.
Keywords: Alcohol, dorsolateral striatum, reward
There is mounting evidence that chronic alcohol causes profound alterations in neural mechanisms mediating plasticity, which may underlie associated changes in behavior (Everitt & Robbins 2005; McCool 2011; Holmes, Spanagel & Krystal 2013). Recent work has shown, e.g. that mice subjected to relatively limited chronic intermittent ethanol (CIE) exposure (eight daily bouts) is sufficient to disrupt synaptic plasticity in the dorsolateral striatum (DLS) and facilitate various forms of operant learning (DePoy et al. 2013). Interestingly, more prolonged CIE exposure (16 daily bouts or more) compromises plasticity-mediating mechanisms in the medial prefrontal cortex and impairs regulation of fear and ethanol-seeking (Holmes et al. 2012; Meinhardt et al. 2013). These previous findings suggest that the impact of CIE on corticostriatal functions may vary depending on the chronicity of exposure. However, the effects of prolonged (16-bout) CIE on DLS plasticity and DLS-mediated behaviors are currently unclear.
To address this gap in the literature, we began with ex vivo electrophysiological recordings made from cortical glutamatergic inputs to synapses in the DLS (Brigman et al. 2013) after mice were exposed to CIE (16 daily bouts) (Fig. 1a). Each daily bout of exposure comprised 16 hours of continuous exposure to vaporized ethanol (achieving 175 mg/dL blood ethanol concentrations) followed by 8 hours of withdrawal, and was designed to mimic drinking patterns characteristic of alcoholism (Koob 2003; Heilig & Egli 2006; Griffin, Lopez & Becker 2009) (for methods in full, see Supporting Information). All recordings (as well as the behavioral tests described below)were performed 3 days after the final bout—a time point at which acute withdrawal has abated and mice do not exhibit signs of heightened anxiety-like behavior, motor impairment or ethanol tolerance (Holmes et al. 2012).
Figure 1.
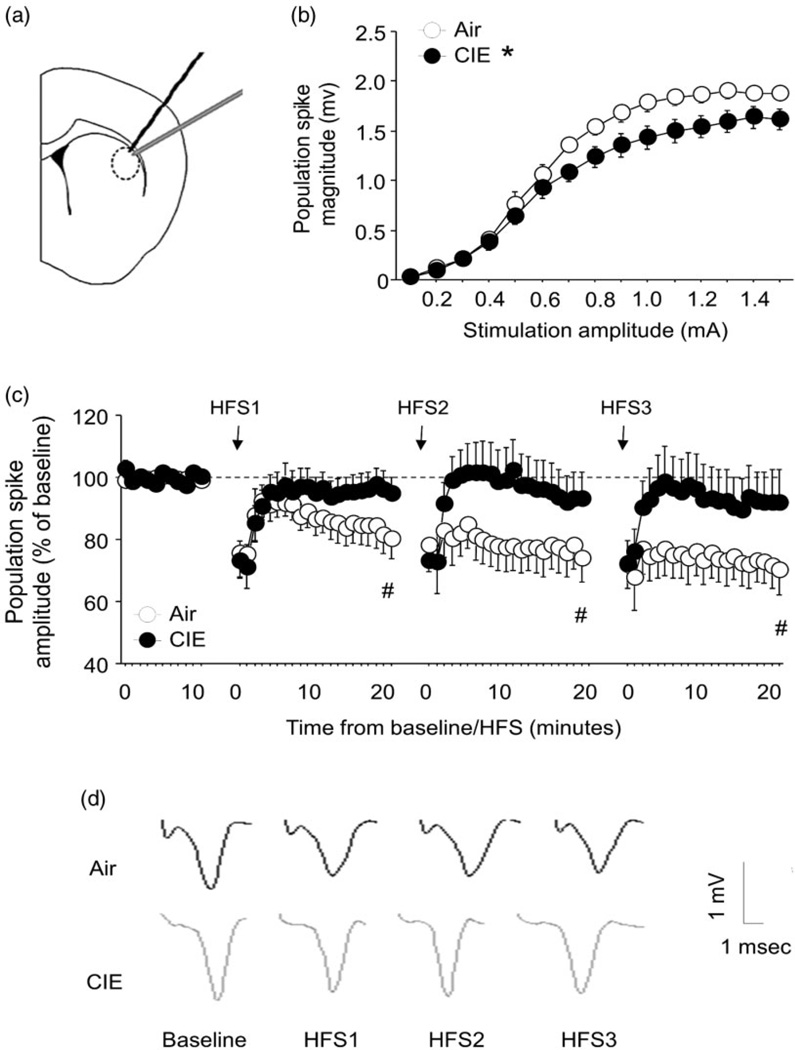
Prolonged CIE impairs dorsal striatal synaptic plasticity. (a) Location of recordings made from synapses in DLS. (b) Population spike magnitude increased with stimulation amplitude, but CIE mice showed significantly lower values than air mice. (c) High-frequency stimulation (HFS) induced LTD in air, but not CIE, mice after each of three sets of trains compared with baseline. (d) Example traces. n = 6–8/group. Data are means ± SEM. *P < 0.05 versus air, #P < 0.05 versus baseline
