Abstract
Microparticles (MPs) are shed from normal blood cells and may contribute to the coagulation potential of plasma. Transfusion of fresh frozen plasma (FFP) is used to correct coagulopathies and blood loss in trauma or major surgery. The role of MPs in FFP clinical efficacy is unknown. Regulations that govern the preparation of FFP vary in different countries. The aim of this study was to determine the effect of whole blood (WB)-hold conditions before FFP preparation on the MP profile. WB units were held at room temperature (RT) or combination of RT and refrigeration for up to 24hr before FFP preparation. The MP content in thawed FFP was measured to reflect transfusion practice. The absolute number of MPs in FFP increased with longer WB hold time. Refrigeration of WB may also promote increased generation of MPs. In particular the number of platelet-derived and phosphatidylserine-containing MPs, which are known to have procoagulant properties, increased. Lipid peroxidation increased with longer WB-hold time. Donor-related factors appear to govern lipid peroxidation levels. Holistic proteomic and coagulant analyses of FFP MPs is warranted. Such information could guide the choice of the optimal handling conditions of WB and the most relevant quality control procedures for FFP.
Keywords: microparticles, phosphatidylserine, plasma, platelets, transfusion, whole blood
INTRODUCTION
Microparticles (MPs) are membrane-encapsulated vesicles of less than 1 μm diameter shed by all types of cells, including platelets, red blood cells (RBC), leukocytes, epithelial cells of various tissue origins, as well as tumor cells [1-3]. MPs are released during cell activation, apoptosis, cell senescence, cell lysis and oxidative stress. For these reasons MPs are regarded as indicators for cell injury, stress and thrombosis and are of particular interest for understanding the mechanisms of vascular pathology [4, 5].
Proteomic analyses of plasma MPs have identified a large array of proteins, whilst flow cytometric protocols have been developed for the enumeration and cell-specific characterization of plasma MPs [5-7]. The premise of many of the reported characterization studies centers on the potential usefulness of plasma MPs as clinical diagnostic biomarkers. Consequently, the collection and processing of the plasma specimen prior to MP analysis are critical factors that can compromise the integrity of the plasma MP profile [8]. Published recommendations for the collection and handling of plasma samples for proteomic analysis differ in type of anticoagulant, types of collection tubes, holding temperature of whole blood (WB), use of protease inhibitors [9, 10]. All agree that plasma should be separated immediately or as soon possible after blood collection.
Plasma is also an important therapeutic transfusion product used for the correction of coagulopathies and massive blood loss in trauma or major surgery. The preparation of plasma for transfusion, referred to as fresh frozen plasma (FFP), is strictly regulated to ensure product quality and safety. However, the procedures used by blood processing centers are significantly different to those used for the collection and handling of diagnostic blood plasma specimens [11].
FFP is prepared from citrated WB collected into soft-plasticized polyvinyl packs. Typically WB units are held at room temperature (RT) (i.e. 20 - 24 °C) for a period of time prior to separation of cellular components and freezing of the plasma [11]. In some countries, including Australia and much of Europe, FFP can be prepared from WB units held at RT for up to 24 hr following phlebotomy. For other countries, such as the United States of America (USA), tighter time limits apply and FFP is prepared from WB units held for a maximum of 8 hr at RT after phlebotomy. In the USA, if processing is delayed beyond 8 hr, WB units can be held at 4 °C for up to a further 18 hr and the product is labelled as “plasma frozen within 24 hours after phlebotomy”, which is considered a different product to FFP principally because of loss of activity of labile coagulation factors [12, 13].
The WB-holding conditions and processing time-frames for FFP have been dictated by the requirement to preserve the levels of labile coagulation factors, particularly Factor VIII. However, recent findings have suggested that the MP content in FFP may also be a clinically relevant hemostatic “active ingredient” of FFP [14]. Lawrie et al [14] showed that the MPs in FFP contributed to the rate of clot formation and depletion of MPs significantly slowed clot formation. The FFP used in the study by Lawrie et al [14] was prepared from WB units held overnight at 4 °C followed by filtration through a specialized WB filter, which removes the majority of unwanted leukocytes and platelets, and conserves the RBCs and plasma for preparation to transfusion products. The FFP processing conditions reflected those used by the national blood service in the United Kingdom, but are not necessarily the same conditions used in other countries.
We hypothesized that different WB-hold and processing conditions, as used in various countries, may influence the MP content of FFP and therefore may potentially contribute to differences in clinical efficacy of FFP transfusion. Specifically, we hypothesized that longer WB-hold times would correlate with progressive accumulation of MPs in the plasma due to shedding of MPs from the different types of blood cells in WB. Here we present the MP enumeration and profile studies, which principally utilized flow cytometry in conjunction with simple proteomic assessment, to investigate the effect of WB-hold conditions on the MP content of FFP as a preliminary to more detailed proteomic investigation.
MATERIALS AND METHODS
Whole blood and FFP
WB units (n = 7) were collected according to standard procedures from healthy volunteer blood donors attending the Australian Red Cross Blood Service, Melbourne. The study was conducted with donor consent and approval from the Blood Service’s Human Research and Ethics Committee. All donors were male, blood group A-positive, mean age 31 ± 6 years (range 20 - 41 years).
WB (470 mL ± 10%) was collected into polyvinyl chloride blood collection packs containing 70 mL ± 10% citrate-phosphate-dextrose anticoagulant (Pall Medical, Portsmouth, UK). Each WB unit was divided equally into four pediatric-size (150 mL) blood packs (Terumo, Tokyo, Japan) using a sterile docking device. One each of the pediatric WB packs was held at RT for 6 or 24 hr following blood collection, or at RT for the first 6 hr and then at 4 °C for a further 18 hr (hereafter referred to as 24hr-RT+4°C). These WB-hold conditions represent the typical pre-processing conditions of WB units used to prepare clinical transfusion products in Australia. At the end of the nominated hold period, the WB was centrifuged at 5,005 × g for 10 min at 22 °C to sediment blood cells and platelets, consistent with standard blood banking procedures for the preparation of clinical-grade FFP. Plasma was carefully collected by aspiration and frozen in multiple aliquots at −80 °C until analysis, which was performed within one month of blood collection.
