Abstract
Prefrontal serotonin 5-HT2 receptors have been linked to the pathogenesis and treatment of affective disorders, yet their function in psychiatric vulnerability is not known. Here, we examine the effects of 5-HT2 receptors in a rat model of psychiatric vulnerability using electrophysiology, gene expression, and behavior. Following the early stress of chronic maternal separation, we found that serotonin has atypical 5-HT2 receptor-mediated excitatory effects in the adult prefrontal cortex that were blocked by the 5-HT2A receptor antagonist MDL 100907. In the absence of a serotonergic agonist, the intrinsic excitability of the prefrontal cortex was not enhanced relative to controls. Yet, in response to stimulation of 5-HT2 receptors, adult animals with a history of early stress exhibit heightened prefrontal network activity in vitro, enhanced immediate early gene expression in vivo, and potentiated head shake behavior. These changes arise in the absence of any major alteration of prefrontal 5-HT2A/C mRNA expression or 5-HT2 receptor binding. Our microarray results and quantitative PCR validation provide insight into the molecular changes that accompany such enhanced 5-HT2 receptor function in adult animals following early stress. We observed persistent prefrontal transcriptome changes, with significant enrichment of genes involved in cellular developmental processes, regulation of signal transduction, and G-protein signaling. Specific genes regulated by early stress were validated in an independent cohort, and several altered genes were normalized by chronic blockade of 5-HT2 receptors in adulthood. Together, our results demonstrate enhanced prefrontal 5-HT2 receptor function and persistent alterations in prefrontal gene expression in a rat model of psychiatric vulnerability.
Introduction
Prefrontal serotonin 5-HT2 receptors have long been implicated in mood and anxiety disorders (Naughton et al., 2000; Stockmeier, 2003). However, it remains controversial whether enhanced 5-HT2 receptor binding in the prefrontal cortex (PFC) predicts the presence of a psychiatric illness (D'haenen et al., 1992; Yatham et al., 2000; Shelton et al., 2009) and whether a change in this measure predicts the effectiveness of treatment (Yatham et al., 1999; Meyer et al., 2001; Zanardi et al., 2001). Instead, it has been hypothesized that 5-HT2 signaling efficiency may be a better predictor of vulnerability to psychiatric illness (González-Maeso and Meana, 2006). Patients with mood disorders have increased coupling efficiency of the 5-HT2 receptor with its associated G-protein in the PFC (Friedman and Wang, 1996) and in blood platelets (Parakh et al., 2008). Furthermore, adding a 5-HT2 antagonist to the current standard treatment for mood and anxiety disorders augments its therapeutic efficacy (Marek et al., 2003) and improves the therapeutic response of treatment-resistant patients (Brawman-Mintzer et al., 2005; Marcus et al., 2008; Sato et al., 2009). However, it has not been directly investigated whether vulnerability to mood and anxiety disorders is associated with an increase in prefrontal 5-HT2 receptor electrophysiological effects and downstream signaling cascades.
Here, we have investigated the function of the prefrontal 5-HT2 receptors in a rat model of increased vulnerability to adult psychopathology. We chose the early stress of maternal separation, since it results in a persistent vulnerability to endophenotypes of anxiety and depression in adulthood (Sánchez et al., 2001). Using a combination of whole-cell electrophysiological recordings, 5-HT2-mediated induction of immediate early gene expression, and behavioral output, we demonstrate that exposure to early stress (ES) increases prefrontal 5-HT2 signaling efficacy in adulthood. We further identified alterations in genes associated with cellular development and signal transduction in the PFC of adult animals previously exposed to ES, using microarray analysis with quantitative PCR (qPCR). Collectively, our findings indicate that a life history of ES enhances the function of prefrontal 5-HT2 receptors. Given the importance of the PFC in modulating emotional behavior (Price, 1999), and the ability of cortical 5-HT2A receptors to influence anxiety states (Weisstaub et al., 2006), this neurobiological abnormality may contribute to the genesis of mood and anxiety disorders.
Materials and Methods
Animals
Sprague Dawley rats were used for all experiments (electrophysiological, behavioral, and molecular experiments) and were maintained on a 12 h light/dark cycle (lights on at 7:00 A.M.) with ad libitum access to food and water. The electrophysiological experiments were performed at the University of Toronto and approved by the local Animal Care and Use Committee. The molecular and behavioral experiments were performed at the Tata Institute of Fundamental Research (TIFR), and were approved by the TIFR Institutional Animal Ethics Committee. All protocols conformed to the National Institutes of Health Guidelines on the Care and Use of Experimental Animals.
Early stress paradigm
As a model of vulnerability to psychiatric disorders, the ES paradigm of maternal separation (Nair et al., 2007) was used. In brief, pregnant primiparous dams delivered pups within the animal housing facility and on postnatal day (P) 1 litters were randomly assigned to ES or control groups. Pups in the ES litters were separated from their mothers for a period of 3 h at the same time in the morning each day from P2 to P14. Control litters were left undisturbed. All litters were handled briefly at 3–4 d intervals to allow for cage cleaning. Once weaned, all pups were housed in same-sex sibling groups of 2–5 rats.
Electrophysiological experiments
Slice preparation.
Sprague Dawley dams (Charles River) delivered pups within the University of Toronto (Toronto, Ontario, Canada) animal housing facility and underwent the ES paradigm described above. The electrophysiological experiments included recordings of 21 rats from 11 control litters and 28 rats from 10 ES litters. In adolescence and adulthood, male and female rats (P21–83) were anesthetized with chloral hydrate (400 mg/kg) and coronal slices (400 μm thick) of the PFC (4.2 to 2.5 mm from bregma) were sliced on a Dosaka linear slicer (SciMedia) and transferred to 32°C oxygenated ACSF (128 mm NaCl, 10 mm d-glucose, 24 mm NaHCO3, 2 mm CaCl2, 2 mm MgSO4, 3 mm KCl, 1.25 mm NaH2PO4, pH 7.4) in a prechamber (Automate). For recording, slices were placed in a modified superfusion chamber (Warner Instruments) and mounted on the stage of an Olympus BX50WI microscope. Oxygenated ACSF at 31–33°C flowed over the slice at a rate of 3–4 ml/min.
Whole-cell recording.
Whole-cell patch electrodes (2–3 MΩ) contained 120 mm potassium gluconate, 5 mm KCl, 2 mm MgCl2, 4 mm K2-ATP, 0.4 mm Na2-GTP, 10 mm Na2-phosphocreatine, and 10 mm HEPES buffer (adjusted to pH 7.3 with KOH). Layer V pyramidal neurons in PFC (prelimbic and anterior cingulate regions) were patched under visual control using infrared differential interference contrast microscopy. Pyramidal neurons were identified based on their pyramidal shape and the presence of a prominent apical dendrite. Currents were recorded using continuous single-electrode voltage-clamp mode with a Multiclamp 700b amplifier (Molecular Devices), acquired at 20 kHz, and low-pass filtered (3 kHz) using pClamp10.2 and Digidata1440 software (Molecular Devices).
Data collection.
After a 1 min period of baseline recording, serotonergic currents were probed by adding 5-hydroxytryptamine creatinine sulfate (5-HT) (10 μm, 30 s) in the bath perfusion followed by at least a 5 min washout period. The amplitude of the dominant 5-HT current was measured using Clampfit software (Molecular Devices) by subtracting the mean current at the peak of the 5-HT response (averaged over 10 s) from the mean current at baseline (averaged over 10 s). There were no significant sex differences in the 5-HT currents within the control and ES groups (unpaired t test, p = 0.9). When applying DOI (3 μm, 15 min) to measure the inward currents elicited by 5-HT2 stimulation, we noticed the spontaneous appearance of network activity or “up” states in almost every recording from the ES animals. These phenomena were identified and quantified based on previous description (Sanchez-Vives and McCormick, 2000).
To measure membrane excitability, neurons were injected with depolarizing current pulses of 500 ms length, increasing in 10 pA increments from 0 to 400 pA and separated by a 1 s interval. Spontaneous postsynaptic currents (sPSCs) were analyzed with MiniAnalysis software (Synaptosoft). Glutamatergic sPSCs were recorded at baseline and during 5-HT application (10 μm, 30 s) under the recording conditions described above. Recording of GABAergic sPSCs were performed with patch electrodes that contained 50 mm K-gluconate, 75 mm KCl, 2 mm MgCl2, 4 mm K2-ATP, 400 μm Na2-GTP, 10 mm Na2-phosphocreatine, and 10 mm HEPES buffer (adjusted to pH 7.3 with KOH). These recordings were performed in the presence of the AMPA/KA glutamate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (20 μm). Under these conditions, GABAergic sPSCs were completely suppressed by application of the GABAA receptor blocker bicuculline (10 μm; 10 min). Analysis of glutamatergic and GABAergic sPSCs was performed using MiniAnalysis software (Synaptosoft).
