Abstract
Endoxifen (4-hydroxyl-N-desmethyl tamoxifen), one of the major active metabolites of tamoxifen, has substantially greater estrogen antagonist properties and antiproliferative effects in breast tumor cells than tamoxifen, a mixed estrogen agonist/antagonist. An associated risk of endometrial cancer and hyperplasia has been linked to the estrogen agonist properties of tamoxifen. We evaluated endoxifen using a classic uterotrophic effects method. Rats were given endoxifen or tamoxifen orally for three days. Estradiol was the positive control. Endoxifen and tamoxifen plasma levels exceeded those previously observed clinically. Uterine weight was three-fold higher in the estradiol group than in the tamoxifen or endoxifen groups, which did not differ from vehicle controls. Tamoxifen and endoxifen caused a greater increase in luminal epithelial cell height than estradiol. Both tamoxifen and endoxifen produced an increase in the stromal BrdU labeling index (LI) that was ≤ estradiol and inversely related to dose, but did not affect luminal epithelial cell BrdU LI. As expected, estradiol increased luminal epithelial cell proliferation. These results indicate that endoxifen induces uterotrophic effects, but is less potent than estradiol in eliciting these effects. Given prior preclinical observations that endoxifen has superior antitumor activity than tamoxifen, the observations of similar uterine effects suggest that the endoxifen risk/benefit ratio may be superior to tamoxifen.
Keywords: endoxifen, tamoxifen, endometrial cell proliferation
Introduction
The investigational anticancer drug endoxifen (4-hydroxy-N-desmethyl-tamoxifen) is generated by CYP2D6-mediated bioactivation of tamoxifen and is thought to be a major contributor to the anticancer activity of tamoxifen (Johnson et al., 2004; Ames et al., 2010; Ahmad et al., 2010a, 2010b; Reid et al., 2010; Safgren et al., 2012, Lim et al., 2006). For several key reasons, primary administration of endoxifen is expected to address limitations associated with CYP2D6-dependent generation of endoxifen from tamoxifen. The response rate in breast cancer patients taking tamoxifen is correlated with the CYP2D6 genotype (Jeppesen et al., 1996; Stearns et al., 2003; Johnson et al., 2004; Goetz et al., 2007). Patients who carry genetic variants of CYP2D6 (i.e. reduced or absent enzyme activity) have a shorter time before recurrence and shorter relapse-free survival compared to patients that extensively metabolize tamoxifen (Goetz et al., 2007; Goetz et al., 2013; Schroth et al., 2009; Karle et al., 2013). Similarly, breast cancer patients taking concomitant medications known to inhibit CYP2D6 activity, such as selective serotonin reuptake inhibitors, have a decreased response to tamoxifen (Jin et al., 2005; Stearns et al., 2003; Kelly et al., 2010).
Tamoxifen is also associated with an increased risk of endometrial cancer in women (Fornander et al., 1993; Bernstein et al., 1999; Iqbal et al., 2012). This risk has been linked to the estrogen agonist properties of tamoxifen and potentially, its active metabolites. Endoxifen has been reported by multiple independent groups to have approximately 100 fold greater affinity for the estrogen receptor as well as substantially greater effects on cell proliferation compared to the parent compound, tamoxifen. However, its effects on the estrogen receptor (ER) and proliferation appear to be similar to 4-OH-tamoxifen (Johnson et al., 2004; Hawse et al., 2013; Wu et al., 2009; Wu et al., 2011) and it has potent activity against tamoxifen-refractory tumors preclinically (Reinicke et al., 2011).
At this time, multiple studies are ongoing to evaluate the antitumor activity of endoxifen in women with hormonally positive breast cancer (NCT01327781 and NCT01273168); therefore, it is critical to evaluate the potential uterotrophic effects of endoxifen. To assess whether endoxifen would have similar effects on the endometrium as tamoxifen, we compared the cell proliferative effects of endoxifen with tamoxifen using a validated rat uterotrophic model (Carthew et al., 1999; Kanno et al., 2001, 2003a, 2003b; Owens and Koëter, 2003). Our results suggest that endoxifen has similar effects compared to tamoxifen in this model, suggesting that it fits the classification of a selective estrogen receptor modulator.
Materials and Methods
Test Articles
Endoxifen hydrochloride (4-hydroxy-N desmethyl tamoxifen; NSC 750393) was obtained from the Developmental Therapeutics Program, National Cancer Institute (Bethesda, MD). Tamoxifen citrate salt and1,3,5(10)-estratriene-3,17β-diol 3-benzoate (estradiol) were purchased from Sigma-Aldrich (St. Louis, MO). Formulations were prepared under yellow light in a sterile laminar flow hood. Endoxifen was prepared in sterile water (VWR International, LLC, Brisbane, CA). Tamoxifen was prepared in 10% Tween 80 (Aldrich Chemical Company, Inc. Allentown, PA), 15% PEG 400 (Spectrum Chemical Mfg. Corp., Gardena, CA), and 75% sterile water (VWR International, LLC, Brisbane, CA). Estradiol was prepared in 1,2,3-tricapryloylglycerol (Tricaprylin, Sigma-Aldrich, St. Louis, MO).
Animals and Treatment
The study was conducted using the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996), and the U.S. Department of Agriculture through the Animal Welfare Act, as well as current AAALAC recommendations. Ovariectomized Sprague-Dawley rats (Harlan, Livermore, CA) were 11–12 weeks old at study initiation. Five females/group were given tamoxifen (20 or 200 mg/kg/day) or endoxifen (5, 80, or 200 mg/kg/day). The test articles were administered once daily on Days 1–3 by oral gavage. The positive control estradiol was given as a subcutaneous injection on Days 1–3 to a separate group of five female rats. Vehicle control groups (5 females/group) were included for endoxifen and tamoxifen. All rats on study were given a continuous 3-day subcutaneous infusion (Days 1–3) of 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich, St. Louis, MO) in 0.9% sodium chloride for injection (VWR International, LLC, Brisbane, CA) for the purpose of measuring cell proliferation in the uterus. BrdU was delivered by a surgically implanted Alzet osmotic pump (Alzet, Cupertino, CA; Cat 2ML1) at an infusion rate of 10 μl/hr (200 μg BrdU/hr or 4.8 mg BrdU/day). Rats were euthanized on Day 4, and the uterus from each rat was weighed, fixed in 10% neutral-buffered formalin and processed for histomorphometric and cell proliferation measurements as described below.
