Summary
The poor clinical outcome in pancreatic ductal adenocarcinoma (PDA) is attributed to intrinsic chemoresistance and a growth-permissive tumor microenvironment. Conversion of quiescent to activated pancreatic stellate cells (PSCs) drives the severe stromal reaction that characterizes PDA. Here we reveal that the vitamin D receptor (VDR) is expressed in stroma from human pancreatic tumors and that treatment with the VDR ligand calcipotriol markedly reduced markers of inflammation and fibrosis in pancreatitis and human tumor stroma. We show that VDR acts as a master transcriptional regulator of PSCs to reprise the quiescent state resulting in induced stromal remodeling, increased intratumoral gemcitabine, reduced tumor volume and a 57% increase in survival compared to chemotherapy alone. This work describes a molecular strategy through which transcriptional reprogramming of tumor stroma enables chemotherapeutic response and suggests Vitamin D priming as an adjunct in PDA therapy.
Introduction
Cancer-associated fibroblast-like cells (CAFs) in the tumor stroma have been shown to exert a profound influence on the initiation and progression of human cancer (Bhowmick et al., 2004; Kalluri and Zeisberg, 2006; Pietras and Ostman, 2010; Rasanen and Vaheri, 2010; Shimoda et al., 2010). Pancreatic ductal adenocarcinoma (PDA) in particular is defined by a prominent stromal compartment, and numerous features ascribed to CAFs promote pancreatic cancer progression and hinder therapeutic efficacy (Mahadevan and Von Hoff, 2007). CAFs enhance PDA growth in allograft models in part via paracrine activation of pro-survival pathways in tumor cells, and inhibition of tumor-stroma interactions limits tumor progression (Hwang et al., 2008; Ijichi et al., 2011; Vonlaufen et al., 2008). Further, the dense extracellular matrix (ECM) associated with PDA obstructs intratumoral vasculature, preventing chemotherapeutic delivery (Olive et al., 2009), leading to new ideas to overcome this stromal “roadblock” (Jacobetz et al., 2012; Provenzano et al., 2012). Beyond drug delivery, recent evidence implicates the tumor stroma in innate drug resistance in numerous tumor types (Straussman et al., 2012; Wilson et al., 2012), and treatment paradigms targeting both neoplastic cells and stromal components are emerging for PDA (Heinemann et al., 2012). While these findings suggest that CAFs in the PDA microenvironment represent a potential therapeutic target, the tumor-supporting features of pancreatic stellate cells (PSCs), the predominant fibroblastic cell type in the tumor microenvironment of the pancreas, remain poorly understood.
PSCs are nestin-positive and resident lipid-storing cells of the pancreas, with an important role in normal ECM turnover (Apte et al., 1998; Phillips et al., 2003). In health, PSCs are in a quiescent state, characterized by abundant cytoplasmic lipid droplets rich in vitamin A, and low levels of ECM component production (Apte et al., 2012). During pancreatic injury, PSCs are activated by cytokines, growth factors, oxidative or metabolic stress and transdifferentiate to a myofibroblast-like cell (Masamune and Shimosegawa, 2009). Activated PSCs lose their cytoplasmic lipid droplets, express the fibroblast activation marker α-smooth muscle actin (αSMA), acquire proliferative capacity, and synthesize abundant ECM proteins. Activated PSCs also acquire an expansive secretome which is starkly subdued in the quiescent state (Wehr et al., 2011). Persistent PSC activation under conditions of chronic injury results in pathological matrix secretion leading to fibrosis, creating a physical barrier to therapy. Further, a reciprocal supportive role for activated PSCs and pancreatic cancer cells has become increasingly appreciated: pancreatic cancer cells produce mitogenic and fibrogenic factors which promote PSC activation, such as platelet-derived growth factor (PDGF), transforming growth factor β (TGFβ), and sonic hedgehog (SHH) (Apte and Wilson, 2012; Bailey et al., 2008). Reciprocally, activated PSCs produce PDGF, insulin-like growth factor 1 (IGF1), connective tissue growth factor (CTGF) and other factors which may promote cancer cell proliferation, survival, and migration (Apte and Wilson, 2012; Feig et al., 2012). Tumor-promoting features are largely restricted to the activated PSC state; the activation process may be reversible as suggested by recent work in hepatic stellate cells (Kisseleva et al., 2012). However, the cellular factors and molecular pathways controlling this process remain elusive.
We hypothesized that pharmacologic means to revert activated cancer-associated PSCs (CAPSCs) to quiescence would hinder tumor-stroma crosstalk and tumor growth, resulting in enhanced clinical efficacy of cancer cell-directed chemotherapy. We show here that the vitamin D receptor (VDR) acts as a master genomic suppressor of the PSC activation state. VDR ligand reduces fibrosis and inflammation in a murine pancreatitis model, and simultaneously undermines multiple tumor-supporting signaling pathways in PDA to enhance the efficacy of a co-administered chemotoxic agent. These results highlight a potentially widely applicable strategy to modulate stroma-associated pathologies including inflammation, fibrosis and cancer.
Results
Identification of Cancer-Associated Gene Signatures in PSCs
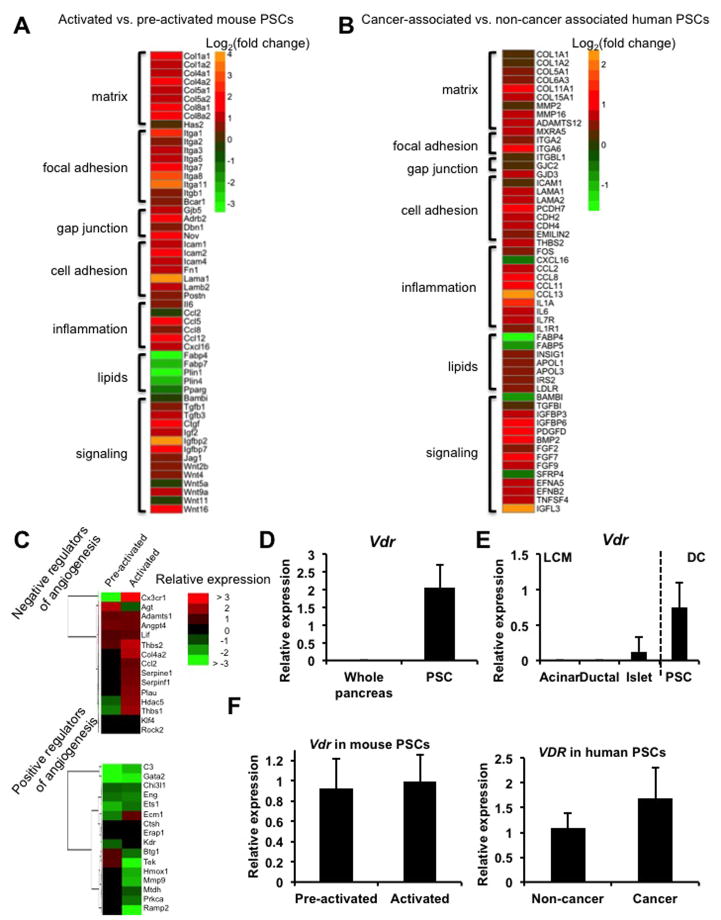
To characterize cancer-associated changes in PSCs, we performed massively parallel sequencing (RNA-Seq) of the PSC transcriptome at various stages of activation. A comparison of the transcriptomes of pre-activated (3-day culture) and culture activated (7-day culture, Omary et al., 2007) PSCs isolated from healthy mouse pancreas revealed that, during activation, PSCs decrease expression of genes implicated in lipid storage and lipid metabolism, consistent with loss of the lipid droplet phenotype associated with quiescence (Figure 1A and Figure S1A–C). Activation also resulted in increased expression of a cadre of genes with tumor-supporting potential including cytokines, growth factors, ECM components, and signaling molecules such as Wnts. Notably, cytokine induction in the stroma has been shown to promote pancreatic cancer initiation and progression in a paracrine manner (Fukuda et al., 2011; Lesina et al., 2011). In addition to the PSC “activation signature” resulting from transdifferentiation in culture, we identified a PSC “cancer signature” by comparing the transcriptomes of PSCs isolated from patients with PDA (CAPSCs) with those from patients undergoing resection for benign conditions (Figure 1B). These human PSCs were cultured (and thus culture-activated) for 15 days to achieve adequate yield and purity. This comparison of activated non-cancer associated PSCs to cancer-associated PSCs reveals changes to the activated phenotype resulting from exposure to the tumor microenvironment. Both the activation and cancer signatures include gene classes from a previously identified stromal signature which predicts poor survival and chemoresistance in PDA (Garrido-Laguna et al., 2011). Lipid storage genes such as fatty acid binding proteins were downregulated in both signatures, and were accompanied by increased expression of genes implicated in the cholesterol biosynthesis and uptake pathway, consistent with an increased proliferative capacity. Given the hypovascular nature of PDA, particularly within stromal regions, the reciprocal induction of negative angiogenic regulators and suppression of angiogenic inducers is auspicious (Figure 1C). In particular we note the induction of thrombospondin-1 (Thbs1), a well-described and potent endogenous inhibitor of angiogenesis (Lawler, 2002). Both gene signatures include ECM components, cell adhesion molecules, inflammatory mediators, paracrine growth and survival factors, genes implicated in lipid/cholesterol metabolism and modulators of signal transduction.