Pharmacology.
In a subset of experiments, pharmacological agents were applied to the slice using oxygenated ACSF: 50 μm D(−)-2-amino-5-phosophonopentanoic acid (APV), 10 μm bicuculline, 20 μm CNQX, 3 μm DOI, 2 μm ketanserin tartrate, 30 nm MDL 100907, and 30 nm WAY 100635. The MDL 100907 was a gift from Dr. George Aghajanian of Yale University (New Haven, CT). All other compounds were obtained from Sigma or Tocris Bioscience. All compounds stored in stock solutions at −20°C before being diluted in oxygenated ACSF.
Statistical analysis.
All statistical comparisons were made at a significance level of 0.05 unless noted otherwise. Statistical comparisons between responses from different experimental groups (control vs ES) were determined using two-tailed unpaired t tests. Analysis of correlations between experimental parameters (e.g., postnatal age and 5-HT currents) were examined using linear regression. Two sets of analysis were performed for sPSCs. Within-cell analysis of 5-HT-elicited change in sPSCs was examined with Kolmogorov–Smirnov test (significance level of 0.01). The average sPSC frequency by group was assessed with parametric two-way ANOVA and post hoc with Bonferroni tests. Comparison between proportions of cells displaying a network activity response or 5-HT-elicited sPSC response was analyzed using Fisher's exact test.
Molecular and behavioral experiments
Experimental design.
Sprague Dawley rats (bred in the animal facility at TIFR) underwent the ES paradigm as described above, and the males were examined in adulthood (P60–90). Using qPCR and radioligand binding for [3H]ketanserin, the influence of ES on 5-HT2A and 5-HT2C receptor mRNA expression and 5-HT2 binding in the PFC (4.2 to 2.5 mm from bregma) was assessed. To examine the functional status of the prefrontal 5-HT2 receptors, both control and ES animals were intraperitoneally administered the 5-HT2 agonist DOI (8 mg/kg, Sigma) or vehicle (0.9% NaCl). The effect of DOI on the expression of the immediate early gene Arc was examined using in situ hybridization. DOI-induced head twitch response, a behavior mediated by prefrontal 5-HT2A receptors (Willins and Meltzer, 1997), was studied in control and ES animals. To determine changes in gene expression that arise in the PFC following a history of ES, a microarray analysis was performed. Furthermore, to address gene expression changes that arise following 5-HT2 stimulation, the DOI-induced transcriptome in the PFC of control animals was analyzed. Candidate genes observed to be regulated in the microarray studies were validated using qPCR on independent tissue samples. Finally, we addressed whether a component of the prefrontal transcriptome regulated by early stress history can be reversed by systemic treatment with the 5-HT2 receptor antagonist ketanserin (Sigma).
5-HT2 receptor autoradiography.
Control (n = 4) and ES animals (n = 7) were rapidly decapitated and the brains were frozen on dry ice and stored at −80°C before processing for receptor autoradiography. Coronal sections (14 μm thick) were cut on the cryostat (Leica), thaw mounted on Probe-on Plus slides (Electron Microscopy Sciences), and stored at −80°C. Receptor autoradiography for [3H]ketanserin (67ci/mmol; PerkinElmer) binding in the PFC of control and ES animals was assessed as described previously (Preece et al., 2004). In brief, two slides from each brain were preincubated in a buffer containing 170 mm Tris, pH 7.7 (binding buffer), followed by incubation in the same buffer containing 2 nm [3H]ketanserin for 2 h at room temperature. Prazosin (1 μm; Sigma) was added to block binding to α1 adrenoceptors. Furthermore, 10 μm ketanserin, along with 2 nm [3H]ketanserin, was used as a nonspecific binding control on separate slides. The slides were washed with binding buffer, air dried overnight, and exposed to 3H-sensitive film (Kodak) for 8–10 weeks. The autoradiograms were developed and binding densities were quantitated using Scion Image software (Scion). The binding density of [3H]ketanserin in the PFC region was determined using optical density measurements (average of 6–8 measurements from three or four sections per animal). The calibration was done using 14C standards, and data were expressed as percentage of control. The slides incubated with 10 μm ketanserin (nonspecific binding control) did not yield any detectable signal confirming the specificity of [3H]ketanserin binding.
In situ hybridization.
To visualize alterations in circuit activity induced by 5-HT2 stimulation with DOI, in situ hybridization was performed for the immediate early gene, activity regulated cytoskeletal-associated protein Arc (Temple et al., 2003). Control and ES animals (n = 4–5 per group) received an i.p. injection of DOI or vehicle and were killed 2 h later. Animals were rapidly decapitated and the brains were immediately frozen on dry ice. In situ hybridization was performed as described previously (Nair et al., 2007). Cryostat-cut (14 μm) coronal sections (4.2 to 2.5 mm from bregma) were fixed, acetylated, and dehydrated before storage at −70°C. Antisense riboprobes to Arc mRNA were generated from a transcription competent plasmid kindly provided by Dr. Oswald Steward (Johns Hopkins University, Baltimore, MD). Slides were incubated with the 35S-UTP-labeled riboprobe (1 × 106 cpm/150 μl) in hybridization buffer (50% formamide, 1× SSC, 25× Denhardt's solution, 40 mm dithiothreitol, 150 μg/ml yeast tRNA, 10% dextran sulfate, 400 μg/ml salmon sperm DNA) for 16 h at 60°C. Following hybridization, all slides were washed in ribonuclease A (20 μg/μl) followed by stringent washes in decreasing concentrations of SSC. Slides were air dried and exposed to Hyperfilm β-max (Kodak) for 5 d. Levels of Arc mRNA were quantified using Scion Image software and calibrated using 14C standards to correct for nonlinearity. Optical density was measured from both hemispheres of the PFC in three or four sections per brain.
DOI-induced head shake behavior.
DOI-induced head shakes are a stereotypical behavior elicited by activation of 5-HT2A receptors in the PFC (Willins and Meltzer, 1997). To address whether a history of ES influenced 5-HT2A receptor-mediated behavior, the frequency of head shakes induced in response to DOI administration was examined in control and ES animals. Adult control and ES rats (n = 4–5 per group) received an i.p. injection of either DOI or vehicle, and behavior was recorded in their home cages for a duration of 20 min commencing 20 min after the injection of DOI or vehicle. The head shakes were defined as a rapid radial movement of the head and were counted by an experimenter blind to the treatment groups.
Microarray.
To understand the mechanistic underpinnings of the altered 5-HT2 function in ES animals, a microarray approach was used to examine the long-lasting transcriptional changes in adult ES animals compared with controls. Furthermore, to assess whether the transcriptome changes observed in adult animals with a history of ES include a component that may be a consequence of altered 5-HT2 receptor signaling, we compared the ES transcriptome to the changes induced in response to 5-HT2 receptor stimulation by DOI. Animals from three experimental groups [control (n = 3), ES (n = 4), DOI (n = 4)] were rapidly decapitated 2 h after either DOI or vehicle injection. The PFC was dissected, frozen in liquid nitrogen, and stored at −80°C. RNA from bilateral prefrontal cortices from each animal was extracted using an Rneasy minikit (Qiagen). The quality and integrity of the RNA was determined using an optical density ratio of 260/280 using the NanoDrop spectrophotometer (NanoDrop Technologies) and bioanalyzer profiles using Agilent 2100 Bioanalyzer (Agilent Technologies). RNA was labeled using the Agilent Quick Amp PLUS kit according to manufacturer's instructions (Agilent Technologies). Agilent standard spike controls were used in all labeling reactions. The labeled RNA (1650 ng) was fragmented and hybridized to a custom rat array 8X15K (Agilent microarray design identifier: 21617) with 15,000 features. The hybridized slides were washed using wash buffers (Agilent Technologies) and scanned using the Agilent microarray scanner G, model G2565BA, at 100% laser power, 30% photomultiplier tube, and 5 μm resolution. Data extraction was performed with Agilent Technologies Feature Extraction software (version 9.1).