Plasma Drug Level Determinations
Blood was collected from all rats on Day 3 for determination of tamoxifen and endoxifen plasma levels at 2 hrs post-dose (Tmax for tamoxifen, where Tmax is the time to reach peak plasma concentration), 4 hr post-dose (Tmax of endoxifen after endoxifen dosing), and 6 hrs post-dose (Tmax of endoxifen after tamoxifen dosing) (Tmax values were obtained from a prior rat pharmacokinetic study conducted for the National Cancer Institute by Southern Research Institute (Birmingham, AL).
Samples were collected in amber tubes and all sample preparation procedures were performed in the dark under minimum exposure to light. Plasma concentrations of tamoxifen, endoxifen, 4-OH-tamoxifen, and N-desmethyl-tamoxifen were measured using a modification of the HPLC assay described by Lee et al., (2003) with a C18 column, fluorescence detection and toremifene as the internal standard.
Necropsy and Histology
The uterus was collected in situ from all rats, weighed with fluid, and placed in 10% neutral-buffered formalin. For each animal, one section of uterine body and three transverse sections from each uterine horn were embedded in paraffin for sectioning and staining. One hematoxylin & eosin (H&E) stained slide and one BrdU immunostained slide were prepared from each block for microscopic evaluation.
Immunohistochemistry for BrdU Incorporation into DNA
Sections (~ 5 μm thick) of paraffin-embedded tissues were placed on positively charged slides (Superfrost Plus, Fisher Scientific, Pittsburgh, PA) to ensure adhesion during processing for BrdU. Standard immunohistochemical methods were used to stain tissues for BrdU (Eldridge et al., 1990). Briefly, tissue sections were deparaffinized in xylene, passed through graded alcohols and treated with 1N HCl for 1 hour at 40°C. In order to further expose antigenic sites, tissues were incubated in citrate buffer for 60 minutes in a pressure cooker. Endogenous peroxidase was inhibited with 1% hydrogen peroxidate for 20 minutes at room temperature. Sections were incubated with normal horse serum for 20 minutes at room temperature followed by incubation with a monoclonal antibody to BrdU diluted 1:25 (Becton Dickenson, Mountain View, CA) for 1 hour at room temperature. After incubation with the primary antibody, slides were incubated with biotinylated horse anti-mouse IgG (1:200) for 30 minutes at room temperature. Slides were then incubated with an avidin-biotin peroxidase complex (Vectastain ABC peroxidase kit, Burlingame, CA) for 30 minutes at room temperature. BrdU incorporation was localized by a final incubation with chromagen 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich, St. Louis, MO). Tissue sections were counterstained with hematoxylin, dehydrated and coverslipped. A negative control slide was included in the staining run and consisted of study tissue that was not incubated with the primary antibody. A positive control slide was included in the staining run that consisted of liver and duodenum from a rat that had been given BrdU prior to necropsy that has been used historically as a positive control for BrdU immunohistochemical staining. Slides from all animals were stained manually in a single staining run to eliminate potential variability between runs.
Quantitation of BrdU Labeling Index
Light microscopy with an eyepiece fitted with a gridded reticle was used for determining the BrdU labeling index (LI) as a measurement of cell proliferation. Positive staining was identified as brown to black nuclear pigment in the nuclei of cells that had incorporated BrdU into the DNA during the S-phase of the cell cycle. Slides were first examined at low magnification (10X) to judge quality of staining, processing and sectioning, pattern of cell labeling, and potential histomorphologic changes. Histomorphologic changes were further assessed by evaluating the corresponding H&E slide from each animal. The BrdU labeling index was then quantified at higher magnification (20X).
BrdU labeling indices for the endometrial stroma were determined by examining 1000 nuclei per transverse section per animal (excluding the glandular epithelium). Three different transverse sections of uterus were quantified per animal representing proximal, medial and distal parts, and the mean value was calculated for each animal as well as each dose group. Thus, a total of 3000 nuclei were scored per animal and the LI expressed as the percentage of BrdU labeled cells in the stroma.
BrdU labeling indices were also evaluated for the luminal epithelium by determining the percentage of BrdU labeled luminal epithelial cells in three different transverse sections of uterus representing proximal, medial and distal parts. At least 100 cells were scored per section for a total of at least 300 luminal epithelial cells per animal. The mean LI, expressed as a percentage, was calculated for each animal as well as for each dose group.
Histomorphometry
From the H&E slides, luminal epithelial cell height was measured manually by light microscopy using an eyepiece fitted with a calibrated gridded reticle. Three measurements of luminal epithelial height were made per transverse uterine cross section for three different sampling sites in the uterus representing the proximal, medial and distal parts. Mean luminal epithelial height values were calculated for each individual animal as well as for each dose group.
Statistical Analysis
Results are presented as the mean and standard error of the mean for each endpoint. The Student’s t-test (two-sided, unequal variance) was used to test for statistical significance in uterine weight, BrdU LI (stroma and luminal epithelium) and luminal cell height comparing tamoxifen and endoxifen treatment groups to their respective vehicle control group. A P value of ≤ 0.05 was considered statistically significant.