For analysis, plasma samples were rapidly thawed at 37 °C to avoid precipitation of cold-precipitating proteins, consistent with blood banking procedure for the thawing of clinical FFP for transfusion.
MP quantitation by flow cytometry
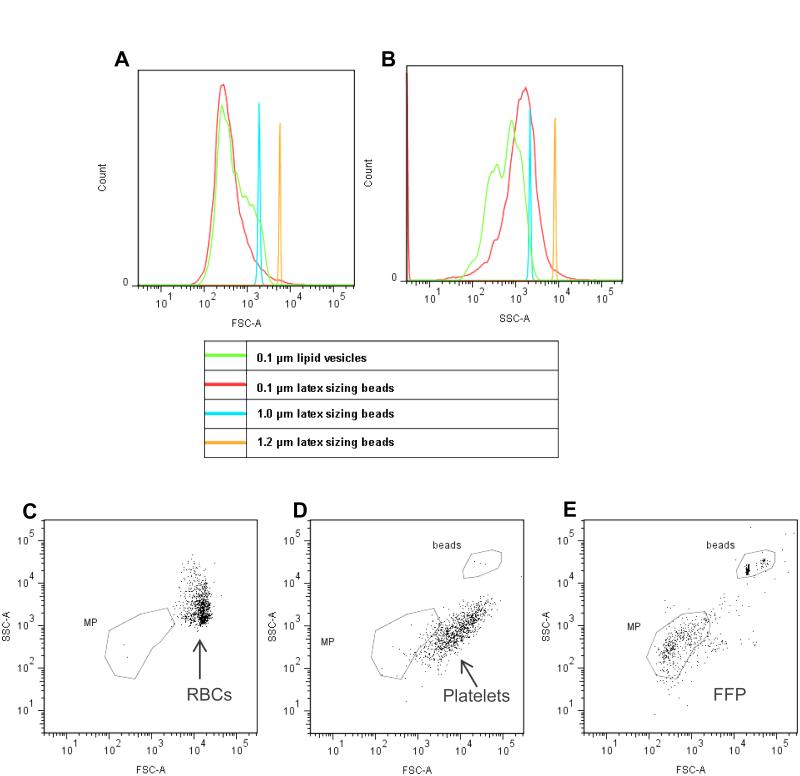
Flow cytometric analyses were performed on a digital flow cytometer (FACSCantoII with Diva software; BD Biosciences, San Jose, CA, USA). The sheath fluid and all buffers were filtered through a 0.2 μm-pore filter to minimize background “noise”. Voltage settings and gating were optimized for MPs using a flow cytometry size calibration kit (Invitrogen Molecular-Probes, Eugene, Oregon, USA) and sulfate latex beads (diameter range 0.1 μm to 1.2 μm) (Invitrogen Molecular-Probes) and lipid vesicles (0.1 μm diameter), which have a closer refractive index to MPs compared to latex beads. As shown in Figure 1, clear discrimination of the 0.1 μm lipid vesicles and the 1.0 and 1.2 um latex beads was equally achieved by the forward scatter and side scatter detectors, which discriminate the size and granularity of particles, respectively (Fig. 1A and B, respectively). The 0.1 μm latex beads were well discriminated by forward scatter, but less well separated by side scatter, suggesting that the material composition of sub-cellular sized particles influences the discriminatory limits of the FACSCanto II flow cytometer. The MP gate was set to include particles of approximately 0.1 μm to 1.0 μm diameter on a log-scale forward scatter versus side scatter plot. The appropriate positioning of the MP gate was confirmed by comparison with freshly collected RBCs (Fig. 1C) and apheresis platelets (Fig. 1D) to ensure that the MP gate excluded intact RBCs and platelets, but captured events present in freshly prepared leukocyte-filtered plasma (Fig 1E). The combined use of both forward scatter and side scatter parameters to define the positioning of the MP gate is consistent with recently published findings from other investigators [15-17]. Enumeration of MPs was determined using absolute count tubes that contain a specified number of fluorescent beads (TruCount tubes, BD Biosciences), according to the manufacturer’s instructions.
Figure 1.
Flow cytometry set-up strategy for defining MPs in FFP.
Log-scale forward scatter (A) and side scatter (B) profiles of latex sizing beads of different diameters, 0.1 μm (red line), 1.0 μm (blue line), 1.2 μm (orange line) and 0.1 μm diameter lipid vesicles (green line) were used to establish and verify that the MP gate captured the appropriate sized events. The position of fresh red blood cells (6 – 8 μm diameter) on a log-scale forward scatter versus side scatter plot (C) and fresh apheresis platelets (diameter 2 - 4 μm) (D) were also used to verify that the MP gate excluded intact cells or larger particulate matter. The forward scatter versus side scatter plot of a fresh leukocyte-filtered plasma sample (E) shows that the position of the MP gate captured the majority of events present in plasma and that there is minimal numbers of intact cells or larger particulate matter in leukocyte-filtered plasma.
MP profile by flow cytometry
The cell source of MPs in FFP and their enumeration were determined by flow cytometry using cell-type specific fluorochrome-conjugated antibodies; anti-CD41 (platelets), anti-CD45 (leukocyte), anti-CD144 (endothelial cells), anti-CD235a (RBCs) and appropriate isotype controls (all from BD BioSciences). A single-label protocol with antibodies conjugated with the same fluorochrome (i.e. phycoerythrin) was used to maximize the accuracy of the results.
Freshly thawed FFP (25 μL) was placed in an absolute bead counting tube (TruCount tubes, BD BioSciences), 2.5 μL of fluorochrome labelled antibody was added and the mixture was diluted to a final volume of 100 μL with 0.2 μm-filtered phosphate buffered saline (PBS), pH 7.2. The samples were incubated for 30 min at RT in the dark, after which 300 μL of filtered PBS was added. The samples were mixed and analyzed immediately by flow cytometry. The sample and reaction volumes were optimized prior to the study. Concentration-matched isotype antibodies were used as controls.
