Abstract
From an engineering perspective, many forms of heart disease can be thought of as a reduction in biomaterial performance, in which the biomaterial is the tissue comprising the ventricular wall. In materials science, the structure and properties of a material are recognized to be interconnected with performance. In addition, for most measurements of structure, properties, and performance, some processing is required. Here, we review the current state of knowledge regarding cardiac tissue structure, properties, and performance as well as the processing steps taken to acquire those measurements. Understanding the impact of these factors and their interactions may enhance our understanding of heart function and heart failure. We also review design considerations for cardiac tissue property and performance measurements because, to date, most data on cardiac tissue has been obtained under non-physiological loading conditions. Novel measurement systems that account for these design considerations may improve future experiments and lead to greater insight into cardiac tissue structure, properties, and ultimately performance.
Keywords: Cardiac myocyte mechanics, Cardiac sarcomere mechanics, Cardiac tissue testing, Review
INTRODUCTION
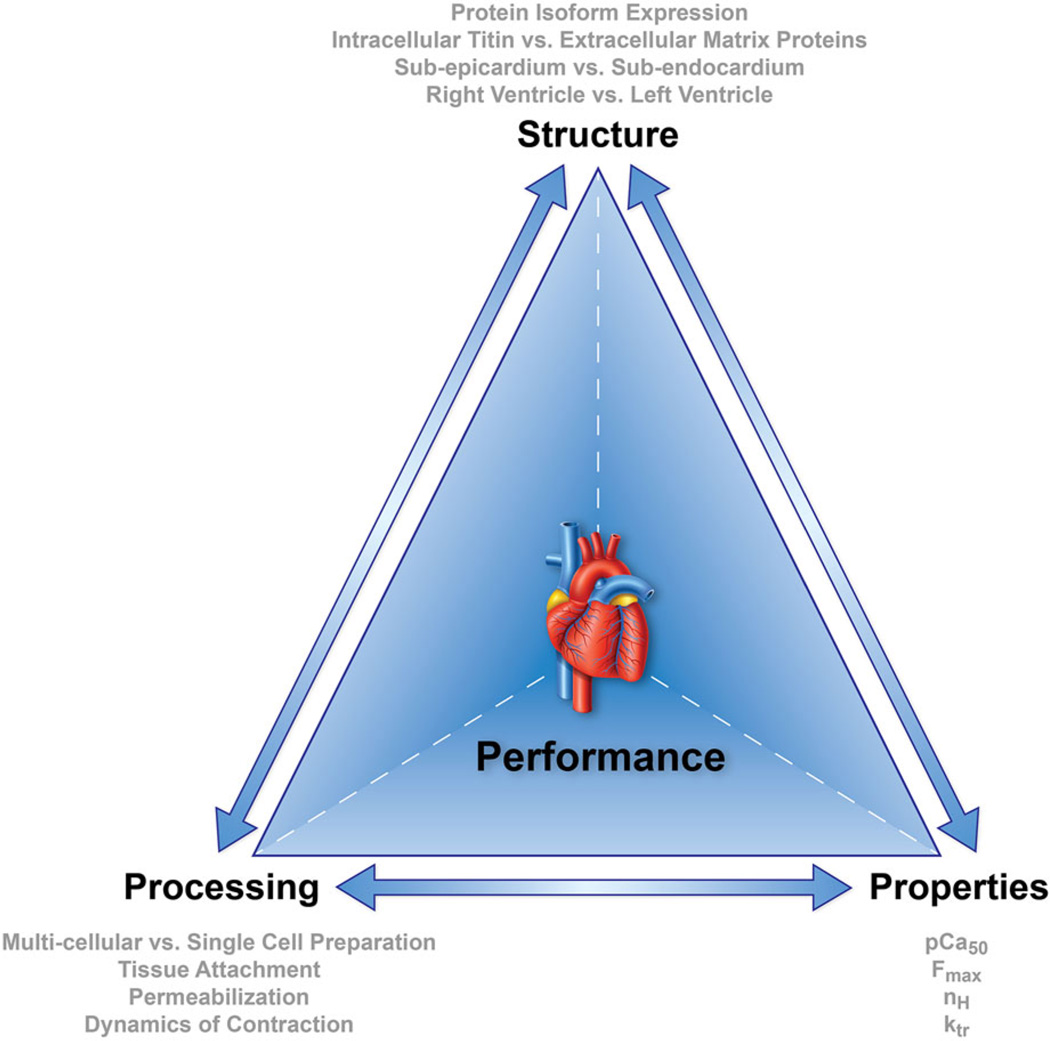
Heart disease is a leading cause of death in the U.S.45 From a clinical perspective, heart disease encompasses a range of pathologies including arrhythmias, ischemic heart disease, and valve dysfunction, all of which potentially lead to heart failure if untreated. From an engineering perspective, many forms of heart disease can be thought of as a reduction in biomaterial performance where the biomaterial is cardiac tissue. The materials science tetrahedron illustrates how the structure, properties, and processing of a material and ultimately its performance are interconnected (Fig. 1). For the ventricular wall tissue that determines heart function, the relevant aspects of structure, defined as the arrangement of internal components, include the amount and arrangement of intra- and extracellular proteins as well as their isoforms. The relevant material properties at the single- and multi-cellular tissue levels include passive and active force–length relationships, and the active force–length relationship sensitivity to calcium. Importantly, most of the techniques used to measure these passive and active responses in vitro subject tissues to non-physiological static or dynamic loading at sub-physiological frequencies. In order for loading of single- and multi-cellular cardiac tissues to mimic in vivo conditions, such that robust inferences about in vivo performance can be drawn, in vitro testing protocols need to be improved. Finally, in order to obtain in vitro measurements of material properties, processing is required, which can affect structure, properties, and performance. Here, we use the material science tetrahedron (structure–property–processing–performance) perspective to consider the factors that affect the cardiac tissue contractile response in healthy states and in heart failure in vitro so that we may gain insight into heart function and heart failure in vivo.
FIGURE 1.
Materials science tetrahedron. Structure, properties, processing, and performance are interconnected and influence the behavior in all biomaterials.
In writing this review, our goals are to (i) summarize the current state of knowledge regarding the structure– property–processing–performance relationships of heart tissue, (ii) highlight the design considerations of novel measurement techniques and protocols to more accurately mimic the physiological loading of cardiac tissues, and (iii) stimulate research to address key knowledge gaps, which will ultimately improve our understanding of cardiac tissue performance and our ability to suggest novel interventions to preserve or restore cardiac function. We first review the fundamentals of cardiac structure and function (“Properties: Fundamentals of Cardiac and Sarcomere Mechanics” section). We then discuss the known impact of cardiac tissue, specifically sarcomere structural differences on properties, which can arise from differential protein isoform expression, intracellular and extracellular protein content, position within the myocardial wall, and location in the left or right ventricle, on the tissue contractile response (“Structure” section). Then, we review the impact of processing, i.e., tissue preparation techniques and loading protocols, on measured parameters (“ Processing and measurement techniques” section). To understand how structure and properties measured in vitro influence function in vivo, computational models are briefly reviewed (“Performance: Integration via Computational Models” section). Finally, we discuss heart failure in this context (“Heart Failure” section) and conclude with suggestions for future studies (“Summary and Suggestions for Future Research” section).