Results
Plasma Drug Levels
Mean plasma levels of tamoxifen, endoxifen, 4-OH-tamoxifen, and N-desmethyl-tamoxifen are reported in Table 1. Tamoxifen plasma levels were dose proportional, i.e., 924 nM and 8380 nM, after a dose of 20 and 200 mg/kg/day, respectively. These plasma levels exceed the plasma levels (200–300 nM) noted in humans who received a therapeutic dose of tamoxifen (20 mg daily) (Jin et al., 2005). Endoxifen plasma levels in rats dosed with tamoxifen were 69.4 nM and 129 nM after a dose of 20 or 200 mg/kg/day, respectively. Plasma levels of 4-OH-tamoxifen and N-desmethyl-tamoxifen were undetectable after administration of endoxifen. Endoxifen plasma levels in rats dosed with endoxifen ranged from 168 nM (5 mg/kg/day) to 9620 nM (200 mg/kg/day). Plasma levels of endoxifen resulting from either tamoxifen or endoxifen treatment were similar to or exceeded plasma levels of endoxifen (20–180 nM) found in humans given a therapeutic dose of tamoxifen (Jin et al., 2005). Endoxifen levels also exceeded the plasma concentrations (35.4–52.3 ng/ml or 86.5–127.9 nM) of endoxifen noted in a clinical trial in which 4 mg/kg/day endoxifen was given orally (Ahmad et al., 2010b).
Table 1.
Mean ± SD Peak Plasma Concentrations of Tamoxifen Metabolites in Rats after Oral Dosing of Tamoxifen and Endoxifen Once Daily for Three Days (n=5)
| Drug | Dose (mg/kg) | Tamoxifen (nM) | Endoxifen (nM) | 4-OH-Tamoxifen (nM) | N-Desmethyl-Tamoxifen (nM) |
|---|---|---|---|---|---|
| Tamoxifena | 20 | 924 ± 111 | 69 ± 9 | 135 ± 18 | 682 ± 104 |
| 200 | 8380 ± 1530 | 129 ± 43 | 768 ± 106 | 4920 ± 730 | |
| Endoxifen* | 5 | --- | 168 ± 102 | --- | --- |
| 80 | --- | 8050 ± 1080 | --- | --- | |
| 200 | --- | 9620 ± 1740 | --- | --- |
Tamoxifen, t = 2 hrs after dosing on Day 3; Endoxifen, t = 4 hrs after dosing on Day 3
Treatment-Related Effects on Body and Organ Weights
All rats survived to scheduled necropsy. Since significant body weight losses were seen in endoxifen dose groups given 80 or 200 mg/kg/day (Table 2), uterine organ wet weights were calculated as a percentage of body weight (Figure 1). There were no significant changes in uterine weight as a percentage of body weight in any tamoxifen or endoxifen treated group compared to the corresponding vehicle control group. Mean percent uterine weight in the positive control (estradiol) group was approximately two-fold or three-fold higher than in the tamoxifen or endoxifen groups, respectively.
Table 2.
Mean ± SEM Body and Organ Weights 24 Hours after Three Daily Treatments with Tamoxifen, Endoxifen or Estradiol in Ovariectomized Sprague-Dawley Rats
| Treatment | Body weight (g) | Uterine weight (g) |
|---|---|---|
| 17-(3-estradiol benzoate (0.05 mg/kg/day) | 301±5.5 | 0.96±0.15 |
| Vehicle control (Tween 80/PEG 400) | 256±4.9 | 0.30±0.03 |
| Tamoxifen (20 mg/kg/day) | 257±4.1 | 0.40±0.05 |
| Tamoxifen (200 mg/kg/day) | 248±12 | 0.34±0.08 |
| Vehicle control (water) | 283±10 | 0.33±0.10 |
| Endoxifen (5 mg/kg/day) | 257±3.4 | 0.28±0.04 |
| Endoxifen (80 mg/kg/day) | 249±4.8a | 0.25±0.01 |
| Endoxifen (200 mg/kg/day) | 244±4.9a | 0.26±0.03 |
Statistical significance compared to vehicle (water) control, P≤0.05
Figure 1.
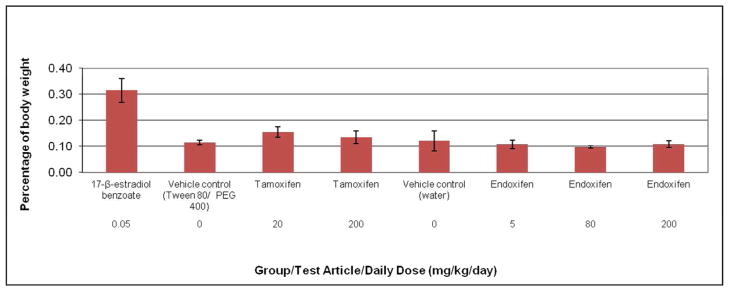
Uterine weight (percentage of body weight) of ovariectomized rats after treatment with estradiol, tamoxifen, or endoxifen. Rats were administered tamoxifen or endoxifen once daily for three days by oral gavage, and euthanized on Day 4. The vehicle control groups for tamoxifen and endoxifen were given 10% Tween 80/15% PEG 400 or sterile water, respectively, via the same route and schedule. The positive control group was given 17-β-estradiol benzoate (estradiol) subcutaneously on the same schedule. No statistically significant changes were noted in uterine weight with tamoxifen or endoxifen compared to respective vehicle controls. Each data point represents the mean and standard error of the mean for five animals per group.
Hypertrophy of Luminal Epithelial Cells of the Uterus in Response to Treatment
All doses of tamoxifen and endoxifen caused an increase in luminal epithelial cell height that was significantly greater than the respective vehicle control group (Figure 2). Luminal epithelial cell height was approximately two-fold greater than vehicle control in the tamoxifen treated groups, and approximately three-fold greater than the corresponding vehicle control in the endoxifen treated groups. Luminal epithelial cell height in the tamoxifen and endoxifen treated groups was less than two-fold greater than the estradiol treated group. Luminal epithelial cell height in the endoxifen treated groups was similar to the 20 mg/kg/day tamoxifen treated group, and slightly lower than the 200 mg/kg/day tamoxifen treated group. Representative photomicrographs showing luminal epithelial cell height are shown in Figure 3.