The expression of phosphatidylserine (PS) on MPs and the number of PS+ MPs in FFP was determined by the binding of fluorochrome-conjugated annexin V-allophycocyanin (BD BioSciences) or lactadherin-fluorescein isothiocyanate (Hematologic Technologies, Essex Junction, Vermont). Labeling was performed in absolute bead counting tubes (TruCount tubes, BD BioSciences) as described for the cell-type antibody labeling.
Lipid peroxidation, TBARS assay
The level of lipid peroxidation in FFP samples was determined by the thiobarbituric acid reactive substances (TBARS) assay kit (Cayman Chemical Company, Ann Arbor, USA) according to the manufacturer’s instructions. TBARS was measured as malondialdehyde (MDA)-equivalent units. The normal range for MDA-equivalent units in human plasma is 1.86 – 3.94 μmol/L [18].
Isolation of MPs and SDS PAGE
MPs were isolated from 250 μL of FFP by two centrifugation procedures: 1) high speed centrifugation at 18,000 × g at 4 °C for 30 min in a microcentrifuge (Eppendorf, Hamburg, Germany); 2) a two-step centrifugation that consisted of high speed centrifugation at 18,000 × g at 4 °C for 30 min in a microcentrifuge, as described above, followed by centrifugation at ~100,000 × g at RT for 30 min, 1 hr, 2 hr or 3 hr in an airfuge (Beckman Coulter, Palo Alto, CA). The MP pellet was washed three times in PBS and care was taken in the aspiration of the wash buffer to minimize disturbance of the MP pellet. MP proteins (20 μL MP pellet) were precipitated by cold acetone (80 μL) at −20 °C for 1 hr. Following centrifugation at 18,000 g, 20 °C for 10 min, the precipitated MP proteins were dissolved in 40 μL reducing SDS-PAGE sample buffer (0.25 M Tris, 5 % SDS, 0.15 M DTT, 10 % Glycerol). FFP sample was diluted 20-fold with reducing sample buffer. Protein electrophoresis was performed by loading 10 μL samples, or molecular weight protein standard (Life Technologies, Carlsbad, USA), on to precast 10 % Bis-Tris mini-gel (NuPAGE; Life Technologies) according to the manufacturer’s instructions. Proteins were detected by silver staining (Silverxpress kit; Life Technologies) and the image was captured by an image analyser (Image Quant LAS 4000 imager with ImageQuant TL software; GE Healthcare, Amersham, UK).
Statistical analysis
Results presented are mean ± standard deviation (SD). The two-tailed paired Student’s t-test was used to determine statistical difference between FFP groups. Linear regression was used to determine the strength of the relationship between various MP parameters. Statistical software (SigmaStat Version 3.0; Systat Software, Richmond, CA) was used for statistical analyses. Statistical significance was defined as p < 0.05.
RESULTS
WB hold conditions contribute to the total number of MPs in FFP
A 24 hr RT-hold of WB resulted in a mean 1.6-fold increase in the total number of MPs present in FFP compared to FFP produced from WB units held for only 6 hr at RT (Table 1). The increase in the total number of MPs for 24hr-RT FFP compared to 6hr-RT FFP did not reach statistical significance (p = 0.08) with this sample size, although may become significant in a larger study. Wide inter-donor variability was noted, as evident by the large standard deviations (Table 1). Transfer of the WB units following 6 hr RT-hold to refrigerated-hold for a further 18 hr (i.e. 24hr-RT+4°C FFP) resulted in significantly higher total number of MPs compared to 6hr-RT FFP (1.9-fold increase; p = 0.024) (Table 1). Compared to WB units held at RT for 24 hr, refrigerated-hold resulted in a mean 1.4-fold increase in the total number of MPs, although the increase did not reach statistical significance.
Table 1.
Numbers and marker profile of MPs in FFP produced from WB held under different conditions
| Antibody Specificity / Ligand |
Cell type / Ligand Specificity |
Number of MPs per μL FFP (% of total MPs) |
||
|---|---|---|---|---|
| 6hr-RT | 24hr-RT | 24hr-RT+4°C | ||
| Total MPs | - | 3,698 ± 1,032 | 5,638 ± 3,707 | 7,370 ± 3,869* |
| CD41 | Platelet | 527 ± 262 (14) |
1,140 ± 831*
(20) |
1,210 ± 639*
(16) |
| CD144 | Endothelial cell | 14 ± 13 | 12 ± 13 | 16 ± 17 |
| CD45 | Leukocyte | 6 ± 2 | 8 ± 2 | 8 ± 3 |
| CD235a | Red blood cell | 299 ± 165 (8) |
278 ± 92 (5) |
341 ± 139 (5) |
| annexin V | Phosphatidylserine | 549 ± 145 (15) |
1,073 ± 586*
(19) |
1,322 ± 486*
#
(18) |
| Lactadherin | Phosphatidylserine | 1,567 ± 1,211 (42) |
2,352 ± 1,739*
&
(42) |
2,897 ± 2,068*
&
(39) |
Mean ±SD (n = 10)
Significantly different (p < 0.05) compared to the respective 6hr-RT FFP
Significantly different (p = 0.04) compared to the respective 24hr-RT FFP
Significantly different (p = 0.05) compared to the number of annexin V-binding MPs in the corresponding FFP sample.
Blood cell source of MPs in FFP and effect of WB hold conditions
Identification of the blood cell types that contributed to the generation of MPs during the holding time of WB units prior to processing to FFP showed that platelet-derived (CD41+) MPs were the most abundant MPs and comprised between 14 to 22 % of the total MPs, followed by RBC-derived (CD235a+) MPs (5 to 8 % of total MPs). Leukocyte-derived (CD45+) MPs and endothelial cell-derived (CD144+) MPs each contributed to less than 1 % of the total MPs (Table 1).
Combined, the cell-specific MPs identified by the panel of antibodies used here (CD41, CD45, CD144, CD235a) accounted for only 20 to 30 % of the total MPs in FFP. This suggests that the majority of MPs in FFP either express different cell-specific membrane antigens to those used here, or are devoid of cell-specific membrane antigens.