Figure 1.
Activated and cancer-associated PSCs exhibit a pro-fibrotic, pro-inflammatory phenotype. (A) Heatmap representing selected genes from RNA-Seq analysis of primary mouse PSCs, demonstrating gene categories with altered expression during activation. Data are represented as log2 fold change, activated (day 7) vs. pre-activated (day 3), n=3 per group. (B) Heatmap representing selected genes from RNA-Seq analysis of primary human PSCs, isolated from PDA patients (n=5) or cancer-free donors (n=4) and cultured for 15 days to achieve adequate yield and purity, expressed as log2 fold change PDA vs. cancer-free. (C) Heatmap showing the relative abundance of negative (top) and positive (bottom) regulators of angiogenesis in pre-activated and activated primary mouse PSCs. (D) Vdr expression in mouse whole-pancreas homogenates and in isolated PSCs, cultured for 3 days to expand and purify, as measured by qRT-PCR. (E) Vdr expression in the indicated pancreatic populations by qRT-PCR (normalized to 36B4; n=5). Acini, ducts, and islets were isolated by laser capture microdissection (LCM); PSCs were isolated by density centrifugation (DC). (F) Vdr expression in pre-activated and activated mouse PSCs (left) and in human non-cancer associated and cancer-associated PSCs (right) determined by qRT-PCR (normalized to 36b4, n=3). Bars indicate the mean; error bars indicate SD.
VDR Regulates the PSC Activation Network
These analyses also revealed that PSCs unexpectedly express high levels of the vitamin D receptor (VDR), previously thought not to be expressed in the exocrine pancreas (Zeitz et al., 2003) (Figures 1D and 1E; Figures S1D and S1E). Importantly, VDR expression is maintained in the cancer-associated PSCs (Figure 1F). We focused on this druggable receptor in light of our previous work implicating VDR as a critical regulator of the fibrogenic gene network in closely related hepatic stellate cells (Ding et al., 2013) and due to the established anti-inflammatory actions of 1,25(OH)2D3 and its analogues (Cantorna et al., 1996, 1998; Cantorna et al., 2000; Ma et al., 2006; Nagpal et al., 2005). Here we used calcipotriol (Cal), a potent and nonhypercalcemic vitamin D analog to control VDR induction (Naveh-Many and Silver, 1993). While not present in any post-surgical CAPSCs, surprisingly, Cal treatment induced lipid droplet formation in 19/27 primary patient samples (Figure 2A, Figure S2A), and decreased expression of αSMA (ACTA2) in 24/27 patient samples (Figure 2B). This strongly supports the idea that the activation state is controllable in a signal dependent fashion. To assess the genome-wide effects of VDR activation in PSCs, we performed transcriptome analysis of pre-activated and activated PSCs grown in the presence or absence of VDR ligand. While Cal treatment affected gene expression in pre-activated PSCs (significantly increased and decreased expression of 307 and 431 genes, respectively), VDR activation had a more widespread transcriptional response in activated PSCs (664 and 1616 genes with significantly increased and decreased expression, respectively). Notably, we observed a Cal-dependent inhibition of the activation and cancer signatures in PSCs (Figure 2C, Table S1), including suppression of negative regulators of angiogenesis such as Thbs1 and induction of positive regulators of angiogenesis like Mmp9 (Bergers et al., 2000) (Figure 2D). Similar effects of Cal treatment were observed on selected candidate genes in human CAPSCs (Figure 2E). Furthermore, these effects were dependent on VDR, as siRNA-mediated knockdown of the receptor abrogated Cal-induced expression changes (Figure 2F). To explain, in part, the broad impact of VDR on the PSC activation program, we assessed genomic crosstalk between VDR and the TGFβ/SMAD pathway (Schneider et al., 2001; Yanagisawa et al., 1999) which we previously demonstrated in hepatic stellate cells (Ding et al., 2013). Consistent with an inhibitory effect on TGFβ/SMAD signaling, Cal increased VDR binding while decreasing SMAD3 binding in the promoter regions of fibrogenic genes (Figures S2B and S2C). To determine whether VDR activation decreased PSC activation in vivo, we induced experimental chronic pancreatitis in wild type mice using the cholecystokinin analog cerulein (Willemer et al., 1992), and co-administered Cal throughout disease progression. Compared to mice receiving cerulein alone, Cal-treated animals displayed attenuated inflammation and fibrosis, consistent with decreased PSC activation (Figures S3A and S3B). Expression of activation and cancer signature genes was decreased in isolated PSCs from mice treated with Cal compared to controls (Figure 3A). Reductions were observed on activation signature genes which are of functional significance in the tumor microenvironment, including ECM components, inflammatory cytokines and growth factors. In addition, Acta2 expression, which is associated with cell motility, trended downwards. Further, reduced induction of phospho-Stat3 was observed in Cal-treated mice (Figure 3B), consistent with decreased inflammatory signaling from the stroma. Notably, Stat3 activation has been established as a mechanistic link between inflammatory damage and initiation of PDA (Fukuda et al., 2011; Lesina et al., 2011). Cal treatment during acute pancreatitis in wild type mice similarly impaired activation-associated changes in PSC gene expression (Figure 3C), and reduced leukocyte infiltration and fibrosis (Figures 3D and 3E). Strikingly, pancreata from Vdr−/− mice displayed spontaneous periacinar and periductal fibrosis (Figure S3C), further supporting a role for VDR in opposing PSC activation. Consistent with this notion, activation-associated changes in PSC gene expression were augmented in cerulein-induced acute pancreatitis in Vdr−/− mice (Figure 3F) and were accompanied by increased fibrosis (Figure 3G). Furthermore, Cal treatment of culture-activated PSCs from Vdr−/− mice demonstrated the VDR dependence of the observed gene expression changes (Figure 3H). Together, these results suggest that VDR acts as a master genomic regulator of the PSC activation program, and that VDR induction by ligand promotes the quiescent PSC state both in vitro and in vivo.
Figure 2.
A VDR-regulated transcriptional network opposes PSC activation. (A) Representative images of primary human CAPSCs treated with vehicle (DMSO) or 100nM calcipotriol (Cal) for 48h and stained with BODIPY 493/503 for detection of neutral lipids. Quantification of percent BODIPY-positive area per cell in 3 patient samples treated with DMSO or Cal appears below, plotted as the mean + SD. Statistical significance determined by Student’s unpaired t-test (*p<0.05). Scale bar = 20 μm. (B) Expression of ACTA2 in 27 primary human CAPSCs treated with vehicle or 100nM Cal for 48h. Values were plotted as DMSO/Cal and normalized to 36B4. (C) Heatmap representing selected genes from RNA-Seq analysis of primary mouse PSCs treated with DMSO (D) or Cal (C) and harvested on day 3 (pre-activated) or day 7 (activated) of culture after isolation (n=3). VDR target genes Cyp24a1 and Vdr are shown as controls. Related to Table S1. (D) Heatmap showing the relative abundance of negative (top) and positive (bottom) regulators of angiogenesis in activated primary mouse PSCs cultured in the presence of vehicle (DMSO) or Cal. (E) Expression levels of selected genes from the PSC activation or cancer signatures in CAPSCs treated with DMSO or 100nM Cal for 48h. Results are representative of 3 patient samples and are plotted as the mean + SD. qRT-PCR was performed in technical triplicate and values were normalized to 36B4. Statistical significance determined by Student’s unpaired t-test (*p < 0.05). (F) CAPSCs were transfected with siRNA pools against VDR (siVDR) or a non-targeting control (siNT). Cells were treated with DMSO or 100nM Cal for 48h and analyzed by qRT-PCR. Values were normalized to 36B4. Results are representative of 3 patient samples and are plotted as the mean + SD. Statistical significance determined by Student’s unpaired t-test (*p<0.05; n.s.=not significant).
