Abstract
Background
Dexrazoxane may reduce anthracycline-associated cardiotoxicity in pediatric cancer patients. However, concerns of secondary acute myeloid leukemia (AML) have led to restrictions on pediatric dexrazoxane use in Europe. Published data about dexrazoxane-associated secondary AML are limited and conflicting. We sought to estimate the secondary AML risk in children receiving dexrazoxane after anthracycline exposure.
Procedure
A retrospective cohort of children with newly identified malignancies (excluding AML) receiving anthracyclines between January 1, 1999 and March 31, 2011 was established using the Pediatric Health Information System (PHIS). Patients were followed for all subsequent admissions to identify dexrazoxane exposures and secondary AML, defined by AML ICD-9 codes and AML induction chemotherapy. Logistic regression was used to model the association of dexrazoxane and secondary AML risk. A propensity score was used to adjust for measurable confounding.
Results
Of 15,532 patients in the cohort exposed to anthracyclines, 1,406 received dexrazoxane. The secondary AML rate was 0.21% (3 of 1,046) in dexrazoxane-exposed and 0.55% (77 of 14,126) in unexposed patients. In a propensity score-adjusted multivariate analysis, dexrazoxane exposure was not associated with an increased risk of secondary AML, OR =0.38, 95% CI 0.11–1.26.
Conclusions
Dexrazoxane was not associated with an increased risk of secondary AML in a large cohort of pediatric cancer patients receiving anthracyclines in US hospitals. While these data support dexrazoxane’s safety in the general pediatric oncology population, additional studies are needed to confirm these findings and to quantify dexrazoxane’s long-term cardioprotective effects. Pediatr Blood Cancer
Keywords: cardiotoxicity after cancer therapy, epidemiology, secondary malignancy, dexrazoxane
INTRODUCTION
Nearly 50% of childhood cancer survivors are exposed to anthracyclines, which are associated with both early and delayed cardiac toxicity [1]. Approximately 5% of survivors of childhood cancer will eventually develop congestive heart failure (CHF), and nearly 10% of children treated with higher cumulative doses of anthracyclines (≥300 mg/m2) will develop CHF [2–4]. Anthracy-cline-associated cardiotoxicity is associated with female sex, younger age at exposure, higher cumulative anthracycline dose, and time from exposure to anthracyclines [5–7].
The mechanism by which anthracyclines and structurally related anthracenediones exert cardiotoxic effects differs from the mechanism primarily responsible for the anti-cancer effect of these drugs. Evidence suggests that anthracyclines produce iron-dependent oxygen free radicals that lead to intracellular damage and cardiac myocyte death [8]. Dexrazoxane is a topoisomerase II inhibitor that also chelates intracellular free iron and iron bound to anthracyclines, thereby reducing the formation of iron-dependent oxygen free radicals [9]. Dexrazoxane has been shown to be an effective cardioprotectant in adults [10,11]. Evidence for the effectiveness of dexrazoxane in children is limited, but the available data support both a short and long-term cardioprotective benefit [12–15]. Dexrazoxane is currently approved by the US Food and Drug Administration (FDA) for use as a cardioprotectant in adult breast cancer patients and as a treatment for extravasation of anthracyclines [16,17].
Despite the cardioprotective benefits of dexrazoxane, its use in children is relatively rare in the United States [18]. Furthermore, the European Medicines Agency (EMA) restricted dexrazoxane use to patients over the age of 18 years in 2011 due to concerns about the risk of secondary acute myeloid leukemia (AML) [19]. This restriction of dexrazoxane use in children is based on data from two randomized trials of dexrazoxane in 239 children with Hodgkin lymphoma from the Pediatric Oncology Group (POG 9425 and 9426) that reported an increased risk of secondary malignant neoplasms (SMN), particularly AML/myelodysplasia (MDS), in a secondary analysis of SMN occurring as a first event [20]. However, long term follow-up data from Dana Farber acute lymphoblastic leukemia (ALL) trials (n =553) and the POG 9404 T cell ALL (n =363) trial showed no increased risk of secondary AML/MDS after dexrazoxane exposure [21–23]. The efficacy of dexrazoxane is currently being studied in the Children’s Oncology Group ALTE11C2 trial to determine whether patients on POG 9404, 9425, and 9426 randomized to receive dexrazoxane have decreased markers of congestive heart failure in long-term follow-up.