WB hold time and temperature had a significant effect on the number of platelet-derived (CD41+) MPs present in FFP, but did not significantly influence the numbers of MPs that expressed CD45, CD144 or CD235a. Compared to FFP prepared after 6 hr RT-hold of WB units, the number of platelet-derived (CD41+) MPs significantly increased after 24 hr RT-hold (p = 0.04) (Table 1). Transfer of the WB units to 4 °C (i.e. 24hr-RT+4°C FFP) resulted in a greater increase in the number of /platelet-derived (CD41+) MPs, which was statistically significant compared to 6 hr-RT FFP (p = 0.01). These results suggest that of the various blood cell types in WB, platelets are the most susceptible to generate MPs, which is influenced by extended holding time of WB and possibly refrigeration.
PS expression by MPs and effect of WB hold conditions
Longer WB hold time and cold temperature resulted in significantly increased numbers of PS+ MPs in FFP compared to 6hr-RT FFP (p < 0.05), as detected by the PS-specific ligands, annexin V and lactadherin (Table 1). Refrigerated storage of WB units (i.e. 24hr-RT+4°C FFP group) resulted in a significant increase in PS+ MPs compared to WB held at RT for 24 hrs (i.e. 24hr-RT FFP group), as detected by annexin V binding (p = 0.04); lactadherin binding showed a similar trend, but did not reach statistical significance compared to 24hr-RT FFP (Table 1). Lactadherin bound to higher numbers of MPs compared to annexin V, which reached significance for the 24hr-RT FFP and 24hr-RT+4°C FFP groups (p = 0.05 for both) (Table 1).
Strong correlations were found between the number of PS+ MPs and platelet-derived (CD41+) MPs. PS detected by annexin V was strongly correlated with the number of platelet-derived (CD41+) MPs ( (r > 0.9 for 24hr-RT FFP and 24hr-RT+4°C FFP; p < 0.006). These findings suggest that WB hold, either at RT or refrigeration, is associated with progressive activation and/or apoptosis of platelets that results in the release and accumulation of platelet-derived, PS+ MPs into the plasma.
Lipid peroxidation and WB hold conditions
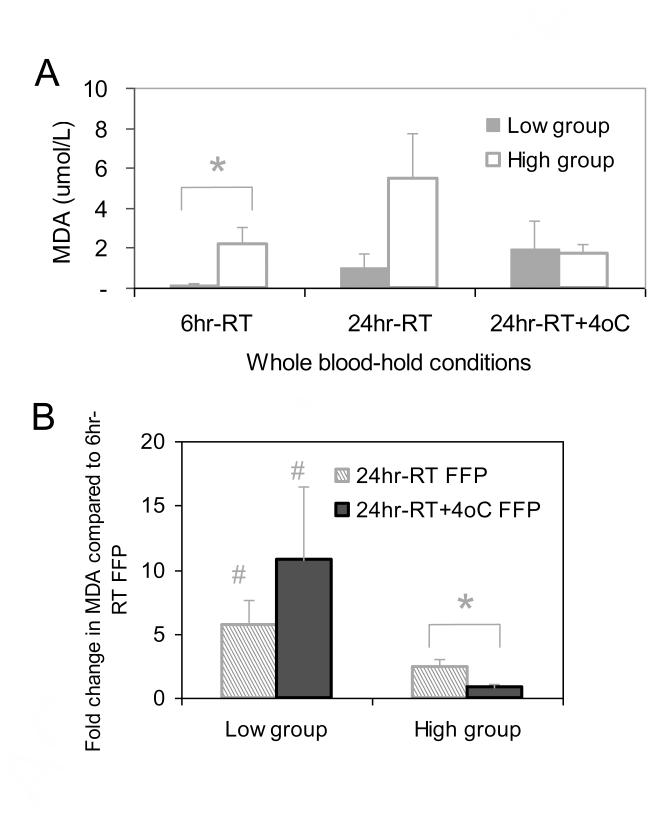
The levels of lipid peroxidation, as measured by the TBARS (MDA) assay, showed divergent results suggestive of donor-related influences. Four of the seven WB units (i.e. henceforth referred to as the ‘low’ group) had low MDA levels in the 6hr RT-hold FFP (mean 0.18 ± 0.08 μmol/L) (Fig. 2A), which markedly increased up to 24 hr RT-hold (mean 5.6-fold increase), and doubled following refrigerated storage (mean 10.8-fold increase compared to 6hr RT-hold FFP) (Fig. 2B), although the concentration did not exceed a mean 2.0 ± 1.4 μmol/L. In contrast, the other three WB units (i.e. henceforth referred to as the ‘high’ group) showed significantly higher levels in the 6hr RT-hold FFP (mean 2.21 ± 0.85 μmol/L) compared to the low group (p = 0.05) (Fig. 2A). The MDAlevels in the high group remained higher than the low group up to 24 hr RT-hold (mean 5.5 ± 2.3 μmol/L and 1.0 ± 0.7 μmol/L, respectively), although the rate of increase for the high group was significantly less than the low group (p = 0.04) (Fig 2B). Refrigerated storage of the high group resulted in significantly lower MDA levels compared to the 24hr RT-hold FFP (p = 0.02) and were not significantly different to the MDA levels in the refrigerated low group 24hr RT+4°C-hold FFP (Fig 2A). These results suggest that MDA levels are significantly influenced by donor-related factors and are further influenced by extended RT-hold of WB.
Figure 2.
Influence of WB-hold conditions on the level of lipid peroxidation in FFP.
Lipid peroxide level was expressed in terms of MDA units in FFP. MDA levels identified two distinct sub-groups of WB donations with either low (n = 4) or high (n = 3) MDA levels in the 6hr-RT FFP samples. (A) MDA levels in the low group (closed bars) and high group (open bars). * Significance difference (p = 0.05) between the low and high groups in the 6hr-RT FFP. (B) Fold-change in the MDA levels of the low and high groups at 24hr-RT WB-hold (striped bars) and 24hr-RT+4°C WB-hold conditions (closed bars) compared to MDA levels in the paired 6hr-RT FFP samples. * Significant difference (p = 0.02) fold-change in MDA levels at 24hr-RT FFP compared to 24hr-RT+4°C FFP. # Significant difference (p < 0.04) in fold-change of MDA levels between the corresponding FFP samples of the low and high groups. Results are mean ± SD.