Figure 2.
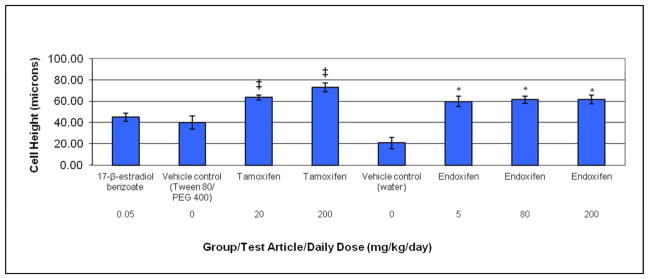
Hypertrophy as determined by luminal epithelial cell height in ovariectomized rats after treatment with estradiol, tamoxifen, or endoxifen. Rats were administered tamoxifen or endoxifen once daily for three days by oral gavage, and euthanized on Day 4. The vehicle control groups for tamoxifen and endoxifen were given 10% Tween 80/15% PEG 400 or sterile water, respectively, via the same route and schedule. The positive control group was given 17-β-estradiol benzoate (estradiol) subcutaneously on the same schedule. ‡Statistically significant at the 5% level compared to vehicle (Tween 80/PEG 400) control; *statistically significant at the 5% level compared to vehicle (water) control. Each data point represents the mean and standard error of the mean for five animals per group.
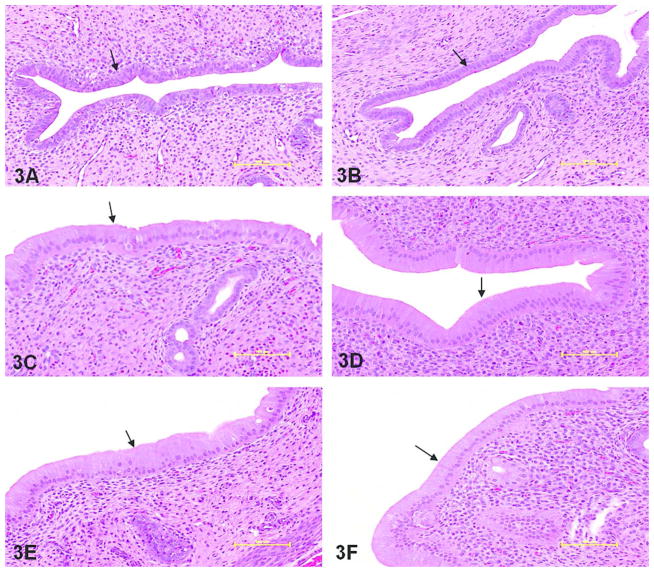
Figure 3.
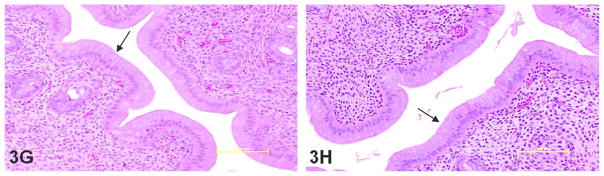
Hematoxylin and eosin stained sections of rat uterus demonstrating luminal epithelial cell hyperplasia as measured by cell height (arrow points to luminal epithelium). Compared to respective vehicle controls (A and B) and positive estradiol control (C), luminal cell height was significantly greater in all tamoxifen and endoxifen dose groups: 20 mg/kg/day tamoxifen (D), 200 mg/kg/day tamoxifen (E), 5 mg/kg/day endoxifen (F), 80 mg/kg/day endoxifen (G) and 200 mg/kg/day endoxifen (H). 20X objective; bar = 100 μm
BrdU Labeling Indices in Stromal and Luminal Compartments of Uterus in Response to Treatment
Compared to the corresponding vehicle control group (Tween 80/PEG 400), the low dose tamoxifen group (20 mg/kg/day) had a significant increase in stromal BrdU LI that was 2.8-fold greater than its vehicle control group, whereas stromal BrdU LI in the high dose tamoxifen group (200 mg/kg/day) did not differ significantly from vehicle control (Figure 4). Stromal BrdU LI was significantly greater than the corresponding vehicle control group (water) in all endoxifen treated groups. Compared to its vehicle group, 5 mg/kg/day, 80 mg/kg/day and 200 mg/kg/day endoxifen resulted in a 6.5-fold, 5.5-fold and 3.7-fold increase in stromal BrdU LI. For both tamoxifen and endoxifen, the stromal BrdU LI was inversely related to dose. The group mean stromal BrdU LI for 20 mg/kg/day and 200 mg/kg/day tamoxifen treated animals was 87% and 39% of estradiol, respectively. In endoxifen treated animals, the mean stromal BrdU LI was 93%, 78% and 53% of estradiol at 5 mg/kg/day, 80 mg/kg/day and 200 mg/kg/day, respectively. The stromal BrdU LI in the 5 mg/kg/day and 80 mg/kg/day endoxifen treated groups was similar to the 20 mg/kg/day tamoxifen treated group. The LI for the 200 mg/kg/day endoxifen treated group was intermediate between the two tamoxifen treated groups.
Figure 4.