PROPERTIES: FUNDAMENTALS OF CARDIAC AND SARCOMERE MECHANICS
Ventricular function, often clinically assessed via cardiac output or ejection fraction, depends on sarcomere function, loading conditions such as preload (i.e., during filling), afterload (i.e., during ejection), and interventricular interactions as well as action potential conduction and other factors. Sarcomere function is affected by calcium concentration and loading conditions including both the length of the sarcomere and the magnitude and frequency with which the load is applied.
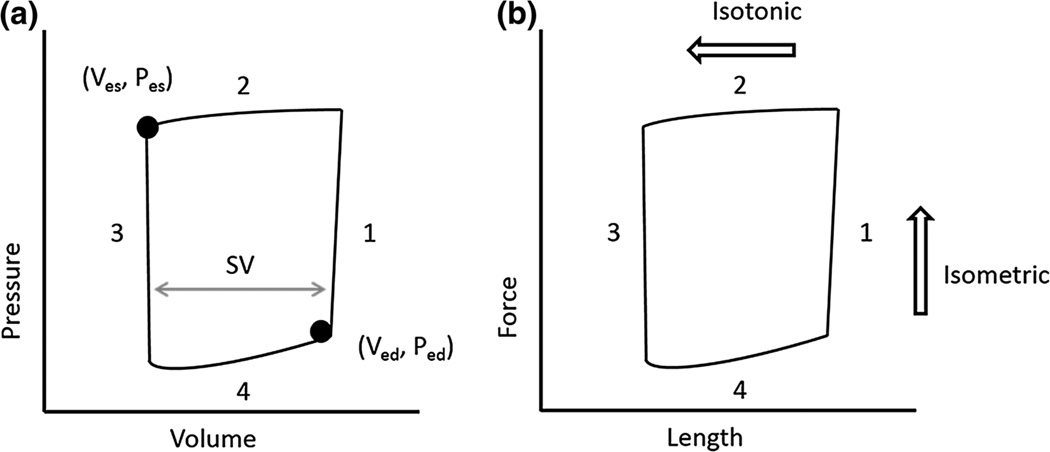
In vivo, over the normal cardiac cycle, during isovolumic ventricular contraction (Fig. 2a, phase 1), an influx of calcium causes sarcomere contraction with isometric loading (Fig. 2b, phase 1) at a sarcomere length (SL) that depends on preload and the support provided by the extracellular matrix (ECM). During ventricular ejection (Fig. 2a, phase 2), calcium concentration, regulated by the sarcoplasmic reticulum, remains high; the amount of blood ejected is dependent on the power generated during sarcomere shortening (Fig. 2b, phase 2). Subsequently, during ventricular relaxation (Fig. 2a, phase 3) and filling (phase 4), intracellular calcium is removed by Ca2+ transporters2 to allow isometric relaxation (Fig. 2b, phase 3). The extent of relaxation determines sarcomere length (phase 4), which depends again on preload and the support (or constraint) of the ECM.
FIGURE 2.
(a) Representative ventricular pressure–volume loop. Systole comprises phases 1 and 2 and ends at the end systolic pressure–volume (Ves, Pes) point. Phases 3 and 4 represent diastole, which ends at the end-diastolic pressure–volume (Ved, Ped) point. Stroke volume (SV) is obtained by subtracting the end-systolic volume from the end-diastolic volume. (b) Representative sarcomere force–length loop. Isometric contractions develop force (phase 1) and isometric relaxation dissipates force (phase 3). Isotonic contraction leads to sarcomere shortening (phase 2) and sarcomere lengthening (phase 4).
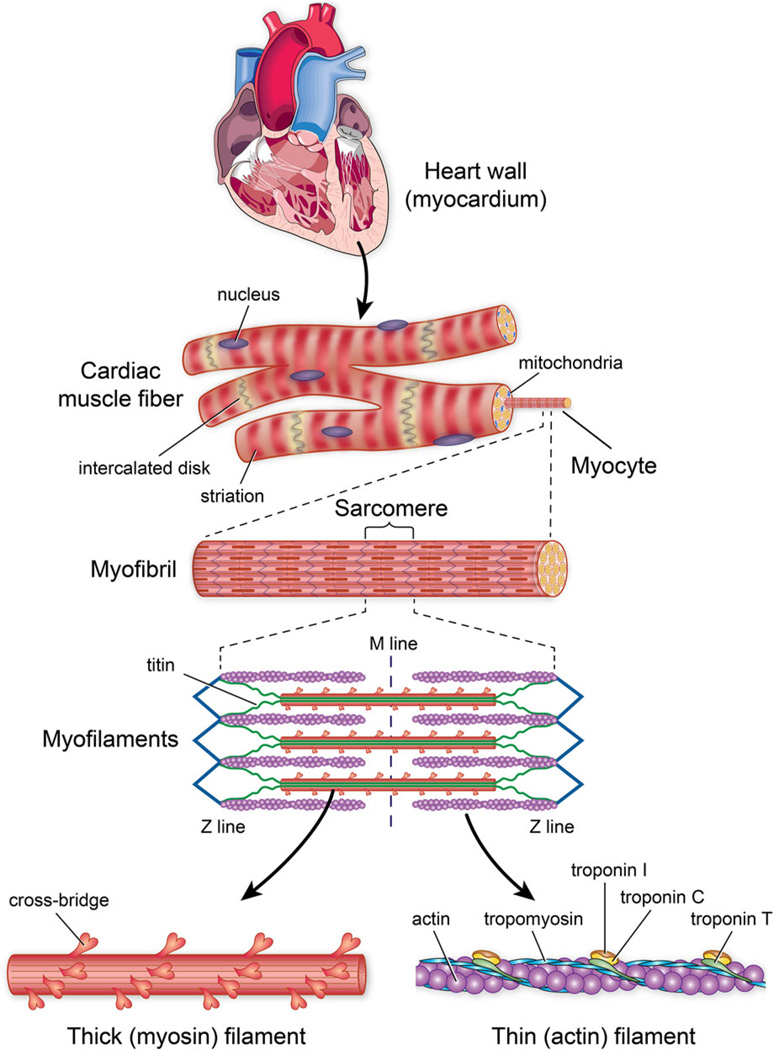
Sarcomere force generation can be measured in vitro by isolating myocytes, i.e., single muscle cells (Fig. 3), or groups of myocytes surrounded by ECM proteins such as trabeculae or papillary muscles, i.e., multicellular preparations. With either the single- or multicellular preparation, the force that results from changes in calcium concentration (defined as pCa = −log[Ca2+]) is typically measured as a function of sarcomere length (Fig. 4) using an experimental setup in which length is controlled with a linear motor and measured with optical imaging, and force is measured with a load cell. Force–length-pCa data are obtained in low pCa and high pCa solutions and active behavior is taken as the difference between total (low pCa) and passive (high pCa) responses.10 The increase in calcium responsiveness that occurs with an increase in sarcomere length is termed length-dependent activation.15
FIGURE 3.