Given these conflicting data, the impact of dexrazoxane exposure on the development of secondary AML was evaluated by analysis of a retrospective cohort of cancer patients treated in 43 children’s hospitals contributing data to the Pediatric Health Information System (PHIS) database from January 1, 1999 to March 31, 2011. The hypothesis was that dexrazoxane exposure would not significantly increase the risk of secondary AML in this cohort.
METHODS
Study Design and Cohort Definition
A retrospective cohort design was used. All children admitted to a PHIS member hospital between January 1, 1999 and March 31, 2011 with International Classification of Diseases, 9th Revision (ICD-9) diagnosis codes consistent with malignancy and with an anthracycline or anthracenedione exposure within one year of first identified cancer admission were eligible for cohort inclusion. Patients with an AML or unspecified leukemia ICD-9 code (140. XX-204.XX; 235.XX-239.XX; excluding 205.XX-208.XX) [24] either as a primary or secondary diagnosis were excluded. All index admissions of patients that had more than one malignancy code or were assigned a non-malignancy code as the primary diagnosis code were individually reviewed (DW). Patients were further excluded if the index admission contained an ICD-9 discharge diagnosis code consistent with: (1) a relapsed malignancy; (2) history of stem cell transplantation; or (3) pre-malignant disorder, such as myelodysplasia, in order to limit the population to patients with new onset malignancies. Patients entered the cohort on the day of the first admission that contained an ICD-9 malignancy code and were followed through all subsequent admissions through March 2011. Patients were censored at death or diagnosis of secondary AML. Length of follow-up was defined as the time from first anthracycline exposure to last observed inpatient day within the study period.
Data Source
Data contained in the PHIS database includes the following: encrypted patient medical record number; demographics; dates of admission and discharge; up to 41 ICD-9 discharge diagnosis and procedure codes per hospital admission; and billing data corresponding to specific resources utilized, including pharmaceutical agents, blood products, laboratory tests, radiology imaging studies, and clinical services utilized. All resource utilization data are associated with a date on which they were billed. Additionally, pharmaceutical data includes medication name and route of administration. Laboratory and radiology results are not available, nor are outpatient ICD-9 or billing data.
Oversight of PHIS data quality is a joint effort between the Children’s Hospital Association (data management center), Truven Health Analytics (data processing partner), and participating hospitals. After submission to Truven Health Analytics, quality checks are performed for data entries (e.g., valid ICD-9 diagnosis codes) and reasonable patient information (e.g., birth weight). Reports are generated that identify errors needing correction by the respective hospitals. Error rates above threshold values require hospitals to review their data and resubmit until error rates fall below the threshold values. Known data quality issues are transparently communicated to all PHIS data users, and data quality reports allow the users to exclude specific portions of the dataset based on concerns of data quality.
The PHIS database was queried to extract each patient’s data for each hospital day identified during the study period of interest. SAS version 9.2 (Cary, NC) and STATA statistical software version 11.0 (College Station, TX) were used to convert the PHIS data into a database format representing information for the daily inpatient experience of each child in final cohort. For each inpatient day, information on medications ordered was available. Disposition at hospital discharge was recorded for each admission.
Study Variables
Demographics
Patient age at admission, gender, race, treating institution, and discharge disposition were collected for all admissions. Age was analyzed as a categorical variable (<1 year, 1 to <5 years, 5 to <10 years, 10 to <15 years, 15 to <20 years and ≥20 years). Race (white, black, Asian, Native American, other, and missing) and insurance status (private, government, self-pay, and other) were also analyzed as categorical variables. Since a substantial number of patients had missing data on Hispanic ethnicity, no analyses by ethnicity were conducted.
Anthracycline, Dexrazoxane, and Etoposide Exposure
Medication exposure was determined by pharmacy billing data. The specific anthracyclines and the anthracenedione evaluated were: doxorubicin, daunorubicin, idarubicin, epirubicin, and mitoxantrone. Dexrazoxane and etoposide exposures were defined using billing codes for either dexrazoxane or etoposide occurring concurrently or after exposure to an anthracycline or anthracenedione but prior to onset of a secondary AML diagnosis.
