Abstract
Using the theory of gender and power (TGP) and data from the Tsogolo la Thanzi (TLT) study, we examined how relationship power shapes young people’s decisions to test for HIV in rural Malawi (N=932), a high-HIV prevalence setting undergoing rapid expansions in testing services. We used generalized estimating equations (GEE) to examine associations among five constructs of relationship power (socioeconomic inequalities, relationship dominance, relationship violence, relationship unity, and mistrust), perceived risk, and receiving an HIV test over a 16-month period. The results indicate that young Malawians are testing for HIV at relatively high rates, repeatedly, and not just during pregnancy. Over the study period, 47.3% of respondents received at least one HIV test outside of TLT (range: 0–4). The GEE analysis revealed that men and women with higher levels of relationship unity were less likely to test for HIV. For men, being a victim of sexual coercion was an additional barrier to testing. Women’s testing decisions were more strongly influenced by perceptions of a partner’s risk for HIV than their own, whereas men relied more on self-assessments. The results highlight that testing decisions are deeply embedded within the relationship context, which should be considered in future HIV testing interventions.
Given the challenges of using condoms (Chimbiri, 2007; Tavory & Swidler, 2009) and the risks of extramarital partnerships (Carpenter, Kamali, Ruberantwari, Malamba, & Whitworth, 1999), it is not surprising that the majority of new HIV infections in sub-Saharan Africa occur within marriage or cohabitation (Dunkle et al., 2008). In response to these challenges, HIV testing and counseling has been promoted as an alternative strategy to reduce HIV transmission through prevention services and timely access to care and treatment (Painter, 2001; VCT Efficacy Group, 2000). Although the availability of HIV testing and counseling has improved throughout the region, many people still do not know their serostatus and may inadvertently transmit the virus to their sexual partners (World Health Organization [WHO], 2007). To date, the majority of research on HIV testing barriers focused on psychosocial factors that occur at the individual level (Obermeyer & Osborn, 2007), even though HIV testing decisions are often made within the broader relationship context.
Failure to consider the interpersonal dimension of HIV testing limits new opportunities to intervene at the couple level and improve uptake of HIV testing services. In this study, we considered whether one relationship dynamic in particular, power, shapes young people’s decisions to test in a high-HIV prevalence community in southern Malawi.
Background
HIV Testing Behavior in Sub-Saharan Africa
In sub-Saharan Africa, the majority of studies on HIV testing have examined whether people have ever tested or recently tested, as if it is a one-time event (deGraft-Johnson, Paz-Soldan, Kasote, & Tsui, 2005; Irungu, Varkey, Cha, & Patterson, 2008; Jean, Anglaret, Moh, Lert, & Dray-Spira, 2012; MacPhail, Pettifor, Coates, & Rees, 2007). These studies limit our understanding of HIV testing behavior and the ability to improve HIV testing programs and policy. As testing shifts to a more normative practice, it is likely that a substantial proportion of testing clients will have tested repeatedly (Bradley, Tsui, Kidanu, & Gillespie, 2011). From a public health perspective, a single HIV test result is not enough information for serodiscordant couples to be able to successfully prevent HIV transmission, especially in a setting where extramarital relationships are common and may increase the risk for HIV infection (Carpenter et al., 1999). From a methodological perspective, cross-sectional studies that simultaneously collect predictor and outcome variables cannot inform conclusions about causation or temporality. Global assessments of testing behavior also make it challenging to determine whether the test occurred before, during, or after the relationship. In the current study, we took advantage of a unique couples data set where HIV testing histories were routinely collected over a 16-month period to prospectively examine uptake of HIV testing in Malawi.
At the time of this study’s conception in 2009, aggregate estimates from 12 high-burden countries in sub-Saharan Africa indicated that only 12% of women and 10% of men in the general population had ever tested for HIV and received their results (WHO, 2007). Frequently cited barriers to testing include fears of stigma and discrimination, confidentiality concerns, and low HIV-related knowledge (Gage & Ali, 2005; Jean et al., 2012; Kalichman & Simbayi, 2003; Weiser et al., 2006). One particularly salient psychosocial factor for HIV testing behavior is perceived risk. Individuals who perceive themselves to be at high risk for HIV may be more likely to test (Creel & Rimal, 2012; deGraft-Johnson et al., 2005; Weiser et al., 2006). However, for women, those who suspect they are HIV infected may refuse or forgo testing out of fear of the consequences (Pool, Nyanzi, & Whitworth, 2001). Perceptions of risk are shaped not only by personal risk behavior but also by what is known or suspected about a partner’s sexual history. In Malawi, Smith, and Watkins (2005) found that women were more worried about getting HIV from unfaithful husbands, while men were more worried about getting HIV from their extramarital partners. Other studies from the region suggest that women who suspect that their main partner has other sexual partners may be more inclined to test for HIV (Luseno & Wechsberg, 2009; Morin et al., 2006). Few studies, however, have obtained data from couples, which could provide clarifying information regarding the effect of perceived risk on both partners’ testing behaviors.
It is conceivable that relationship dynamics such as power play an important role in partners’ decisions to test for HIV. One of the challenges of studying power is the variety of definitions used to measure power and its effects on health (Blanc, 2001). More than a decade ago, Pulerwitz, Gortmaker, and DeJong (2000) developed the first theoretically based measure of power called the Sexual Relationship Power Scale (SRPS). The authors defined relationship power as the ability to control a partner’s actions, act independently, dominate decision making, or engage in behavior against the other partner’s wishes. Other researchers have adapted the SRPS to African samples, such as Pettifor, Measham, Rees, and Padiant (2004), who measured relationship power as relationship control and recent experience of forced sex. Among women, studies demonstrate that relationship power imbalances are associated with key risk factors for HIV, including less condom use, increased number of sexual partners, and physical violence (Dunkle et al., 2007; Dunkle et al., 2004; Harrison, O’Sullivan, Hoffman, Dolezal, & Morrell, 2006; Pettifor et al., 2004). In several studies, a direct association has been established between power and HIV infection (Dunkle et al., 2004; Pettifor et al., 2004). Although it has been noted that relationship power plays a role in the use of sexual health services (Blanc, 2001), little research has explicitly studied the intersection of power and HIV testing. In one exception conducted in the United States, Longmore, Johnson, Manning, and Giordano (2013) found that power does play a role in young women’s ability to test for HIV such that women with higher levels of power were more likely to have tested during their relationship. In sub-Saharan Africa, the ongoing shift toward biomedical approaches to HIV and AIDS underscores the need to understand how relationship power affects use of HIV services.
The Theory of Gender and Power
The theory of gender and power (TGP) (Connell, 1987; Wingood & DiClemente, 2002) provides a useful theoretical lens to understand how relationship power may relate to HIV testing. This theory proposes power inequities arise from three overlapping social structures that interact to generate different exposures and risk factors for HIV: the sexual division of labor, the sexual division of power, and cathexis. The sexual division of labor functions at the societal level through the different occupations allocated to men and women, which manifests as economic exposures and risk factors for HIV/ AIDS. The sexual division of power is maintained by social mechanisms, such as the abuse of authority and control in relationships. Finally, the structure of cathexis dictates appropriate sexual behavior on the basis of gender and reinforces cultural taboos regarding female sexuality. For example, in Africa, there exists a nearly universal sexual double standard that rewards men for infidelity and multiple sexual partners but punishes women for the same behaviors (for an example in South Africa, see Wood & Jewkes, 1997).