Figure 3.
VDR ligand modulates PSC activation in vivo. (A) Expression levels of selected genes in PSCs isolated from mice injected with cerulein (Cer) or cerulein + Cal for 12 weeks (n=10). Values were normalized to 36b4 and are plotted as the mean + SD. (B) Quantification of immunofluorescent staining for phospho-Stat3 (p-Stat3) on frozen sections from wild-type mice treated with cerulein or cerulein + Cal for 12 weeks (n=5). (C) Expression levels of selected genes in PSCs isolated from mice injected with Cer or Cer + Cal to induce acute pancreatitis (for details see Supplemental Experimental Procedures; n=5). Values were normalized to 36b4 and are plotted as the mean + SD. (D) Leukocyte recruitment, as measured by CD45-positive cells, in mice with acute pancreatitis (immunofluorescent staining of frozen sections, positive cells in 20X field, n=5). (E) Fibrosis, as measured by Sirius red staining, in mice with acute pancreatitis (per 20X field, n=5). (F) Expression levels of selected genes in PSCs isolated from Vdr+/+ and Vdr−/− mice injected with cerulein to induce acute pancreatitis (n=5). Means + SD are shown; values normalized to B2M. (G) Sirius red-positive area in Vdr+/+ and Vdr−/− mice with acute pancreatitis (per 20X field, n=5). Statistical significance determined by Student’s unpaired t-test (*p<0.05). (H) Expression levels of selected genes in PSCs isolated from Vdr+/+ and Vdr−/− mice after treatment with DMSO or 100 nM Cal for 48 h. Statistical significance determined by Student’s unpaired t-test (*p<0.05; n.s.=not significant).
Stromal VDR Activation Inhibits Tumor-Supportive Signaling Events
We next assessed the impact of VDR activation in PSCs on crosstalk to tumor cells. While CAPSCs consistently expressed VDR and responded to ligand, pancreatic cancer cell lines displayed varying VDR expression, and typically low VDR activity (Figures S4A and S4B). This was observed in human PDA samples as well (Figure S4C). To assess the contribution of stromal VDR activation on the epithelial compartment, we examined the effects of CAPSC-derived secreted factors on the MIAPaCa-2 cell line, which has extremely low VDR expression and no significant response to VDR ligand (Figure S4A and S4B). Primary CAPSCs were grown to confluency, and cultured in the presence or absence of Cal for the final 48 hours of culture. CAPSC conditioned media (CM) collected from these cultures was transferred to MIAPaCa-2 cells for 48 hours. Volcano plot analysis of gene expression in MIAPaCa-2 cells incubated in CAPSC CM revealed broad changes (center panel), which were largely abrogated (right panel) when CM from Cal-treated CAPSCs was used (Figure 4A). CAPSC CM induced gene expression changes in epithelial cells implicated in proliferation (Table S2), survival, epithelial-mesenchymal transition, and chemoresistance. These changes were broadly inhibited by stromal, but not epithelial, VDR activation (Figure 4B), though direct anti-proliferative and pro-apoptotic effects of VDR activation in pancreatic cancer cells have been reported in other experimental systems (Persons et al., 2010; Yu et al., 2010). Importantly, this sensitivity to stromal but not epithelial VDR activation was replicated in pancreatic cancer cell lines with variable VDR expression (Figure 4C–G). Of note, stromal VDR activation significantly reduced CSF2 expression, implicated in pancreatic tumor progression and evasion of antitumor immunity (Bayne et al., 2012; Pylayeva-Gupta et al., 2012). Gene expression changes were accompanied by decreased induction of phospho-STAT3 (Figure 4H) and decreased resistance to chemotherapy in vitro (Figure 4I). These results demonstrate that VDR activation in PSCs negatively regulates the tumor-supporting PSC secretome.
Figure 4.
Stromal VDR activation decreases pro-tumorigenic paracrine signaling. (A) Volcano plots representing gene expression changes detected by RNA-Seq in MIAPaCa-2 cells treated with 100nM Cal for 48h vs. media alone (left), with CAPSC conditioned media (CM) for 48h vs. media alone (middle), or with CM from Cal-treated CAPSC (100nM, 48h) for 48h vs. media alone. Blue indicates significant change; red indicates no significant change. (B) Heatmap representing selected genes from the RNA-Seq analyses described in (A), plotted as fold change vs. media alone (DMEM). (C–G) The indicated cell lines were incubated with Cal directly, or with CM from CAPSC with or without Cal treatment, as described above. Expression levels of candidate genes CXCL1, CSF2, and AURKB were determined by qRT-PCR. Values were normalized to 36B4; means + SD are shown. Statistical significance determined by Student’s unpaired t-test (*p < 0.05). Results are shown as replicates with 1 patient sample, and are representative of results from multiple patient samples (n=4), though sample-to-sample variability was noted. (H) Immunoblot for p-STAT3 from MIAPaCa-2 cells treated for 24h with 100nM Cal, CAPSC CM, Cal + CAPSC CM, or Cal + CAPSC (Cal-treated) CM. Actin served as a loading control. Values indicate densitometric ratios (p-STAT3/Actin). (I) Viability of MIAPaCa-2 cells, treated as described above, incubated with the indicated doses of gemcitabine for 48h. Results are representative of 3 CAPSC CM samples and are plotted as the mean ± SD. Statistical significance determined by Student’s unpaired t-test (*p<0.05). Asterisks designate statistically significant differences in viability between CM and CM (PSC+Cal) samples at the indicated dose of gemcitabine.
VDR Ligand Plus Gemcitabine Shows Efficacy Against PDA In Vivo
A principal goal for PSC targeted therapy is to exploit the inhibition of tumor-stroma crosstalk to enhance efficacy of a cytotoxic (or immunologic) agent, which in the case of gemcitabine, though standard of care, offers minimal (1.5 month) benefit to PDA patients (Burris et al., 1997). To explore the potential of vitamin D combination therapy we first explored Cal treatment in an orthotopic allograft model utilizing immune-competent hosts (Collisson et al., 2012). The tumor cells for transplantation were derived from p48-Cre; KrasLSL-G12D/+; p53lox/+ mice (Bardeesy et al., 2006), and express low levels of Vdr (Figures S5A and S5B). Two other mouse PDA-derived cell lines demonstrated low VDR expression and activity as well (Figures S5A and S5B). This suggests that any observed therapeutic effect would likely result from host-derived stromal VDR activation, though some contribution from the epithelial compartment is not excluded. Though the stromal reaction in transplant models of PDA is subdued compared to the spontaneous KPC (KrasLSL-G12D/+;Trp53LSL-R172H/+;Pdx-1-Cre) model (Hingorani et al., 2005; Olive et al., 2009), measureable PSC activation of Col1a1, Col1a2, and Acta2 was observed in allograft recipients, accompanied by fibrosis (Figures S5C and S5D). Cal treatment decreased stromal activation and fibrosis in transplanted mice (Figure S5E). Though transplant models are responsive to gemcitabine, we also compared mice treated with gemcitabine to those treated with a combination of gemcitabine and Cal. Importantly, in combination therapy recipients, we observed a clear improvement in gemcitabine responsiveness with respect to inhibition of proliferation and expression of stromal and epithelial genes from our signatures for PSC activation (Figures S5F and S5G).