Sarcomere structure. Sarcomeres are composed of thin and thick filaments and titin arranged in repeating units. Thin filaments contain actin, a globular protein that is polymerized into a filament, tropomyosin, a rod-shaped molecule that binds to actin and troponin, and troponin, which hinders myosin binding to actin by fixing tropomyosin over the actin binding site. The thick filament contains myosin and is composed of a rod-like domain (backbone) and globular domains (cross-bridges). Titin extends from the edge of the sarcomere to the middle and acts like a spring during contraction and relaxation.
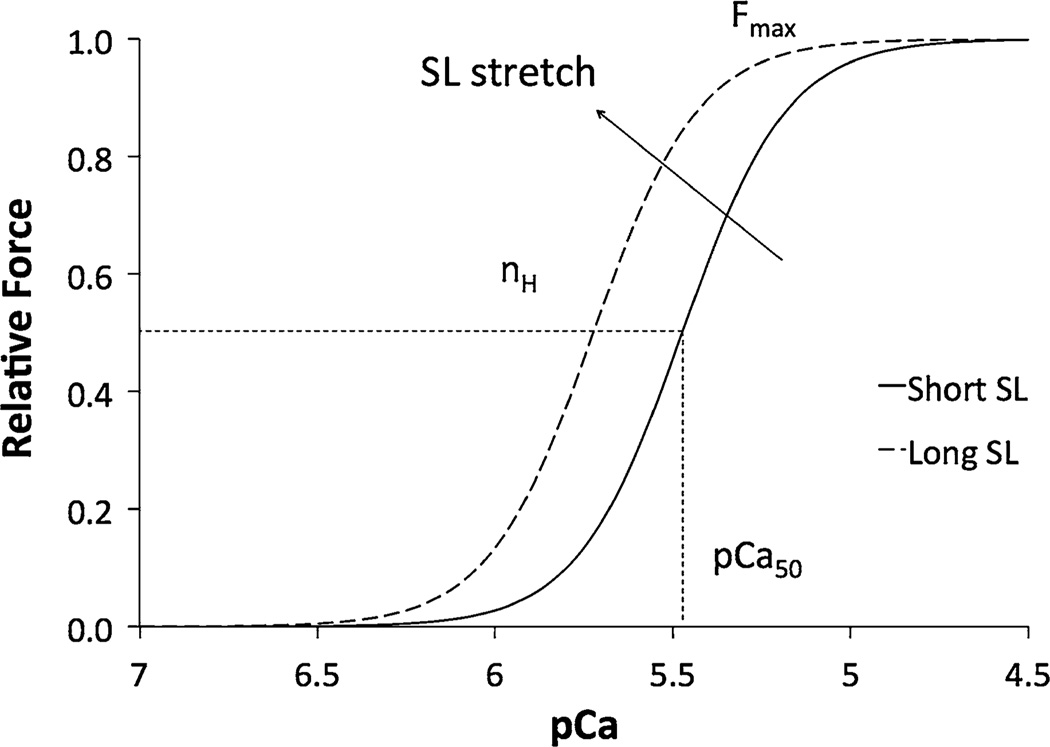
FIGURE 4.
Force-pCa curve. As the pCa decreases, the submaximal force increases progressively to maximal force as modeled by the Hill Equation. Upon an increase in SL, the parameters of the Hill equation are changed, which is characteristic of length-dependent activation.
The properties typically used to assess sarcomere function from in vitro experiments are listed in Table 1. They are quantified by fitting force-pCa data to a nonlinear sigmoid function modeled by the Hill Equation:
| (1) |
where the force generated in response to a given calcium concentration, F, or the submaximal Ca2+ activated force, is normalized to the maximum force, Fmax; pCa50 represents the concentration of Ca2+ ions required for half-maximal force generation; and the Hill coefficient, nH, is the slope of the fitted curve and represents the degree of cooperative binding of Ca2+ to regulatory sites on the thin filament, also called myofilament cooperativity. The rate constant of force redevelopment, ktr, is measured using a release/restretch protocol in solutions with high and low Ca2+ concentrations and is used to quantify the kinetics of sarcomere contraction.
TABLE 1.
Myofilament calcium response parameters
| Parameter | Definition | Index of | Explanation |
|---|---|---|---|
| pCa50 | Calcium concentration required for half-maximal activation | Calcium sensitivity of force | Quantifies the amount calcium it takes to reach half-maximal force |
| nH | Hill coefficient | Myofilament cooperativity of force development | Slope of the force-pCa curve; it is the result of increased receptor affinity for Ca2+ after a Ca2+ binds to the target troponin subunit |
| Fmax | Maximal Ca2+ activated force | Maximal sarcomere generated force | Maximum sarcomere force generated by exposing myocytes to Ca2+ solutions |
| ktr | Rate constant of force redevelopment | Myosin cross-bridge kinetics | Characterizes transitions from non-force generating to force generating states |
Despite decades of research into cardiac tissue mechanics, our understanding is still incomplete, largely due to differences in the published literature regarding the structural components analyzed, the material properties measured, and the processing steps used in a given experiment. Computational models are an ideal approach for synthesizing the existing Cardiac Tissue Structure, Properties, and Performance knowledge at the cell and cell–matrix level to the whole organ level, but even these are still hampered by limitations in the available experimental data. These limitations are highlighted by the materials science tetrahedron, which nicely illustrates how structure, material properties, processing (for in vitro measurements), and performance are inter-related and interdependent. That is, processing affects structure; structure affects material properties; and all of these factors affect performance. By reviewing the current literature in light of sarcomere structure, material properties, processing required to conduct in vitro tests on cardiac tissue, and, if known, their interactions, we hope to contribute to a more comprehensive and consistent understanding of cardiac tissue performance and ultimately cardiac function.
STRUCTURE
The arrangement of intra- and extracellular proteins as well as their isoforms contribute to ventricular wall material properties. From single myocytes studies, differences between sub-endocardium and sub-epicardium tissues, and between left and right ventricle tissues, have been found to affect sarcomere mechanics. Each of these aspects of structure is briefly described, and its effect on mechanics is discussed below.
Components of Sarcomeres
Myofilaments, the force-generating fibers of sarcomeres, are composed of titin filaments and repeating units of thin and thick filaments as illustrated in Fig. 3. Titin, an intracellular anchor protein, works as a spring both at rest and during contraction.66 Thin filaments consist of actin and the regulatory proteins tropomyosin and troponin, and thick filaments are composed of myosin and accessory proteins. Rod domains of myosin heavy chains comprise the backbone, while globular heads called cross-bridges protrude outward from that backbone. Light chain accessory proteins bound to the myosin globular heads provide structural reinforcement of cross-bridges but in some contractile systems, particularly smooth muscle and non-muscle systems, serve a regulatory function. The interaction of myosin cross bridges with actin generates contractile force.