Feature extracted data were analyzed using GeneSpring GX, version 7.3.1 software from Agilent Technologies. Normalization of the data was done in GeneSpring GX using the recommended one color per chip, and per gene data were as follows: (1) transformation: set measurements <0.01 to 0.01; (2) per chip: normalize to 50th percentile; (3) per gene: normalize to specific samples (control or ES). Differentially regulated genes were filtered with a cutoff of >1.5 for upregulation and <0.66 for downregulation. Statistical analysis was done using a t test with a significance level of 0.05 and corrected for multiple comparisons using the Benjamini and Hochberg method with a false discovery rate of 0.05 for all arrays (Benjamini and Hochberg, 1995). Hierarchical clustering was done based on fold change values for each gene using the GeneSpring software. The microarray data discussed in this manuscript have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus (GEO accession number: GSE14720). Upregulated and downregulated genes in the ES animals (compared with control) were subjected to functional analysis using DAVID (Database for Annotation, Visualization, and Integrated Discovery; http://david.abcc.ncifcrf.gov/) functional annotation tools (Dennis et al., 2003; Huang et al., 2009).
Quantitative PCR.
To validate candidate genes from our array studies, qPCR was conducted on tissue samples from an independent experimental cohort of animals. qPCR was also performed to determine the influence of ES on 5-HT2A and 5-HT2C receptor mRNA expression. Tissue samples from the PFC were prepared as described above, RNA was extracted, and quality control was performed to confirm RNA integrity using spectroscopic analysis. RNA was then reverse transcribed and subjected to qPCR with TaqMan probes (Applied Biosystems). Quantification was determined using the ΔΔCt method as described previously (Bookout and Mangelsdorf, 2003; Tsankova et al., 2004). Data from all groups were normalized to an average of four endogenous housekeeping genes (18s rRNA, Gapdh, Hprt, β-actin). Results were compared with the control group and expressed as a fold change ± SEM. See supplemental Table 5, available at www.jneurosci.org, for a list of primers used.
Blockade with the 5-HT2 antagonist, ketanserin.
To address whether chronic blockade of 5-HT2 receptor signaling is capable of restoring gene expression changes observed in the PFC of ES animals, we treated animals with the 5-HT2 antagonist ketanserin. Control and ES animals were treated with ketanserin for 6 d, receiving ketanserin (2 mg/kg) (Pei et al., 2000) or vehicle (10% DMSO) via i.p. injections twice daily. On day 6, the animals were killed 1 h after receiving the first injection of the day. The PFC was dissected, frozen in liquid nitrogen, and stored at −80°C. RNA was extracted, subjected to quality control analysis, reverse transcribed, and subjected to qPCR using SYBR Green (Applied Biosystems) as described above. See supplemental Table 6, available at www.jneurosci.org, for a list of primers used.
Statistical analysis.
Statistical analysis was performed using the software Prism (Graphpad). Experiments with two groups were analyzed using the unpaired Student's t test. Experiments with four groups were subjected to a two-way ANOVA, followed by a Bonferroni post hoc test with a significance level of 0.05.
Results
Early stress changes the adult pattern of responses to serotonin in prefrontal neurons
To assess the effects of ES on the response to serotonin in the PFC, we performed whole-cell recordings of layer V pyramidal neurons in the PFC from control and ES rats. In control animals, we found that bath application of 5-HT (10 μm; 30 s) elicited a dominant outward current in almost all layer V pyramidal neurons (n = 30/35; 86%), with only a small percentage of neurons displaying no response (n = 5/35; 14%). These findings are summarized in Figure 1A and are consistent with previous reports showing that the net serotonergic response of adult layer V pyramidal neurons is dominated by an inhibitory 5-HT1A-mediated outward current (Gartside et al., 2000; Béïque et al., 2004; Puig et al., 2005).
Figure 1.
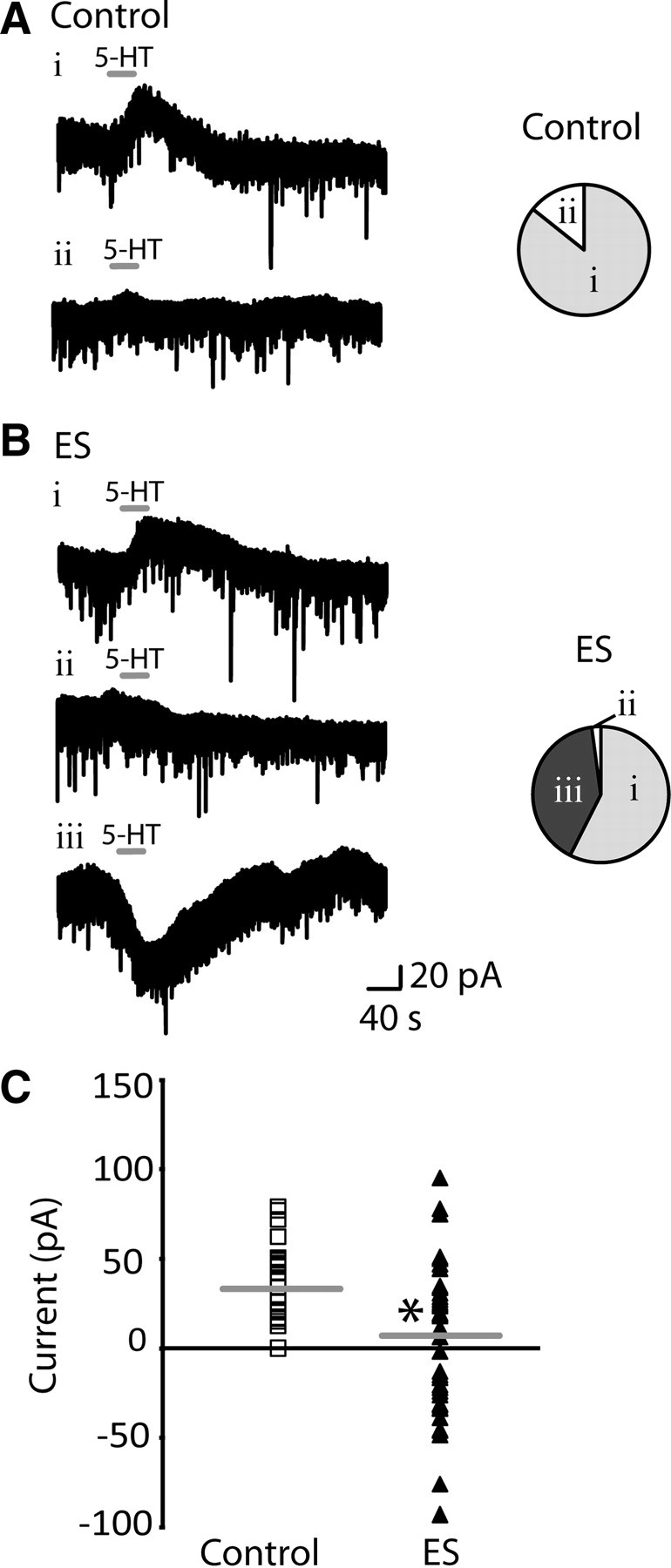
Serotonin elicits a different pattern of adult responses in layer V pyramidal neurons after early stress. A, Examples of voltage-clamp traces from control animals show that bath application of 5-HT (10 μm; 30 s) elicited either a dominant outward current (Ai) or no net response (Aii) in layer V pyramidal neurons in the prefrontal cortex. The horizontal line denotes application of 5-HT. The pie chart shows that a large proportion of layer V pyramidal neurons in control animals displayed a 5-HT-induced outward current (Ai; n = 30/35), and a small subset of neurons showed no response (Aii; n = 5/35). B, Examples of voltage-clamp traces from ES animals show that the same application of 5-HT elicited one of three responses: a dominant outward current (Bi), no net response (Bii), or a dominant inward current (Biii) in layer V pyramidal neurons. The dominant inward current was only observed in brain slices from ES animals. The pie chart represents the proportion of layer V pyramidal neurons that displayed outward currents (Bi; n = 27/47), no response (Bii; n = 1/47), or inward currents in response to 5-HT (Biii; n = 19/47) in ES animals. C, Scattergram shows the range of responses in layer V pyramidal neurons to 5-HT in control (n = 35) and ES animals (n = 47). Horizontal line denotes the mean response in layer V pyramidal neurons from control (34.2 ± 3.7 pA) and ES animals (5.9 ± 5.6 pA; *p = 0.0002, unpaired t test).