We next tested the efficacy of gemcitabine plus Cal combination therapy in the KPC model, which recapitulates human PDA in poor uptake of and response to gemcitabine (Olive et al., 2009). Combination therapy significantly reduced tumor volume with transient or sustained reduced tumor growth observed in ~70% of mice (Figure 5A and Figure S6A). In agreement with the induction of stromal remodeling, reduced tumor-associated fibrosis was observed in mice which received combination therapy compared to controls (Figure 5B). Further, combination-treated mice demonstrated significantly altered expression of genes from our stromal and epithelial gene signatures associated with PSC activation (Figure 5C). The decreased expression of PSC activation genes and induction of quiescence marker Fabp4 suggests that the tumor-associated PSCs are shifting from an activated toward a quiescent state. The observed differential sensitivity of individual genes to the drug treatment regimens may be the result of specific perturbations to stromal-tumor paracrine signaling in vivo.
Figure 5.
Stromal VDR activation shows efficacy against pancreatic carcinoma in vivo when combined with gemcitabine. KPC mice were treated for 9 days with gemcitabine (Gem), calcipotriol (Cal), or Gem + Cal (Gem: n=4; Cal: n=7; Gem+Cal: n=7 unless otherwise indicated). (A) Percent change in tumor volume at study endpoint, measured by high-resolution ultrasound. Plots indicate range, median, and quartiles. *p<0.02; Kruskal-Wallis and Dunn’s nonparametric comparison test. (B) Aniline blue-stained collagen fibers were quantified as positive pixels per 20X field. Plots indicate range, median, and quartiles. *p<0.05 by Mann-Whitney U test. (C) Gene expression in tumor homogenates was determined by qRT-PCR. Values were normalized to 36b4. Bars indicate mean + SD. *p<0.05 by Student’s unpaired t-test (compared to Gem alone). (D) Intratumoral concentrations of gemcitabine triphosphate (dFdCTP, measured by LC-MS/MS) in Gem- and Gem+Cal-treated mice 2h after the final dose of gemcitabine (n=4 and 7 respectively). Plots indicate range, median, and quartiles. *p<0.05 by Mann-Whitney U test. (E) IHC for cleaved caspase-3 (CC3) was quantified as %CC3-positive tumor cells per 20X field. Plots indicate range, median, and quartiles. *p<0.05 by Mann-Whitney U test.
Combination therapy also increased intratumoral concentration and efficacy of gemcitabine (Figure 5D and Figure S6B), with ~500% increase in the median concentration of dFdCTP, an active metabolite of gemcitabine, in mice that received combination therapy compared to gemcitabine alone. No drug-induced changes were seen in the expression levels of the gemcitabine degrading enzyme cytidine deaminase (Cda), the rate-limiting deoxycytidine kinase (dCK) or the nucleoside transporter Ent1 (Figure 6A), though allosteric effects are possible. Increased dFdCTP was accompanied by increased positivity for apoptotic marker CC3, indicating improved chemotherapeutic efficacy (Figure 5E). Furthermore, intratumoral vasculature was significantly increased by combination therapy, evidenced by increased CD31 positivity and apparent vessel patency (Figures 6B and 6C). While the combination of Cal with gemcitabine markedly improved therapeutic efficacy, in the absence of gemcitabine, Cal alone showed no measurable beneficial effects (data not shown). Importantly, gemcitabine plus Cal combination therapy significantly prolonged survival of KPC mice compared to chemotherapy alone, with median survival increased by 57% (median survival: Gem=14 days, Gem+Cal=22 days) (Figure 6D). In addition, in the Cal +Gem arm only, 29% of the mice were ‘long term’ survivors (>30days) with an average survival of 52.8 days.
Figure 6.

VDR ligand enhances delivery and efficacy of gemcitabine. KPC mice were treated for 9 days with gemcitabine (Gem), calcipotriol (Cal), or Gem + Cal (Gem: n=4; Cal: n=7; Gem+Cal: n=7 unless otherwise indicated), or treated with Gem (n=12) or Gem+Cal (n=15) until moribund. (A) Dck, Cda and Slc29a1/Ent1 gene expression in tumor homogenates determined by qRT-PCR. Values were normalized to 36b4. Bars indicate mean + SD. (B) IHC for CD31 was quantified as CD31 (NovaRed)-positive area per 40X field. Plots indicate range, median, and quartiles. *p<0.05 by Mann-Whitney U test. (C) Representative CD31 IHC from Gem- and Gem+Cal-treated KPC tumors. Arrows indicate a collapsed vessel in a gemcitabine-treated tumor (top), and a vessel with an apparent lumen in a Gem + Cal-treated tumor (bottom). Scale bar = 50 μm. (D) Kaplan-Meier survival analysis for KPC mice treated with Gem or Gem+Cal. p=0.0186 by Mantel-Cox (log rank) test.
Discussion
Despite numerous attempts, the 5 year survival rate (6%) for pancreatic cancer has not changed in decades (Rahib et al., 2014). In part, this is because treatments targeting tumor cells have largely failed. The emerging role for tumor stroma as the ‘fuel supply-line’ for cancer offers an opportunity to redirect the singular focus on the cancer cell itself to the greater tumor micro-environment (Figure 7). Indeed, by targeting VDR to transcriptionally reprogram the stroma we simultaneously suppress inflammatory cytokines and growth factors, enhance angiogenesis, increase the efficacy of gemcitabine treatment in PDA and, most importantly, significantly improve survival.
Figure 7.

Model depicting a role for VDR in signal-dependent stromal remodeling, limiting pancreatic tumor-stroma crosstalk. PSCs progressively acquire tumor-supporting functions during activation, a process that is driven by pancreatic injury and tumor progression via secreted factors from the epithelial compartment (and possibly from immune/inflammatory cells). VDR activation drives reversion of PSCs to a more quiescent, less tumor-supportive state. As such, co-treatment of pancreatic tumors with gemcitabine to target the tumor cells and VDR ligand to deactivate PSCs leads to an overall decrease in the reciprocal tumor-stroma crosstalk that presents a major barrier to the delivery and efficacy of gemcitabine alone.
VDR directed therapy has a dual benefit as it reduces fibrosis and inflammation in both acute and chronic murine pancreatitis. This is significant as pancreatitis lacks any mechanistic based therapy, is a seriously disease, and is a known risk factor for pancreatic cancer. Recently, we have shown that VDR achieves these effects by blocking TGFβ/SMAD signaling via genomic competition (Ding et al., 2013). In the acute setting this could preclude damaging effects of an unchecked wound healing response. This balance may be tipped unfavorably by chronic tissue damage or by vitamin D deficiency, which may explain in part the inverse correlation between plasma vitamin D levels or vitamin D intake and pancreatic cancer risk (Skinner et al., 2006; Wolpin et al., 2012) and the link between vitamin D deficiency and chronic pancreatitis (Mann et al., 2003).
Our work illustrates that transcriptional remodeling of pancreatic tumor stroma via VDR activation broadly weakens the capacity of PSCs to support tumor growth. VDR genomic targets of importance in PDA include the extracellular matrix (Jacobetz et al., 2013; Provenzano et al., 2012), the Shh pathway (Olive et al., 2009), cytokines/chemokines such as IL6 (Fukuda et al., 2011; Ijichi et al., 2011; Lesina et al., 2011), growth factors such as CTGF (Aikawa et al., 2006; Neesse et al., 2013) and Cxcl12, a mediator of the T cell blockade (Ding et al., 2013; Feig et al., 2012). This gains significance in light of recent work demonstrating that inhibition of stroma-derived survival factor CTGF potentiates the antitumor response to gemcitabine (Neesse et al., 2013) and that CXCL12 inhibition can restore T-cell response. Notably, important differences exist between stromal ablation and stromal remodeling therapeutic strategies. The notion that cellular and structural components of a “normal” microenvironment exert tumor-suppressive forces and signals has been discussed previously (Bissell and Hines, 2011), though this has not been demonstrated in the pancreas and leaves in question the potential benefits of reprogrammed stroma. As VDR ligand pushes activated PSCs toward a more quiescent phenotype, it is conceivable that remodeled PSCs re-establish a physiologic and metabolic environment adverse to tumor growth, a benefit not achievable by stromal ablation. The role of VDR in tissue vitality and resilience is supported by the fact that absence of VDR in normal stroma is sufficient to promote tissue fibrosis and a hyper-inflammatory response. This potential benefit of VDR-mediated stromal remodeling, to restore normal stroma, offers a conceptual advantage over stromal depletion which could leave a tissue without a critical control mechanism.