In Malawi, some women complain of limited control over their relationships, such as the ability to choose their husbands, to bear children, and to have sex or not (Lindgren, Rankin, & Rankin, 2005). Through the division of power, male dominance over women may also extend into the realm of health decision making. Indeed, a common reason provided by women who refuse testing in Malawi and elsewhere is the need to discuss the issue with their husband or because the husband refused testing himself (Baiden et al., 2005; Dahl, Mellhammar, Bajunirwe, & Bjorkman, 2008; Kranzer et al., 2009; Perez, Zvandaziva, Engelsmann, & Dabis, 2006). Qualitative research suggests that a double standard exists around HIV testing (i.e., cathexis) such that women need to request permission from their husbands, but men are free to make testing decisions on their own (Maman, Hogan, & Kilonza, 2001). Women’s reported obligation to seek permission may occur through the division of labor, if men control the economic resources needed to test (for an example in Zimbabwe, refer to Morin et al., 2006). Through the division of power, worry about physical abuse may hinder women’s ability to test if they suspect they could be HIV positive (Pool et al., 2001). In Malawi, intimate partner violence is a common event during marriage (Malawi Demographic Health Survey [MDHS], 2011; Mkandawire-Valhmu et al., 2013) and may be connected with aspects of cathexis. Many men, and women, perceive that it is acceptable for a husband to beat his wife for her supposed gender transgressions, such as disobedience, failing to perform household duties, or marital infidelity (Kim & Motsei, 2002). Fears of divorce or partner abandonment have also been noted as barriers to testing (Irungu et al., 2008; Mlay, Lugina, & Becker, 2008). Through the division of labor, unemployed or underemployed women who are economically dependent on their partners may have limited agency and negotiating power in the relationship (Foa & Foa, 1980).
While the TGP has been primarily applied to study women’s vulnerabilities to HIV/AIDS, the same social structures may also influence men’s use of testing services. In Malawi, evidence suggests that women will leave a partner who threatens to bring HIV into the household through their extramarital relations (Schatz, 2005). To circumvent an admission of infidelity (i.e., cathexis), men may avoid HIV testing even if they are dying of AIDS (Simpson, 2009). Through the division of labor, men’s breadwinner role may buffer any negative consequences of testing, thus making testing a more riskworthy venture. However, being employed means having less time to wait in lines at crowded health centers at the risk of lost wages. Indeed, employment migration (Weiser et al., 2006) and logistical barriers (Bwambale, Ssali, Byaruhanga, Kalyango, & Karamagi, 2008) have been shown to limit men’s use of testing services. Through the intersection of power and cathexis, men who adhere to patriarchal ideals around masculinity and male dominance may be less likely to test for HIV. Several studies from the region demonstrate how masculinities that require men to be strong, disease free, and economically productive conflict with the act of HIV testing itself (Izugbara, Undie, Mudege, & Ezeh, 2009; Skovdal et al., 2011). To conclude, men face similar barriers around gender and power that affects uptake of HIV testing; however, their position has largely been neglected in this body of research.
One important relationship dynamic not explicitly covered in the TGP but related to the structure of cathexis is relationship unity. In a previous study, we set out to develop a new measure of relationship power for the Malawi context (Conroy, 2014). Unexpectedly, a factor analysis revealed that relationship unity, or aspects of couple communication and reciprocity, was a salient construct of power for Malawian couples. Qualitative couple interviews confirmed this; for example, when respondents were asked about what makes people feel powerful in their relationships, many of them mentioned when their partners listened to their opinions (Conroy, 2013). It has been demonstrated elsewhere that aspects of unity such as spousal communication foster a more supportive environment for discussions around testing (Gage & Ali, 2005).
Given this background literature and theory, we hypothesized that the following relationship power and risk constructs would be associated with uptake of HIV testing over a 16-month period.
Socioeconomic inequalities (division of labor)
Women in a lower socioeconomic position relative to their partners will be less likely to test because they will have stronger fears around partner abandonment and loss of financial support. With less economic power, these women may also be in a disadvantaged position to negotiate testing with their partners. Conversely, women with greater socioeconomic power relative to their partners will be more likely to test. For men, we do not specify any a priori hypotheses, as both directions are possible.
Relationship dominance (division of power)
For women, being in a male-dominated relationship will be negatively associated with testing due to male control over testing decision making. For men, male dominance will be negatively associated with testing if these men adhere to traditional beliefs about masculinity and therefore are disinclined to test.
Relationship violence (division of power)
For women, having a history of relationship violence (physical and sexual) is a proxy for fear of abuse, which has been shown to be a barrier to testing. Women in violent relationships will therefore be less likely to test for HIV. Similarly, men who have been victims of violence will be less likely to test.
Relationship unity (cathexis)
For both women and men, relationship unity will be positively associated with HIV testing.
Mistrust/perceived partner infidelity
Individuals who perceive that their partners are cheating will be more likely to get tested, as suggested by qualitative research from Malawi (Conroy, 2013). This association will be less pronounced for men, whose perception of risk may be more influenced by extramarital relationships than by their spouse (Smith & Watkins, 2005).
Perceived risk of self and partner
Women who perceive themselves or their partners at higher risk for HIV will be less likely to test out of fear of the consequences. Conversely, men who perceive they are at higher risk for HIV will be more likely to test. Perceptions of a partner’s risk for HIV will be a less salient predictor of testing for men.
Method
Setting and Study Participants
Malawi has some of the highest rates of HIV infection in sub-Saharan Africa; 11% of adults are HIV infected (MDHS, 2011). HIV prevalence is higher among women than men (13% and 8%, respectively) and is the highest in the southern region (15%) (MDHS, 2011), where this study was situated. HIV testing and counseling is offered through integrated heath services, including antenatal care, and at stand-alone testing centers, clients’ homes, and workplace sites. As of 2010, 73% of women and 53% of men had ever been tested for HIV, which reflects a dramatic increase from 2005, when only 17% of adults knew their HIV status (MDHS, 2005, 2011).
The data come from Tsologo la Thanzi (TLT; “healthy futures” in Chichewa), a population-based panel study on reproduction and AIDS among young adults. Study procedures for TLT have been described elsewhere.1 To summarize, a random sample of women aged 15 to 25 was selected from a household listing of the Balaka town in southern Malawi. Women were asked to recruit up to three male partners through the use of incentive-based tokens. Longitudinal survey data were collected at four-month intervals over a period of approximately three years (for a total of eight waves). In wave 3, respondents were asked to respond to a series of statements on relationship power if they reported a current serious sexual partner, including a spouse, live-in partner, or boyfriend/girlfriend. The relationship power questions were asked with regard to the most serious relationship. For married respondents, their spouse automatically served as the reference partner. This baseline set of heterosexual couples (466 couples or 932 individuals) was merged with their corresponding data from waves 4 to 7. This allowed us to evaluate whether relationship power measured at wave 3 had an effect on HIV testing uptake over each subsequent four-month period.
At the start of the study, the women and their partners were also assigned to three equal groups to assess how knowledge of HIV status influences sexual behavior. Group 1 received regular HIV testing at every wave. Group 2 received an HIV test at the end of the first year and then again at the end of the study. Group 3 received an HIV test only at the end of the study. We included all three groups in the analysis, because respondents who tested via TLT could also have tested outside of the study. Respondents were not told when they would be tested, and thus if they desired to learn their status, knowledge of the TLT testing schedule would have had little impact on decisions to test.
Measures
Dependent variable
Having an HIV test was the dependent variable for this study. At each wave, respondents were asked: “When were you last tested for HIV?” If they had previously tested, respondents specified the date of their last HIV test and whether it was conducted through TLT or at a local health care center. Using the date of the previous survey, a binary variable was created to track whether respondents received a new HIV test (outside of the TLT study) at each wave (4 through 7).
Socioeconomic inequalities
Three variables were created to measure socioeconomic inequality between partners: age inequality, education inequality, and employment inequality. Given that men normally marry when they are approximately three years older than women (MDHS, 2011), we considered an age gap of five years to be a meaningful difference. Thus, age inequality was captured as a binary variable, where 0 referred to less than or equal to five years age difference and 1 referred to greater than five years age difference. We considered four years of education to be a meaningful difference in education between partners, which could distinguish between a primary and secondary school education. Thus, education inequality was captured as a three-level categorical variable, where 0 referred to similar education between partners, 1 referred to the man having at least four more years of education, and 2 referred to the woman having at least four more years of education. Using the responses for women and their partners, we created a four-level categorical variable for employment inequality, where 0 referred to both unemployed; 1 referred to man employed, woman unemployed; 2 referred to woman employed, man unemployed; and 3 referred to both employed.