Protein Isoform Expression
Cardiac sarcomeres expressing different thick and thin filament protein isoforms have been shown to have different mechanical properties.35 For example, the thick filament myosin heavy chain isoform shifts with age and disease progression as well as animal size.63 In particular, larger mammals have a higher expression of β-myosin heavy chain whereas smaller mammals contain more α-myosin heavy chain.49 These thick filament protein isoform differences likely explain previously reported species-specific mechanics differences. 9,18 Different thin filament protein isoforms can also affect sarcomere properties. For example, myocytes isolated from mice expressing β- vs. α-tropomyosin exhibited a greater pCa50 and ktr at submaximal calcium concentrations.43 Similarly, when troponin was replaced with a mutant isoform, calcium sensitivity increased,34 Fmax decreased at all sarcomere lengths and nH decreased at short sarcomere lengths.19 Thus, structural changes in the thick and thin filaments independently affect myocardial properties, although the mechanisms are still not understood.
Differential expression of isoforms of the intracellular spring-like protein titin has also been shown to alter contractile properties. With excision of the N2B titin isoform, pCa50 changed more readily with sarcomere length and passive tension increased.37 With a smaller N2B isoform, Fmax, pCa50, and nH were less dependent on sarcomere length changes compared to controls.44 Since titin isoform expression varies with species and location within the heart,66 some species-specific and location-specific differences in cardiac tissue material properties may be explained on this basis.
Since thick, thin, and titin protein isoforms each influence sarcomere properties and since sarcomere proteins interact internally, the overall ventricular contractile response may further be influenced by interactions of multiple proteins expressing different isoforms. However, interaction effects between isoforms of different intracellular proteins have yet to be determined.
Intracellular Titin vs. Extracellular Matrix
The intracellular protein titin and the ECM proteins provide support and load bearing and are also important contributors to sarcomere mechanics. Titin influences length dependent activation, and the underlying mechanism may involve titin exerting a radial force on myofilaments.10 The ECM is a scaffold composed predominantly of collagen, which surrounds and interconnects myofilaments. The ECM maintains the structural integrity of the heart and transmits sarcomere-generated force to the ventricles to produce pressure.13 One important limitation of the single-cell preparation to assess sarcomere function, discussed below in “Processing and Measurement Techniques” section, is that isolated myocytes do not have ECM and thus the impact of collagen and other proteins on the contractile response can be difficult to assess.
To differentiate the contributions of titin from collagen, specific proteases such as trypsin have been used to degrade titin. Tension-sarcomere length relations can then be obtained in the presence and absence of titin, and the stiffness can be determined from the slope of the tension-sarcomere length relation.66 Studies focusing on titin vs. collagen have established that myocardial tension, both passive and active, is influenced by both proteins, but the individual contributions of each are still debated.10,23,66 Furthermore, contributions of titin and collagen are related to other factors, such as sarcomere length. For example, a consistent finding has been that titin dominates passive tension at shorter SLs while the contribution of collagen is higher at longer SLs.11,23,66 19 Therefore, sarcomere length determines the extent to which each protein contributes to passive tension. In addition, titin-based and collagen-based tension varies between myocytes isolated from the atria and those isolated from the ventricles.66 These data establish that (i) mechanical properties are affected by titin and collagen proteins, and (ii) the contribution of titin vs. collagen is dependent on sarcomere length and location within the heart.
Sub-Epicardium vs. Sub-Endocardium
While the underlying structural differences have not yet been identified, sarcomeres isolated from the subendocardium exhibit differences in material properties from those isolated from the sub-epicardium of the ventricular wall.1,8,16 In particular, active and passive tension is greater in sub-endocardial cells,9 which may be due to differences in sarcomere rest length and subsequently different levels of titin and collagen loading. In support of this suggestion, Chung and Granzier11 found that the working sarcomere length range was smaller in sub-epicardial cells. Also, length-dependent responses differ between the layers. For example, at large sarcomere lengths, pCa50 and maximum tension were significantly higher in sub-endocardial cell preparations compared to sub-epicardial cell preparations.1 Furthermore, with increases in sarcomere length, nH increased in sub-endocardial preparations but decreased in sub-epicardial, and the magnitude of the length-dependent response of pCa50 and ktr was greater in sub-endocardial cells.1 To better account for and quantify the effect of ventricular wall location on sarcomere structure, properties, and performance, measurements should be taken at multiple sarcomere lengths, with and without trypsin degradation of titin, and from additional locations within the ventricular wall.
Right vs. Left Ventricle
The right ventricle is embryologically,68 structurally 48,62 and functionally47 distinct from the left ventricle, which may lead to differences in sarcomere structure.20 Differences between LV and RV sarcomeres may be dominated by a single structural difference (i.e., protein isoform expression), caused by multiple structural differences or due to interactions between aspects of structure. An example of the latter, interactions between aspects of structure, is that sarcomere shortening is highest in LV endocardial cells, intermediate in LV epicardial cells, and lowest in RV cells.33 The structural differences responsible for the fact that calcium sensitivity of force is greater in sarcomeres obtained in the rat LV than that from the rat RV45 remain unknown. Similarly, it is unknown why higher shortening velocities have been found in RV tissue compared to LV tissue, despite no differences in total or passive stress.26 Overall, these results suggest that: (i) baseline mechanical properties are different in LV and RV tissues, which may be attributed to underlying structural differences, and (ii) LV and RV sarcomere properties depend on ventricular wall position. The aspects of sarcomere structure responsible for these differences remain unclear.
PROCESSING AND MEASUREMENT TECHNIQUES
As illustrated in Fig. 1, cardiac tissue properties and performance measured in vitro are largely dependent on the processing steps performed and the measurement techniques used. In a sense, the measurement technique can be considered an element of processing because it can induce structural changes, alter measured properties, and determine feasible loading conditions, which ultimately affect performance. The choices of multi-cellular vs. single cellular tissue preparation and whether or not tissue is permeabilized (skinned) also critically affect response parameters. Sarcomere mechanics test systems use force transducers, typically simultaneously with optical imaging. Design specifications for force transducers include resonance frequency, damping capacity, compliance, and force resolution. Several techniques have been developed to measure mechanics at the myofibril level including motility assay systems53 and a setup including a magnetic field and wire current.30 Improvement in instrumentation and processing can only be accomplished by accounting for critical design considerations including whether single or multi-cellular preparations are used, tissue attachment, permeabilization, and the dynamics of contraction (i.e., isometric, isotonic or both). These aspects are reviewed below.
Multi-Cellular vs. Single Cell Preparation
Cardiac sarcomere mechanics can be measured with single myocytes or multi-cellular preparations. Single cardiac myocytes are advantageous due to their homogeneity and independence from the ECM. Without ECM or cell membranes, the response of single cardiac myocytes is dependent only on the delivery of Ca2+ to the myoplasm and the interactions of the actin and myosin that mediate contraction. However, single myocytes require more excessive processing compared to multi-cellular tissue, which includes enzymatic digestion, perfusion, sieving to remove debris, and centrifugation,20,22,67 all of which can alter structure, properties, and ultimately performance. There are multiple types of multi-cellular preparations, including trabeculae, which are muscular fibers on the surface of the endocardium, and papillary muscles, which are muscular projections from the ventricular walls to the atrioventricular valves. The contractile responses of trabeculae tend to be more consistent due to their smaller radii (which ensures gas and chemical diffusion to interior cells) and more uniform myocyte spacing.54 Disadvantages of multicellular tissue testing include variability in diffusion of chemicals from the extracellular to intracellular spaces and sample heterogeneity.