Outcome Definition
The primary outcome was onset of secondary AML. A patient was determined to have secondary AML if an ICD-9 code for AML (205.xx) was assigned in a hospitalization starting at least 90 days after the first observed hospitalization containing an anthracycline exposure and manual review of the chemotherapy billed during the hospitalization inclusive of an AML ICD-9 code was consistent with a standard AML chemotherapy induction regimen [25].
Statistical Analysis
Patient demographics, diagnosis group, and length of follow-up were summarized with standard summary statistics. Patients exposed and not exposed to dexrazoxane were compared on these characteristics using a Chi-square test or Wilcoxon test. The incidence of secondary AML and death in the two groups and unadjusted odds ratios (OR) were estimated and 95% confidence intervals (CI) provided.
A logistic regression model was built with occurrence of secondary AML as the outcome and dexrazoxane exposure as the exposure of interest. A propensity score was established to balance patient-level confounders that may have altered the likelihood of exposure to dexrazoxane. The propensity score represents the probability that a patient would receive dexrazoxane based on specified observed covariates [26]. The propensity scores were calculated using multivariable logistic regression with dexrazoxane exposure as the outcome, and covariates including age, gender, race, insurance, diagnosis group, and hospital as predictors. The scores were then grouped into quintiles, and the distributions of the five propensity score categories in the two exposure groups were described. The five-strata propensity score was included as a categorical covariate in the logistic regression model to adjust for possible confounding. Etoposide exposure was also included in the final logistic model as an individual covariate. This allowed for an estimate of the association of etoposide with the occurrence of secondary AML and adjustment for etoposide as a possible confounder of any association of dexrazoxane and secondary AML. The same analyses were also conducted in the subgroups of patients with lymphomas and non-lymphoma diagnoses separately.
A post hoc power analysis was conducted to investigate the minimal detectable increase of secondary AML rate for dexrazoxane exposed vs. non-dexrazoxane exposed groups, with 80% power, assuming the current study sample size and the observed baseline secondary AML rate in the non-dexrazoxane group. Sensitivity analyses were performed to evaluate the impact of potential misclassification of dexrazoxane exposure on the results.
RESULTS
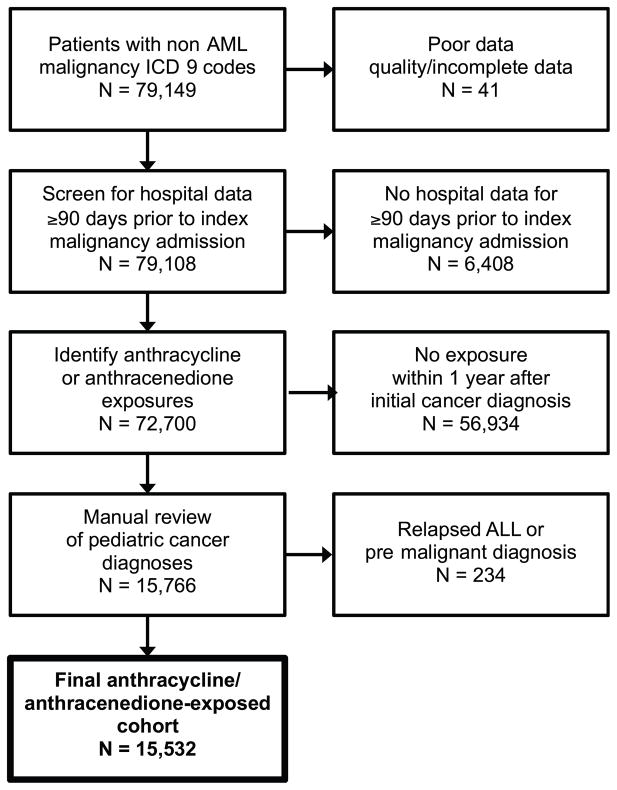
Initially 79,149 patients were identified in the PHIS database with an index admission for malignancy other than AML between January 1, 1999 and March 31, 2011. After excluding patients who had not been billed for an anthracycline and/or anthracenedione during any PHIS admission, had incomplete or missing data, or had any ICD-9 code suggesting the index admission was for a relapsed cancer or pre-malignant diagnosis excluding them from the cohort on individual review, 15,532 patients remained (Fig. 1).
Fig. 1.