Relationship dominance
Relationship dominance was measured by asking respondents: “In your relationship, who would you say is generally in charge?” Answer choices were respondent, equal control, or partner. Because less than 2% of women and less than 1% of men responded that their relationship was female dominated, a binary variable was created where 0 referred to egalitarian or female-dominated and 1 referred to male dominated. During the measure development phase of this study in 2009, face validity was assessed through cognitive interviewing techniques to ensure the concept captured its intended meaning. Construct validity was assessed through a separate logistic regression analysis (results not shown), which revealed that being in a male-dominated relationship was negatively associated with using condoms and positively associated with sexual coercion.
Relationship violence
Relationship violence measures were derived from Pulerwitz and colleagues (2000) and then were adapted to the Malawian context to ensure validity. Respondents were asked if they were victims (but not perpetrators) of intimate partner violence (IPV) in relation to their reference partner. Sexual IPV was captured as a binary variable that asked respondents: “Has your partner ever forced you to have sex when you did not want to?” Here, forced referred to verbal pressure to have sex (not rape) when one did not want to (i.e., sexual coercion). Physical IPV was captured with a binary variable that asked respondents: “Has your partner ever hurt you by beating you?” While multiple forms of physical abuse are possible, such as hitting, kicking, or punching, we restricted the item to beating to reflect the predominant local term used to describe physical abuse in Malawi.
Relationship unity
Relationship unity was a scale that consisted of three items: “My partner shows they care about me”; “When I need my partner’s assistance, he/she is there to help me”; and “My partner and I discuss important matters together.” Response options included Strongly agreed (1), Agreed (2), Disagreed (3), and Strongly disagreed (4). Responses were reverse scored so that higher scores meant more unity. Composite reliability for relationship unity was 0.68 (women: 0.76; men: 0.58); refer to (Conroy, 2014) for more details on the unity scale.
Mistrust/perceived partner infidelity
Mistrust, or perceived partner infidelity, was measured with the following statement: “My partner is probably having sex with someone else.” Response options included Strongly agreed (1), Agreed (2), Disagreed (3), or Strongly disagreed (4). A binary variable was created for mistrust by collapsing Agreed/strongly agreed (set to 1) and Disagreed/strongly disagreed (set to 0).
Perceived risk of HIV
Perceived risk for HIV, of self and of partner, were included as predictors of HIV testing and modeled as categorical variables. Perceived risk of self was captured with the statement: “Pick the number of beans that reflect how likely it is that you are infected with HIV now.” There were 10 beans for respondents to use. We created a five-level categorical variable where 0 equaled no likelihood (0 beans), 1 equaled low likelihood (1 to 4 beans), 2 equaled a medium likelihood (5 beans), 3 equaled high likelihood (6 to 9 beans), and 4 equaled certain likelihood (10 beans). We included “no likelihood of infection” and “certain likelihood of infection” as separate categories to account for actual knowledge of HIV status. Perceived risk of partner was captured with the statement: “What is the likelihood that your partner is currently infected with HIV?” Response options included no likelihood, low, medium, high, and “I know she/he is” (infected with HIV). We created a three-level categorical variable for perceived risk of partner, where 0 referred to no or low likelihood of infection, 1 referred to medium likelihood of infection, and 2 referred to high or certain likelihood of infection. For the statistical models, the medium likelihood was later collapsed with the high/certain category given the low number of medium responses.
Sociodemographic control variables
Several socio-demographic variables were included in all multivariate models as statistical controls: age, years of education, household economic status, marital status, and relationship duration. Age and years of education were modeled as continuous variables. Up to and including 8 years of education was considered primary school, 9 to 12 years was considered secondary school, and greater than 12 years was considered tertiary school. An index of nine common household goods (e.g., bicycle, television, bed with mattress, radio) was used to approximate household economic status. Marital status was a four-level categorical variable that consisted of the following states: married/cohabitating, separated/divorced, widowed, or unmarried. Relationship duration (in years) was computed by subtracting the date of the survey from the date the couple first started spending time together as more than friends.
HIV testing control variables
Three additional controls were included in the multivariate models: new antenatal care HIV test (for women only), previous TLT testing, and previous external testing. First, we wanted to control for women who tested as part of antenatal care and thus would be less likely to test elsewhere. Second, we controlled for previous TLT testing by including a continuous variable that tracked the number of previous TLT tests (cumulative) at each wave. Finally, we anticipated that previous external testing could influence future uptake of testing and therefore included a continuous variable to control for the number of previous external tests (cumulative) at each wave.
Statistical Analysis
For the independent variables, missing data were minimal with the exception of perceived risk of partner (63 missing; 6.7%). For the outcome variable new HIV test, 799 of the 932 respondents had complete data at all waves. Using the “mi ice” command in Stata 11.2, we performed multiple imputation via chained equations to build an imputation model containing all predictors, covariates, and outcome variables. After generating six multiply imputed data sets corresponding to the percentage of missing data, we dropped observations that had an imputed value for new HIV test. Unless there are substantial missing data for the independent variables, imputing the dependent variable adds no additional information to the regression equation and may result in larger standard errors than listwise deletion (von Hippel, 2007). Thus, dropping values should improve the efficiency of standard error estimates (Acock, 2012). The final models used complete data for all independent variables but included only individuals who participated in a given wave.
For the main analysis, we used generalized estimating equations (GEE) with robust standard errors to produce more efficient estimates of the coefficients as compared to fixed or random effects models (Allison, 2004). The GEE method specifies how the average of a response variable of a subject changes with covariates, while allowing for the correlation between repeated measurement on the same subject over time. To determine the best fitting model, we computed the QIC statistic (quasi-likelihood under the independence model criterion) for three different correlation structures: exchangeable, unstructured, and autoregressive (Pan, 2001). Using the autoregressive correlation structure, we found that the full model had the smallest QIC and thus it was chosen as the preferred model. The autoregressive correlation structure indicates that two observations taken closer in time within an individual tend to be more highly correlated than two observations taken far apart, which was consistent with our belief that people would be more likely to test in clusters. The final odds ratios were computed using the “xtgee” command in Stata 11.2. All variables, with the exception of marital status, perceived risk, antenatal care testing for HIV, previous TLT testing, and previous external testing, were modeled as time-invariant variables. Models were fitted separately for men and for women to allow for different effects to emerge as statistically significant for each gender, as would be expected by the TGP.
Results
Characteristics of the Baseline Sample
A total of 932 men and women (466 couples) were asked the relationship power questions at TLT’s wave 3. We have described the demographic and relationship characteristics of this sample in depth elsewhere (Conroy, 2014). To summarize, the mean age for the sample was 24.8 years and the mean years of education was 7.3 years, reflecting a primary school education level. The sample was biased toward more stable partnerships, with the majority of the sample reporting either being married or cohabitating (91.6%). Serious male partners were more likely than casual sexual partners to participate in the study. Of the married respondents at wave 3, 85.7% of those who participated in all five waves remained married over the 16-month period. The majority (73.5%) of couples had at least one child together and had been together, on average, for approximately five years. The distribution of the relationship power and perceived risk variables by gender are provided in Table 1.
Table 1.