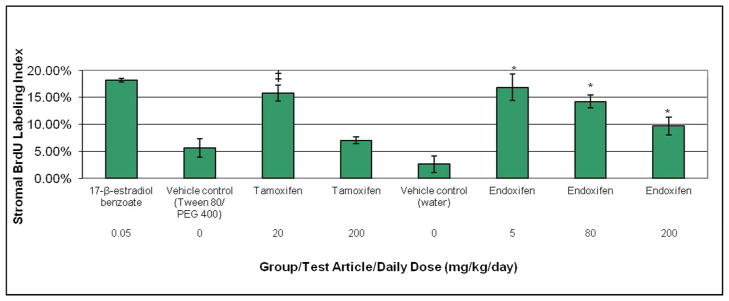
BrdU labeling index in the endometrial stroma of ovariectomized rats after treatment with estradiol, tamoxifen, or endoxifen. Rats were administered tamoxifen or endoxifen once daily for three days by oral gavage, and euthanized on Day 4. The vehicle control groups for tamoxifen and endoxifen were given 10% Tween 80/15% PEG 400 or sterile water, respectively, via the same route and schedule. The positive control group was given 17-β-estradiol benzoate (estradiol) subcutaneously on the same schedule. A continuous 3-day subcutaneous infusion of BrdU was given on Days 1–3 to all rats. ‡Statistically significant at the 5% level compared to vehicle (Tween 80/PEG 400) control; *statistically significant at the 5% level compared to vehicle (water) control. Each data point represents the mean and standard error of the mean for five animals per group.
In the luminal epithelium, with the exception of the 20 mg/kg/day tamoxifen treated group, the BrdU LI in all tamoxifen and endoxifen dose groups did not differ significantly compared to the respective vehicle control groups (Figure 5). Although not statistically significant compared to vehicle controls, there was an apparent dose-related increase in luminal cell BrdU LI for both tamoxifen and endoxifen; however, the mean group values for all tamoxifen and endoxifen treated groups were less than the estradiol group by two- to three-fold. The BrdU LI for the 5 mg/kg/day endoxifen treated group was intermediate between the two tamoxifen treated groups, whereas the 80 mg/kg/day and 200 mg/kg/day endoxifen group had a LI similar to the 200 mg/kg/day tamoxifen treated group. It is worthy to note that in both vehicle control groups, there was one animal with a luminal epithelial cell BrdU LI that approached 100%. The reason for this is unclear. Given that the stromal BrdU LI for these two animals was within the same range as other vehicle control animals, the high BrdU LI observed in the luminal epithelium in these two animals does not appear to be a staining artifact. Furthermore, all slides from all animals on study were stained in a single staining run to prevent potential variability between staining runs.
Figure 5.
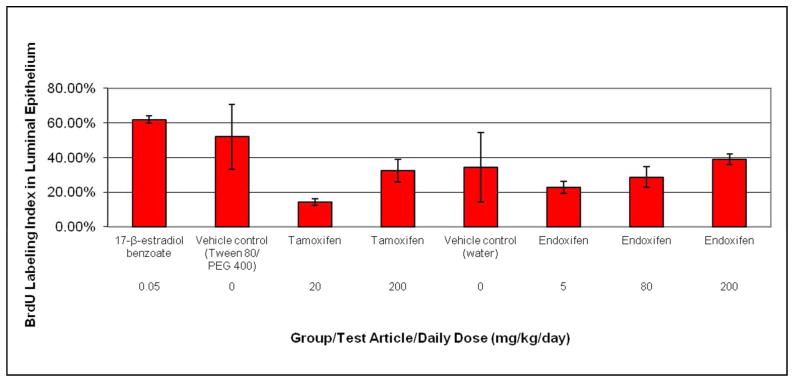
BrdU labeling index in the luminal epithelium of ovariectomized rats after treatment with estradiol, tamoxifen, or endoxifen. Rats were administered tamoxifen or endoxifen once daily for three days by oral gavage, and euthanized on Day 4. The vehicle control groups for tamoxifen and endoxifen were given 10% Tween 80/15% PEG 400 or sterile water, respectively, via the same route and schedule. The positive control group was given 17-β-estradiol benzoate (estradiol) subcutaneously on the same schedule. A continuous 3-day subcutaneous infusion of BrdU was given on Days 1–3 to all rats. Each data point represents the mean and standard error of the mean for five animals per group.
Representative photomicrographs demonstrating BrdU immunohistochemical staining in the endometrial stroma and luminal epithelium of treated animals are shown in Figure 6.
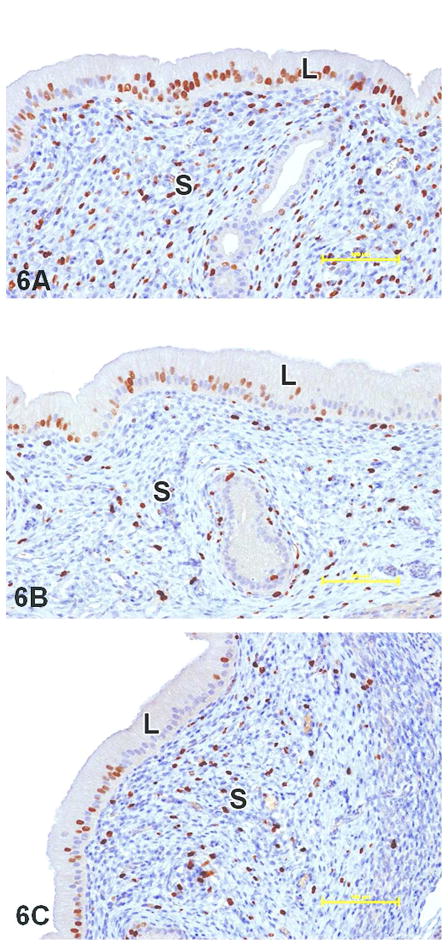
Figure 6.