In brain slices from ES animals, by contrast, bath application of 5-HT (10 μm; 30 s) elicited a different pattern of responses in layer V pyramidal neurons (Fig. 1B). A group of the neurons responded to 5-HT with dominant outward currents (n = 27/47; 58%), one showed no response (n = 1/47; 2%), and almost half the neurons displayed dominant excitatory inward currents (n = 19/47; 40%), an effect not observed in control neurons. These unexpected, 5-HT-elicited inward currents were observed in ES animals throughout adolescence and adulthood (P21–83) with no significant change with age (n = 19; linear regression: R2 = 0.09; F(1,17) = 1.66; p = 0.2). The existence of the dominant 5-HT inward currents in ES neurons significantly shifted the mean of the 5-HT current toward an excitatory response: 34.2 ± 3.7 pA in controls (n = 35) versus 5.9 ± 5.6 pA in ES (n = 47; p = 0.0002, unpaired t test with Welch's correction) (Fig. 1C).
Adult prefrontal neurons show greater 5-HT2-mediated currents following early stress
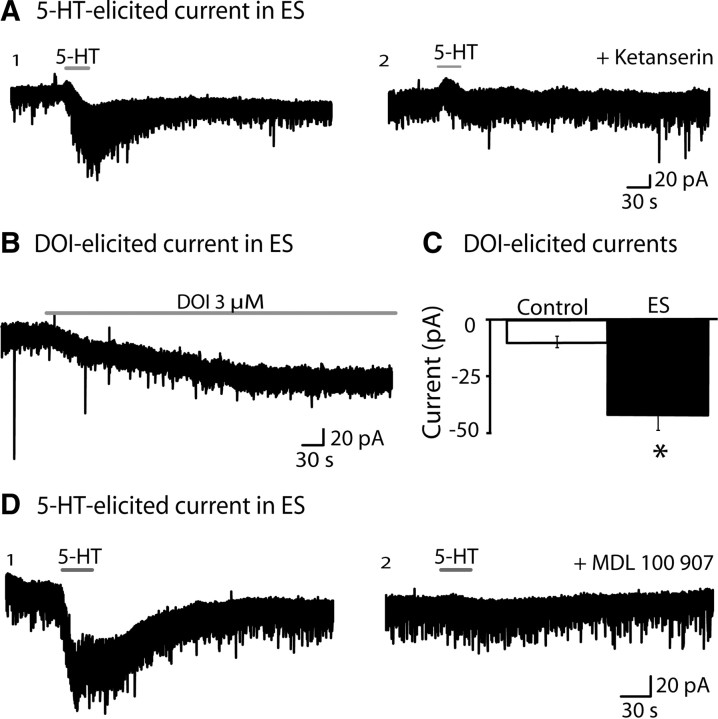
Since 5-HT2A receptors are expressed in prefrontal layer V neurons in adulthood (Burnet et al., 1995; Amargós-Bosch et al., 2004; Santana et al., 2004; Weber and Andrade, 2010), we hypothesized that the inward currents in ES animals were mediated by the 5-HT2A receptor. Notably, the excitatory 5-HT2 current is the dominant serotonergic regulator of prefrontal layer V neurons during the first 2 weeks of normal postnatal development (Zhang, 2003; Béïque et al., 2004). In adult ES rats, we found that the unusual, 5-HT-elicited inward currents were completely blocked in the presence of the 5-HT2 antagonist, ketanserin (2 μm; 10 min, n = 5), as illustrated in Figure 2A. To compare the magnitude of the 5-HT2 current across the groups, we examined neuronal responses to the partial 5-HT2 agonist, DOI (3 μm; 15 min). DOI elicited a significantly larger inward current in layer V pyramidal neurons from ES animals: −9.9 ± 2.7 pA in controls (n = 9) versus −42.4 ± 6.1 pA in ES (n = 11; unpaired t test with Welch's correction; p = 0.0003) (Fig. 2B,C). Finally, to determine whether the 5-HT-elicited inward current was mediated by the 5-HT2A or 5-HT2C receptor, we used the selective 5-HT2A antagonist MDL 100907 (30 nm; 10 min). To examine the inward current in both control and ES neurons, we first had to block the 5-HT1A outward current (note that this inhibitory receptor dominates the net response to 5-HT in control neurons and a large portion of the ES neurons) with the 5-HT1A antagonist WAY 100635 (30 nm; 10 min). Under these conditions, 5-HT-elicited inward currents (10–30 μm; 30 s) could be observed in both groups and were completely blocked by MDL 100907: ES animals (100 ± 0% blockade, n = 5, paired t test, p = 0.01) (Fig. 2D) and controls (100 ± 0% suppression; n = 4; paired t test, p = 0.01). Together, these findings suggest that the potentiated excitatory responses to 5-HT in the adult ES prefrontal cortex are mediated by 5-HT2 receptors of the 5-HT2A subtype.
Figure 2.
Serotonin-elicited inward currents in ES animals are mediated by 5-HT2A receptors. A, A voltage-clamp trace from an ES animal shows that the dominant 5-HT-elicited inward current (A1) is completely blocked by the 5-HT2 antagonist, ketanserin (A2) (2 μm; 10 min). B, A voltage-clamp trace shows the 5-HT2 partial agonist DOI (3 μm; 15 min) eliciting an inward current in an ES layer V pyramidal neuron. C, The bar chart shows that the mean peak amplitude of the inward current elicited by bath application of DOI was significantly larger in ES animals (n = 9) relative to control animals (n = 11; *p = 0.0003, paired t test). D, A voltage-clamp trace from an ES animal shows that the 5-HT-elicited inward current (D1) is completely blocked by the selective 5-HT2A antagonist MDL 100907 (D2) (30 nm, 10 min).
Influence of early stress on 5-HT2 receptor binding, 5-HT2A and 5-HT2C mRNA
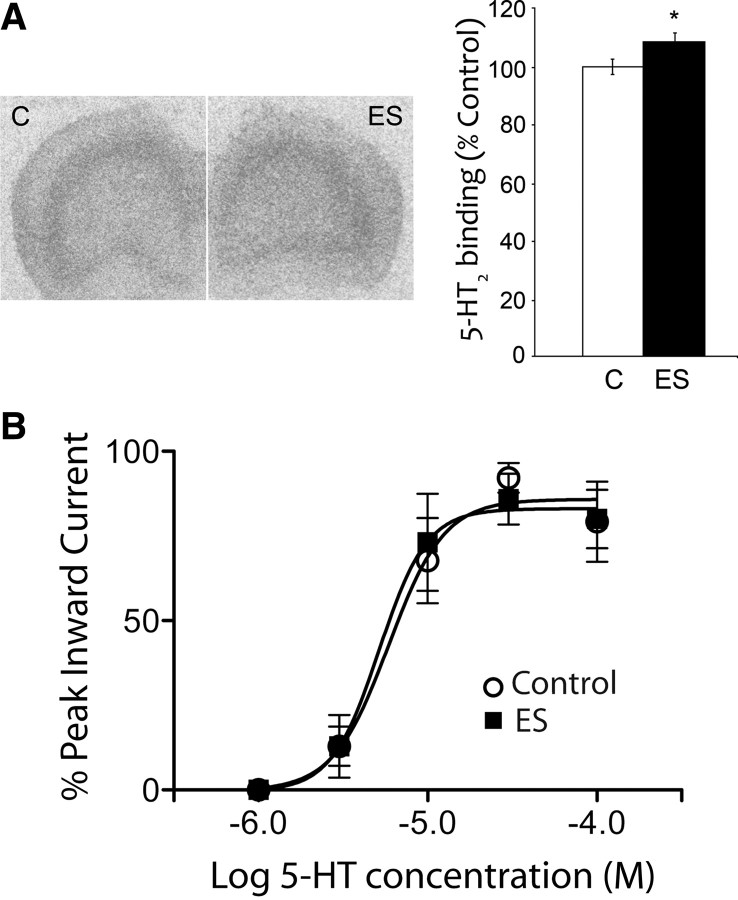
Next, we investigated potential mechanisms mediating the enhanced 5-HT2 receptor currents in ES animals. Consistent with work in nonhuman primates (Law et al., 2009), qPCR results showed no significant difference in 5-HT2A receptor (ES vs control fold change: 0.84 ± 0.09; p = 0.3, unpaired t test; control, n = 8; ES, n = 10) or 5-HT2C receptor (ES vs control fold change: 0.98 ± 0.10; p = 0.9, unpaired t test; control, n = 4; ES, n = 9) mRNA expression in the PFC of ES animals. However, quantitative receptor autoradiography using [3H]ketanserin revealed a small but significant, increase in 5-HT2 receptor density in the prefrontal cortex of ES animals (p = 0.02, unpaired t test; control, n = 4; ES, n = 7) (Fig. 3A). It is noteworthy that despite substantial changes in 5-HT2-evoked responses observed in ES animals, these are not accompanied by commensurate alterations in 5-HT2 receptor binding or mRNA expression.