PDA stroma is believed to limit chemotherapeutic efficacy by blocking drug delivery, a result of severe hypovascularity attributable in part to dense extracellular matrix. VDR ligand significantly reduced the fibrotic content of the tumor and increased intratumoral vasculature. We also demonstrate here that activated PSCs express anti-angiogenic factors such as thrombospondin-1, known to contribute to the hypovascularity in other contexts (Kazerounian et al., 2008). The anti-angiogenic subset of PSC activation signature genes was suppressed by VDR ligand in vitro and, importantly, combination therapy induced improvement of tumor vascularity and drug delivery in vivo. Matrix degradation strategies which increase intratumoral blood flow and gemcitabine delivery have been shown to improve survival in PDA (Jacobetz et al., 2012; Provenzano et al., 2012). However, the significance of VDR-mediated stromal remodeling and improved vascularity with respect to long-term tumor growth and metastatic potential are currently under investigation. Indeed, the recent failure of clinical trials exploring the therapeutic potential of Shh pathway inhibition in combination with gemcitabine in pancreatic cancer bring to light potential limitations of stromal depletion therapy in the context of current treatment strategies (Amakye et al., 2013). Conceptually, reprogramming the tumor stroma and increasing functional vasculature could create a window for therapeutic delivery as well as heighten the potential for dissemination of tumor cells through the bloodstream. While activated stroma is generally considered to enhance tumor growth, two recent papers suggest that eliminating stroma by targeted deletion results in undifferentiated, aggressive pancreatic cancer and conclude that activated stroma is beneficial not harmful (Rhim et al., 2014 and Ozdezmir et al., 2014). Our work is not inconsistent with these studies as quiescent, Vitamin A and lipid droplet-positive stromal cells are a hallmark of healthy tissue and stromal depletion strategies run the risk of eliminating key stromal components needed for tissue homeostasis. As we show, addition of Calcipotriol to gemcitabine treatment enhances survival of KPC mice by 58% while also generating significant (29%) long term survivors. Thus, in contrast to stromal depletion, we advocate that stromal reprogramming not only reduces the fuel supply line for the tumor, but it also restores normal function while allowing for enhanced chemotherapeutic efficacy and potential T cell response. Thus, in our view, coupling signal dependent stromal reprogramming with tumor directed cytotoxic and immunologic drugs should be the goal of new PDA therapies.
Experimental Procedures
Cell lines
The human pancreatic cancer cell lines MIAPaCa-2 (CRL-1420), BxPC-3 (CRL-1687), HPAC (CRL-2119), Panc1 (CRL-1469), and AsPC1 (CRL-1682) were acquired from ATCC and cultured according to supplier’s instructions. The mouse pancreatic cancer cell lines p53 2.1.1, p53 4.4, and Ink 2.2 were derived from PDA in KrasLSL-G12D/+; Trp53lox/+; p48-Cre mice or KrasLSL-G12D/+; Ink4a/Arflox/lox; p48-Cre mice (Bardeesy et al., 2006; Collisson et al., 2011) and cultured as described previously (Collisson et al., 2011; Collisson et al., 2012). The spontaneously immortalized human pancreatic stellate cell line hPSC was isolated and established from a pancreatic cancer patient after surgical resection, as previously described (Mantoni et al., 2011). Description of primary PSC isolation and culture can be found in Supplemental Experimental Procedures.
Animals
KrasLSL-G12D/+;Trp53LSL-R172H/+;Pdx-1-Cre (KPC) mice were described previously (Hingorani et al., 2005), as were Vdr−/− mice (Yoshizawa et al., 1997). All animal protocols were reviewed and approved by the Institute of Animal Care and Use Committee (IACUC) of their respective institutes, and studies were conducted in compliance with institutional and national guidelines.
RNA-Seq
Total RNA (human, biological quadruplicates; mouse biological triplicates) was isolated using Trizol (Invitrogen) and the RNeasy mini kit with on-column DNase digestion (Qiagen). For transcriptome studies, PSCs were treated with vehicle (DMSO) or 100nM calcipotriol (Tocris) and harvested at the indicated time points. Sequencing libraries were prepared from 100–500ng total RNA using the TruSeq RNA Sample Preparation Kit v2 (Illumina). Further details can be found in Supplemental Experimental Procedures.
Lipid droplet accumulation assay
Primary human CAPSCs, allowed to attach to glass coverslips overnight, were treated with vehicle (DMSO) or 100nM calcipotriol for 48h. Washed cells were fixed (10% buffered formalin at room temperature for 15 min), then stained with 1μg/ml 4,4-Difluoro-1,3,5,7,8-Pentamethyl-4-Bora-3a,4a-Diaza-s-Indacene (BODIPY 493/503, Molecular Probes) for 1h at room temperature, protected from light. Washed, stained cells were mounted using Vectastain mounting media (Vector Labs), and fluorescence visualized through the GFP filter on a Leica DM5000B microscope and quantified using ImageJ.
Conditioned media experiments
Primary CAPSCs were grown to 100% confluency. Fresh media was added to the cultures, and at this time, CAPSCs were treated with 100nM calcipotriol. After 48h, conditioned media was harvested, sterile-filtered through 0.45μm pores, and added to pancreatic cancer cells (PCCs) at 50–60% confluency. PCCs were treated directly with 100nM calcipotriol at the onset of conditioned media incubation. After 48h, PCCs were harvested and RNA and protein isolated for analysis. For STAT3 phosphorylation experiments, MIAPaCa-2 cells were serum starved for 12h prior to incubation in serum-free DMEM or serum-free CM for 24h before cell lysis.
Orthotopic transplant/allograft model
The orthotopic transplant model used here was described previously (Collisson et al., 2012). Briefly, 1 × 103 p53 2.1.1 cells were orthotopically injected into 6–8 week old FVB/n mice in 50% Matrigel. After bioluminescent imaging on day 7, mice were randomized into one of four treatment groups: saline, calcipotriol (60μg/kg i.p., QDX20), gemcitabine (20mg/kg i.p., Q3DX4), or calcipotriol + gemcitabine. For combination-treated mice, calcipotriol treatment began on day 7 and gemcitabine treatment began on day 14. Mice were euthanized on day 26 or when distressed, and pancreata were harvested, sliced, and flash frozen in liquid nitrogen or immediately fixed in formalin.
KPC study design
KPC mice with pancreatic ductal adenocarcinoma were enrolled in the study based on tumor size, as described previously (Olive et al., 2009). For the experiments in Figures 5 and 6, enrollment was restricted to mice with tumors of a mean diameter between 6 and 9mm, as determined by high resolution ultrasound imaging. Suitable mice were assigned to a treatment group: gemcitabine; calciptriol; or gemcitabine and calciptriol combination. Gemcitabine was administered as a saline solution at 100mg/kg by intraperitoneal injection, once every three days; when appropriate, a final dose was given two hours prior to euthanasia. Calcipotriol was administered as a saline solution daily at 60μg/kg by intraperitoneal injection. Cal was administered daily for the nine-day regimen, and administered every three days (injected with gemcitabine) for the survival study. Mice were euthanized after nine days of treatment or at the onset of clinical signs such as abdominal ascites, severe cachexia, significant weight loss or inactivity. Tumors were imaged by high resolution ultrasound up to twice during the nine day treatment study.
Imaging and quantification of KPC tumors
High resolution ultrasound imaging of mouse pancreas was carried out using a Vevo 770 system with a 35MHz RMV scanhead (Visual Sonics, Inc.) as described previously (Dowell and Tofts, 2007). Serial 3D images were collected at 0.25mm intervals. Tumors were outlined on each 2D image and reconstructed to measure the 3D volume using the integrated Vevo 770 software package.
Quantification of intratumoral dFdC, dFdU, and dFdCTP by LC-MS/MS
LC-MS/MS was performed as described by Bapiro et al. (Bapiro et al., 2011). Further details can be found in Supplemental Experimental Procedures.