Measurement techniques used on single- and multicellular preparations also vary somewhat. Typically, sarcomere length is measured optically in single myocytes 28,40 and with laser diffraction in multi-cellular preparations.20,67 From a single measurement of width, cross-sectional area is assumed to be either elliptical39,42,67 or rectangular3,20 for single myocytes and cylindrical for trabeculae.45,48 Differences in tissue attachment for single and multi-cellular preparations are discussed below.
Tissue Attachment
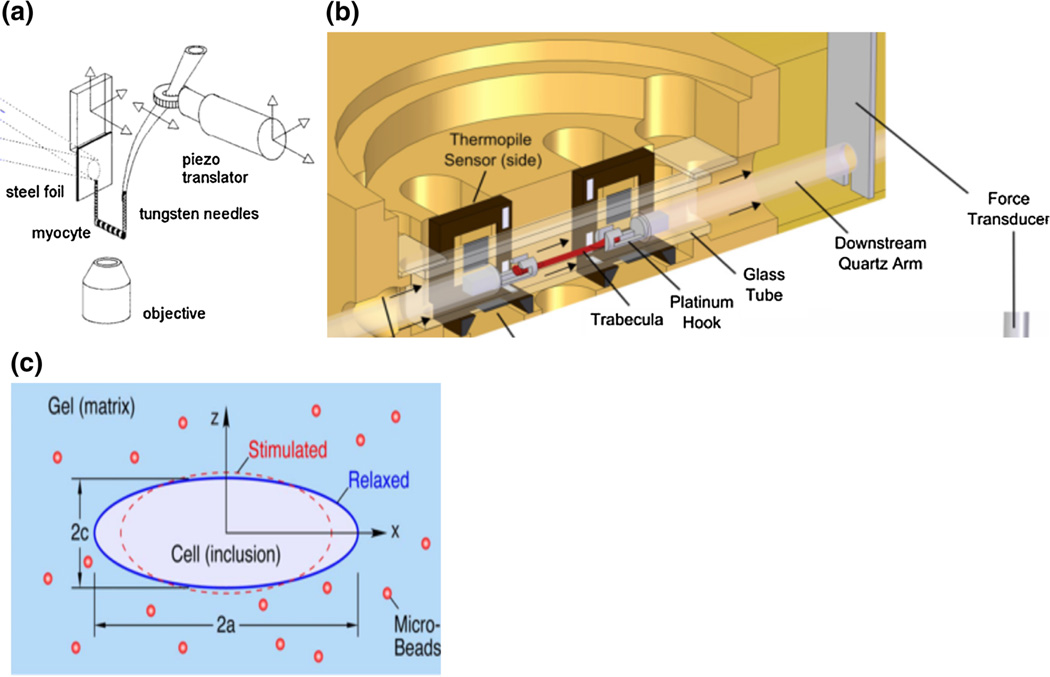
Tissue attachment in an in vitro cardiac tissue mechanics testing system is an important design consideration. Preparations are often mounted to a linear motor for length control on one end and a force transducer on the other,25 both of which can be attached in a variety of ways. Direct attachments including adhesives and suction pipettes are the simplest approaches but the former can be difficult to remove while the latter can induce an inertial artifact into the system.6,56 Stiff or compliant carbon fibers, clips, pins, or hooks are attached to preparations, 14,23,28,40 but these methods cause varying amounts of tissue damage, induce stress concentrations, and increase variability. Figure 5 illustrates single myocytes connected to a force transducer via pins (panel a) and trabeculae attached via platinum hooks (panel b). In more advanced techniques, carbon fibers have been combined with electrostatic67 and electrochemical 36 binding to reduce tissue damage. No tissue damage appears to occur when cardiac myocytes are embedded in an agarose gel46 or in a 3D elastic hydrogel where the myocyte surface adheres to the gel by crosslinking the hydroxyl groups in the matrix51 (Fig. 5c). However, these non-direct attachment systems have not been widely used to date for either single or multi-cellular preparations. A final consideration for tissue attachment is the liquid–air interface. The attachment device is often submerged in medium, and the surface tension forces at the interface may be greater than the cell forces.5 In at least one case, a microtransducer system was fabricated to remove the surface tension artifact.39
FIGURE 5.
Measurement techniques for cardiac mechanics experiments. (a) In vitro experimental setup to characterize sarcomere properties with force transducers and optical imaging from Tasche et al.56 (b) Setup to generate force–length loops developed by Han et al.25 (c) Schematic of myocyte embedded in 3D matrix designed by Shaw et al.51 All figures reproduced with permission (pending).
Permeabilization
Permeabilization, which entails cellular membrane removal, is advantageous in that it removes force contributions from connective tissue and time-varying changes in intracellular Ca2+ but disadvantageous because it can cause myofilament lattice swelling,29 which moves myosin farther from actin binding sites. A study that directly compared permeabilized to intact trabeculae determined that the slope and calcium responsiveness decreased in the permeabilized group.21 This loss of calcium sensitivity may be explained by reduced actin-myosin interactions32 or losses of regulatory proteins in the permeabilized tissue.21 In another study, nH was found to be unaffected by this process.17 Fmax, pCa50, and nH also had similar trends in intact and skinned tissues in heart failure,14 suggesting these aspects of sarcomere mechanics may be unaffected by permeabilization. We conclude that skinning can affect aspects of sarcomere mechanics, which may be due to a non-physiological increase in filament lattice spacing or possibly the loss of regulatory proteins from the thick and thin filaments.
Dynamics of Contraction
Most studies of sarcomere mechanics use non-physiological loading to create a force-pCa curve in which tissues are held at one or one of several fixed sarcomere lengths (isometric contraction). Isotonic and sinusoidal length interventions are also used, but none of these protocols accurately mimic in vivo, dynamic, physiological loading. To address this, several novel techniques use transitions between isometric and isotonic loading to create a force–length work loop in single28,40 or multi-cellular54 preparations that is analogous to the ventricular pressure–volume loop. Initially, force development occurs during an isometric contraction stimulated by an electrical pulse (Fig. 2b; phase 1). The tissue is then allowed to shorten isotonically (phase 2). Then, length is fixed at this shortened distance for force dissipation (isometric relaxation; phase 3) and finally the tissue is lengthened and the force of contraction is measured (a passive re-lengthening; phase 4). The principal limitation for these protocols is that testing is conducted at a sub-physiological frequency; improved instrumentation would allow dynamic loading at physiological frequencies.