Cohort selection process. Patients with non-AML malignancy ICD-9 codes were identified in PHIS. Patients with poor data quality, from hospitals joining PHIS less than 90 days prior to the index admission, without anthracycline/anthracenedione exposures, with relapsed ALL, or without malignant diagnoses were excluded.
Table I displays the patient characteristics, length of follow-up, and time to secondary AML for the entire cohort and by dexrazoxane exposure status. Leukemia (excluding AML) was the most common primary cancer (36.57%), followed by lymphoma (22.5%) and malignancies of the bone/joints (15.18%). Overall, 1,406 (9.1%) patients were exposed to dexrazoxane. All dexrazoxane exposures were intravenous, as expected for cardioprotection rather than for anthracycline extravasation, which would be administered subcutaneously. Patients who received dexrazoxane were more likely to be between the ages of 10 and 20 years and have a malignant bone tumor. Black patients accounted for a higher percentage of children receiving dexrazoxane than among those who did not receive it or among the cohort as a whole; the reverse is true for white patients. Patients with a dexrazoxane exposure had a longer median length of follow-up than those who were unexposed (295 days, interquartile range [IQR] 178–667, vs. 249 days, IQR 95–570, P <0.0001) and were more likely to have an etoposide exposure (51.3% vs. 46.2%, P =0.0003).
TABLE I.
Patient Demographics, Overall and by Dexrazoxane Exposure
| All patients (N =15,532)
|
No dexrazoxane (n =14,126)
|
Dexrazoxane (n =1,406)
|
P-value | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age | <0.0001 | ||||||
| <1 year | 968 | 6.23 | 924 | 6.54 | 44 | 3.13 | |
| 1 to <5 years | 3,706 | 23.86 | 3,491 | 24.71 | 215 | 15.29 | |
| 5 to <10 years | 2,868 | 18.47 | 2,647 | 18.74 | 221 | 15.72 | |
| 10 to <15 years | 4,357 | 28.05 | 3,878 | 27.45 | 479 | 34.07 | |
| 15 to <20 years | 3,302 | 21.26 | 2,906 | 20.57 | 396 | 28.17 | |
| ≥20 years | 331 | 2.13 | 280 | 1.98 | 51 | 3.63 | |
| Gender | 0.83 | ||||||
| Male | 9,051 | 58.27 | 8,228 | 58.25 | 823 | 58.53 | |
| Female | 6,481 | 41.73 | 5,898 | 41.75 | 583 | 41.47 | |
| Race | <0.0001 | ||||||
| White | 11,216 | 72.21 | 10,271 | 72.71 | 945 | 67.21 | |
| Black | 1,760 | 11.33 | 1,554 | 11.00 | 206 | 14.65 | |
| Asian | 444 | 2.86 | 399 | 2.82 | 45 | 3.20 | |
| American Indian | 171 | 1.10 | 155 | 1.10 | 16 | 1.14 | |
| Other | 1,403 | 9.03 | 1,246 | 8.82 | 157 | 11.17 | |
| Missing | 538 | 3.46 | 501 | 3.55 | 37 | 2.63 | |
| Primary insurance at first admission | 0.14 | ||||||
| Private | 6,357 | 40.93 | 5,774 | 40.87 | 583 | 41.47 | |
| Government | 5,964 | 38.40 | 5,459 | 38.65 | 505 | 35.92 | |
| Self-pay | 389 | 2.50 | 346 | 2.45 | 43 | 3.06 | |
| Other | 2,713 | 17.47 | 2,446 | 17.32 | 267 | 18.99 | |
| Unknown | 109 | 0.70 | 101 | 0.71 | 8 | 0.57 | |
| Diagnosis group | <0.0001 | ||||||
| Leukemia | 5,680 | 36.57 | 5,439 | 38.50 | 241 | 17.14 | |
| Lymphoma | 3,494 | 22.50 | 3,379 | 23.92 | 115 | 8.18 | |
| Bones/joints | 2,358 | 15.18 | 1,721 | 12.18 | 637 | 45.31 | |
| Soft tissue | 802 | 5.16 | 673 | 4.76 | 129 | 9.17 | |
| Endocrine | 796 | 5.12 | 764 | 5.41 | 32 | 2.28 | |
| Brain/NS | 191 | 1.23 | 169 | 1.20 | 22 | 1.56 | |
| Urinary | 502 | 3.23 | 485 | 3.43 | 17 | 1.21 | |
| Other | 1,709 | 11.00 | 1,496 | 10.59 | 213 | 15.15 | |
| Etoposide exposurea | 7,251 | 46.68 | 6,530 | 46.23 | 721 | 51.28 | 0.0003 |
| Median | IQR | Median | IQR | Median | IQR | ||
|
| |||||||
| Time to AML among affected patients, days | 553 | 367–1,031 | 538 | 359–1024 | 864 | 552–1563 | 0.25 |
NS, nervous system; IQR, inter-quartile range.