Centrifugation sediments a proportion of MPs only
High speed- or ultra- centrifugation has been used by many investigators to sediment and enrich plasma MPs for subsequent proteomics analysis. As a preliminary optimization of sample preparation methods for detailed proteomic characterization of FFP MPs, centrifugation under different conditions followed by flow cytometric light scatter analysis of the residual FFP following MP sedimentation and SDS-PAGE analysis of the MP pellet was performed.
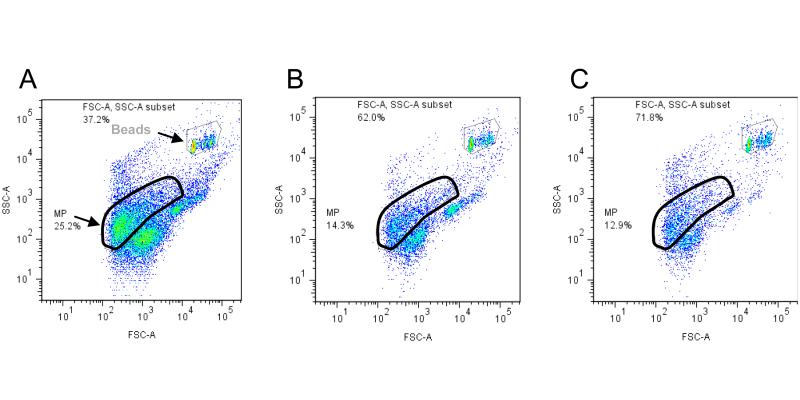
Figure 2 shows the flow cytometric light scatter plots of a representative FFP sample (Fig. 3A) and the same FFP sample following centrifugation at 18,000 × g for 30 min (Fig. 3B) and further centrifugation at ~100,000 × g for 30 min (Fig. 3C). Compared to the events shown in Figure 3A, high speed centrifugation of FFP at 18,000 × g for 30 min resulted in a 44 % depletion of events from the MP gate and 37 % depletion of other undefined/ungated events outside of the MP gate compared to untreated FFP (Fig. 3B) (Table 2). Further centrifugation of the FFP sample at ~100,000 × g for 30 min in an airfuge resulted in an additional 4% depletion of events from the MP gate (i.e. total MP depletion, 48 %), and an additional 23 % depletion of the undefined/ungated events (total depletion of undefined events, 61 %) (Fig. 3C) (Table 2). Thus the centrifugation procedures used in this study achieved sequential depletion of MPs, albeit that the second centrifugation at 100,000 × g yielded only a small incremental depletion compared to the first 18,000 × g centrifugation step. Extended centrifugation in the airfuge of up to 3 hr, failed to achieve further depletion of events from the MP gate or the undefined/ungated events (results not shown).
Figure 3.
Flow cytometric light scatter plots of a representative FFP sample following sequential centrifugation to sediment the MPs.
The MP and counting beads gates are indicated. Undefined events are those events outside of the MP and beads gates. Refer to the Materials and Methods for further details about the gating set-up. (A) Replete 6hr-RT FFP; (B) FFP following high-speed centrifugation at 18,000 × g for 30 min; (C) FFP following high-speed centrifugation as in plot B, and airfuge-centrifugation at 100,000 × g for 30 min. Reduction in the number of events in the FFP samples following centrifugation, as shown in plots B and C, is indicative that the centrifugation steps were effective in sedimenting MPs and other particulate matter present in the replete FFP sample shown in plot A. Approximately 48 % of MPs and 61 % of undefined events were sedimented by the combination of high-speed- and airfuge-centrifugation.
Table 2.
Ability of centrifugation to deplete MPs from FFP as determined by change to the FFP flow cytometric light scatter profile
| FFP treatment | % Flow Cytometric Light Scatter Events (% change compared to untreated FFP) |
|||
|---|---|---|---|---|
| Counting Beads* |
MP-gated Events |
Ungated Events |
FFP Total Events |
|
| Untreated | 37 | 25 | 38 | 63 |
| High speed centrifugation# | 62 (+25) | 14 (−44) | 24 (−37) | 38 (−28) |
| High speed + airfuge centrifugation& |
72 (+35) | 13 (−48) | 15 (−61) | 28 (−47) |
Absolute counting beads (TruCount tubes) added to the FFP sample for enumeration of flow cytometric events; refer to Materials and Methods for details.
18,000 × g for 30 min in a microcentrifuge.
18,000 × g for 30 min in a microcentrifuge followed by ~100,000 × g for 30 min in an airfuge.
+ = enrichment compared to untreated FFP
− = enrichment compared to untreated FFP
The cell source of the MPs remaining in the FFP following high speed centrifugation alone or with airfuge-centrifugation showed ~50 % depletion of platelet (CD41+) MPs, RBC (CD235a+) MPs and PS+ MPs (annexin V-binding and lactadherin-binding MPs), which was consistent with the 44 – 48 % depletion of total MPs (results not shown). These results suggest that high speed centrifugation of FFP does not favor the sedimentation of MPs derived from any particular cell type.
Protein profile of FFP MPs
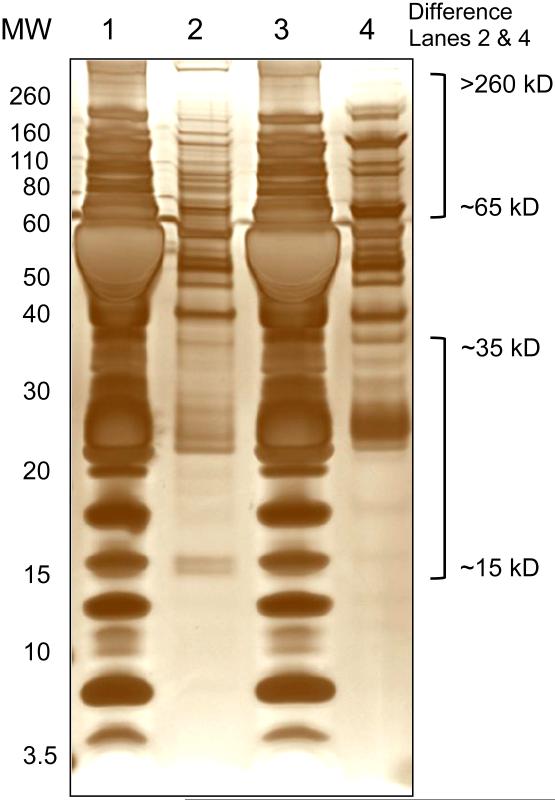
The proteins in the MP pellet obtained following high speed centrifugation were extracted with acetone and separated by SDS-PAGE. The MP pellets were not washed extensively, and consequently may have contained some residual plasma proteins. Nevertheless, SDS-PAGE analysis showed clear differences in the protein band profile of the MP pellet compared to untreated FFP (Fig. 4, lane 1 compared to lanes 2 and 4, respectively). Furthermore differences in the protein band profiles were noted between the FFP MP pellet obtained following high speed centrifugation compared to the MP pellet obtained from FFP that had been subjected to high speed centrifugation and airfuge centrifugation (Fig. 4, lane 2 and 4, respectively). The most apparent differences between the two types of MP preparations appeared to be for proteins in the molecular weight ranges of 15 to 35 kD and 65 to >260 kD. Further studies are required to determine the relevance of these differences in order that the most appropriate methods can be identified for the preparation of FFP MP samples to be used for detailed proteomic investigation.