As expected, population spike magnitude increased in response to the application of higher stimulation amplitudes (F14,182 = 205.30, P < 0.01). However, CIE mice showed significantly lower values than controls exposed to air vapors (F1,13 = 5.44, P < 0.05) (Fig. 1b), consistent with a CIE-induced loss in efficacy of synaptically induced activation in the DLS. Next, we examined long-term depression (LTD) induced by local afferent high-frequency stimulation (HFS) of glutamatergic inputs to DLS. Indicating LTD in air controls, population spike amplitude was significantly decreased after the first [t(13) = 2.49, P < 0.05], second [t(13) = 2.34, P < 0.05] and third [t(13) = 2.29 P < 0.05] set of three HFS trains, as compared with pre-HFS baseline. By contrast, HFS completely failed to induce LTD in CIE mice (Fig. 1c,d), similar to the effects of more limited (eight-bout) CIE found previously (DePoy et al. 2013). Loss of LTD could reflect constrained rescaling of DLS synapses in response to cortical input and, together with adaptations in other regions, notably enhanced N-methyl-D-aspartic acid receptor-dependent long-term potentiation in dorsal medial striatum (DMS; Wang et al. 2010), may aggregate to cause changes in dorsal striatum (DS)-mediated behaviors.
To test whether changes in DS plasticity were associated with alterations in behaviors known to be mediated by the DS (Wang et al. 2010), we first assessed ethanol drinking using a 24-hour access two-bottle choice ethanol drinking procedure (Holmes et al. 2012). We found that CIE mice showed significantly higher ethanol consumption (CIE × time interaction: F6,78 = 3.21, P < 0.01) and preference (CIE × time interaction: F6,72 = 2.50, P < 0.05), as compared with air controls, specifically on the second, third and fourth 2-day blocks following vapor exposure (Fig. 2a,b). These data replicate prior studies showing that CIE, including more limited (eight-bout) exposure, increases ethanol drinking and seeking (Griffin et al. 2009; DePoy et al. 2013; Meinhardt et al. 2013) and establish a behavioral correlate of the altered striatal plasticity produced by the chronic ethanol exposure.
Figure 2.
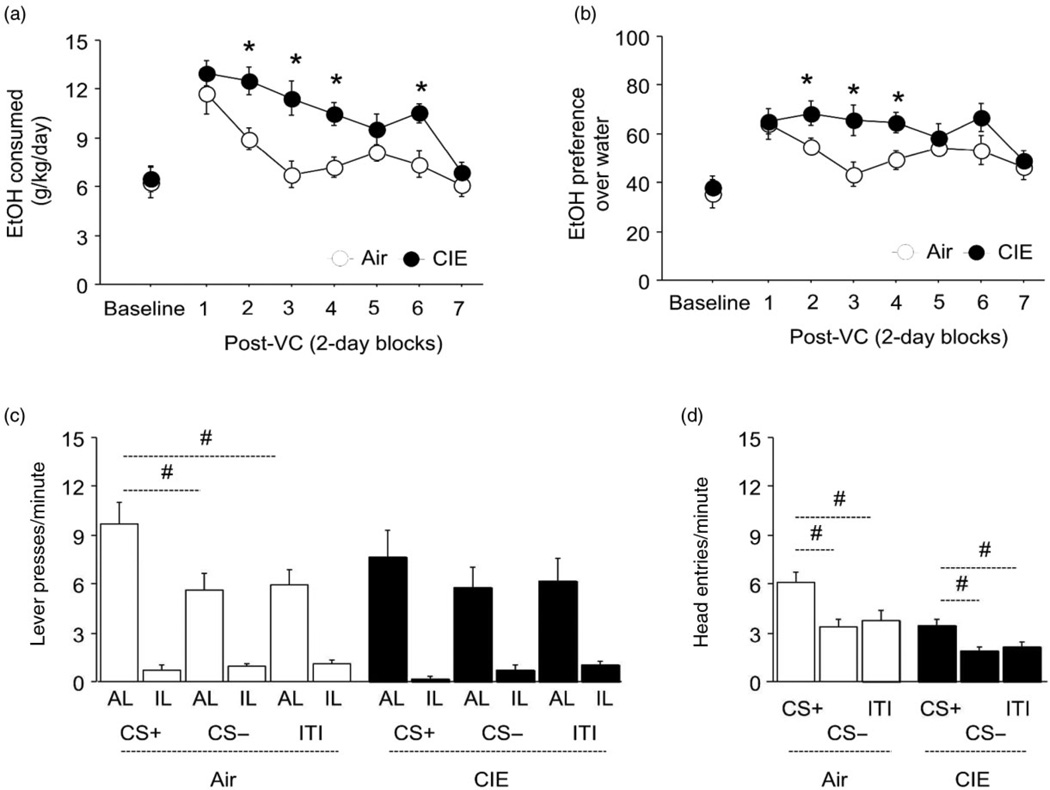
Prolonged CIE impairs PIT and increases ethanol drinking. (a,b) CIE mice had significantly higher ethanol consumption and preference than air mice after the vapor chambers. (c) Air, but not CIE, mice pressed the active lever more in the presence of the CS+ than the CS− or during the intertrial interval (ITI), and both groups pressed the active lever more than the inactive lever regardless of CS. (d) Air and CIE mice made more head entries into the magazine in the presence of the CS+ than the CS− or during the ITI, although air mice made more head entries than CIE mice. n = 6–12/group. Data are means ± SEM. *P < 0.05 versus air, #P < 0.05 versus CS+