Using dynamic loading protocols that impose work loops, additional contractile response parameters have been established. The Frank–Starling Gain index is the ratio between slopes in the end systolic and end diastolic force–length relationships derived from the work loops,4 and the net mechanical efficiency is the ratio of work done to the total energy expenditure (heat and Cardiac Tissue Structure, Properties, and Performance work).54 These metrics help to further characterize the performance of cardiac tissue in a more physiological loading pattern.
Improving the measurement techniques for sarcomere mechanics can be accomplished by: (i) designing novel attachment mechanisms to prevent tissue damage which may be specific to single vs. multi-cellular preparations and (ii) subjecting tissue to dynamic protocols which more accurately mimic physiological loading. In the future, it will be important to make these novel systems widely available so that data can be collected from multiple species and in multiple conditions to better improve our understanding of cardiac mechanics.
PERFORMANCE: INTEGRATION VIA COMPUTATIONAL MODELS
Sarcomere generated force underlies pressure development in the ventricles. Understanding the dynamics of the latter from the details of the former requires integrating information at multiple scales. Computational models allow measurements made at the single and multi-cellular levels to be related to whole heart performance through the integration of myofilament mechanics, myofilament and ECM orientation, and overall ventricular anatomy (wall thickness, chamber volume, etc.).12,38,52
Computational models of the heart, and insights gained from them, have recently been comprehensively reviewed.41,57 In brief, at the subcellular level, cuttingedge myofilament models simulate actin-myosin interactions as well as calcium based activation;57 models also exist of electrical communication across cell–cell junctions.27 Sarcomere dynamics, including changes in sarcomere length and calcium binding,65 have been incorporated into finite element models of the human ventricles.64 Furthermore, the availability of oxygenated blood via the coronary arteries and the impact of fluid– structure interactions on myocardial perfusion have also been simulated.41 A great advantage of models is the ability to predict the effects of disease progression and novel therapies on heart function.41,57 The next big advance may be models that integrate not only spatial and temporal scales, structure and properties to predict performance, but also incorporate advanced clinical imaging of structure and function that enable personalized computational models to be developed.
HEART FAILURE
Heart failure is frequently assessed by mechanical pump function parameters including ejection fraction, cardiac output, stroke volume, and measures of contractility including end-systolic elastance and pre-load recruitable stroke work.31,42,55,58 While some of these metrics can be obtained from clinical right heart catheterization (ejection fraction, cardiac output and stroke volume), the afterload independent metrics of heart function (end systolic elastance and preload recruitable stroke work) require simultaneous pressure and volume measurements and can involve preload reduction in order to obtain the measurements.
Understanding the structural and material property changes in end-stage heart failure may lead to the development of novel prevention therapies. Structural changes during heart failure can include maladaptive ECM remodeling, cardiac myocyte hypertrophy, and alterations in protein isoform expression such as a shift in expression from the α- myosin heavy chain isoform to the β-myosin heavy chain isoform.24 Material property changes with heart failure include calcium sensitivity.60 When measured under isometric conditions, pCa50 is higher (i.e., a lower [Ca2+]) in failing compared to healthy ventricular wall tissue.45,59,61 Also, the length dependent change in calcium sensitivity (ΔpCa50) is smaller in failing vs. non-failing hearts,7,50 which indicates that the length-dependence of pCa50, which underlies heart function, is impaired in heart failure. Interestingly, heart failure appears to manifest differently in the RV vs. the LV. In particular, Fmax and pCa50 increase in the failing RV but not the failing LV.45 Future studies on the specific relations between structural and material property changes during heart failure will contribute to a more holistic understanding of the ventricular contractile response with the hope of preventing and treating heart failure.
SUMMARY AND SUGGESTIONS FOR FUTURE RESEARCH
The materials science tetrahedron illustrates the important inter-relations between properties (“Properties: Fundamentals of Cardiac and Sarcomere Mechanics” section), structure (“Structure” section), processing required for in vitro measurements (“Processing and Measurement Techniques” section) and performance (Sect. Performance: Integration via Computational Models” section). For example, as discussed above, protein isoforms affect material properties and some isoforms shift during heart failure. Importantly, different isoforms of various proteins may interact during various phases of the cardiac cycle, which is a direction for future studies. Differential isoforms have been shown to have varying effects on properties at different sarcomere lengths, which in turn influence length-dependent properties. Depending on the sarcomere length, the structural proteins titin (which is intracellular) and collagen (which is extracellular) will have different contributions to properties. Also, the working range of sarcomere length differs between the endocardium and epicardium, and may differ between the RV and LV, which is yet another way that structure influences properties and possibly vice versa. Finally, in vitro, processing steps such as permeabilization, the choice of attachment method, and loading protocol can impact measured properties as well as measured structure. To relate all of these measurements at the single and multi-cellular level to whole heart performance, computational models are critical.
Based on this review of the literature from the materials science perspective, we have the following recommendations for future research into cardiac tissue structure, properties and performance:
Tissue must be excised from different wall layers and ventricles since structure and material properties vary with location in the heart.
The specific age and size of the animal model must be considered as protein isoforms are age and size dependent.
Measurements should be obtained at several sarcomere lengths since the sarcomere working length varies with heart wall layers and will be differently affected by titin and collagen at different sarcomere lengths.
Processing steps should be minimal, with no tissue damage due to attachment to measurement apparatus, no alternation of contractile proteins via permeabilization, and using measurement techniques that permit dynamic loading protocols at physiological frequencies.
Computational models should incorporate cellular and whole organ function and enable patient-specific prediction of disease progression and efficacy of therapy.
Overall, improving our understanding of the structure– property–processing–performance tetrahedron of heart tissue will improve our understanding of heart health and heart failure.
ACKNOWLEDGEMENTS
Our work was supported by NIH grants 1R01HL086939 (NCC) and 1R37HL82900 (RLM). The authors thank Carol Dizack for preparing the illustrations and Dr. Jitandrakumar Patel for unpublished force-pCa data.