Characteristics of the Couples Sample, Tsogolo La Thanzi Wave 3
| Variable | Total (N= 932)
|
Women (N= 466)
|
Men (N= 466)
|
|||
|---|---|---|---|---|---|---|
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | |
| Individual characteristics | ||||||
| Age (16–57) | 24.8 (4.8)* | 22.1 (2.7) | 27.6 (4.9) | |||
| Years of education (0–13) | 7.3 (3.0)* | 6.6 (2.6) | 7.9 (3.1) | |||
| Household goods (0–7) | 3.0 (1.4) | 3.0 (1.4) | 3.0 (1.4) | |||
| Couple characteristics | ||||||
| Married | 91.6 | 91.6 | 91.6 | |||
| Relationship duration, years (0.5–12.9) | 5.0 (2.7) | 5.0 (2.7) | 5.0 (2.7) | |||
| At least one living child with partner | 73.5 | 74.7 | 72.3 | |||
| Age difference (age inequality) | ||||||
| 0–5 years | 58.6 | |||||
| 6 + years | 41.4 | |||||
| Education inequality | ||||||
| Similar education | 70.2 | |||||
| Man has 4 + more years of education | 22.7 | |||||
| Woman has 4 + more years of education | 7.1 | |||||
| Employment inequality | ||||||
| Both unemployed | 8.4 | |||||
| Woman employed, man unemployed | 2.4 | |||||
| Man employed, woman unemployed | 54.7 | |||||
| Both employed | 34.6 | |||||
| Relationship unity (total score) | 3.77 (0.41) | 3.77 (0.45) | 3.77 (0.37) | |||
| My partner shows that they care about me | 3.87 (0.40) | 3.86 (0.44) | 3.88 (0.35) | |||
| When I need my partner’s assistance, he or she is there to help me | 3.63 (0.69) | 3.65 (0.67) | 3.60 (0.70) | |||
| My partner and I discuss important matters together | 3.80 (0.47) | 3.78 (0.51) | 3.82 (0.43) | |||
| Relationship dominance | ||||||
| Male-dominated | 85.0* | 81.5 | 88.4 | |||
| Female-dominated/egalitarian | 15.0 | 18.5 | 11.6 | |||
| Relationship violence | ||||||
| Ever experienced forced sex by partner | 16.5* | 21.5 | 11.6 | |||
| Ever been physically abused by partner | 4.0* | 6.0 | 1.9 | |||
| Perceived risk of self for HIV (0–10) | 1.73 (2.43) | 1.63 (2.48) | 1.82 (2.39) | |||
| Perceived risk of partner for HIV | ||||||
| No or low likelihood of infection | 93.8* | 91.3 | 96.3 | |||
| Medium likelihood of infection | 2.6 | 3.9 | 1.3 | |||
| High or certain likelihood of infection | 3.6 | 4.8 | 2.4 | |||
| Perceptions that partner is having affair | ||||||
| Strongly disagree/disagree | 85.7* | 81.8 | 89.7 | |||
| Strongly agree/agree | 14.3 | 18.2 | 10.3 | |||
Note. Unity: 1 = Strongly disagree; 2 = Disagree; 3 = Agree; 4 = Strongly agree. Higher scores indicate more unity. Unity scores were created by taking the mean across all unity items.
Chi-square and ANOVA differences for gender were significant at p <0.05.
HIV Testing Histories
At baseline, respondents were asked the following question: “I don’t want to know the results, but have you ever tested for HIV and received the results?” Almost three-quarters (71.5%) of the total sample had previously been tested for HIV. More women than men had previously tested for HIV (79.8% versus 63.2%). We calculated the percentage of respondents who had a new HIV test (outside of TLT) since the previous wave. At wave 4, approximately 24% of the 902 respondents who participated at that wave received a new HIV test since wave 3. These percentages increased slightly from waves 5 to 7. Approximately 20% had a new test from waves 4 to 5, 22% had a new test from waves 5 to 6, and 25% had a new test from waves 6 to 7. Figure 1 illustrates the breakdown of testing (outside of TLT) by gender. Higher rates of external testing among women across all four waves are attributed, in part, to antenatal care testing. For example, between waves 3 and 4, 30 pregnant women (13.8% of all who tested) had tested for HIV through antenatal care. Finally, we calculated the distribution of cumulative new HIV tests (outside of TLT) recorded at wave 7 over the course of 16 months. These data include only respondents who had complete data for all waves (N = 799). The number of cumulative new HIV tests ranged from zero (no new tests) to a maximum of four. More than half (52.7%) of participating respondents had no new tests over the 16-month period, 21.8% had one new test, and 25.5% had more than one new test.
Figure 1.
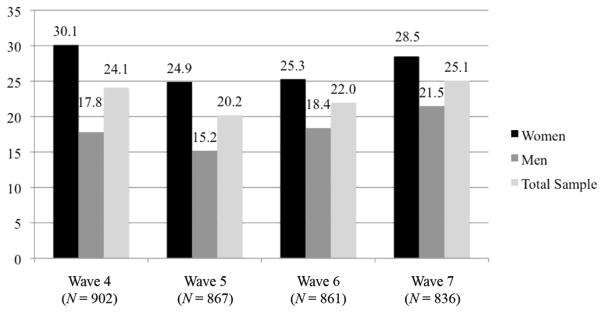
Percentage of participating respondents who had an external HIV test since the previous wave, Tsogolo La Thanzi waves 4 through 7.
Predictors of HIV Testing Uptake
Table 2 presents the crude and adjusted odds of having an HIV test from waves 4 to 7.
Table 2.
Odds Ratios From Generalized Estimating Equations (GEE) Models Predicting an HIV Test, Tsogolo La Thanzi (TLT) Waves 4–7
| Variable | Women
|
Men
|
||
|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted OR (95% CI)a | Crude OR (95% CI) | Adjusted OR (95% CI)a | |
| Socioeconomic inequality | ||||
| Age difference | ||||
| 0–5 years difference (ref) | ref | ref | ref | ref |
| 6+ years difference | 0.71 (0.53, 0.95)* | 0.78 (0.59, 1.03)† | 0.70 (0.47, 1.03)† | 1.04 (0.67, 1.61) |
| Education inequality | ||||
| Similar education (ref) | ref | ref | ref | ref |
| Man has 4+ years more education | 0.91 (0.65, 1.27) | 0.89 (0.62, 1.30) | 0.86 (0.56, 1.33) | 0.85 (0.57, 1.28) |
| Woman has 4+ years more education | 0.80 (0.44, 1.43) | 0.77 (0.42, 1.41) | 0.68 (0.33, 1.39) | 1.22 (0.58, 2.56) |
| Employment inequality | ||||
| Both members unemployed (ref) | ref | ref | ref | ref |
| Woman employed, man unemployed | 0.89 (0.41, 1.93) | 1.12 (0.50, 2.48) | 1.09 (0.35, 3.36) | 2.04 (0.71, 5.90) |
| Man employed, woman unemployed | 0.53 (0.33, 0.87) * | 0.99 (0.58, 1.68) | 0.60 (0.33, 1.09)† | 1.20 (0.69, 2.09) |
| Both members employed | 0.70 (0.42, 1.16) | 1.27 (0.73, 2.21) | 0.62 (0.33, 1.18) | 1.39 (0.77, 2.50) |
| Relationship dominance | ||||
| Female-dominated/egalitarian (ref) | ref | ref | ref | ref |
| Male-dominated | 1.28 (0.89, 1.84) | 1.37 (0.96, 1.95)† | 0.93 (0.59, 1.47) | 1.16 (0.72, 1.89) |
| Relationship violence | ||||
| Ever been sexually coerced by partner | 1.37 (1.00, 1.87) * | 1.16 (0.83, 1.64) | 0.49 (0.27, 0.88) * | 0.53 (0.31, 0.90) * |
| Ever been physically abused by partner | 0.85 (0.49, 1.50) | 0.70 (0.43, 1.14) | 0.52 (0.15, 1.88) | 0.78 (0.19, 3.21) |
| Relationship unity | 0.69 (0.52, 0.91) * | 0.68 (0.52, 0.90) ** | 0.62 (0.39, 0.99) * | 0.70 (0.46, 1.06)† |
| Perception that partner is having an affair | ||||
| Strongly disagree/disagree (ref) | ref | ref | ref | ref |
| Strongly agree/agree | 0.95 (0.67, 1.36) | 0.95 (0.67, 1.33) | 0.90 (0.47, 1.71) | 1.02 (0.57, 1.85) |
| Perceived risk for HIV (self) | ||||
| No likelihood (ref) | ref | ref | ref | ref |
| Low likelihood | 0.73 (0.55, 0.96) * | 0.75 (0.54, 1.05)† | 0.78 (0.56, 1.09) | 0.82 (0.56, 1.18) |
| Medium likelihood | 0.59 (0.41, 0.83) ** | 0.72 (0.48, 1.09) | 0.58 (0.40, 0.86) ** | 0.64 (0.41, 1.00) * |
| High likelihood | 0.85 (0.49, 1.48) | 0.67 (0.35, 1.28) | 0.72 (0.32, 1.60) | 0.90 (0.36, 2.24) |
| Certain likelihood | 0.90 (0.54, 1.50) | 1.00 (0.55, 1.83) | 0.45 (0.20, 1.03)† | 0.52 (0.23, 1.16) |
| Perceived risk for HIV (partner) | ||||
| No or low likelihood (ref) | ref | ref | ref | ref |
| Medium, high, or certain likelihood | 0.70 (0.42, 1.17) | 0.57 (0.36, 0.88) * | 0.67 (0.28, 1.59) | 1.15 (0.42, 3.13) |
| N (respondents), N (observations) | 440, 1729 | 413, 1608 | ||
Adjusted models control for all predictor variables, marital status, age, education, household goods index, relationship duration, previous testing through the TLT, previous testing outside of TLT, and antenatal care testing (women only). Time-varying predictors include perceived risk, marital status, and the testing control variables. Unity scores ranged from 1 to 4, with higher values indicating more unity.