Any exposure at or after time of first anthracycline.
The rate of secondary AML was 0.52% for the entire cohort. The incidence of secondary AML in the dexrazoxane-exposed and unexposed groups was 0.21% (95% CI 0.04–0.62) and 0.55% (95% CI 0.43–0.68), respectively, with a resultant unadjusted OR of 0.39 (95% CI 0.12–1.24). In an unadjusted subgroup analysis exclusive to patients with lymphoma, there was no difference in the incidence of secondary AML in dexrazoxane-exposed versus unexposed patients (0.87% and 0.56%, respectively; P =0.6675). Among patients with diagnoses other than lymphoma, there was also no difference in secondary AML incidence (0.15% and 0.54%, respectively; P =0.0638). A time-to-event analysis showed similar results (not shown).
Table II displays the distribution of the quintiles of the propensity score by dexrazoxane-exposed and unexposed groups. In the dexrazoxane-exposed group, the majority of patients (73%) were in the highest quintile of likelihood for dexrazoxane exposure. In contrast, patients in the dexrazoxane-unexposed group were more equally distributed among propensity score quintiles. After including etoposide exposure and the propensity score as a categorical covariate in the primary model, there was an association between etoposide exposure and secondary AML (OR =2.36, 95% CI 1.48–3.79, P =0.0003) but still no observed association between dexrazoxane exposure and secondary AML (OR =0.38, 95% CI 0.12–1.27, P =0.1166). Subgroup analyses in the lymphoma-only subgroup and lymphoma-excluded subgroup also did not show a statistically significant association (OR =1.41, 95% CI 0.17–11.46, P =0.75, and OR =0.25, 95% CI 0.06–1.07, P =0.0608, respectively).
TABLE II.
Distribution of Patients by Propensity Score Quintile and Dexrazoxane Exposure Status
| Propensity Score Quintile | No dexrazoxane (n =14,126)
|
Dexrazoxane (n =1,406)
|
||
|---|---|---|---|---|
| n | % | n | % | |
| 1 | 3,092 | 21.89 | 16 | 1.14 |
| 2 | 3,051 | 21.60 | 53 | 3.77 |
| 3 | 3,013 | 21.33 | 95 | 6.76 |
| 4 | 2,894 | 20.49 | 212 | 15.08 |
| 5 | 2,076 | 14.70 | 1,030 | 73.26 |
Given the low incidence of secondary AML in this cohort, we conducted a post-hoc power analysis to determine the detectable difference in secondary AML rates between patients with and without dexrazoxane exposure. The cohort sample size provides 80% power to detect an increase in incidence from 0.55% in the dexrazoxane-unexposed group to 1.23% in the dexrazoxane-exposed group, or an absolute increase in incidence of 0.68%.
Since dexrazoxane could have been given in the outpatient setting and therefore not observed, sensitivity analyses were performed to estimate the magnitude of dexrazoxane exposure misclassification necessary to prevent the observation of a statistically significant increased risk of secondary AML after dexrazoxane exposure. If patients were classified as “unexposed” but actually received dexrazoxane and if these misclassified patients had an increased secondary AML rate of 0.75% (50% increase above the observed rate), approximately 5,650 patients (40%) would need to be misclassified as “unexposed” to prevent detection of a statistically significant association of dexrazoxane exposure with increased risk of secondary AML. Likewise, if the misclassified patients had a secondary AML rate of 1% (100% increase), then approximately 2,400 (17%) of dexrazoxane-unexposed patients would need to have been misclassified to prevent observation of a statistically significant association. For these analyses, the secondary AML rate was held fixed at the observed rate of 0.55% in the dexrazoxane-unexposed group.