BrdU immunostained sections demonstrating cell proliferation (brown staining) in luminal epithelial (L) and stromal (S) compartments of rat uterus. Compared to the positive estradiol control (A), cell proliferation was significantly less in both the luminal epithelium and stroma at 200 mg/kg/day tamoxifen (B) and 200 mg/kg/day endoxifen (C). Cell proliferation in the luminal epithelium was also significantly less than the positive control in the lower dose groups of both tamoxifen (20 mg/kg/day) and endoxifen (80 and 5 mg/kg/day) (see Figure 5 for data). Stromal cell proliferation was significantly less than the positive control at 200 mg/kg/day tamoxifen, but not at 20 mg/kg/day. Additionally, stromal cell proliferation was significantly less than the positive control at 80 mg/kg/day endoxifen, but not at 5 mg/kg/day (see Figure 4 for data). 20X objective; bar = 100 μm
Discussion
Our data show that endoxifen and tamoxifen have similar biological effects on the uterus of ovariectomized rats implying a similar uterotrophic effect. Therefore, despite endoxifen’s greater anti-estrogenic effects in breast cancer cells compared to tamoxifen (Hawse et al., 2013; Wu et al., 2009; Wu et al., 2011), its uterotrophic effects are similar to tamoxifen, suggesting a potential improved risk/benefit ratio with endoxifen compared to tamoxifen. Luminal cell height, cell proliferation via BrdU labeling index in both the luminal epithelium and endometrial stroma of the uterus, and uterine weight were evaluated in ovariectomized rats after oral treatment with tamoxifen or endoxifen for three days (modified from Carthew et al., 1999).
Endometrial changes that have been reported in rats given tamoxifen subcutaneously or orally using this model include luminal epithelial cell hypertrophy, increased uterine weight, increased cell proliferation in the endometrial stroma and myometrium, and increased expression of nuclear estrogen receptor α and nuclear progesterone receptor (Carthew et al., 1999; Stygar et al., 2003; Kwekel et al., 2009).
Although uterine wet weight increased in rats in our study given the positive control (estradiol), no changes were noted after tamoxifen or endoxifen treatment. This result differs from several previous studies in ovariectomized rats, in which tamoxifen given orally or subcutaneously for two or three days increased uterine wet weight (Carthew et al., 1999; Stygar et al., 2003; Kwekel et al., 2009). The lack of increased uterine weight in our study is probably unrelated to tamoxifen plasma levels, since uterine wet weight increased in a study in which tamoxifen was given orally daily for 3 days to ovariectomized rats at a much lower dose (0.1 mg/kg/day) than what was used in our study (20 or 200 mg/kg/day) (Kwekel et al., 2009). However, in other studies using ovariectomized rats, estradiol produced fluid accumulation in the uterus, whereas tamoxifen and other partial estrogen agonists did not (Carthew et al., 1999; O’Connor et al., 1996). A number of factors may account for study differences in uterine weight, including water retention, cell proliferation, vascular permeability, estrogen agonist properties, route of drug exposure, and animal strain (Barton et al., 1998; Clark and Peck, 1979; Reel et al., 1996; Carthew et al., 1999; Bailey and Nephew 2002).
There was an inverse relationship between dose and stromal cell proliferation associated with tamoxifen or endoxifen administration. In contrast, increased doses of tamoxifen or endoxifen were associated with a dose-dependent increase in luminal epithelial cell proliferation. Cell proliferation in both the stroma and luminal epithelium was similar between the endoxifen and tamoxifen treated groups. Cell proliferation in both compartments was lower in the tamoxifen and endoxifen treated groups compared to the estradiol group, with the exception of low dose tamoxifen and endoxifen, which produced a stromal cell proliferative response similar to estradiol. The increases in luminal epithelial cell height and stromal cell proliferation were not reflected by a concomitant increase in relative uterine weight. It is not known how much of an increase in cell hypertrophy and/or proliferation is needed to translate to an increase in organ weight. Furthermore, in most uterotrophic assays, an increase in uterine weight typically results from water imbibition. Thus, uterotrophic responses may consist of either cell proliferation, hypertrophy and/or water imbibition. The reduction in BrdU-labeled cells in the stroma at higher doses of tamoxifen and endoxifen may be associated with cells being arrested in S-phase or blocking entry of these cells into the next cell cycle. However, this study was not designed to investigate this hypothesis.
Our histomorphology study results agree with previously published data for tamoxifen and estradiol; i.e. increased luminal epithelial cell height (i.e., hypertrophy) has been documented previously in ovariectomized rats treated with these compounds (Stygar et al., 2003; Carthew et al., 1999; Kwekel et al., 2009). Also, the cell proliferation results in our study are similar to those of Carthew et al. (1999), who found less cell proliferation at 72 hrs in the uterine stroma of Wistar rats given tamoxifen (1 mg/kg/day subcutaneously, once daily for three days) compared to estradiol. However, our results for the proliferative effects of tamoxifen and estradiol on luminal epithelium differ from those of Carthew et al., who found that the BrdU labeling index did not differ at 72 hrs in rats given tamoxifen or estradiol. The route of administration (subcutaneous vs. oral) may explain the dissimilar results for tamoxifen, since metabolite levels and biodistribution likely differ after oral dosing vs. subcutaneous dosing.
The plasma levels of tamoxifen and N-desmethyl-tamoxifen in rats given 200 mg/kg tamoxifen orally are similar to those in humans administered a standard (20 mg) daily tamoxifen dose (Robinson et al. 1991); however, 4-OH-tamoxifen levels are much higher in rats than in humans. Given that endoxifen and 4-OH-tamoxifen are equipotent in terms of binding affinity for the estrogen receptor (Johnson et al., 2004), and the binding affinity of 4-OH-tamoxifen is 30–100-fold greater than tamoxifen, endoxifen and 4-OH-tamoxifen may be out-competing tamoxifen for the estrogen receptor in the rat. This limitation in the model regarding tamoxifen dosing does not affect data interpretation resulting from endoxifen dosing. Our results suggest that, in terms of organ weight (uterus), histomorphology (luminal epithelial cell height), and cell proliferation in the stroma and luminal epithelium of the uterus, endoxifen has similar uterotrophic effects than tamoxifen when administered orally to rats. Given prior preclinical observations that endoxifen has superior antitumor activity than tamoxifen, these observations of similar uterine effects suggest that the endoxifen risk/benefit ratio may be superior to tamoxifen.