Figure 4.
Protein band profile of FFP compared to proteins in MPs isolated from FFP.
Representative silver-stained 10% SDS-PAGE gel of a FFP sample (Lane 1 and 3), FFP MP pellet obtained following high-speed centrifugation at 18,000 × g for 30 min (Lane 2); FFP MP pellet obtained following high-speed centrifugation as in Lane 2 and airfuge-centrifugation at 100,000 × g for 30 min (Lane 4). Samples were prepared and run under reducing conditions. Position of the molecular weight standards (MW) is indicated on the left side. The MW ranges of the most apparent differences between the protein profiles of the MP samples in Lanes 2 and 4 are indicated on the right side.
DISCUSSION
This study showed that the MP content of FFP is influenced by the holding conditions of WB units prior to the preparation of blood components for transfusion. In particular, the number of platelet-derived MPs increased along with a concomitant increase in the number of PS+ MPs, which are known to have procoagulant properties. To our knowledge, this is the first report to describe the effect of WB-hold conditions prior to blood component preparation on the number and profile of MPs present in FFP.
PS+ MPs detected by annexin V comprised 15 to 19 % of the total number of MPs in FFP, whilst lactadherin, which also binds to PS, detected 39 to 42 % of the total number of MPs in FFP. The proportion of PS+ MPs approximated the combined total of 20 to 30 % of MPs that expressed the cell type-specific membrane proteins (i.e. CD41, CD45, CD144 and CD235a). It cannot be confirmed that PS+ MPs co-expressed cell type-specific membrane proteins, which would have required a dual-label flow cytometric protocol to be used. A dual-labeling protocol was not used in this study due to concerns of potential technical inaccuracy arising from interference and steric hindrance of multiple bound fluorochrome-labeled reagents to very small particles, such as MPs.
Increased binding of lactadherin compared to annexin V is consistent with findings reported by other investigators, who suggested that lactadherin is a more sensitive marker of PS exposure at the cell membrane of intact cells compared to annexin V [19]. Shi et al [20] suggested that lactadherin can bind to surfaces with greater curvature and lower density of PS compared to annexin V. Such binding characteristics could favor the binding of lactadherin to PS expressed by MPs.
Increased numbers of platelet-derived MPs in FFP following refrigeration of WB units is consistent with the well documented “cold storage lesion” suffered by platelets when exposed to temperatures below approximately 20 °C [21]. Refrigeration of WB is known to induce significant changes to platelets similar to platelet activation, including shedding of bioactive molecules [21-23]. To our knowledge a detailed comparative investigation of the constituents shed from platelets following chilled and RT storage of WB has not been reported. Comparison of the platelet and plasma proteome databases has previously identified at least 41 platelet-derived proteins present in the plasma proteome database [24].
A major proportion of the MPs in FFP did not express the cell-specific markers used in this study (i.e. CD41, CD45, CD144, CD235a), which suggests that MPs in FFP have different cell-specific membrane antigens to the ones measured here or are devoid of cell-type specific membrane antigens. The latter is consistent with the characteristics of exosomes, which are normal constituents of blood plasma [25]. Exosomes (or nanovesicles) are very small vesicles of less than 0.1 μm diameter, compared to the MPs, which are approximately 0.1 μm - 1 μm diameter [1]. The flow cytometric gating strategy used in this study was designed to identify MPs of less than 1 μm diameter. It is possible that a proportion of exosomes were captured in the gate along with MPs. The large numbers of undefined/ungated events with low forward and side scatter below the MP gate in Figure 3A support this notion. Whether a differential role exists for plasma MPs and exosomes in hemostasis and coagulation remains to be elucidated.
The proportions of cell type specific MPs reported here differ from those reported by Lawrie e al [14] who found that RBC-derived (CD235a+) MPs were significantly more abundant than platelet-derived (CD41+) MPs. The reason for the difference between the two studies is not clear, but may relate to differences in processing and handling of the WB/FFP units, and/or the protocol for MP flow cytometric quantitation or the cell type-specific antibodies used. A potentially important difference is that the WB units used by Lawrie et al [14] were filtered to deplete leukocytes prior to the separation of FFP. We are currently investigating the effect of WB leukocyte-filtration on FFP MP content and profile. Preliminary findings suggest that leukoreduction of WB can reduce the total number of MPs as well as the relative proportions of cell-specific MPs (unpublished data).
Plasma lipid peroxides and reactive aldehydes, expressed as MDA units, increase as a result of oxidative stress. In the absence of sufficient anti-oxidant activity, lipid peroxidation products and free radicals can lead to irreparable cell damage and protein modification [26]. The MDA level in normal plasma has been variously suggested to be less than 1.5 μmol/L and 1.9 – 4 μmol/L. The results of the ‘low’ and ‘high’ groups reported here were consistent with the two normal ranges and suggest that susceptibility to oxidative stress is governed by donor-related factors. No association of MDA concentration with any of the MP parameters measured was apparent, however a larger sample size is needed to determine whether or not a relationship exists.