DISCUSSION
Our study demonstrated no increased risk of secondary AML in children with cancer that received dexrazoxane, either with standard bivariate or propensity score-adjusted multivariate analyses. Furthermore, significant associations were not observed in subgroup analyses both limited to and excluding patients with lymphoma.
Our findings are consistent with the work of Vrooman et al. [23] who studied the effects of dexrazoxane on the development of SMNs in patients receiving anthracyclines for high-risk ALL. With 3.8 years’ median follow-up, only one out of 553 patients developed secondary AML, for an estimated 5-year cumulative incidence of SMNs of 0.24 ± 0.24% (95% CI 0.02–1.29). Additionally, Salzer et al. [22] reported no significant difference either in event-free survival or in the cumulative incidence of SMNs in 363 patients treated on the Pediatric Oncology Group (POG) T cell ALL trial 9404, in which patients were randomized to receive dexrazoxane versus no dexrazoxane.
Our findings are in contrast to a POG study that randomized dexrazoxane use in 478 patients with Hodgkin lymphoma [20]. The overall 4-year cumulative incidence rates (CIR) for SMNs and AML/MDS were not significantly different; however, when development of AML/MDS and secondary malignant neoplasms as a first event were evaluated in a secondary analysis, the 4-year CIR of AML/MDS was 2.1% in the dexrazoxane-exposed group versus 0.42% in the unexposed group (P =0.1052). The CIRs of all SMNs as first event in the two groups were 2.98% and 0.42%, respectively (P =0.0355). In a follow-up paper, the authors hypothesize that the use of three topoisomerase inhibitors concurrently (etoposide, doxorubicin, and dexrazoxane) may be the source of this observed increase in SMNs in their studies [27]. Our data supported the well-known association of etoposide exposure with secondary AML, but in the adjusted model including etoposide, dexrazoxane was still not significantly associated with secondary AML. Importantly, both etoposide and dexrazoxane exposures are analyzed as binary coefficients measuring any exposure to either drug during the study period. Timing of administration of the three topoisomerase inhibitors and cumulative dosing may be relevant but are not evaluated in the present model.
While PHIS data offer many advantages for conducting pharmacoepidemiology studies, several limitations should be recognized. First, the outcome of secondary AML was not confirmed histologically but rather was ascertained by a combination of ICD-9 codes and manual chemotherapy review. This approach is associated with a high positive predictive value but may fail to identify some cases of AML [25]. The assessment of secondary AML was done without knowledge of dexrazoxane exposure status. Therefore, ascertainment rates of secondary AML should not have differed between dexrazoxane-exposed and unexposed patients. Such a non-differential bias should primarily impact study power [28]. Given the available sample size and the concordance between secondary AML rates in our study and other studies, this impact is likely modest.
Second, PHIS data are limited to inpatient data, thus outpatient administration of anthracyclines and dexrazoxane was not captured, resulting in a potential misclassification bias of exposure status. Specifically, patients who received dexrazoxane in the outpatient setting would have been classified as dexrazoxane-unexposed. If dexrazoxane were truly associated with an increased risk of secondary AML, this direction of exposure misclassification could result in an underestimation of the secondary AML risk after dexrazoxane exposure. However, a simulation of the rate of misclassification needed to alter estimates of secondary AML risk demonstrated that a very substantial misclassification would be required to mask a true association between dexrazoxane exposure and secondary AML. As a related potential bias, patient transfers between PHIS institutions and admissions to non-PHIS hospitals are not available in PHIS data. The expected magnitude of this bias is modest given low rates of transfer between PHIS centers and the low rates of provision of care for serious medical conditions such as secondary AML at non-PHIS sites. As noted previously, both these biases would need to occur non-differentially in dexrazoxane-exposed and unexposed patients to result in biased effect size ratios. Although the median follow-up time was relatively short, the median follow-up time for patients who developed AML is significantly longer than for the entire cohort.