Supplementary Material
Table S1. Identity and relative expression of genes displayed in Figure 2C heatmap, related to Figure 2
Table S2. VDR activation in PSCs broadly impacts stroma→tumor crosstalk, related to Figure 4. Metacore pathways analysis determined canonical pathway maps significantly altered in MIAPaCa-2 cells treated with CM from untreated or Cal-treated CAPSCs. The top ten pathways are shown here.
Figure S1. Primary mouse PSCs transdifferentiate to an activated phenotype between days 3 and 7 of culture, related to Figure 1. PSCs were isolated from pancreata of wild-type C57BL6/J mice at 8 weeks of age and cultured for 7 days (see Supplemental Experimental Procedures). (A) Brightfield microscopy displays cytoplasmic lipid droplets (indicated by arrow) in pre-activated PSCs on day 3 of culture, which give rise to myofibroblast-like activated PSCs by culture day 7. Scale bar = 50 μm. (B) RNA was harvested on days 3 and 7 and quantitative RT-PCR (qRT-PCR) performed for fibroblast activation marker Acta2. (C) RNA was harvested immediately after PSC isolation (day 0), and on days 3 and 7 of culture. Quantitative RT-PCR was performed for α-amylase to determine the degree of acinar cell contamination, which was no longer detectable by day 3 of culture. (D) Whole-cell lysates were prepared and analyzed by Western blot to determine protein levels of Vdr in whole mouse pancreas and in isolated PSCs. Actin was a loading control. Lysates from 2 representative mice are shown here. (E) Purity of the isolated pancreatic populations assessed by relative expression of cell-type specific genes determined by qRT-PCR. Data normalized to 36b4. Bars indicate mean + SD.
Figure S2. VDR activation antagonizes the TGFβ/SMAD pathway in PSCs, related to Figure 2. (A) Primary human CAPSCs treated with vehicle (DMSO, not shown) or 100nM calcipotriol (Cal) for 48h were fixed and stained with BODIPY 493/503 for detection of neutral lipids. Six images (representing 19/27 samples) represent Cal-treated cells and contain cytoplasmic lipid droplets, a hallmark of the quiescent state. (B) The hPSC cell line was acutely activated with 1ng/ml TGFβ for 4h, and pretreated with 100nM calcipotriol (Cal) for 16h. Cells were fixed and subject to chromatin immunoprecipitation (ChIP) for SMAD3 and VDR, and rabbit IgG as an isotype control. Chromatin immunoprecipitates were analyzed by QPCR to assess binding of VDR and SMAD3 to the promoter regions of the HAS2 and (C) COL1A1 genes. Rabbit IgG served as an isotype control for both antibodies. Bars indicate mean + SD. *p<0.05 by Student’s t-test.
Figure S3. VDR activation reduced inflammation and fibrosis during cerulein-induced pancreatitis, related to Figure 3. For details of chronic (n=10) and acute (n=5) pancreatitis methods, see Supplemental Experimental Procedures. Pancreata were harvested, sliced, and immediately fixed in formalin or embedded in OCT and frozen. (A) H&E staining of FFPE sections from the indicated treatment groups. Scale bar = 100 μm. (B) Co-immunofluorescence for Collagen I and PSC marker GFAP on frozen sections from the indicated treatment groups. Scale bar = 100 μm. (C) Pancreata from wild-type and Vdr−/− littermates at 6 months of age were harvested and collagen was stained with Sirius Red. Two representative samples are shown per genotype (n=8). Scale bar = 500μm.
Figure S4. VDR is consistently expressed and ligand-responsive in PSCs, but expression is variable and transcriptional activity is lower in pancreatic cancer cells, related to Figure 4. (A) VDR expression was measured by qRT-PCR in the 5 indicated pancreatic cancer cell lines, and in 3 CAPSC samples (0051, 0052, and 0056). (B) The indicated cell lines or samples were incubated with vehicle or Cal (100nM, 16h) and expression of VDR target gene CYP24A1 was measured by qRT-PCR. Values were normalized to 36B4. Bars represent mean + SD. (C) Resected sections of human PDA were used for double immunoflorescent staining of VDR and a-SMA (a marker of activated PSCs). Nuclei were counterstained with DAPI. Bar: 40μm.
Figure S5. VDR ligand decreases stromal activation in PDA in vivo, related to Figure 5. Three pancreatic cancer cell lines derived from mouse PDA were compared to mouse PSCs with respect to VDR expression and activity. (A) RNA was isolated from the indicated cell lines and activated mouse PSCs (culture day 7), and Vdr expression was measured by qRT-PCR. (B) The indicated cell types were treated with vehicle (DMSO) or 100nM calcipotriol for 16h. RNA was isolated, and expression of Vdr target gene Cyp24a1 was measured by qRT-PCR. Values were normalized to 36b4. Bars indicate mean + SD. (C) PSCs were isolated from mock surgery and allograft recipients. RNA was isolated and qRT-PCR performed to measure expression of PSC activation markers. Values were normalized to 36b4. Bars indicate mean + SD. (D) Pancreata from mock surgery or allograft recipients were harvested and formalin-fixed. FFPE sections were used for Masson’s trichrome staining. A representative trichrome stain from PDA in a KPC mouse is shown for comparison. Scale bar = 100 μm. (E) PDA allograft recipients received daily intraperitoneal injections of 60μg/kg calcipotriol or saline for 21 days. Pancreata were harvested and fixed in formalin. FFPE sections were stained with Masson’s trichrome (quantification per 20X field below; n=5, *p < 0.05 by Student’s t-test). Scale bar = 100 μm. (F&G) PDA allograft recipients received daily intraperitoneal injections of 60μg/kg calcipotriol or saline for 21 days, and intraperitoneal injections of 20mg/kg gemcitabine Q3DX4 for the final 12 days of treatment. Pancreata were harvested, sliced, and immediately fixed in formalin or frozen in liquid nitrogen. (F) FFPE sections were used for immunohistochemical staining of phospho-histone H3 and subsequent quantification (see Experimental Procedures). Plot indicates range, median, and quartiles. *p<0.05 by Student’s t-test. (G) Flash-frozen pancreata were homogenized, RNA isolated, and expression of stromal and epithelial genes from our gene signatures were measured by qRT-PCR. Values were normalized to 36b4. Bars indicate mean + SD. *p<0.05 by Student’s t-test. n.s.=not significant.
Figure S6. VDR ligand increases gemcitabine efficacy in vivo, related to Figure 6. (A) PDA-bearing KPC mice were treated as indicated and imaged by high-resolution ultrasound before the start of treatment, on day 4 of treatment, and at study endpoint (see Experimental Procedures). One mouse in the gemcitabine cohort was not imaged on day 4, and 2 mice from both the Cal and Gem+Cal cohorts were sacrificed before the endpoint per institutional guidelines. Waterfall plots indicate % volume increase from pre-treatment tumor volumes on treatment day 4 (top) and at study endpoint (bottom). (B) Tumors were harvested from KPC mice treated with the indicated regimen, and homogenates were analyzed by LC-MS/MS to determine intratumoral concentrations of gemcitabine (dFdC; top left), and its deaminated metabolite (dFdU; top right). Ratio of dFdC/dFdU appears in the bottom row. Lines indicate mean ± SD. Outliers were identified and appear in red boxes.
Highlights.
VDR is a master transcriptional regulator in pancreatic stellate cells
VDR ligands suppress pancreatitis
Stromal VDR activation overcomes chemotherapeutic drug resistance
VDR ligand plus gemcitabine enhances survival in a PDA mouse model
Acknowledgments
We thank E. Ong and C. Brondos for administrative support; M. Baran, T. Guerin, J. Schlomer, J. Kalen, L. Riffel, P. Mackin, S. Kaufman, J. Alvarez, and H. Juguilon for technical assistance; and D. von Hoff and T. Bapiro for discussion. We thank T. Guerin and J. Schlomer for efficacy studies done at the Center for Advanced Preclinical Research (CAPR), the Center for Cancer Research, the National Cancer Institute. M.H.S. was supported by a Ruth L. Kirchstein National Research Service Award (T32-CA009370). This work was funded by grants from the National Institutes of Health (HL105278, DK0577978, DK090962, CA014195, and ES010337), the Helmsley Charitable Trust and the Samuel Waxman Cancer Research Foundation. R.M.E. and M.D. are supported in part by a Stand Up to Cancer Dream Team Translational Cancer Research Grant, a Program of the Entertainment Industry Foundation (SU2C-AACR-DT0509). C.L. and M.D. are funded by grants from the National Health and Medical Research Council of Australia Project Grants 512354, 632886 and 1043199. M. Apte and J. Wilson are funded by grants from the Cancer Council of NSW. A. Masamune is supported by Grant-in-Aid from Japan Society for the Promotion of Science (23591008). R.M.E. is an investigator of the Howard Hughes Medical Institute and March of Dimes Chair in Molecular and Developmental Biology at the Salk Institute and supported by a grant from The Lustgarten Foundation.