p <0.10.
p <0.05.
p <0.01.
Socioeconomic inequalities
We hypothesized that women in a lower socioeconomic position as compared to their partner would be less likely to test. In the unadjusted models, women whose partners were much older had lower odds of testing as compared to women paired with men of a similar age (OR = 0.71, p < 0.05). This association was slightly attenuated and became less statistically significant after controlling for other covariates in the model (OR = 0.78, p <0.10). Regarding education inequality, having significantly more or less education as compared to a partner did not play a significant role in women’s uptake of testing; both the crude and adjusted odds ratios were nonsignificant. Regarding employment inequality, women who were unemployed when their male partner was employed had lower odds of testing than when both partners were unemployed (OR = 0.53, p <0.05). However, this association was attenuated and became nonsignificant when other variables were added to the model. Finally, in both the crude and adjusted models for women, being employed when a partner was unemployed or being in a dual-income-earning couple did not give women the advantage to test.
For men, the results for the socioeconomic inequalities are as follows. In the unadjusted models, there was a trend toward significance between men who were significantly older than their partners and lower odds of testing (OR = 0.70, p <0.10). Yet this association was attenuated and became nonsignificant after controlling for other covariates. Regarding education inequality, having more or less education than a partner did not influence men’s uptake of HIV testing in either the unadjusted or adjusted models. Regarding employment inequalities, the unadjusted models showed a marginally significant association between employed men paired with unemployed women and a lower odds of testing (OR = 0.60, p <0.10); however, this association became nonsignificant after controlling for other covariates.
Relationship dominance
It was hypothesized that if men dominated the relationship, women would be less likely to get tested for HIV through the division of power. To the contrary, the adjusted models showed that women in male-dominated relationships had 37% higher odds of testing as compared to women in egalitarian/female-dominated relationships, although it did not reach statistical significance (p <0.10). For men, it was hypothesized that male dominance would be negatively associated with men’s uptake of testing. However, being in a male-dominated relationship was not significantly associated with HIV testing uptake for men in the crude or adjusted models.
Relationship violence
We expected that relationship violence would serve as a barrier to testing. In the unadjusted models, women who had experienced sexual IPV had 37% higher odds of testing (p <0.05). However, this association was attenuated after controlling for perceived risk and other covariates, and was no longer statistically significant. In accordance with the hypothesis for physical IPV, women with a history of physical violence had a lower odds of testing in both the unadjusted and adjusted models; however, the associations failed to achieve statistical significance. Interestingly, men who experienced sexual IPV had lower odds of testing (OR = 0.49, p <0.05), which persisted even after controlling for all other covariates (OR = 0.53, p <0.05). The same association did not hold for physical abuse, which may be an artifact of low levels of physical abuse reported among men in the sample.
Relationship unity
In contradiction with the hypothesis that individuals with higher levels of unity in their relationships would be more likely to test, the unadjusted results for women showed that unity was associated with lower odds of having a new HIV test (OR = 0.69, p <0.05). This association held after adjusting for all covariates. For each one-unit increase in relationship unity, the odds of having an HIV test decreased by 32% (p <0.01). In the unadjusted models for men, similar associations were found. For each one-unit increase in relationship unity, the odds of having an HIV decreased by 38% (p <0.05). This association was attenuated only slightly when all other covariates were added to the model (OR = 0.70, p <0.10).
Mistrust/perceived partner infidelity
Contrary to the hypothesis that women, and to a lesser extent men, would be more likely to test if they suspected partner infidelity, the results did not indicate that such a relationship exists—even before adjusting for perceived risk.
Perceived risk of HIV
Regarding perceived risk of self, we hypothesized that women would be less likely to test if they perceived themselves to be at a higher risk for HIV. In the unadjusted models, women who reported a low (OR = 0.73, p <0.05) or medium (OR = 0.59, p <0.01) likelihood of HIV infection had lower odds of testing as compared to women who reported no likelihood of HIV infection. These associations were attenuated after controlling for other covariates (OR = 0.75, p <0.10; OR = 0.72, p >0.10). Regarding perceived risk of partner, women who believed that their partner was likely to be HIV infected had 43% lower odds of testing than women who perceived their partners were at no or low risk for HIV, even after controlling for all other covariates (p <0.05). In the models for men, we hypothesized that those who reported a higher perceived risk of self would be more likely to test. However, in the unadjusted analysis, men who reported a medium likelihood of HIV infection had lower odds of testing as compared to men who reported no likelihood of HIV infection (OR = 0.58, p <0.01). This association held after adjusting for all other covariates (OR = 0.64, p <0.05). In addition, we did not find a relationship between perceptions of a partner’s risk for HIV and testing for HIV among men.
Discussion
The results of this study demonstrate that rural Malawians are getting tested at relatively high rates, repeatedly, and not just during pregnancy. In stark contrast to a host of studies from sub-Saharan Africa that show low levels of lifetime testing among nationally representative samples (e.g., MacPhail et al., 2007; Peltzer, Matseke, Mzolo, & Majaja, 2009; Sambisa, Curtis, & Mishra, 2012), most respondents in this study had received at least one HIV test prior to enrolling in the TLT study. Rates of testing exceeded population-based estimates from the Malawi Demographic Health Survey, which found that 64% of women and 43% of men aged 15 to 24 had ever tested for HIV in 2010 (MDHS, 2011). Higher rates of testing in this study may, in part, reflect the younger age of the sample, who may be more likely to test as part of their transitions to marriage and then parenthood. The findings indicate that young people in southern Malawi currently have good access to HIV testing and counseling services, which is likely to improve in upcoming years as testing becomes more integrated into routine health care.
The use of longitudinal HIV testing history data, as opposed to a one-time event of ever testing was a unique feature of this study that allowed for the investigation of predictors of HIV testing over a 16-month period. This is an important contribution to the literature, above and beyond the ability to circumvent methodological problems associated with cross-sectional data. With the recent scale-up of HIV health services in sub-Saharan Africa, more people are returning to health care centers for additional HIV tests (e.g., refer to Fiorillo et al., 2012). Repeated HIV testing and counseling is crucial for “treatment as prevention” interventions that rely on high rates of regular HIV testing to identify new seroconversions (Isingo et al., 2012). Still, it is unusual for studies from sub-Saharan Africa to measure the number of times people have ever been tested for HIV in non-clinic-based samples—with a few exceptions. For example, in South Africa, Kalichman and Simbayi (2003) reported that 29% of their cross-sectional sample of men and women had tested twice and 19% had tested three times for HIV. In Tanzania, Venkatesh and colleagues (2011) reported that approximately 58% of respondents had ever received multiple HIV tests. While the current study captured HIV testing histories in a relatively short time period (as compared to a lifetime), high rates of repeat testing were reported: Almost 13% had reported two new HIV tests and another 13% had tested more than twice. This indicates that lifetime history of repeat testing is likely to be even higher in this population.