Further investigations are underway to understand the implications of donor-related oxidative stress levels at the time of blood donation on the quality and efficacy of clinical FFP for transfusion. The WB donations used in this study came from young male donors (mean 31 ± 6 years, n = 7). There was no apparent medical history to explain the difference in MDA levels between the ‘low’ and ‘high’ groups. All of the donors had previously donated (mean 2.3 ± 1.3 times; time duration since previous donation, median 22 weeks, range 8 – 302 weeks). Lifestyle differences, such as diet, smoking, exercise are known to affect plasma oxidant/anti-oxidant levels [27-29]. Information about lifestyle is not collected from normal volunteer blood donors donating to public blood transfusion services. Oxidative stress levels at the time of blood donation may also have implications for the extent of storage-related effects on the cellular components, RBCs and platelets, and this is also currently under investigation.
Although identification of specific proteins was not conducted in this study, SDS-PAGE analysis clearly showed different protein band profiles of FFP MPs compared to replete FFP. Detailed proteomics analysis of FFP MPs is therefore likely to be an informative approach to better understand the effects of WB hold conditions on the quality and efficacy of clinical FFP transfusion products. Improved procedures to isolate MPs will be required, including differential characterization of exosomes, to ensure that future the proteomic analyses of MPs present in FFP are accurate and informative.
CONCLUSION
The hold time and temperature of WB units prior to the preparation of FFP has a significant effect on the MP content of FFP. In particular the numbers of platelet-derived MPs and PS+ MPs increased with extended hold time of WB units, as well as MPs (and/or exosomes) of undefined cell origin. Refrigerated temperature may also promote increased generation of MPs. The influence of MP content on FFP coagulant potential needs to be further investigated. Donor-related factors, such as oxidative stress, may also contribute to the quality of FFP products. FFP is frequently used for trauma resuscitation and in massive transfusion where coagulopathy is an inherent morbidity [30-32]. However, the underlying clinical evidence for FFP transfusion is a topic of current debate [33-36]. Further studies to better understand the effects of WB hold conditions and processing variables, such as leukoreduction, on the quality and efficacy of clinical FFP transfusion products, including holistic proteomic and coagulant analyses, are therefore warranted to help fill the current gap in knowledge of the clinical efficacy of FFP transfusion. This combined approach could provide important new information to guide the choice of the optimal handling conditions of WB units prior to blood component preparation and the most relevant quality control procedures for clinical FFP, such as the MP content and/or specific coagulation factors. Similar studies may also be warranted to determine the effect of different collection methodologies, such as plasmapheresis, on MP content and the clinical efficacy of apheresis-derived FFP.
HIGHLIGHTS.
Hold conditions of whole blood affects the fresh frozen plasma microparticle content.
Platelet-derived microparticles increase with longer hold time.
Phoshatidylserine-positive microparticles increase with longer hold time.
Plasma lipid peroxidation is donor-related.
Effect on clinical efficacy of plasma transfusion needs further investigation.
ACKNOWLEDGEMENTS
We thank the Donor Services and Processing laboratory staff at Australian Red Cross Blood Service, Melbourne for assistance with the collection and processing of whole blood units. Australian governments fully fund the Australian Red Cross Blood Service for the provision of blood products and services to the Australian community. This work was funded in part by NIH Grant 1R01 HL095470-01A1.
Abbreviations
- FFP
fresh frozen plasma
- MDA
malondialdehyde
- MP
microparticle
- PS
phosphatidylserine
- RBC
red blood cell
- TBARS
thiobarbituric acid reactive substances
- WB
whole blood
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No conflict of interest to disclose.
REFERENCES
- 1.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 2.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107:1047–57. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 3.Rak J. Microparticles in cancer. Sem Thromb Hemost. 2010;36:888–906. doi: 10.1055/s-0030-1267043. [DOI] [PubMed] [Google Scholar]
- 4.Amabile N, Rautou PE, Tedgui A, Boulanger CM. Microparticles: key protagonists in cardiovascular disorders. Sem Thromb Hemost. 2010;36:907–16. doi: 10.1055/s-0030-1267044. [DOI] [PubMed] [Google Scholar]
- 5.Little KM, Smalley DM, Harthun NL, Ley K. The plasma microparticle proteome. Sem Thromb Hemost. 2010;36:845–56. doi: 10.1055/s-0030-1267038. [DOI] [PubMed] [Google Scholar]
- 6.Gelderman MP, Simak J. Flow cytometric analysis of cell membrane microparticles. Methods Mol Biol. 2008;484:79–93. doi: 10.1007/978-1-59745-398-1_6. [DOI] [PubMed] [Google Scholar]
- 7.Orozco AF, Lewis DE. Flow cytometric analysis of circulating microparticles in plasma. Cytometry Part A. 2010;77A:502–14. doi: 10.1002/cyto.a.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuana Y, Bertina RM, Osanto S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb Haemost. 2010:105. doi: 10.1160/TH10-09-0595. [DOI] [PubMed] [Google Scholar]
- 9.Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8:113–7. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi J, Craft D, Gelfand CA. Minimizing preanalytical variation of plasma samples by proper blood collection and handling. Methods Mol Biol. 2011;728:137–49. doi: 10.1007/978-1-61779-068-3_8. [DOI] [PubMed] [Google Scholar]
- 11.Greening DW, Glenister KM, Sparrow RL, Simpson RJ. International blood collection and storage: clinical use of blood products. J Proteomics. 2010;73:386–95. doi: 10.1016/j.jprot.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Roback JD, Coombs MR, Grossman BJ, Hillyer CD. Technical manual. 16th AABB; Bethesda, USA: 2008. [Google Scholar]