Figure 3.
Influence of early stress on 5-HT2 receptor binding and the sensitivity of the 5-HT2A-elicited inward current in layer V pyramidal neurons. A, ES caused a small increase in [3H]ketanserin binding in the PFC in adulthood. Shown are representative autoradiograms and graphical representation of the 5HT2 receptor binding density from the PFC of control (C) and ES animals. Data are percentage of control and are mean ± SEM (*p = 0.02, unpaired t test, n = 4 control, n = 7 ES). B, Concentration response curves for the 5-HT2A-elicited inward current in layer V pyramidal neurons from control and ES animals. These experiments assessed the inward current mediated directly by 5-HT2A receptors on layer V pyramidal cells by recording after the application of WAY 100635 (30 nm, 10 min) to block 5-HT1A receptors and in the presence of antagonists to block glutamate and GABA synaptic transmission (CNQX, 20 μm; APV, 50 μm; bicuculline, 10 μm). The EC50 values are not significantly different between groups: (control, 5.6 ± 1.9 μm, n = 5; ES, 5.1 ± 1.9 μm, n = 5; unpaired t test, p = 0.8).
Early stress does not alter the sensitivity of the 5-HT2A-mediated inward current
A small increase in density of 5-HT2 receptors could significantly shift the 5-HT concentration–response curve to the left if the increased density reflects an enhanced number of spare 5-HT2A receptors (Bourne and von Zastrow, 2007). Therefore, we compared the 5-HT2A concentration–response curves from control and ES layer V neurons. These recordings were performed in the presence of a 5-HT1A receptor antagonist (30 nm WAY 100635) as well as blockers for ionotropic glutamatergic (20 μm CNQX and 50 μm AP-5) and GABAergic (10 μm bicuculline) neurotransmission. As illustrated in Figure 3B, there was no significant difference in the 5-HT2A concentration–response relationships between control (EC50, 5.6 ± 1.9 μm; n = 5) and ES neurons (EC50, 5.1 ± 1.9 μm; n = 5; unpaired t test, p = 0.8). These results suggest that the number of spare receptors and the sensitivity of the 5-HT2A receptor for 5-HT is unaltered by a history of early stress.
Early stress increases spontaneous network activity in adult prefrontal slices exposed to DOI
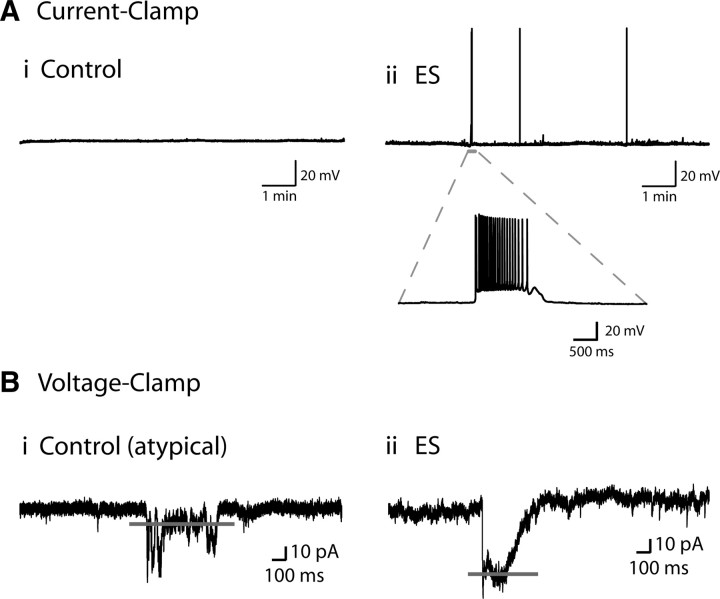
Surprisingly, following the application of the 5-HT2 receptor partial agonist DOI (3 μm; 15 min), we observed the occurrence of spontaneous network activity in ES animals, as illustrated in Figure 4. These network activity events produced a temporary excited state in the layer V neurons, often accompanied by the discharge of action potentials when observed in current-clamp (Fig. 4A). To quantify the amplitude of the network activity, voltage-clamp recordings were performed (Fig. 4B; Table 1).We found that the majority of layer V pyramidal neurons from ES animals displayed spontaneous network activity events in the presence of DOI (n = 12/13; 92%) (Table 1). In brain slices from control animals, by contrast, the presence of DOI (3 μm; 15 min) rarely produced spontaneous network activity in layer V pyramidal neurons (n = 2/13; 15%; p = 0.0002, Fisher's exact test). The scarcity of spontaneous network activity in the control slices following DOI is consistent with the results of previous work (Lambe and Aghajanian, 2007). These results illustrated that a history of ES significantly increased the probability of 5-HT2-mediated network activity measured in layer V pyramidal neurons of the PFC.
Figure 4.
Early stress increases the prevalence of spontaneous network activity (up states) in the presence of DOI. A, Examples from control (Ai) and ES (Aii) recordings in current-clamp showing the lack (control) and the occurrence (ES) of episodes of spontaneous network activity detected by layer V pyramidal neurons after application of the 5-HT2 partial agonist, DOI (3 μm; 15 min), to the brain slice. One episode is enlarged to show detail of the spontaneously occurring up state observed. These phenomena were identified based on previous description of network activity in cortical brain slices (see Materials and Methods). B, To quantify the amplitude of the network activity, voltage-clamp recording were performed. Examples of control (rare) (Bi) and ES (Bii) network activity are shown in voltage-clamp. The horizontal gray line denotes the measured amplitude of the up state.
Table 1.
Early stress increases the prevalence of spontaneous network activity in the presence of the 5-HT2 receptor agonist DOI
| Control | Early stress | p value | |
|---|---|---|---|
| Number of cells displaying network activity/total number of cells | 2/13 (15%) | 12/13 (92%) | 0.0002 |
| Amplitude (pA) | −17.4 ± 9.5 | −67.9 ± 8.3 | 0.06 |
| Duration (ms) | 450 ± 50 | 651 ± 205 | 0.7 |
Voltage-clamp recordings (10 min per neuron) were made from control and ES neurons following application of DOI (3 μm; 15 min) to the brain slice. Under these conditions, activity patterns consistent with network activity (Sanchez-Vives and McCormick, 2000) were observed in very few neurons from control animals and in almost all neurons from ES animals (significance assessed with Fisher's exact test). The amplitude and duration of the measured network events in each group are shown as mean ± SEM (controls: 2 events; ES: 17 events) and were compared with Student's unpaired t test.