Using this testing history data, we sought to investigate whether several theoretical constructs of relationship power influenced young people’s decisions to test for HIV. We found mixed support for the role of the TGP in explaining HIV testing behavior. Through the sexual division of labor, socioeconomic inequalities between partners could either enable or inhibit uptake of testing depending on a couple member’s relative position. There was weak evidence that women paired with significantly older partners were less likely to test for HIV. Similarly, in the unadjusted analysis, older men were less likely to test for HIV than men closer in age to their partners. In sub-Saharan Africa, age confers social status and respect, and this differential is likely to influence an individual’s communication with his or her partner. Younger women may elect not to bring up HIV testing with older partners. Older men, on the other hand, may be less pressured or persuaded by their younger female partners to test for HIV during the childbearing years. In addition, some evidence in the bivariate analysis suggested that being an unemployed woman paired with an income-generating man creates a situation of economic dependence that could limit women’s use of HIV testing services. This association, however, was attenuated and became nonsignificant after controlling for other factors.
Through the sexual division of power, male dominance was hypothesized to act as a barrier to testing through the mechanism laid out by Maman and colleagues (2001). In contradiction, we found that being in a male-dominated relationship was associated with higher rates of HIV testing for women. This conflicts with other research that suggests male control over women’s use of HIV testing services prevents women from getting tested (Baiden et al., 2005; Dahl et al., 2008; Kranzer et al., 2009; Perez et al., 2006). It is possible that traditional marital power structures facilitate testing, for example, if men take responsibility for the family’s health and well-being by escorting their wives for testing. In qualitative studies from Malawi and Uganda, escorting a wife to the clinic was a common practice and considered a sign of a loving and responsible husband (Kululanga, Sundby, Malata, & Chirwa, 2012; Larsson et al., 2010; Medley & Kennedy, 2010). Future programs could harness the positive aspects of masculinity to encourage the adoption of healthy behaviors in couples (Mankowski & Maton, 2010).
Relationship violence is one of the most widely studied manifestations of the sexual division of power. Interestingly, for women, a history of physical abuse was not predictive of HIV testing. This differs from studies that cite fear of abuse as a possible reason for why women may refuse testing (Irungu et al., 2008; Maman et al., 2001). It is possible that violence does not necessarily prevent women from testing but rather from disclosing their results. Interestingly, we found that men who had experienced sexual IPV were significantly less likely to get tested—net of perceived risk. This finding underscores the need for further research regarding men’s experiences of IPV in this setting, which have not been examined outside of a few qualitative accounts of younger men paired with older women (e.g., Dunkle et al., 2007; Simpson, 2009). There may be other unmeasured characteristics of men who are unable to refuse sex that make them less likely to test. Future studies should explore men’s experiences of sexual coercion in more depth and how this may affect use of HIV services.
Unexpectedly, relationship unity was associated with lower rates of HIV testing for both men and women. It is possible that individuals who report more unity with their partners (i.e., caring for partner, working together, and good communication) find less reason to test for HIV in the first place. Supporting qualitative study from the same region of southern Malawi suggests that people test for HIV when they perceive there to be problems in the relationship, not during times of harmony (Conroy, 2013). Parallels can also be drawn from the literature on condom use in Malawi, which finds that condoms are largely incompatible with serious relationships because they go against ideals of love, trust, and intimacy (Chimbiri, 2007; Tavory & Swidler, 2009). Similar mechanisms may shape HIV testing behavior within intimate relationships.
Finally, in Malawi, people are still fearful of testing positive for HIV. Both men and women who reported a higher likelihood of their own HIV infection were less likely to test. For women, beliefs about a partner’s risk for HIV played a stronger role in decisions to test than perceptions of their own risk. Men’s decisions to test, on the other hand, were more likely to be prompted by personal risk assessments. This suggests that women may take on more of a relationship-centered orientation to risk and testing as compared to men. Similar findings were found in a sample of young adults from the United States. Men were less reliant on the relationship context of risk when making decisions to test as opposed to their own personal sexual histories; For women, both their own and their partner’s risk influenced their decisions to test for HIV (Longmore et al., 2013).
Several limitations of this study are noteworthy. First, our HIV testing outcome measure included HIV testing through multiple different settings such as home-based testing, workplace testing, routine health services, and antenatal care testing. Thus, since it was difficult to disentangle the testing modality, it is unclear how relationship power influences decisions to test across different venues. This limitation is not necessarily limited to the current study; a large number of published studies that examined “ever tested” did not inquire further about where the test occurred and who initiated it (deGraft-Johnson et al., 2005; Irungu et al., 2008; Weiser et al., 2006). However, we minimized the effect of testing modality on our estimates by controlling for antenatal care testing, which is one of the more prevalent modes of testing in Malawi (Weir, Hoffman, & Muula, 2008). Second, many of our predictor variables were included only at a single time point although we know that relationship factors are dynamic and may even change in response to testing outcomes. Future research could examine how changing relationship patterns or characteristics over the course of the relationship motivate or constrain decisions to test for HIV. Third, despite our use of a subsample of randomly selected women from TLT, the results cannot be generalized to the larger population. Our baseline sample self-selected to be mostly married or cohabitating heterosexual couples, thereby making it difficult to draw conclusions about other types of relationships. Finally, we point out the limitations of the TGP, which inherently takes on a Western feminist perspective that portrays women as victims of male dominance, control, and abuses. The concept of relationship unity emerged inductively through our interviews with rural Malawians as well as in our factor analysis, and this brings attention to the need to expand the TGP to include other culturally relevant forms of power. In addition, we acknowledge that we may be missing aspects of the TGP that were not measured in this study but could potentially explain HIV testing behavior.
Despite these limitations, our study strengths include the relatively large sample size of couples and the use of an innovative measure of HIV testing. To the best of our knowledge, this study is also one of the first to use dyadic data to test whether a theoretical model of relationship power affects decisions to test in this context. Our findings suggest that HIV testing programs and couple-based interventions need to consider how different facets of power and perceived risk shape HIV testing behavior within young, married couples. Higher-functioning couples, such as those with less conflict, better communication, and higher relationship satisfaction, should be given special attention to ensure they can overcome testing barriers that may be related to aspects of intimacy and relationship quality.
Acknowledgments
We are grateful to Sheana Bull, Sara Yeatman, Jean Scandlyn, Jennifer Harman, Torsten Neilands, Lynae Darbes, and several anonymous reviewers for their valuable feedback on earlier versions of this manuscript.
Funding
The research was supported by grants F31-MH093260 and T32-MH19105 from the National Institutes of Mental Health and grant R01-HD058366 from the National Institute of Child Health and Human Development.
Footnotes
Refer to http://projects.pop.psu.edu/tlt for more information about the TLT data set, to request data access, and for replication files.
An earlier version of this manuscript was presented at the Annual Meeting of the American Public Health Association (APHA) on October 30, 2012, in San Francisco, CA.