- 13.Yazer MH, Cortese-Hassett A, Triulzi DJ. Coagulation factor levels in plasma frozen within 24 hours of phlebotomy over 5 days of storage at 1 to 6 degrees C. Transfusion. 2008;48:2525–30. doi: 10.1111/j.1537-2995.2008.01913.x. [DOI] [PubMed] [Google Scholar]
- 14.Lawrie AS, Harrison P, Cardigan RA, Mackie IJ. The characterization and impact of microparticles on haemostasis within fresh-frozen plasma. Vox Sang. 2008;95:197–204. doi: 10.1111/j.1423-0410.2008.01081.x. [DOI] [PubMed] [Google Scholar]
- 15.Mullier F, Bailly N, Chatelain C, Dogné JM, Chatelain B. More on: calibration of the measurement of microparticles: needs, interests, and limitations of calibrated polystyrene beads for flow cyotmetry-based quantitation of biological microparticles. J Thromb Hemost. 2011;9:1679–81. doi: 10.1111/j.1538-7836.2011.04386.x. [DOI] [PubMed] [Google Scholar]
- 16.Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJP, Hole P, Carr B, Redman CWG, Harris AL, Dobson PJ, Harrison P, Sargent IL. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine. 2011;7:780–8. doi: 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Pol E, van Gemert MJC, Sturk A, Nieuwland R, van Leewen TG. Single vs. swarm detection of microparticles and exosomes by flow cytometry. J Thromb Hemost. 2012;10:919–30. doi: 10.1111/j.1538-7836.2012.04683.x. [DOI] [PubMed] [Google Scholar]
- 18.Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol. 1998;108:101–6. doi: 10.1385/0-89603-472-0:101. [DOI] [PubMed] [Google Scholar]
- 19.Hou J, Fu Y, Zhou J, Li W, Xie R, Cao F, et al. Lactadherin functions as a probe for phosphatidylserine exposure and as an anticoagulant in the study of stored platelets. Vox Sang. 2011;100:187. doi: 10.1111/j.1423-0410.2010.01375.x. [DOI] [PubMed] [Google Scholar]
- 20.Shi J, Heegaard CW, Rasmussen JT, Gilbert GE. Lactadherin binds selectively to membranes containing phosphatidyl-L-serine and increased curvature. Biochim Biophys Acta. 2004;1667:82–90. doi: 10.1016/j.bbamem.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Egidi MG, D'Alessandro A, Mandarello G, Zolla L. Troubleshooting in platelet storage temperature and new perspectives through proteomics. Blood Transfus. 2010;8(Suppl 3):s73–81. doi: 10.2450/2010.012S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayukawa O, Nakamura K, Kariyazono H, Ikeda R, Arima J, Shinkawa T, et al. Enhanced platelet responsiveness due to chilling and its relation to CD40 ligand level and platelet-leukocyte aggregate formation. Blood Coag Fibrin. 2009;20:176–84. doi: 10.1097/MBC.0b013e328322ffd5. [DOI] [PubMed] [Google Scholar]
- 23.Rumjantseva V, Hoffmeister KM. Novel and unexpected clearance mechanisms for cold platelets. Transfus Aph Sci. 2010;42:63–70. doi: 10.1016/j.transci.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greening DW, Glenister KM, Kapp EA, Moritz RL, Sparrow RL, Lynch GW, et al. Comparison of human platelet membrane-cytoskeletal proteins with the plasma proteome: Towards understanding the platelet-plasma nexus. Proteomics Clin Appl. 2008;2:63–77. doi: 10.1002/prca.200780067. [DOI] [PubMed] [Google Scholar]
- 25.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Exp Rev Proteomics. 2009;6:267–83. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 26.Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 27.van den Berg R, van Vliet T, Broekmans WM, Cnubben NH, Vaes WH, Roza L, et al. A vegetable/fruit concentrate with high antioxidant capacity has no effect on biomarkers of antioxidant status in male smokers. J Nutr. 2001;131:1714–22. doi: 10.1093/jn/131.6.1714. [DOI] [PubMed] [Google Scholar]
- 28.Prior RL. Plasma antioxidant measurements. J Nutr. 2004;134:3184S–5S. doi: 10.1093/jn/134.11.3184S. [DOI] [PubMed] [Google Scholar]
- 29.Prior RL, Gu L, Wu X, Jacob RA, Sotoudeh G, Kader AA, et al. Plasma antioxidant capacity changes following a meal as a measure of the ability of a food to alter in vivo antioxidant status. J Am Coll Nutr. 2007;26:170–81. doi: 10.1080/07315724.2007.10719599. [DOI] [PubMed] [Google Scholar]
- 30.Brown LM, Aro SO, Cohen MJ, Holcomb JB, Wade CE, Brasel KJ, et al. A high fresh frozen plasma: packed red blood cell transfusion ratio decreases mortality in all massively transfused trauma patients regardless of admission international normalized ratio. J Trauma. 2011;71:S358–63. doi: 10.1097/TA.0b013e318227f152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kautza BC, Cohen MJ, Cuschieri J, Minei JP, Brackenridge SC, Maier RV, et al. Changes in massive transfusion over time: An early shift in the right direction? J Tauma. 2012;72:106–11. doi: 10.1097/TA.0b013e3182410a3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizoli SB, Scarpelini S, Callum J, Nascimento B, Mann KG, Pinto R, et al. Clotting factor deficiency in early trauma-associated coagulopathy. J Trauma. 2011;71:S427–34. doi: 10.1097/TA.0b013e318232e5ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho AM, Dion PW, Yeung JH, Holcomb JB, Critchley LA, Ng CS, et al. Prevalence of survivor bias in observational studies on fresh frozen plasma: erythrocyte ratios in trauma requiring massive transfusion. Anesthesiology. 2012;116:716–28. doi: 10.1097/ALN.0b013e318245c47b. [DOI] [PubMed] [Google Scholar]
- 34.Kozek-Langenecker S, Sorensen B, Hess JR, Spahn DR. Clinical effectiveness of fresh frozen plasma compared with fibrinogen concentrate: a systematic review. Crit Care. 2011;15:R239. doi: 10.1186/cc10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitra B, Cameron PA, Gruen RL. Aggressive fresh frozen plasma (FFP) with massive blood transfusion in the absence of acute traumatic coagulopathy. Injury. 2012;43:33–7. doi: 10.1016/j.injury.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Yang L, Stanworth S, Hopewell S, Doree C, Murphy M. Is fresh-frozen plasma clinically effective? An update of a systematic review of randomized controlled trials. Transfusion. 2012 Jan 18; doi: 10.1111/j.1537-2995.2011.03515.x. [Epub ahead of print] doi: 10.1111/j.1537-2995.03515.x. [DOI] [PubMed] [Google Scholar]