Footnotes
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Ait Mou Y, et al. Differential contribution of cardiac sarcomeric proteins in the myofibrillar force response to stretch. Pflugers Arch. 2008;457:25–36. doi: 10.1007/s00424-008-0501-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bers DM. Calcium fluxes involved in control of cardiac myocyte contraction. Circ. Res. 2000;87:275–281. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
- 3.Bluhm WF, McCulloch AD, Lew WY. Active force in rabbit ventricular myocytes. J. Biomech. 1995;28:1119–1122. doi: 10.1016/0021-9290(94)00018-y. [DOI] [PubMed] [Google Scholar]
- 4.Bollensdorff C, Lookin O, Kohl P. Assessment of contractility in intact ventricular cardiomyocytes using the dimensionless ‘frank-starling gain’ index. Pflugers Arch. 2011;462:39–48. doi: 10.1007/s00424-011-0964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brady AJ. Mechanical properties of isolated cardiac myocytes. Physiol. Rev. 1991;71:413–428. doi: 10.1152/physrev.1991.71.2.413. [DOI] [PubMed] [Google Scholar]
- 6.Brady AJ, Tan ST, Ricchiuti NV. Contractile force measured in unskinned isolated adult rat heart fibres. Nature. 1979;282:728–729. doi: 10.1038/282728a0. [DOI] [PubMed] [Google Scholar]
- 7.Brixius K, et al. Reduced length-dependent cross-bridge recruitment in skinned fiber preparations of human failing myocardium. Eur. J. Appl. Physiol. 2003;89:249–256. doi: 10.1007/s00421-002-0782-2. [DOI] [PubMed] [Google Scholar]
- 8.Bryant SM, Shipsey SJ, Hart G. Regional differences in electrical and mechanical properties of myocytes from guinea-pig hearts with mild left ventricular hypertrophy. Cardiovasc. Res. 1997;35:315–323. doi: 10.1016/s0008-6363(97)00111-9. [DOI] [PubMed] [Google Scholar]
- 9.Cazorla O, Le Guennec JY, White E. Length-tension relationships of sub-epicardial and sub-endocardial single ventricular myocytes from rat and ferret hearts. J. Mol. Cell. Cardiol. 2000;32:735–744. doi: 10.1006/jmcc.2000.1115. [DOI] [PubMed] [Google Scholar]
- 10.Cazorla O, et al. Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circ. Res. 2001;88:1028–1035. doi: 10.1161/hh1001.090876. [DOI] [PubMed] [Google Scholar]
- 11.Chung CS, Granzier HL. Contribution of titin and extracellular matrix to passive pressure and measurement of sarcomere length in the mouse left ventricle. J. Mol. Cell. Cardiol. 2011;50:731–739. doi: 10.1016/j.yjmcc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa KD, Holmes JW, McCulloch AD. Modelling cardiac mechanical properties in three dimensions. Philos. Trans. R. Soc. Lond. 2001;359:1233–1250. [Google Scholar]
- 13.de Tombe PP. Altered contractile function in heart failure. Cardiovasc. Res. 1998;37:367–380. doi: 10.1016/s0008-6363(97)00275-7. [DOI] [PubMed] [Google Scholar]
- 14.de Tombe PP, et al. Right ventricular contractile protein function in rats with left ventricular myocardial infarction. Am. J. Physiol. 1996;271:H73–H79. doi: 10.1152/ajpheart.1996.271.1.H73. [DOI] [PubMed] [Google Scholar]
- 15.de Tombe PP, et al. Myofilament length dependent activation. J. Mol. Cell. Cardiol. 2010;48:851–858. doi: 10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diffee GM, Nagle DF. Regional differences in effects of exercise training on contractile and biochemical properties of rat cardiac myocytes. J. Appl. Physiol. 2003;95:35–42. doi: 10.1152/japplphysiol.00951.2002. (1985) [DOI] [PubMed] [Google Scholar]
- 17.Dobesh DP, Konhilas JP, de Tombe PP. Cooperative activation in cardiac muscle: impact of sarcomere length. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1055–H1062. doi: 10.1152/ajpheart.00667.2001. [DOI] [PubMed] [Google Scholar]
- 18.Edes IF, et al. Rate of tension redevelopment is not modulated by sarcomere length in permeabilized human, murine, and porcine cardiomyocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R20–R29. doi: 10.1152/ajpregu.00537.2006. [DOI] [PubMed] [Google Scholar]
- 19.Farman GP, et al. The role of thin filament cooperativity in cardiac length-dependent calcium activation. Biophys. J. 2010;99:2978–2986. doi: 10.1016/j.bpj.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedberg MK, Redington AN. Right versus left ventricular failure: differences, similarities, and interactions. Circulation. 2014;129:1033–1044. doi: 10.1161/CIRCULATIONAHA.113.001375. [DOI] [PubMed] [Google Scholar]
- 21.Gao WD, et al. Myofilament Ca2+ sensitivity in intact versus skinned rat ventricular muscle. Circ. Res. 1994;74:408–415. doi: 10.1161/01.res.74.3.408. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Webb MG, et al. A modular instrument for exploring the mechanics of cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H866–H874. doi: 10.1152/ajpheart.01055.2006. [DOI] [PubMed] [Google Scholar]
- 23.Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys. J. 1995;68:1027–1044. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamdani N, et al. Sarcomeric dysfunction in heart failure. Cardiovasc. Res. 2008;77:649–658. doi: 10.1093/cvr/cvm079. [DOI] [PubMed] [Google Scholar]
- 25.Han JC, et al. A unique micromechanocalorimeter for simultaneous measurement of heat rate and force production of cardiac trabeculae carneae. J. Appl. Physiol. 2009;107:946–951. doi: 10.1152/japplphysiol.00549.2009. [DOI] [PubMed] [Google Scholar]
- 26.Han JC, et al. Interventricular comparison of the energetics of contraction of Trabeculae Carneae isolated from the rat heart. J. Physiol. 2013;591:701–717. doi: 10.1113/jphysiol.2012.242719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hand PE, Peskin CS. Homogenization of an electrophysiological model for a strand of cardiac myocytes with gap-junctional and electric-field coupling. Bull. Math. Biol. 2010;72:1408–1424. doi: 10.1007/s11538-009-9499-2. [DOI] [PubMed] [Google Scholar]
- 28.Iribe G, Helmes M, Kohl P. Force-length relations in isolated intact cardiomyocytes subjected to dynamic changes in mechanical load. Am. J. Physiol. Heart Circ Physiol. 2007;292:H1487–H1497. doi: 10.1152/ajpheart.00909.2006. [DOI] [PubMed] [Google Scholar]
- 29.Irving TC, et al. Myofilament lattice spacing as a function of sarcomere length in isolated rat myocardium. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H2568–H2573. doi: 10.1152/ajpheart.2000.279.5.H2568. [DOI] [PubMed] [Google Scholar]
- 30.Iwazumi T. High-speed ultrasensitive instrumentation for myofibril mechanics measurements. Am. J. Physiol. 1987;252:C253–C262. doi: 10.1152/ajpcell.1987.252.2.C253. [DOI] [PubMed] [Google Scholar]
- 31.Joho S, et al. Left ventricular pressure-volume relationship in conscious mice. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H369–H377. doi: 10.1152/ajpheart.00704.2006. [DOI] [PubMed] [Google Scholar]
- 32.King NMP, et al. Mouse intact cardiac myocyte mechanics: cross-bridge and titin-based stress in unactivated cells. J. Gen. Physiol. 2011;137:81–91. doi: 10.1085/jgp.201010499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondo RP, et al. Comparison of contraction and calcium handling between right and left ventricular myocytes from adult mouse heart: a role for repolarization waveform. J. Physiol. 2006;571:131–146. doi: 10.1113/jphysiol.2005.101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konhilas JP, et al. Troponin I in the murine myocardium: Influence on length-dependent activation and interfilament spacing. J. Physiol. 2003;547:951–961. doi: 10.1113/jphysiol.2002.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korte FS, McDonald KS. Sarcomere length dependence of rat skinned cardiac myocyte mechanical properties: dependence on myosin heavy chain. J. Physiol. 2007;581:725–739. doi: 10.1113/jphysiol.2007.128199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Guennec JY, et al. A new method of attachment of isolated mammalian ventricular myocytes for tension recording: length dependence of passive and active tension. J. Mol. Cell. Cardiol. 1990;22:1083–1093. doi: 10.1016/0022-2828(90)90072-a. [DOI] [PubMed] [Google Scholar]