Footnotes
Accession numbers
The Gene Expression Omnibus accession number for the RNA-Seq data is GSE43770.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aikawa T, Gunn J, Spong SM, Klaus SJ, Korc M. Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Molecular cancer therapeutics. 2006;5:1108–1116. doi: 10.1158/1535-7163.MCT-05-0516. [DOI] [PubMed] [Google Scholar]
- Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte MV, Pirola RC, Wilson JS. Pancreatic stellate cells: a starring role in normal and diseased pancreas. Frontiers in physiology. 2012;3:344. doi: 10.3389/fphys.2012.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte MV, Wilson JS. Dangerous liaisons: pancreatic stellate cells and pancreatic cancer cells. Journal of gastroenterology and hepatology. 2012;27(Suppl 2):69–74. doi: 10.1111/j.1440-1746.2011.07000.x. [DOI] [PubMed] [Google Scholar]
- Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, Ouellette MM, Hollingsworth MA. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapiro TE, Richards FM, Goldgraben MA, Olive KP, Madhu B, Frese KK, Cook N, Jacobetz MA, Smith DM, Tuveson DA, et al. A novel method for quantification of gemcitabine and its metabolites 2′,2′-difluorodeoxyuridine and gemcitabine triphosphate in tumour tissue by LC-MS/MS: comparison with (19)F NMR spectroscopy. Cancer chemotherapy and pharmacology. 2011;68:1243–1253. doi: 10.1007/s00280-011-1613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, Feng B, Brennan C, Weissleder R, Mahmood U, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5947–5952. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, Vonderheide RH. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature cell biology. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nature medicine. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. The Journal of nutrition. 1998;128:68–72. doi: 10.1093/jn/128.1.68. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. The Journal of nutrition. 2000;130:2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nature medicine. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collisson EA, Trejo CL, Silva JM, Gu S, Korkola JE, Heiser LM, Charles RP, Rabinovich BA, Hann B, Dankort D, et al. A central role for RAF-->MEK-->ERK signaling in the genesis of pancreatic ductal adenocarcinoma. Cancer discovery. 2012;2:685–693. doi: 10.1158/2159-8290.CD-11-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, Leblanc M, Coulter S, He M, Scott C, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell. 2013;153:601–613. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell NG, Tofts PS. Fast, accurate, and precise mapping of the RF field in vivo using the 180 degrees signal null. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2007;58:622–630. doi: 10.1002/mrm.21368. [DOI] [PubMed] [Google Scholar]
- Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A, Wang SC, Morris JPt, Folias AE, Liou A, Kim GE, Akira S, Boucher KM, Firpo MA, Mulvihill SJ, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Laguna I, Uson M, Rajeshkumar NV, Tan AC, de Oliveira E, Karikari C, Villaroel MC, Salomon A, Taylor G, Sharma R, et al. Tumor engraftment in nude mice and enrichment in stroma- related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:5793–5800. doi: 10.1158/1078-0432.CCR-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann V, Haas M, Boeck S. Systemic treatment of advanced pancreatic cancer. Cancer treatment reviews. 2012;38:843–853. doi: 10.1016/j.ctrv.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer research. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijichi H, Chytil A, Gorska AE, Aakre ME, Bierie B, Tada M, Mohri D, Miyabayashi K, Asaoka Y, Maeda S, et al. Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. The Journal of clinical investigation. 2011;121:4106–4117. doi: 10.1172/JCI42754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2012 doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature reviews Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. Journal of cellular and molecular medicine. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, Yoshimura A, Reindl W, Sipos B, Akira S, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Ma Y, Khalifa B, Yee YK, Lu J, Memezawa A, Savkur RS, Yamamoto Y, Chintalacharuvu SR, Yamaoka K, Stayrook KR, et al. Identification and characterization of noncalcemic, tissue-selective, nonsecosteroidal vitamin D receptor modulators. The Journal of clinical investigation. 2006;116:892–904. doi: 10.1172/JCI25901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Molecular cancer therapeutics. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB. Pancreatic stellate cells radioprotect pancreatic cancer cells through beta1-integrin signaling. Cancer research. 2011;71:3453–3458. doi: 10.1158/0008-5472.CAN-10-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamune A, Shimosegawa T. Signal transduction in pancreatic stellate cells. Journal of gastroenterology. 2009;44:249–260. doi: 10.1007/s00535-009-0013-2. [DOI] [PubMed] [Google Scholar]
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocrine reviews. 2005;26:662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- Naveh-Many T, Silver J. Effects of calcitriol, 22-oxacalcitriol, and calcipotriol on serum calcium and parathyroid hormone gene expression. Endocrinology. 1993;133:2724–2728. doi: 10.1210/endo.133.6.8243296. [DOI] [PubMed] [Google Scholar]
- Neesse A, Frese KK, Bapiro TE, Nakagawa T, Sternlicht MD, Seeley TW, Pilarsky C, Jodrell DI, Spong SM, Tuveson DA. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proceedings of the National Academy of Sciences of the United States of America; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. The Journal of clinical investigation. 2007;117:50–59. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persons KS, Eddy VJ, Chadid S, Deoliveira R, Saha AK, Ray R. Anti-growth effect of 1,25-dihydroxyvitamin D3-3-bromoacetate alone or in combination with 5-amino-imidazole-4-carboxamide-1-beta-4-ribofuranoside in pancreatic cancer cells. Anticancer research. 2010;30:1875–1880. [PubMed] [Google Scholar]
- Phillips PA, McCarroll JA, Park S, Wu MJ, Pirola R, Korsten M, Wilson JS, Apte MV. Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover. Gut. 2003;52:275–282. doi: 10.1136/gut.52.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Experimental cell research. 2010;316:1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasanen K, Vaheri A. Activation of fibroblasts in cancer stroma. Experimental cell research. 2010;316:2713–2722. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Schneider E, Schmid-Kotsas A, Zhao J, Weidenbach H, Schmid RM, Menke A, Adler G, Waltenberger J, Grunert A, Bachem MG. Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. American journal of physiology Cell physiology. 2001;281:C532–543. doi: 10.1152/ajpcell.2001.281.2.C532. [DOI] [PubMed] [Google Scholar]
- Shimoda M, Mellody KT, Orimo A. Carcinoma-associated fibroblasts are a rate-limiting determinant for tumour progression. Seminars in cell & developmental biology. 2010;21:19–25. doi: 10.1016/j.semcdb.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HG, Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Vitamin D intake and the risk for pancreatic cancer in two cohort studies. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:1688–1695. doi: 10.1158/1055-9965.EPI-06-0206. [DOI] [PubMed] [Google Scholar]
- Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonlaufen A, Joshi S, Qu C, Phillips PA, Xu Z, Parker NR, Toi CS, Pirola RC, Wilson JS, Goldstein D, et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer research. 2008;68:2085–2093. doi: 10.1158/0008-5472.CAN-07-2477. [DOI] [PubMed] [Google Scholar]
- Wehr AY, Furth EE, Sangar V, Blair IA, Yu KH. Analysis of the human pancreatic stellate cell secreted proteome. Pancreas. 2011;40:557–566. doi: 10.1097/MPA.0b013e318214efaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemer S, Elsasser HP, Adler G. Hormone-induced pancreatitis. European surgical research Europaische chirurgische Forschung Recherches chirurgicales europeennes. 1992;24(Suppl 1):29–39. doi: 10.1159/000129237. [DOI] [PubMed] [Google Scholar]
- Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–509. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpin BM, Ng K, Bao Y, Kraft P, Stampfer MJ, Michaud DS, Ma J, Buring JE, Sesso HD, Lee IM, et al. Plasma 25-hydroxyvitamin D and risk of pancreatic cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:82–91. doi: 10.1158/1055-9965.EPI-11-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa J, Yanagi Y, Masuhiro Y, Suzawa M, Watanabe M, Kashiwagi K, Toriyabe T, Kawabata M, Miyazono K, Kato S. Convergence of transforming growth factor-beta and vitamin D signaling pathways on SMAD transcriptional coactivators. Science. 1999;283:1317–1321. doi: 10.1126/science.283.5406.1317. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nature genetics. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- Yu WD, Ma Y, Flynn G, Muindi JR, Kong RX, Trump DL, Johnson CS. Calcitriol enhances gemcitabine anti-tumor activity in vitro and in vivo by promoting apoptosis in a human pancreatic carcinoma model system. Cell Cycle. 2010;9:3022–3029. doi: 10.4161/cc.9.15.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:509–511. doi: 10.1096/fj.02-0424fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Identity and relative expression of genes displayed in Figure 2C heatmap, related to Figure 2
Table S2. VDR activation in PSCs broadly impacts stroma→tumor crosstalk, related to Figure 4. Metacore pathways analysis determined canonical pathway maps significantly altered in MIAPaCa-2 cells treated with CM from untreated or Cal-treated CAPSCs. The top ten pathways are shown here.