Acknowledgments
The authors would like to acknowledge Kim Claggett of Charles River, Pathology Associates for histology and immunohistochemistry support.
Funding
Supported by National Cancer Institute contracts N01-CM-42203, N01-CM-52206, and N02-CM-27009. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. This research was supported [in part] by the Developmental Therapeutics Program in the Division of Cancer Treatment and Diagnosis of the National Cancer Institute.
Abbreviations
- LI
labeling index
- BrdU
5-bromo-2′-deoxyuridine
- Tmax
time to reach peak plasma concentration
- H&E
hematoxylin & eosin
References
- Ahmad A, Ali SM, Ahmad MU, Sheikh S, Ahmad I. Orally administered endoxifen is a new therapeutic agent for breast cancer. Breast Cancer Res Treat. 2010a;122:579–84. doi: 10.1007/s10549-009-0704-7. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Shahabuddin A, Sheikh S, Kale P, Krishnappa M, Rane RC, Ahmad I. Endoxifen, a new cornerstone of breast cancer therapy: demonstration of safety, tolerability, and systemic bioavailability in healthy human subjects. Clin Pharmacol Ther. 2010b;88:814–7. doi: 10.1038/clpt.2010.196. [DOI] [PubMed] [Google Scholar]
- Ames MM, Reid JM, Buhrow SA, Walden CA, Safgren SL, Goetz MP. Endoxifen pharmacokinetics and bioavailability in female mice. Proc Amer Assoc Cancer Res. 2010;51:874. (A3603) [Google Scholar]
- Bailey JA, Nephew KP. Strain differences in tamoxifen sensitivity of Sprague-Dawley and Fischer 344 rats. Anticancer Drugs. 2002;13:939–48. doi: 10.1097/00001813-200210000-00006. [DOI] [PubMed] [Google Scholar]
- Bernstein L, Deapen D, Cerhan JR, Schwartz SM, Liff J, McGann-Maloney E, Perlman JA, Ford L. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999;91:1654–62. doi: 10.1093/jnci/91.19.1654. [DOI] [PubMed] [Google Scholar]
- Carthew P, Edwards RE, Nolan BM, Tucker MJ, Smith LL. Compartmentalized uteroptrophic effects of tamoxifen, toremifene, and estradiol in the ovariectomized Wistar (Han) rat. Toxicol Sci. 1999;48:197–205. doi: 10.1093/toxsci/48.2.197. [DOI] [PubMed] [Google Scholar]
- Clark JH, Peck EJ., Jr . Female Sex Steroids. Springer-Verlag; New York: 1979. [Google Scholar]
- Eldridge SR, Tilbury LF, Goldsworthy TL, Butterworth BE. Measurement of chemically-induced cell proliferation in rodent liver and kidney: a comparison of 5-bromo-2′-deoxyuridine and [3H]thymidine administered by injection or osmotic pump. Carcinogenesis. 1990;11:2245–51. doi: 10.1093/carcin/11.12.2245. [DOI] [PubMed] [Google Scholar]
- Fornander T, Hellstrom AC, Moberger B. Descriptive clinicopathologic study of 17 patients with endometrial cancer during or after adjuvant tamoxifen in early breast cancer. J Nat Cancer Inst. 1993;85:1850–55. doi: 10.1093/jnci/85.22.1850. [DOI] [PubMed] [Google Scholar]
- Goetz MP, Know SK, Suman VJ, Rae JM, Safgren SL, Ames MM, Visscher DW, Reynolds C, Couch FJ, Lingle WL, Weinshilboum RM, Fritcher EG, Nibbe AM, Desta Z, Nguyen A, Flockhart DA, Perez EA, Ingle JN. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101:113–21. doi: 10.1007/s10549-006-9428-0. [DOI] [PubMed] [Google Scholar]
- Goetz MP, Suman VJ, Hoskin TL, Gnant M, Filipits M, Safgren SL, Kuffel M, Jakesz R, Rudas M, Greil R, Dietze O, Lang A, Offner F, Reynolds CA, Weinshilboum RM, Ames MM, Ingle JN. CYP2D6 metabolism and patient outcome in the Austrian Breast and Colorectal Cancer Study Group trial (ABCSG) 8. Clin Cancer Res. 2013;19:500–507. doi: 10.1158/1078-0432.CCR-12-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawse JR, Subramaniam M, Cicek M, Wu X, Gingery A, Grygo SB, Sun Z, Pitel KS, Lingle WL, Goetz MP, Ingle JN, Spelsberg TC. Endoxifen’s molecular mechanisms of action are concentration dependent and different than that of other anti-estrogens. PLoS One. 2013;8(1):e54613. doi: 10.1371/journal.pone.0054613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J, Ginsburg OM, Wijeratne TD, Howell A, Evans G, Sestak I, Narod SA. Endometrial cancer and venous thromboembolism in women under age 50 who take tamoxifen for prevention of breast cancer: a systematic review. Cancer Treat Rev. 2012;38:318–28. doi: 10.1016/j.ctrv.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Jeppesen U, Gram LF, Vistisen K, Loft S, Poulsen HE, Brøsen K. Dose-dependent inhibition CyP1A2, CYP2C19 and CYP2D6 by citalopram, fluoxetine, fluvoxamine and paroxetine. Eur J Clin Pharmacol. 1996;51:73–78. doi: 10.1007/s002280050163. [DOI] [PubMed] [Google Scholar]
- Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH, Skaar T, Storniolo AM, Li L, Araba A, Blanchard R, Nguyen A, Ullmer L, Hayden J, Lemler S, Weinshilboum RM, Rae JM, Hayes DF, Flockhart DA. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, Desta Z, Flockhart DA, Skaar TC. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85:151–59. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- Kanno J, Onyon L, Haseman J, Fenner-Crisp P, Ashby J, Owens W. The OECD program to validate the rat uterotrophic bioassay to screen compounds for in vivo estrogenic responses: Phase I. Environ Health Perspect. 2001;109:785–94. doi: 10.1289/ehp.01109785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno J, Onyon L, Peddada S, Ashby J, Jacob E, Owens W. The OECD program to validate the rat uterotrophic bioassay. Phase 2: dose-response studies. Environ Health Perspect. 2003a;111:1530–49. doi: 10.1289/ehp.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno J, Onyon L, Peddada S, Ashby J, Jacob E, Owens W. The OECD program to validate the rat uterotrophic bioassay. Phase 2: coded single-dose studies. Environ Health Perspect. 2003b;111:1550–58. doi: 10.1289/ehp.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karle J, Bolbrinker J, Vogl S, Kreutz R, Denkert C, Eucker J, Wischnewsky M, Possinger K, Regierer AC. Influence of CYP2D6-genotype on tamoxifen efficacy in advanced breast cancer. Breast Cancer Res Treat. 2013;139:553–60. doi: 10.1007/s10549-013-2565-3. [DOI] [PubMed] [Google Scholar]
- Kelly CM, Juurlink DN, Gomes T, Duong-Hua M, Pritchard KI, Austin PC, Paszat LF. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. BMJ 2010. 2010 Feb 8;340:c693. doi: 10.1136/bmj.c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwekel JC, Forgacs AL, Burgoon LD, Williams KJ, Zacharewski TR. Tamoxifen-elicited uterotrophy: cross-species and cross-ligand analysis of the gene expression program. BMC Medical Genomics. 2009;2:19. doi: 10.1186/1755-8794-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Ward BA, Desta Z, Flockhart DA, Jones DR. Quantification of tamoxifen and three metabolites in plasma by high-performance liquid chromatography with fluorescence detection: application to a clinical trial. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;791(1–2):245–53. doi: 10.1016/s1570-0232(03)00218-6. [DOI] [PubMed] [Google Scholar]
- Lim CK, Yan SX, Lamb JH, White IN, De Matteis F, Smith LL. A comparative study of tamoxifen metabolism in female rat, mouse, and human microsomes. Carcinogenesis. 1994;15:589–93. doi: 10.1093/carcin/15.4.589. [DOI] [PubMed] [Google Scholar]
- Lim YC, Li L, Desta Z, Zhao Q, Rae JM, Flockhart DA, Skaar TC. Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells. J Pharmacol Exp Ther. 2006;318:503–12. doi: 10.1124/jpet.105.100511. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- O’Connor JC, Cook JC, Craven SC, Van Pelt CS, Obourn JD. An in vivo battery for identifying endocrine modulators that are estrogenic or dopamine regulators. Fundam Appl Toxicol. 1996;33:182–95. [PubMed] [Google Scholar]
- Owens W, Koëter HBWM. The OECD program to validate the rat uterotrophic bioassay: an overview. Environ Health Perspect. 2003;111:1527–29. doi: 10.1289/ehp.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reel JR, Lamb JC, IV, Neal BH. Survey and assessment of mammalian estrogen biological assays for hazard characterization. Fundam Appl Toxicol. 1996;34:288–305. doi: 10.1006/faat.1996.0198. [DOI] [PubMed] [Google Scholar]
- Reid JM, Buhrow SA, Safgren SL, Jia L, Schweikart K, Noker PE, Davis M, Collins JM, Goetz MP, Ames MM. Endoxifen pharmacokinetics and bioavailability in female rats. Proc Amer Assoc Cancer Res. 2010;51:632. (A2607) [Google Scholar]
- Reinicke KE, Hou X, Goetz M, Suman VJ, Kuffel MJ, Haluska P, Reid JM, Ames MM. Endoxifen exhibits potent in vitro and in vivo antitumor activity in ER+/HER2+ breast cancer and tamoxifen refractory tumors. Proc Amer Assoc Cancer Res. 2011;52:547. (A2283) [Google Scholar]
- Robinson SP, Langan-Fahey SM, Johnson DA, Jordan VC. Metabolites, pharmacodynamics, and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug Metab Dispos. 1991;19:36–43. [PubMed] [Google Scholar]
- Safgren SL, Buhlow SA, Walden C, Kuffel MJ, Reinicke KE, Reid JM, Goetz MP, Ames MM. Pharmacokinetics of endoxifen and tamoxifen in female mice: implications for comparative in vivo activity studies. Proc Amer Assoc Cancer Res. 2012;53:917. doi: 10.1007/s00280-014-2605-7. (A3781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroth W, Goetz MP, Hamann U, Fasching PA, Schmidt M, Winter S, Fritz P, Simon W, Suman VJ, Ames MM, Safgren SL, Kuffel MJ, Ulmer HU, Boländer J, Strick R, Beckmann MW, Koelbl H, Weinshilboum RM, Ingle JN, Eichelbaum M, Schwab M, Brauch H. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302:1429–1436. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–64. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- Stygar D, Muravitskaya N, Eriksson B, Eriksson H, Sahlin L. Effects of SERM (selective estrogen receptor modulator) treatment on growth and proliferation in the rat uterus. Reproductive Biol Endocrinol. 2003;1:40. doi: 10.1186/1477-7827-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Hawse JR, Subramaniam M, Goetz MP, Ingle JN, Spelsbert TC. The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor α for degradation in breast cancer cells. Cancer Res. 2009;69:1722–27. doi: 10.1158/0008-5472.CAN-08-3933. [DOI] [PubMed] [Google Scholar]
- Wu X, Subramaniam M, Grygo SB, Sun Z, Negron V, Lingle WL, Goetz MP, Ingle JN, Spelsberg TC, Hawse JR. Estrogen receptor-beta sensitizes breast cancer cells to the anti-estrogenic actions of endoxifen. Breast Cancer Res. 2011;13(2):R27. doi: 10.1186/bcr2844. [DOI] [PMC free article] [PubMed] [Google Scholar]