References
- Acock A. What to do about missing values. In: Cooper H, editor. APA handbook of research methods in psychology: Vol. 3. Data analysis and research publication. Washington, DC: American Psychological Association; 2012. pp. 27–50. [Google Scholar]
- Allison P. Event history analysis. In: Hardy M, Bryman A, editors. Handbook of data analysis. London, UK: Sage; 2004. pp. 369–385. [Google Scholar]
- Baiden F, Remes P, Baiden R, Williams J, Hodgson A, Boelaert M, Buve A. Voluntary counseling and HIV testing for pregnant women in the Kassena-Nankana district of northern Ghana: Is couple counseling the way forward? AIDS Care. 2005;17(5):648–657. doi: 10.1080/09540120412331319688. [DOI] [PubMed] [Google Scholar]
- Blanc A. The effect of power in sexual relationships on sexual and reproductive health: An examination of the evidence. Studies in Family Planning. 2001;32(3):189–213. doi: 10.1111/j.1728-4465.2001.00189.x. [DOI] [PubMed] [Google Scholar]
- Bradley H, Tsui A, Kidanu A, Gillespie D. Client characteristics and HIV risk associated with repeat HIV testing among women in Ethiopia. AIDS and Behavior. 2011;15:725–733. doi: 10.1007/s10461-010-9765-1. [DOI] [PubMed] [Google Scholar]
- Bwambale FM, Ssali SN, Byaruhanga S, Kalyango JN, Karamagi CAS. Voluntary HIV counselling and testing among men in rural western Uganda: Implications for HIV prevention. BMC Public Health. 2008;8:263. doi: 10.1186/1471-2458-8-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LM, Kamali A, Ruberantwari A, Malamba SS, Whitworth JAG. Rates of HIV-1 transmission within marriage in rural Uganda in relation to the HIV sero-status of the partners. AIDS. 1999;13(9):1083–1089. doi: 10.1097/00002030-199906180-00012. [DOI] [PubMed] [Google Scholar]
- Chimbiri AM. The condom is an “intruder” in marriage: Evidence from rural Malawi. Social Science and Medicine. 2007;64:1102–1115. doi: 10.1016/j.socscimed.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Connell RW. Gender and power. Stanford, CA: Stanford University Press; 1987. [Google Scholar]
- Conroy A. Doctoral dissertation. Denver, CO: University of Colorado–Denver; 2013. Gender, relationship power, and HIV testing in rural Malawi. [Google Scholar]
- Conroy A. Gender, power, and intimate partner violence: A study on couples from rural Malawi. Journal of Interpersonal Violence. 2014;29(5):866–888. doi: 10.1177/0886260513505907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creel AH, Rimal RN. Factors related to HIV-testing behavior and interest in testing in Namibia. AIDS Care. 2012;23(7):901–907. doi: 10.1080/09540121.2010.540227. [DOI] [PubMed] [Google Scholar]
- Dahl V, Mellhammar L, Bajunirwe F, Bjorkman P. Acceptance of HIV testing among women attending antenatal care in south-western Uganda: Risk factors and reasons for test refusal. AIDS Care. 2008;20:746–752. doi: 10.1080/09540120701693990. [DOI] [PubMed] [Google Scholar]
- deGraft-Johnson J, Paz-Soldan V, Kasote A, Tsui A. HIV voluntary counseling and testing service preferences in a rural Malawi population. AIDS and Behavior. 2005;9(4):475–484. doi: 10.1007/s10461-005-9018-x. [DOI] [PubMed] [Google Scholar]
- Dunkle KL, Jewkes RK, Brown HC, Gray GE, McIntyre JA, Harlow SD. Gender-based violence, relationship power, and risk of HIV infection in women attending antenatal clinics in South Africa. Lancet. 2004;363:1415–1421. doi: 10.1016/S0140-6736(04)16098-4. [DOI] [PubMed] [Google Scholar]
- Dunkle KL, Jewkes R, Nduna M, Jama N, Levin J, Sikweyiya Y, Ross MP. Transactional sex with casual and main partners among young South African men in the rural Eastern Cape: Prevalence, predictors, and associations with gender-based violence. Social Science and Medicine. 2007;65(6):1235–1248. doi: 10.1016/j.socscimed.2007.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkle KL, Stephenson R, Karita E, Chomba E, Kayitenkore K, Vwalika C, Allen S. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: An analysis of survey and clinical data. Lancet. 2008;371:2183–2191. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- Fiorillo SP, Landman KZ, Tribble AC, Mtalo A, Itemba DK, Ostermann J, Crump JA. Changes in HIV risk behavior and seroincidence among clients presenting for repeat HIV counseling and testing in Moshi, Tanzania. AIDS Care. 2012;24(10):1264–1271. doi: 10.1080/09540121.2012.658751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E, Foa U. Resource theory: Interpersonal behavior as exchange. In: Gergen K, Greenberg M, Willis R, editors. Social exchange: Advances in theory and research. New York, NY: Plenum Press; 1980. pp. 77–94. [Google Scholar]
- Gage AJ, Ali D. Factors associated with self-reported HIV testing among men in Uganda. AIDS Care. 2005;17(2):153–165. doi: 10.1080/09540120512331325635. [DOI] [PubMed] [Google Scholar]
- Harrison A, O’Sullivan LF, Hoffman S, Dolezal C, Morrell R. Gender role and relationship norms among young adults in South Africa: Measuring the context of masculinity and HIV risk. Journal of Urban Health. 2006;83(4):709–723. doi: 10.1007/s11524-006-9077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irungu TK, Varkey P, Cha S, Patterson JM. HIV voluntary counselling and testing in Nakuru, Kenya: Findings from a community survey. HIV Medicine. 2008;9:111–117. doi: 10.1111/j.1468-1293.2007.00538.x. [DOI] [PubMed] [Google Scholar]
- Isingo R, Wringe A, Todd J, Urassa M, Mbata D, Maiseli G, Zaba B. Trends in the uptake of voluntary counselling and testing for HIV in rural Tanzania in the context of the scale up of antiretroviral therapy. Tropical Medicine and International Health. 2012;17(8):e15–e25. doi: 10.1111/j.1365-3156.2011.02877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izugbara CO, Undie CC, Mudege NN, Ezeh AC. Male youth and voluntary counseling and HIV-testing: The case of Malawi and Uganda. Sex Education: Sexuality, Society, and Learning. 2009;9:243–259. [Google Scholar]
- Jean K, Anglaret X, Moh R, Lert F, Dray-Spira R. Barriers to HIV testing in Côte d’Ivoire: The role of individual characteristics and testing modalities. PLOS One. 2012;7(7):e41353. doi: 10.1371/journal.pone.0041353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Simbayi LC. HIV testing attitudes, AIDS stigma, and voluntary HIV counselling and testing in a Black township in Cape Town, South Africa. Sexually Transmitted Infections. 2003;79(6):442–447. doi: 10.1136/sti.79.6.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Motsei M. “Women enjoy punishment”: Attitudes and experiences of gender-based violence among PHC nurses in rural South Africa. Social Science and Medicine. 2002;54:1243–1254. doi: 10.1016/s0277-9536(01)00093-4. [DOI] [PubMed] [Google Scholar]
- Kranzer K, McGrath N, Saul J, Crampin AC, Jahn A, Malema S, Glynn JR. Individual, household, and community factors associated with HIV test refusal in rural Malawi. Tropical Medicine and International Health. 2009;13(11):1341–1350. doi: 10.1111/j.1365-3156.2008.02148.x. [DOI] [PubMed] [Google Scholar]
- Kululanga LI, Sundby J, Malata A, Chirwa E. Male involvement in maternity health care in Malawi. African Journal of Reproductive Health. 2012;16(1):145–157. [PubMed] [Google Scholar]
- Larsson EC, Thorson A, Nsabagasani X, Namusoko S, Popenoe R, Ekström AM. Mistrust in marriage: Reasons why men do not accept couple HIV testing during antenatal care: A qualitative study in eastern Uganda. BMC Public Health. 2010;10:769. doi: 10.1186/1471-2458-10-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren T, Rankin S, Rankin W. Malawi women and HIV: Socio-cultural factors and barriers to prevention. Women and Health. 2005;41(1):69–86. doi: 10.1300/J013v41n01_05. [DOI] [PubMed] [Google Scholar]