PHIS data do not include drug dosage or radiation therapy details, thus preventing analysis of dose-dependent interactions between dexrazoxane and specific chemotherapy agents or radiation exposure. This is of particular concern as certain medication combinations with overlapping mechanisms of action (e.g., the cumulative topoisomerase activity of etoposide, doxorubicin, and dexrazoxane used in combination, as posited by Tebbi et al. [27]) may increase the risk of secondary AML in a dose-dependent manner. This limitation is compounded by the modest sample sizes for specific diagnoses such as Hodgkin lymphoma in the PHIS data set. While neither our subgroup analysis for the lymphoma-only nor the lymphoma-excluded group had an elevated rate of secondary AML in the dexrazoxane-exposed patients, conclusions about the safety of this intervention in patients with lymphoma should be drawn with caution, given the small number of patients in our analysis with this diagnosis. Finally, lack of access to laboratory and radiology data limited our ability to identify patients with disseminated disease who might have received higher-intensity therapy potentially resulting in either a higher risk of secondary malignancy or a higher risk of dying before developing a SMN.
Despite these limitations, this analysis of PHIS administrative and billing data provides an important complement to the data available from clinical trials. First, this analysis presents an estimation of the absolute secondary AML risk after dexrazoxane exposure in the general pediatric oncology population in a large sample set. The large sample size enables detection of modest increases in secondary AML risk after dexrazoxane exposure, and post-hoc power estimates indicate that this study was powered to detect a less than 1% absolute increase in secondary AML risk. These results provide evidence that dexrazoxane exposure does not substantively increase the risk of secondary AML in the general pediatric oncology population.
Future work should include analyses to validate our estimate of the risk of dexrazoxane-associated secondary AML and to define anthracycline dose-specific risks of cardiac toxicity with current pediatric oncology treatment regimens. The currently open COG ALTE11C2 study is designed to study the long-term efficacy of dexrazoxane as a cardioprotectant in pediatric patients who were randomized to therapy on POG 9404, 9425, and 9426 and will provide critical additional data to guide future treatment decisions. More precise estimates of these competing risks are needed if clinicians and regulatory authorities wish to provide patients with evidence-based treatment recommendations. While obtaining precise risk estimates is difficult, the integration of cooperative group clinical trial data and administrative/billing data may facilitate this work, particularly if such work occurs within an international collaborative framework.
Acknowledgments
Grant sponsor: National Cancer Institute, National Institutes of Health; Grant number: 1 R01 CA3881-01; Grant sponsor: The Alex’s Lemonade Stand Foundation, Center for Childhood Cancer Research Seed Grant; Grant sponsor: American Cancer Society, Mentored Research Scientist Grant in Applied and Clinical Research; Grant number: MRSG-12-215-01-LIB
The authors wish to thank Frank M. Balis, M.D., for critical review of this manuscript. This work was supported by the National Cancer Institute at the National Institutes of Health (1 R01 CA3881-01 to R.A.); the Alex’s Lemonade Stand Foundation (Center for Childhood Cancer Research Seed Grant to A.E.S.); and the American Cancer Society (Mentored Research Scientist Grant in Applied and Clinical Research, MRSG-12-215-01-LIB to A.E.S.).
Footnotes
Conflict of interest: Author Brian T. Fisher receives research support from Pfizer Pharmaceuticals. All other authors declare that they have no conflicts of interest.