Early stress does not increase the intrinsic excitability of prefrontal layer V pyramidal neurons
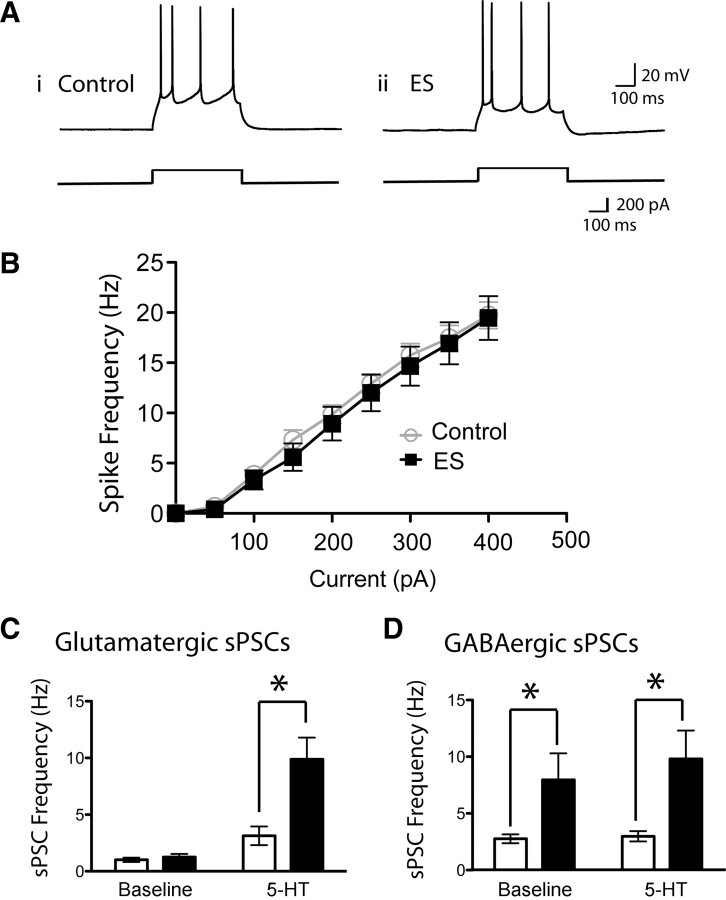
The increased prefrontal 5-HT2-mediated excitability in ES animals occurred without notable change to the intrinsic excitability of the prefrontal layer V neurons. As shown in Figure 5B, layer V pyramidal neurons from ES animal did not display altered neuronal excitability, as quantified by the relationship between current injection and the frequency of action potential firing: slope of 5.42 ± 0.07 Hz/100 pA in controls (n = 15) versus slope of 5.40 ± 0.08 Hz/100 pA (n = 15; unpaired t test, p = 0.9) in ES animals. Consistent with these findings, we observed no difference in the input resistance of layer V neurons between control and ES animals (Table 2). However, we did observe a small, albeit significant, depolarization in ES layer V pyramidal neurons (unpaired t test, p = 0.03) (Table 2). Interestingly, when we repeated the input–output experiments after injecting positive or negative current to bring each neuron to a membrane potential of −75 mV, ES neurons displayed a trend toward reduced excitability compared with controls: slope of 5.70 ± 0.08 Hz/100 pA in controls (n = 14) versus slope of 5.55 ± 0.05 Hz/100 pA in ES animals (n = 15; unpaired t test, p = 0.054) (supplemental Fig. 1, available at www.jneurosci.org).
Figure 5.
Early stress does not appear to enhance the intrinsic excitability of the prefrontal cortical slice as measured by input–output experiments and baseline levels of glutamatergic and GABAergic synaptic activity. A, The intrinsic excitability of layer V pyramidal neurons was assessed by examining the frequency of action potentials elicited by injections of depolarizing current. Representative examples are shown from control (Ai) and ES (Aii) animals. B, A graph of the input–output relationships shows that layer V pyramidal cells from both groups have similar excitability when assessed from their resting potentials. C, The frequency of glutamatergic sPSCs measured in layer V pyramidal neurons at baseline and in response to 5-HT (10 μm, 30 s) in controls and ES. Both groups have a similar frequency of glutamatergic sPSCs at baseline. In the presence of 5-HT, the frequency of glutamatergic sPSCs is significantly enhanced in both controls and ES animals, and two-way ANOVA revealed an interaction whereby the 5-HT-elicited increase in glutamatergic sPSCs is significantly enhanced in ES neurons (*p = 0.0006). D, The frequency of GABAergic sPSCs measured (with high Cl− patch solution in the presence of CNQX, 20 μm) in layer V pyramidal neurons at baseline and in response to 5-HT (10 μm, 30 s) in controls and ES. Interestingly, ES layer V neurons display a significantly higher frequency of GABAergic sPSCs at baseline (two-way ANOVA reveals a significant main effect of postnatal group, *p = 0.001). Bath application of 5-HT (10 μm, 30 s) did not significantly alter the frequency of GABAergic sPSCs in either the control or ES animals. A higher concentration of 5-HT may be required to increase the frequency of GABAergic sPSCs overall (Zhou and Hablitz, 1999).
Table 2.
Layer V pyramidal neurons from ES animals are significantly more depolarized, but display no change in spike amplitude or input resistance
| Control (n = 74) | Early stress (n = 87) | p value | |
|---|---|---|---|
| Resting membrane potential (mV) | −82.4 ± 0.6 | −80.5 ± 0.6 | 0.03 |
| Spike amplitude (mV) | 86.4 ± 0.7 | 85.8 ± 0.8 | 0.5 |
| Input resistance (MΩ) | 102.2 ± 6.3 | 99.9 ± 5.1 | 0.7 |
Values are shown as mean ± SEM and were compared with Student's unpaired t test.
Early stress enhances 5-HT2-elicited glutamatergic sPSCs and baseline GABAergic sPSCs
To examine further the excitability of the prefrontal brain slices, we measured fast glutamatergic synaptic transmission at baseline and in the presence of 5-HT (10 μm; 30 s). At baseline, we observed no difference in baseline frequency of glutamatergic sPSCs (Fig. 5C). However, ES animals displayed a significantly higher frequency of glutamatergic sPSCs in the presence of 5-HT (10 ± 1 Hz; n = 21) than control animals (3 ± 1 Hz; n = 20). As illustrated in Figure 5C, two-way ANOVA revealed a significant interaction between group and the effect of 5-HT (F(2,96) = 8.0, p = 0.0006). Consistent with previous work demonstrating that cortical 5-HT2A receptors mediate this effect normally (Weisstaub et al., 2006), the 5-HT2 antagonist ketanserin (2 μm; 10 min) completely blocked the 5-HT-elicited increase in glutamatergic sPSCs in ES animals (1 ± 1 Hz; n = 10, p = 0.004). There were no significant group differences in the amplitude of glutamatergic sPSCs at baseline or in the presence of 5-HT, as illustrated in supplemental Figure 2 (available at www.jneurosci.org). Interestingly, similar proportions of neurons showed significant 5-HT-elicited increases in glutamatergic sPSCs (Kolmogorov–Smirnov test, p < 0.01) in both control and ES animals (supplemental Table 1, available at www.jneurosci.org). Together, these results suggest that ES animals have increased glutamatergic synaptic activity in the presence of 5-HT, but not at baseline.
To compare prefrontal inhibition in control and ES animals, we examined fast GABAergic synaptic transmission at baseline and in the presence of 5-HT (10 μm; 30 s). To measure GABAergic sPSCs, we used high Cl− patch solution (77 mm) in the presence of continuous application of AMPA/KA receptor blocker, CNQX (20 μm). Under these recording conditions, ES layer V neurons displayed significantly more frequent GABAergic sPSCs at baseline, compared with control (two-way ANOVA revealed a significant main effect of ES, p = 0.001). Bath application of 5-HT at 10 μm (30 s) did not significantly alter the frequency of GABAergic sPSCs in either the control or ES animals (two-way ANOVA revealed no main effect of 5-HT application, p = 0.5). A higher concentration of 5-HT may be required to increase the frequency of GABAergic sPSCs overall (Zhou and Hablitz, 1999). There was no significant group difference in the amplitude of GABAergic sPSCs at baseline or in the presence of 5-HT, as illustrated in supplemental Figure 2 (available at www.jneurosci.org), and the proportions of neurons showing significant 5-HT-elicited increases in GABAergic sPSCs were not different (supplemental Table 1, available at www.jneurosci.org). These results show that the frequency of spontaneous GABAergic synaptic activity is significantly higher at baseline in brain slices of ES animals and that GABAergic synaptic activity is not increased to the same degree as glutamatergic synaptic activity by this concentration of 5-HT.