- Longmore MA, Johnson WL, Manning WD, Giordano PC. HIV testing among heterosexual young adults: The influence of partners’ risk behaviors and relationship dynamics. Journal of Sex Research. 2013;50(5):489–501. doi: 10.1080/00224499.2012.661101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luseno W, Wechsberg WM. Correlates of HIV testing among South African women with high sexual and substance-use risk behaviors. AIDS Care. 2009;21(2):178–184. doi: 10.1080/09540120802017594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhail CL, Pettifor A, Coates T, Rees H. “You must do the test to know your status”: Attitudes to HIV voluntary counseling and testing for adolescents among South African youth and parents. Health Education and Behavior. 2007;35(1):87–104. doi: 10.1177/1090198106286442. [DOI] [PubMed] [Google Scholar]
- Malawi Demographic Health Survey. Malawi demographic and health survey. Calverton, MD: NSO and ORC Macro; 2005. [Google Scholar]
- Malawi Demographic Health Survey. Malawi demographic and health survey 2010. Calverton, MD: NSO and ORC Macro; 2011. [Google Scholar]
- Maman S, Hogan NM, Kilonza GP. Women’s barriers to HIV-1 testing and disclosure: Challenges for HIV-1 voluntary counselling and testing. AIDS Care. 2001;13(5):595–603. doi: 10.1080/09540120120063223. [DOI] [PubMed] [Google Scholar]
- Mankowski ES, Maton KI. A community psychology of men and masculinity: Historical and conceptual review. American Journal of Community Psychology. 2010;45:73–86. doi: 10.1007/s10464-009-9288-y. [DOI] [PubMed] [Google Scholar]
- Medley AM, Kennedy CE. Provider challenges in implementing antenatal provider-initiated HIV testing and counseling programs in Uganda. AIDS Education and Prevention. 2010;22(2):87–99. doi: 10.1521/aeap.2010.22.2.87. [DOI] [PubMed] [Google Scholar]
- Mkandawire-Valhmu L, Wendland C, Stevens PE, Kako PM, Dressel A, Kibicho J. Marriage as a risk factor for HIV: Learning from the experiences of HIV-infected women in Malawi. Global Public Health. 2013;8(2):187–201. doi: 10.1080/17441692.2012.761261. [DOI] [PubMed] [Google Scholar]
- Mlay R, Lugina H, Becker S. Couple counselling and testing for HIV at antenatal clinics: Views from men, women and counsellors. AIDS Care. 2008;20(3):356–360. doi: 10.1080/09540120701561304. [DOI] [PubMed] [Google Scholar]
- Morin SF, Khumalo-Sakutukwa G, Charlebois ED, Routh J, Fritz K, Lane T, Coates TJ. Removing barriers to knowing HIV status: Same-day mobile HIV testing in Zimbabwe. Journal of Acquired Immune Deficiency Syndromes. 2006;41(2):218–224. doi: 10.1097/01.qai.0000179455.01068.ab. [DOI] [PubMed] [Google Scholar]
- Obermeyer CM, Osborn M. The utilization of testing and counseling for HIV: A review of the social and behavioral evidence. American Journal of Public Health. 2007;97(10):1762–1774. doi: 10.2105/AJPH.2006.096263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter T. Voluntary counseling and testing for couples: A high-leverage intervention for HIV/AIDS prevention in sub-Saharan Africa. Social Science and Medicine. 2001;53(11):1397–1411. doi: 10.1016/s0277-9536(00)00427-5. [DOI] [PubMed] [Google Scholar]
- Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Peltzer K, Matseke G, Mzolo T, Majaja M. Determinants of knowledge of HIV status in South Africa: Results from a population-based HIV survey. BMC Public Health. 2009;9(1):174. doi: 10.1186/1471-2458-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F, Zvandaziva C, Engelsmann B, Dabis F. Acceptability of routine HIV testing (“opt-out”) in antenatal services in two rural districts of Zimbabwe. Journal of Acquired Immune Deficiency Syndromes. 2006;41:514–520. doi: 10.1097/01.qai.0000191285.70331.a0. [DOI] [PubMed] [Google Scholar]
- Pettifor AE, Measham DM, Rees HV, Padiant NS. Sexual power and HIV risk, South Africa. Emerging Infectious Diseases. 2004;10(11):1996–2004. doi: 10.3201/eid1011.040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool R, Nyanzi S, Whitworth JAG. Attitudes to voluntary counselling and testing for HIV among pregnant women in rural south-west Uganda. AIDS Care. 2001;13(5):605–615. doi: 10.1080/09540120120063232. [DOI] [PubMed] [Google Scholar]
- Pulerwitz J, Gortmaker SL, DeJong W. Measuring relationship power in HIV/STD research. Sex Roles. 2000;42(7/8):637–660. [Google Scholar]
- Sambisa W, Curtis S, Mishra V. AIDS stigma as an obstacle to uptake of HIV testing: Evidence from a Zimbabwean national population-based survey. AIDS Care. 2012;22(2):170–186. doi: 10.1080/09540120903038374. [DOI] [PubMed] [Google Scholar]
- Schatz E. “Take your mat and go!” Rural Malawian women’s strategies in the HIV/AIDS era. Culture Health and Sexuality. 2005;7(5):479–492. doi: 10.1080/13691050500151255. [DOI] [PubMed] [Google Scholar]
- Simpson A. Boys to men in the shadow of AIDS: Masculinities and HIV risk in Zambia. New York, NY: Palgrave Macmillan; 2009. [Google Scholar]
- Skovdal M, Campbell C, Madanhire C, Mupambireyi Z, Nyamukapa C, Gregson S. Masculinity as a barrier to men’s use of HIV services in Zimbabwe. Globalization and Health. 2011;7(13):1–14. doi: 10.1186/1744-8603-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KP, Watkins SC. Perceptions of risk and strategies for prevention: Responses to HIV/AIDS in rural Malawi. Social Science and Medicine. 2005;60(3):649–660. doi: 10.1016/j.socscimed.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Tavory I, Swidler A. Condom semiotics: Meaning and condom use in rural Malawi. American Sociological Review. 2009;74(2):171–189. [Google Scholar]
- VCT Efficacy Group. Efficacy of voluntary HIV-1 counseling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: A randomized controlled trial. Lancet. 2000;356(9224):103–112. [PubMed] [Google Scholar]
- Venkatesh KK, Madiba P, De Bruyn G, Lurie MN, Coates TJ, Gray GE. Who gets tested for HIV in a South African urban township? Implications for test and treat and gender-based prevention interventions. Journal of Acquired Immune Deficiency Syndromes. 2011;56(2):151–165. doi: 10.1097/QAI.0b013e318202c82c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel PT. Regression with missing Y’s: An improved strategy for analyzing multiple imputed data. Sociological Methodology. 2007;37:83–117. [Google Scholar]
- Weir S, Hoffman I, Muula A. Final Report: Malawi Prevalence Study. Lilongwe, Malawi: UNC Project-Malawi; 2008. [Google Scholar]
- Weiser SD, Heisler M, Leiter K, Percy-de Korte F, Tlou S, DeMonner S, Iacopino V. Routine HIV testing in Botswana: A population-based study on attitudes, practices, and human rights concerns. PLoS Medicine. 2006;3(7):e261. doi: 10.1371/journal.pmed.0030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingood GM, DiClemente RJ. The theory of gender and power: A social structural theory for guiding public health interventions. In: DiClemente RJ, Crosby RA, Kegler MC, editors. Emerging theories in health promotion practice and research. San Francisco, CA: Jossey-Bass; 2002. pp. 313–346. [Google Scholar]
- Wood K, Jewkes R. Violence, rape, and sexual coercion: Everyday love in a South African township. Gender and Development. 1997;5(2):41–46. doi: 10.1080/741922353. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Progress report. Geneva, Switzerland: World Health Organization; 2007. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. [Google Scholar]