References
- 1.Ginsberg JP, Cnaan A, Zhao H, et al. Using health-related quality of life measures to predict cardiac function in survivors exposed to anthracyclines. J Clin Oncol. 2004;22:3149–3155. doi: 10.1200/JCO.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 2.van Dalen EC, van der Pal HJ, Kok WE, et al. Clinical heart failure in a cohort of children treated with anthracyclines: A long-term follow-up study. Eur J Cancer. 2006;42:3191–3198. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Steinherz LJ, Steinherz PG, Tan CT, et al. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266:1672–1677. [PubMed] [Google Scholar]
- 4.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 6.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children’s Oncology Group long-term follow-up guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 7.Silber JH, Jakacki RI, Larsen RL, et al. Increased risk of cardiac dysfunction after anthracyclines in girls. Med Pediatr Oncol. 1993;21:477–479. doi: 10.1002/mpo.2950210704. [DOI] [PubMed] [Google Scholar]
- 8.Cvetkovic RS, Scott LJ. Dexrazoxane: A review of its use for cardioprotection during anthracycline chemotherapy. Drugs. 2005;65:1005–1024. doi: 10.2165/00003495-200565070-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hasinoff BB. The interaction of the cardioprotective agent ICRF-187 [+)-1,2-bis(3,5-dioxopiperazinyl-1-yL)propane); its hydrolysis product (ICRF-198); and other chelating agents with the Fe(III) and Cu(II) complexes of adriamycin. Agents Actions. 1989;26:378–385. doi: 10.1007/BF01967305. [DOI] [PubMed] [Google Scholar]
- 10.Speyer JL, Green MD, Kramer E, et al. Protective effect of the bispiperazinedione ICRF-187 against doxorubicin-induced cardiac toxicity in women with advanced breast cancer. N Engl J Med. 1988;319:745–752. doi: 10.1056/NEJM198809223191203. [DOI] [PubMed] [Google Scholar]
- 11.Swain SM. Adult multicenter trials using dexrazoxane to protect against cardiac toxicity. Semin Oncol. 1998;25:43–47. [PubMed] [Google Scholar]
- 12.Wexler LH, Andrich MP, Venzon D, et al. Randomized trial of the cardioprotective agent ICRF-187 in pediatric sarcoma patients treated with doxorubicin. J Clin Oncol. 1996;14:362–372. doi: 10.1200/JCO.1996.14.2.362. [DOI] [PubMed] [Google Scholar]
- 13.Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145–153. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- 14.Lipshultz SE, Scully RE, Lipsitz SR, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: Long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010;11:950–961. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiavetti A, Castello MA, Versacci P, et al. Use of ICRF-187 for prevention of anthracycline cardiotoxicity in children: Preliminary results. Pediatr Hematol Oncol. 1997;14:213–222. doi: 10.3109/08880019709009491. [DOI] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration. [Accessed September 12, 2013];Dexrazoxane hydrochloride (ANDA 200752) approval letter, Octobter 19, 2011. 2011 http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory-apphist.
- 17.US Food and Drug Administration. [Accessed September 12, 2013];Dexrazoxane hydrochloride (ANDA 076068) approval letter, September 28, 2004. 2004 http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory-apphist.
- 18.Walker DM, Fisher BT, Seif AE, et al. Dexrazoxane use in pediatric patients with acute lymphoblastic or myeloid leukemia from 1999 and 2009: Analysis of a national cohort of patients in the Pediatric Health Information Systems database. Pediatr Blood Cancer. 2013;60:616–620. doi: 10.1002/pbc.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Committee for Medicinal Products for Human Use. [Accessed September 15, 2013];European Medicines Agency recommends restricting the use of dexrazoxane-containing medicines. 2011 http://www.ema.europa.eu.
- 20.Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/ myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol. 2007;25:493–500. doi: 10.1200/JCO.2005.02.3879. [DOI] [PubMed] [Google Scholar]
- 21.Barry EV, Vrooman LM, Dahlberg SE, et al. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J Clin Oncol. 2008;26:1106–1111. doi: 10.1200/JCO.2007.12.2481. [DOI] [PubMed] [Google Scholar]
- 22.Salzer WL, Devidas M, Carroll WL, et al. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984–2001: A report from the children’s oncology group. Leukemia. 2010;24:355–370. doi: 10.1038/leu.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vrooman LM, Neuberg DS, Stevenson KE, et al. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: A report from the Dana-Farber Cancer Institute ALL Consortium. Eur J Cancer. 2011;47:1373–1379. doi: 10.1016/j.ejca.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feudtner C, Hays RM, Haynes G, et al. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107:E99. doi: 10.1542/peds.107.6.e99. [DOI] [PubMed] [Google Scholar]
- 25.Kavcic M, Fisher BT, Torp K, et al. Assembly of a cohort of children treated for acute myeloid leukemia at free-standing children’s hospitals in the United States using an administrative database. Pediatr Blood Cancer. 2013;60:508–511. doi: 10.1002/pbc.24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 27.Tebbi CK, Mendenhall NP, London WB, et al. Response-dependent and reduced treatment in lower risk Hodgkin lymphoma in children and adolescents, results of P9426: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2012;59:1259–1265. doi: 10.1002/pbc.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim MY, Goldberg JD. The effects of outcome misclassification and measurement error on the design and analysis of therapeutic equivalence trials. Stat Med. 2001;20:2065–2078. doi: 10.1002/sim.847. [DOI] [PubMed] [Google Scholar]