Figure S1. Primary mouse PSCs transdifferentiate to an activated phenotype between days 3 and 7 of culture, related to Figure 1. PSCs were isolated from pancreata of wild-type C57BL6/J mice at 8 weeks of age and cultured for 7 days (see Supplemental Experimental Procedures). (A) Brightfield microscopy displays cytoplasmic lipid droplets (indicated by arrow) in pre-activated PSCs on day 3 of culture, which give rise to myofibroblast-like activated PSCs by culture day 7. Scale bar = 50 μm. (B) RNA was harvested on days 3 and 7 and quantitative RT-PCR (qRT-PCR) performed for fibroblast activation marker Acta2. (C) RNA was harvested immediately after PSC isolation (day 0), and on days 3 and 7 of culture. Quantitative RT-PCR was performed for α-amylase to determine the degree of acinar cell contamination, which was no longer detectable by day 3 of culture. (D) Whole-cell lysates were prepared and analyzed by Western blot to determine protein levels of Vdr in whole mouse pancreas and in isolated PSCs. Actin was a loading control. Lysates from 2 representative mice are shown here. (E) Purity of the isolated pancreatic populations assessed by relative expression of cell-type specific genes determined by qRT-PCR. Data normalized to 36b4. Bars indicate mean + SD.
Figure S2. VDR activation antagonizes the TGFβ/SMAD pathway in PSCs, related to Figure 2. (A) Primary human CAPSCs treated with vehicle (DMSO, not shown) or 100nM calcipotriol (Cal) for 48h were fixed and stained with BODIPY 493/503 for detection of neutral lipids. Six images (representing 19/27 samples) represent Cal-treated cells and contain cytoplasmic lipid droplets, a hallmark of the quiescent state. (B) The hPSC cell line was acutely activated with 1ng/ml TGFβ for 4h, and pretreated with 100nM calcipotriol (Cal) for 16h. Cells were fixed and subject to chromatin immunoprecipitation (ChIP) for SMAD3 and VDR, and rabbit IgG as an isotype control. Chromatin immunoprecipitates were analyzed by QPCR to assess binding of VDR and SMAD3 to the promoter regions of the HAS2 and (C) COL1A1 genes. Rabbit IgG served as an isotype control for both antibodies. Bars indicate mean + SD. *p<0.05 by Student’s t-test.
Figure S3. VDR activation reduced inflammation and fibrosis during cerulein-induced pancreatitis, related to Figure 3. For details of chronic (n=10) and acute (n=5) pancreatitis methods, see Supplemental Experimental Procedures. Pancreata were harvested, sliced, and immediately fixed in formalin or embedded in OCT and frozen. (A) H&E staining of FFPE sections from the indicated treatment groups. Scale bar = 100 μm. (B) Co-immunofluorescence for Collagen I and PSC marker GFAP on frozen sections from the indicated treatment groups. Scale bar = 100 μm. (C) Pancreata from wild-type and Vdr−/− littermates at 6 months of age were harvested and collagen was stained with Sirius Red. Two representative samples are shown per genotype (n=8). Scale bar = 500μm.
Figure S4. VDR is consistently expressed and ligand-responsive in PSCs, but expression is variable and transcriptional activity is lower in pancreatic cancer cells, related to Figure 4. (A) VDR expression was measured by qRT-PCR in the 5 indicated pancreatic cancer cell lines, and in 3 CAPSC samples (0051, 0052, and 0056). (B) The indicated cell lines or samples were incubated with vehicle or Cal (100nM, 16h) and expression of VDR target gene CYP24A1 was measured by qRT-PCR. Values were normalized to 36B4. Bars represent mean + SD. (C) Resected sections of human PDA were used for double immunoflorescent staining of VDR and a-SMA (a marker of activated PSCs). Nuclei were counterstained with DAPI. Bar: 40μm.
Figure S5. VDR ligand decreases stromal activation in PDA in vivo, related to Figure 5. Three pancreatic cancer cell lines derived from mouse PDA were compared to mouse PSCs with respect to VDR expression and activity. (A) RNA was isolated from the indicated cell lines and activated mouse PSCs (culture day 7), and Vdr expression was measured by qRT-PCR. (B) The indicated cell types were treated with vehicle (DMSO) or 100nM calcipotriol for 16h. RNA was isolated, and expression of Vdr target gene Cyp24a1 was measured by qRT-PCR. Values were normalized to 36b4. Bars indicate mean + SD. (C) PSCs were isolated from mock surgery and allograft recipients. RNA was isolated and qRT-PCR performed to measure expression of PSC activation markers. Values were normalized to 36b4. Bars indicate mean + SD. (D) Pancreata from mock surgery or allograft recipients were harvested and formalin-fixed. FFPE sections were used for Masson’s trichrome staining. A representative trichrome stain from PDA in a KPC mouse is shown for comparison. Scale bar = 100 μm. (E) PDA allograft recipients received daily intraperitoneal injections of 60μg/kg calcipotriol or saline for 21 days. Pancreata were harvested and fixed in formalin. FFPE sections were stained with Masson’s trichrome (quantification per 20X field below; n=5, *p < 0.05 by Student’s t-test). Scale bar = 100 μm. (F&G) PDA allograft recipients received daily intraperitoneal injections of 60μg/kg calcipotriol or saline for 21 days, and intraperitoneal injections of 20mg/kg gemcitabine Q3DX4 for the final 12 days of treatment. Pancreata were harvested, sliced, and immediately fixed in formalin or frozen in liquid nitrogen. (F) FFPE sections were used for immunohistochemical staining of phospho-histone H3 and subsequent quantification (see Experimental Procedures). Plot indicates range, median, and quartiles. *p<0.05 by Student’s t-test. (G) Flash-frozen pancreata were homogenized, RNA isolated, and expression of stromal and epithelial genes from our gene signatures were measured by qRT-PCR. Values were normalized to 36b4. Bars indicate mean + SD. *p<0.05 by Student’s t-test. n.s.=not significant.
Figure S6. VDR ligand increases gemcitabine efficacy in vivo, related to Figure 6. (A) PDA-bearing KPC mice were treated as indicated and imaged by high-resolution ultrasound before the start of treatment, on day 4 of treatment, and at study endpoint (see Experimental Procedures). One mouse in the gemcitabine cohort was not imaged on day 4, and 2 mice from both the Cal and Gem+Cal cohorts were sacrificed before the endpoint per institutional guidelines. Waterfall plots indicate % volume increase from pre-treatment tumor volumes on treatment day 4 (top) and at study endpoint (bottom). (B) Tumors were harvested from KPC mice treated with the indicated regimen, and homogenates were analyzed by LC-MS/MS to determine intratumoral concentrations of gemcitabine (dFdC; top left), and its deaminated metabolite (dFdU; top right). Ratio of dFdC/dFdU appears in the bottom row. Lines indicate mean ± SD. Outliers were identified and appear in red boxes.