Early stress enhances in vivo DOI-induced Arc expression in the adult prefrontal cortex
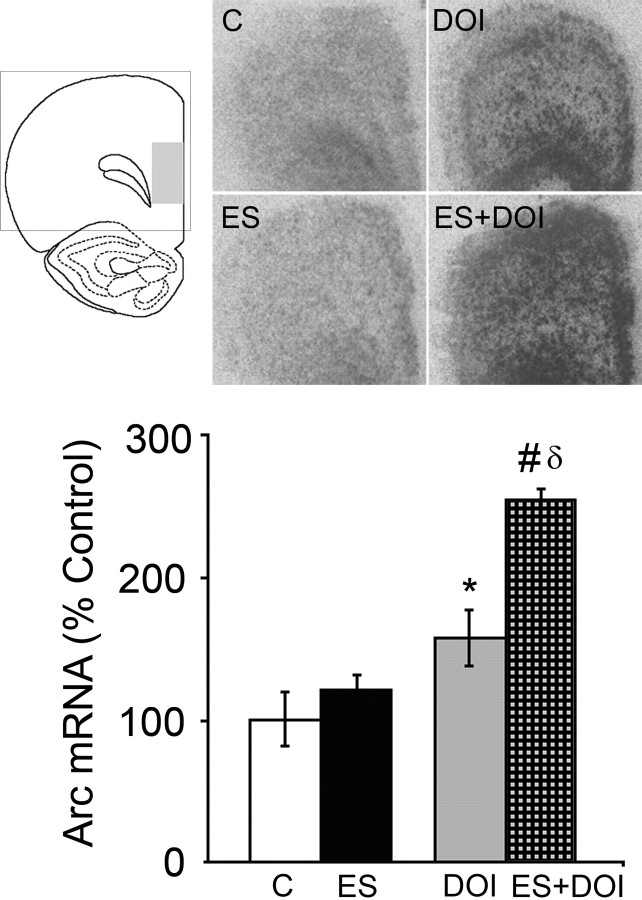
Based on our electrophysiological data demonstrating enhanced DOI responses in prefrontal brain slices of ES animals, we sought to determine whether systemic DOI administration in vivo also increased the activity of the PFC circuit. To visualize in vivo PFC circuit activity, we examined the expression of the immediate early gene, Arc (Temple et al., 2003), which has been previously shown to be strongly upregulated following systemic DOI administration (Pei et al., 2000). Consistent with the electrophysiological data showing that ES animals do not exhibit intrinsic differences in excitability, we observed no difference of baseline Arc mRNA expression between control and ES animals (Fig. 6). However, we observed that the DOI-elicited Arc mRNA expression in ES animals was significantly potentiated compared with controls (two-way ANOVA revealed a significant ES × DOI interaction; F(1,15) = 11.39, p = 0.004) (Fig. 6). Collectively, these experiments indicated that a history of ES resulted in enhanced circuit activation in the PFC in response to 5-HT2 stimulation in the absence of changes to baseline PFC excitability.
Figure 6.
Early stress potentiates the DOI-induced Arc expression in the prefrontal cortex. A history of ES potentiated the DOI-induced increase in Arc mRNA levels within the PFC. Shown are representative autoradiograms of PFC sections and graphical representation of the densitometric analysis from control (C), ES, DOI, and ES plus DOI groups. Results are expressed as a percentage of control and are the mean ± SEM. Two-way ANOVA revealed a significant ES × DOI interaction (F(1,15) = 11.39, p = 0.004). *p < 0.05, significantly different from control; #p < 0.05, significantly different from ES; δp < 0.05, significantly different from DOI (ANOVA and Bonferroni post hoc test); n = 4–5 per group.
A history of early stress enhances DOI-induced head shake behavior
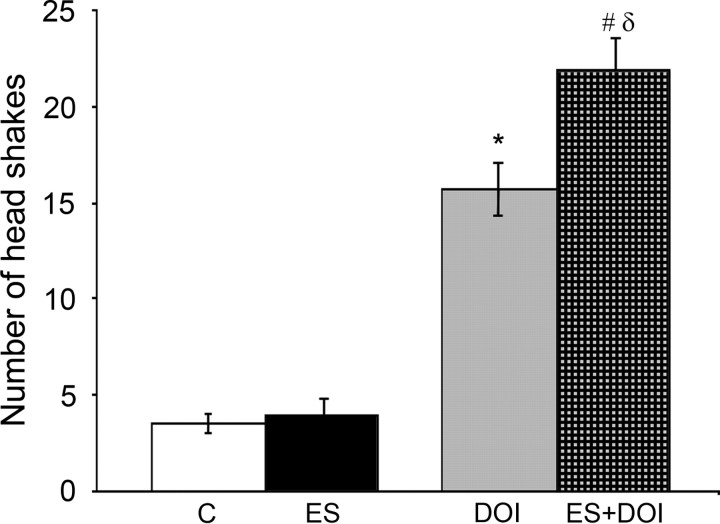
Activation of 5-HT2A receptors in the PFC is thought to mediate DOI-induced head shake behavior (Willins and Meltzer, 1997). To address whether the altered electrophysiological and immediate early gene expression responses to DOI in ES animals are also reflected at the level of 5-HT2-mediated behaviors, we measured DOI-induced head shakes. We found that the number of head shakes induced by systemic administration of DOI was significantly increased in ES animals (two-way ANOVA revealed a significant ES × DOI interaction; F(1,14) = 7.01, p = 0.02) (Fig. 7). Spontaneous head shakes observed in the absence of DOI across the 20 min observation period did not differ between control and ES animals. Our results showed that ES animals exhibit enhanced 5-HT2-mediated behavioral responses in adulthood.
Figure 7.
A history of early stress-enhanced DOI-induced head shake behavior. The induction of head shakes by the 5-HT2 partial agonist DOI was potentiated in ES animals. Results are expressed as the mean ± SEM. Two-way ANOVA analysis indicated a significant ES × DOI interaction effect (F(1,14) = 7.01, p = 0.02). *p < 0.05, significantly different from control; #p < 0.05, significantly different from ES; δp < 0.05, significantly different from DOI (ANOVA and Bonferroni post hoc test); n = 4–5 per group.
Early stress alters the transcriptome in the prefrontal cortex
To gain an understanding into the molecular mechanisms that accompany enhanced prefrontal 5-HT2 signaling in ES animals, we performed a transcriptome analysis on ES animals in adulthood. A history of ES significantly upregulated 666 genes and downregulated 232 genes in the PFC (ES vs control) (supplemental Table 2, available at www.jneurosci.org). To identify functionally clustered gene sets significantly regulated by ES, we used DAVID (Dennis et al., 2003; Huang et al., 2009). This analysis revealed that in the transcriptome of ES animals there was significant enrichment in functional categories of genes such as stress–response pathways, transmembrane components, cellular development processes, and genes involved in transduction of extracellular signal and ion homeostasis (Fig. 8A; supplemental Table 3, available at www.jneurosci.org).
Figure 8.
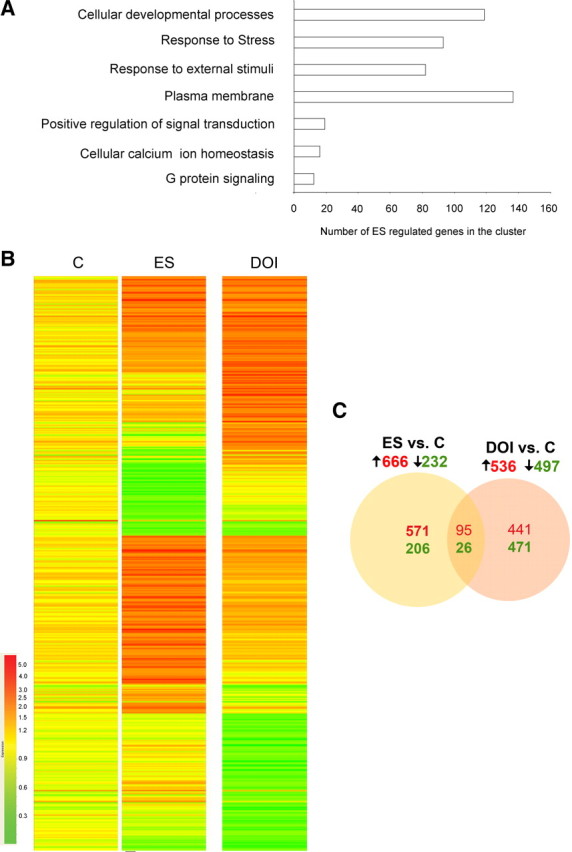
Exposure to early stress alters the adult prefrontal transcriptome. A, Functional analysis of ES-regulated genes reveals enrichment of cellular development processes, stress-response pathways, transmembrane components, transduction of extracellular signal, G protein signaling, and ion homeostasis pathways in the PFC. B, Hierarchical cluster of significantly regulated genes (p < 0.05, t test corrected for multiple comparisons; upregulated genes are shown in red, downregulated genes are shown in green) across the control (C) and ES groups (n = 3 control; n = 4 ES). Each row represents a single gene and each column represents the average fold change for each group. The data are log-transformed, normalized intensity values. C, The numbers of upregulated (red) and downregulated (green) genes in the ES and DOI groups (fold change cutoff: 1.5 for upregulated and 0.66 for downregulated genes, p < 0.05 compared with control; t test corrected for multiple comparisons) are represented using a Venn diagram. As represented in the Venn diagram, ∼13% of the genes regulated by ES were similarly regulated in the DOI group.