Based on prior evidence that eight-bout CIE produces alterations in DLS-mediated operant learning paradigms (DePoy et al. 2013), we next examined the effects of the 16-bout CIE procedure on the DS-dependent, Pavlovian-to-instrumental transfer (PIT) task (Corbit & Janak 2007). The ‘general PIT’ paradigm we used assays the ability of an auditory stimulus (CS+) previously associated with availability of a food reward to increase instrumental responding for the reward (Lederle et al. 2011). We found that on a non-rewarded PIT probe test conducted 3 days after vapor exposure, air controls pressed the active lever significantly more in the presence of the CS+ than the CS− or the no-CS intertrial interval (ITI) (F2,22 = 9.60, P < 0.01) and generally favored the active lever over the inactive lever (Fig. 2c). This discriminated PIT effect was absent in the CIE group, which generally showed more active than inactive lever responding, but did not respond more vigorously during the CS+ than the CS− or ITI (Fig. 2c). However, analysis of the number of head entries into the food magazine, a measure related to discriminated Pavlovian approach behavior, revealed that both the air controls (F2,22 = 8.45, P < 0.01) and the CIE mice (F2,22 = 8.20, P < 0.01) made more head entries during the CS+ than the CS− or ITI (Fig. 2d).
The current PIT data indicate deficient PIT, but intact discriminated approach, following CIE, a finding which is in close agreement with that previously reported in rats repeatedly exposed and withdrawn from a liquid ethanol diet (Ripley et al. 2004). A similar loss of PIT is also produced by inactivation of the DLS (Corbit & Janak 2007), raising the possibility that CIE-induced abnormalities in DLS plasticity might contribute to the deficits in this behavior. It should be noted, however, that while drug addiction is typically associated with increased drug-seeking in the presence of reward-predictive cues (Everitt & Robbins 2005), the effects of exposure to drugs of abuse on PIT in rodents have been found to be complex. For example, PIT can be either enhanced or impaired by a psychostimulant such as amphetamine, depending on when the drug is given relative to conditioning and testing (e.g. Wyvell & Berridge 2001; Hall & Gulley 2011).