- 37.Lee E-J, et al. Calcium sensitivity and the frank-starling mechanism of the heart are increased in titin n2b region-deficient mice. J. Mol. Cell. Cardiol. 2010;49:449–458. doi: 10.1016/j.yjmcc.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LeGrice I, et al. The architecture of the heart: a data–based model. Philos. Trans. R. Soc. Lond. 2001;359:1217–1232. [Google Scholar]
- 39.Lin G, et al. Miniature heart cell force transducer system implemented in MEMS technology. IEEE Trans. Biomed. Eng. 2001;48:996–1006. doi: 10.1109/10.942589. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura S, et al. Single cell mechanics of rat cardiomyocytes under isometric, unloaded, and physiologically loaded conditions. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H196–H202. doi: 10.1152/ajpheart.00948.2003. [DOI] [PubMed] [Google Scholar]
- 41.Nordsletten DA, et al. Coupling multi-physics models to cardiac mechanics. Prog. Biophys. Mol. Biol. 2011;104:77–88. doi: 10.1016/j.pbiomolbio.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Pacher P, et al. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat. Protoc. 2008;3:1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel JR, et al. Pka accelerates rate of force development in murine skinned myocardium expressing α-or β-tropomyosin. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2732–H2739. doi: 10.1152/ajpheart.2001.280.6.H2732. [DOI] [PubMed] [Google Scholar]
- 44.Patel JR, et al. Magnitude of length-dependent changes in contractile properties varies with titin isoform in rat ventricles. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H697–H708. doi: 10.1152/ajpheart.00800.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perreault CL, et al. Differential effects of cardiac hypertrophy and failure on right vs. left ventricular calcium activation. Circ. Res. 1990;67:707–712. doi: 10.1161/01.res.67.3.707. [DOI] [PubMed] [Google Scholar]
- 46.Petroff MG, et al. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+release in cardiomyocytes. Nat. Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- 47.Redington AN, et al. Characterisation of the normal right ventricular pressure-volume relation by biplane angiography and simultaneous micromanometer pressure measurements. Br. Heart J. 1988;59:23–30. doi: 10.1136/hrt.59.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roche SL, Redington AN. The failing right ventricle in congenital heart disease. Can. J. Cardiol. 2013;29:768–778. doi: 10.1016/j.cjca.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Rundell VLM, et al. Impact of beta-myosin heavy chain isoform expression on cross-bridge cycling kinetics. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H896–H903. doi: 10.1152/ajpheart.00407.2004. [DOI] [PubMed] [Google Scholar]
- 50.Schwinger RH, et al. The failing human heart is unable to use the frank-starling mechanism. Circ. Res. 1994;74:959–969. doi: 10.1161/01.res.74.5.959. [DOI] [PubMed] [Google Scholar]
- 51.Shaw J, Izu L, Chen-Izu Y. Mechanical analysis of single myocyte contraction in a 3d elastic matrix. PLoS ONE. 2013;8:e75492–e75492. doi: 10.1371/journal.pone.0075492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevens C, Hunter PJ. Sarcomere length changes in a 3d mathematical model of the pig ventricles. Prog. Biophys. Mol. Biol. 2003;82:229–241. doi: 10.1016/s0079-6107(03)00023-3. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki M, Fujita H, Ishiwata S. A new muscle contractile system composed of a thick filament lattice and a single actin filament. Biophys. J. 2005;89:321–328. doi: 10.1529/biophysj.104.054957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taberner AJ, et al. An innovative work-loop calorimeter for in vitro measurement of the mechanics and energetics of working cardiac trabeculae. J. Appl. Physiol. 2011;111:1798–1803. doi: 10.1152/japplphysiol.00752.2011. [DOI] [PubMed] [Google Scholar]
- 55.Tabima DM, Hacker TA, Chesler NC. Measuring right ventricular function in the normal and hypertensive mouse hearts using admittance-derived pressure-volume loops. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H2069–H2075. doi: 10.1152/ajpheart.00805.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tasche C, Meyhofer E, Brenner B. A force transducer for measuring mechanical properties of single cardiac myocytes. Am. J. Physiol. 1999;277:H2400–H2408. doi: 10.1152/ajpheart.1999.277.6.H2400. [DOI] [PubMed] [Google Scholar]
- 57.Trayanova NA, Rice JJ. Cardiac electromechanical models: from cell to organ. Front. Physiol. 2011;2:43. doi: 10.3389/fphys.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Umar S, et al. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1606–H1616. doi: 10.1152/ajpheart.00590.2009. [DOI] [PubMed] [Google Scholar]
- 59.van der Velden J, et al. Effect of protein kinase a on calcium sensitivity of force and its sarcomere length dependence in human cardiomyocytes. Cardiovasc. Res. 2000;46:487–495. doi: 10.1016/s0008-6363(00)00050-x. [DOI] [PubMed] [Google Scholar]
- 60.van der Velden J, et al. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc. Res. 2003;57:37–47. doi: 10.1016/s0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 61.van der Velden J, et al. The effect of myosin light chain 2 dephosphorylation on Ca2+-sensitivity of force is enhanced in failing human hearts. Cardiovasc. Res. 2003;57:505–514. doi: 10.1016/s0008-6363(02)00662-4. [DOI] [PubMed] [Google Scholar]
- 62.Walker LA, Buttrick PM. The right ventricle: biologic insights and response to disease. Curr. Cardiol. Rev. 2009;5:22–28. doi: 10.2174/157340309787048077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker LA, et al. Biochemical and myofilament responses of the right ventricle to severe pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H832–H840. doi: 10.1152/ajpheart.00249.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Washio T, Okada J-I, Hisada T. A parallel multilevel technique for solving the bidomain equation on a human heart with purkinje fibers and a torso model. SIAM Rev. 2010;52:717–743. [Google Scholar]
- 65.Washio T, et al. Approximation for cooperative interactions of a spatially-detailed cardiac sarcomere model. Cell. Mol. Bioeng. 2012;5:113–126. doi: 10.1007/s12195-011-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu Y, et al. Changes in titin and collagen underlie diastolic stiffness diversity of cardiac muscle. J. Mol. Cell. Cardiol. 2000;32:2151–2162. doi: 10.1006/jmcc.2000.1281. [DOI] [PubMed] [Google Scholar]
- 67.Yasuda S-I, et al. A novel method to study contraction characteristics of a single cardiac myocyte using carbon fibers. Am. J. Physiol. Heart Circ. Physiol. 2001;281:1442–1446. doi: 10.1152/ajpheart.2001.281.3.H1442. [DOI] [PubMed] [Google Scholar]
- 68.Zaffran S, et al. Right ventricular myocardium derives from the anterior heart field. Circ. Res. 2004;95:261–268. doi: 10.1161/01.RES.0000136815.73623.BE. [DOI] [PubMed] [Google Scholar]