The chronicity of drug exposure appears to be another critical factor. We previously found that an eight-bout CIE regimen facilitated, rather than impaired, certain behaviors that are dependent upon the dorsal striatum, including a form of visual choice learning (DePoy et al. 2013). Taken together with the current findings, these data suggest that whereas relatively limited CIE may drive adaptive alterations in the dorsal striatum that favor certain behaviors, including choice learning, more prolonged CIE results in a degradation of some dorsal striatum functions and associated behaviors, such as PIT. This model is likely to be an oversimplification, however. First, both the eight- and 16-bout CIE regimens produced loss of DLS LTD, despite the differing behavioral outcomes. Second, the complex behavioral processes affected by CIE are known to recruit multi-region brain networks that encompass not just the dorsal striatum, but also the prefrontal cortex, nucleus accumbens and amygdala (Holmes, Marchand & Coutureau 2010; Graybeal et al. 2011). Indeed, preliminary work has already shown that 12- or 16-bout CIE regimens disrupt orbitofrontal and medial prefrontal cortex-mediated behaviors (Badanich, Becker & Woodward 2011; Holmes et al. 2012; Kroener et al. 2012). Thus, the behavioral effects of CIE probably reflect the emergent consequences of functional alterations across multiple brain regions. The challenge for future work will be to further parse these effects to determine how chronic ethanol dynamically alters how the brain regulates rewarded behavior.
Supplementary Material
Acknowledgements
We are very grateful to Miss B. Hurd for valuable assistance with the CIE procedure and Mr. R.E. Cruz for facilitating the writing of the manuscript. Research supported by the NIAAA Intramural Research Program.
Footnotes
Authors Contribution
LDP performed experiments, analyzed data and helped write the manuscript. RD performed experiments, analyzed data and helped write the manuscript. TW, NC and BN performed experiments and analyzed data. MC performed experiments, analyzed data and helped write the manuscript. DL helped write the manuscript. AH wrote the manuscript.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Supporting Information Experimental methods in full.
References
- Badanich KA, Becker HC, Woodward JJ. Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behav Neurosci. 2011;125:879–891. doi: 10.1037/a0025922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, Jiang Z, Saksida LM, Jinde S, Pease M, Bussey TJ, Lovinger DM, Nakazawa K, Holmes A. GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nat Neurosci. 2013;16:1101–1110. doi: 10.1038/nn.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. Inactivation of the lateral but not medial dorsal striatum eliminates the excitatory impact of Pavlovian stimuli on instrumental responding. J Neurosci. 2007;27:13977–13981. doi: 10.1523/JNEUROSCI.4097-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL, Macpherson K, Crowley N, Gunduz-Cinar O, Pickens CL, Cinar R, Saksida LM, Kunos G, Lovinger DM, Bussey TJ, Camp MC, Holmes A. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci USA. 2013;110:14783–14788. doi: 10.1073/pnas.1308198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, Holmes A. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat Neurosci. 2011;14:1507–1509. doi: 10.1038/nn.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res. 2009;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA, Gulley JM. Disruptive effect of amphetamines on Pavlovian to instrumental transfer. Behav Brain Res. 2011;216:440–445. doi: 10.1016/j.bbr.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, Macpherson KP, Debrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Spanagel R, Krystal JH. Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl) 2013;229:539–554. doi: 10.1007/s00213-013-3226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes NM, Marchand AR, Coutureau E. Pavlovian to instrumental transfer: a neurobehavioural perspective. Neurosci Biobehav Rev. 2010;34:1277–1295. doi: 10.1016/j.neubiorev.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS ONE. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederle L, Weber S, Wright T, Feyder M, Brigman JL, Crombag HS, Saksida LM, Bussey TJ, Holmes A. Reward-related behavioral paradigms for addiction research in the mouse: performance of common inbred strains. PLoS ONE. 2011;6:e15536. doi: 10.1371/journal.pone.0015536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA. Ethanol modulation of synaptic plasticity. Neuropharmacology. 2011;61:1097–1108. doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M, Harper C, Drescher KU, Spanagel R, Sommer WH. Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci. 2013;33:2794–2806. doi: 10.1523/JNEUROSCI.4062-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley TL, Borlikova G, Lyons S, Stephens DN. Selective deficits in appetitive conditioning as a consequence of ethanol withdrawal. Eur J Neurosci. 2004;19:415–425. doi: 10.1111/j.0953-816x.2003.03114.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010;30:10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered ‘wanting’ for sucrose reward. J Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.