The regulation of the expression of several genes in ES animals was then validated by qPCR in tissue samples from an independent cohort of animals (Table 3), indicating a strong reproducibility of the array expression results. Notably, ion channels such as potassium channels (Kcna3, Kcnh2, Kcnh7), which are modulated by Gαq-protein stimulation (Chung and Schlichter, 1997; Schledermann et al., 2001), were regulated in adult ES animals. This is particularly interesting given that we found altered electrophysiological and network activity responses to 5-HT2 receptor stimulation (Figs. 2, 4; Table 1). In addition, we also found that cellular developmental and neuronal plasticity-related genes, such as the NR2D NMDA receptor subunit (Grin2d), neuroligin 1 (Nlgn1), CamKinase1γ (Camk1g), and calcineurin (Ppp3r2, Ppp3ca), are upregulated in adult ES animals. Genes capable of altering signal transduction, like ADP-ribosyltransferase 5 (Art5) and guanine nucleotide binding protein 1 (Gnb1), are also regulated by ES, potentially contributing to the altered responses following 5-HT2-receptor stimulation. Further, we also observed regulation of genes that are targets for antidepressants and mood stabilizers, such as the β3 adrenergic receptor (Adrb3) (Stemmelin et al., 2008) and inositol monophosphatase-1 (Impa1) (Parthasarathy et al., 2003). The fold changes and significance levels for these validated genes are shown in Table 3.
Table 3.
Validation of microarray results using quantitative PCR
| Gene | ES versus C fold change | p value |
|---|---|---|
| Upregulated | ||
| Art5 | 2.88 ± 0.78 | 0.03 |
| Camk1g | 1.65 ± 0.31 | 0.04 |
| Grin2d | 1.23 ± 0.03 | 0.003 |
| Impa1 | 1.21 ± 0.04 | 0.006 |
| Kcnh2 | 1.45 ± 0.16 | 0.004 |
| Nlgn1 | 1.58 ± 0.23 | 0.03 |
| Ppp3ca | 1.29 ± 0.01 | 0.006 |
| Ppp3r2 | 2.86 ± 0.64 | 0.03 |
| Tnfrsf1b | 1.22 ± 0.01 | 0.01 |
| Downregulated | ||
| Adrb3 | 0.66 ± 0.11 | 0.02 |
| Gnb1 | 0.82 ± 0.09 | 0.06 |
| Kcna3 | 0.75 ± 0.09 | 0.09 |
| Kcnh7 | 0.70 ± 0.04 | 0.006 |
Genes of particular interest implicated in cellular signaling, excitability, and neuronal plasticity were chosen for qPCR to validate the microarray results. The validation of the microarray by qPCR was carried out in tissue samples from an independent cohort of experimental animals. All genes were normalized to an average of four housekeeping genes (18s rRNA, Gapdh, Hprt, β-actin), which were not regulated in the microarray. Genes up-regulated in the early life stress group are shown in alphabetical order at the top of the table and down-regulated genes are shown in alphabetical order at the bottom. Data are expressed as fold change (mean ± SEM). The p values were obtained using an unpaired t test, (Nlgn1, Ppp3ca, Ppp3r2, Tnfrsf1b, Adrb3, Gnb1, Kcna3, Kcnh7, n = 4–7 per group; Art5, Grin2d, Kcnh2, n = 6–10 per group; Camk1g, Impa1, n = 13–16 per group).
Since our data suggest enhanced 5-HT2 signaling in ES animals, we sought to address whether the ES transcriptome exhibits gene expression changes indicative of enhanced 5-HT2 signaling. To test this hypothesis, we used microarray analysis to compare gene expression changes induced by ES to those that arise following systemic stimulation with the 5-HT2 agonist, DOI. The heat maps highlight the overlap observed between the ES and DOI-induced transcriptomes in the PFC (Fig. 8B). We found ∼13% overlap, with 95 genes upregulated in common between untreated ES and DOI-treated controls and 26 genes downregulated in common (Fig. 8C). Again, we used qPCR in an independent cohort of animals to validate these findings. Similar to the expression differences seen in ES animals at baseline (Table 3), stimulation of the 5-HT2 receptor with DOI in control animals also resulted in a significant increase in the expression of Nlgn1 (DOI vs control fold change: 2.29 ± 0.19; p = 0.0001, unpaired t test; n = 4 per group) and Impa1 (DOI vs control fold change: 1.32 ± 0.09; p = 0.04, unpaired t test; n = 8 control, n = 7 DOI) and a downregulation of Kcna3 (DOI vs control fold change: 0.79 ± 0.07; p = 0.05, unpaired t test with Welch correction; n = 5 control, n = 7 DOI). These results indicate that the changes in gene expression observed in ES animals at baseline contain a component that is found in common with 5-HT2 receptor-evoked gene expression changes.
Together, our findings indicated that adult animals with a history of ES exhibit an altered prefrontal transcriptome, a component of which overlaps with gene expression changes that arise from 5-HT2 stimulation. Furthermore, the ES-induced transcriptome showed a significant enrichment of genes associated with cellular development and signaling that may be associated with the enhanced 5-HT2 responses we observe in ES animals.
Treatment with a 5-HT2 antagonist reverses a component of the early stress-induced gene expression changes
Next, we examined whether chronic blockade of 5-HT2 receptors in adulthood would normalize gene expression in the prefrontal cortex of ES animals. Controls and ES animals in adulthood were treated with the 5-HT2 receptor antagonist ketanserin or vehicle (n = 3–5 animals per group) as described in Materials and Methods, and the expression of a subset of ES-regulated genes (Nlgn1, Impa1, Adrb3, Kcnh2, CamK1g, Gnb1, Tnfrsf1b) was analyzed. We observed that chronic treatment with ketanserin reversed the ES-induced changes in the expression of Nlgn1, Impa1, Adrb3, and Kcnh2. Two-way ANOVA analysis revealed a significant interaction between ES × ketanserin for the following: Nlgn1 (fold change: control, 1.0 ± 0.05; ES, 1.71 ± 0.06; control plus ketanserin, 1.63 ± 0.82; ES plus ketanserin, 0.72 ± 0.06; F(1,10) = 9.72, p = 0.01); Impa1 (fold change: control, 1.00 ± 0.24; ES, 1.19 ± 0.19; control plus ketanserin, 1.32 ± 0.36; ES plus ketanserin, 0.34 ± 0.11; F(1,12) = 15.40, p = 0.002); Adrb3 (fold change: control, 1.0 ± 0.12; ES, 0.63 ± 0.07; control plus ketanserin, 0.52 ± 0.16; ES plus ketanserin, 0.84 ± 0.16; F(1,11) = 6.54, p = 0.03); and Kcnh2 (fold change: control, 1.00 ± 0.17; ES, 1.91 ± 0.20; control plus ketanserin, 1.33 ± 0.29; ES plus ketanserin, 0.78 ± 0.09; F(1,13) = 13.18, p = 0.003). In contrast, we found that ketanserin treatment did not specifically alter the ES-regulated changes in expression of Camk1g, Tnfrsf1b, and Gnb1. Two-way ANOVA revealed no significant ES × ketanserin interaction for Camk1g (fold change: control, 1.0 ± 0.21; ES, 2.14 ± 0.32; control plus ketanserin, 0.53 ± 0.10; ES plus ketanserin, 1.39 ± 0.36; F(1,10) = 0.25, p = 0.6; main effect of ES, p = 0.002; main effect of ketanserin, p = 0.003); Gnb1 (fold change: control, 1.0 ± 0.15; ES, 0.73 ± 0.09; control plus ketanserin, 0.71 ± 0.19; ES plus ketanserin, 0.35 ± 0.01; F(1,11) = 1.34, p = 0.2; main effect of ES, p = 0.01; main effect of ketanserin, p = 0.009); and Tnfrsf1b (fold change: control, 1.0 ± 0.19; ES, 2.24 ± 0.41; control plus ketanserin, 0.94 ± 0.49; ES plus ketanserin, 1.37 ± 0.07; F(1,11) = 0.45, p = 0.5; main effect of ES, p = 0.06). In both controls and ES animals, ketanserin treatment exerted significant effects on the expression of Camk1g and Gnb1. Our results suggest that 5-HT2 receptor blockade in adulthood is capable of restoring some of the gene expression changes in the PFC of ES animals and that systemic 5-HT2 receptor blockade may exert distinct effects on prefrontal gene transcription, depending on early life experience. Further experiments are necessary to ascertain whether the effects of systemic ketanserin treatment on the expression of specific PFC genes arise directly through the blockade of prefrontal 5-HT2 receptors or involve possible indirect effects of ketanserin on raphe firing (Wright et al., 1990; Martín-Ruiz et al., 2001).
Discussion
A history of early life stress is linked to enhanced vulnerability for adult psychopathology. The major finding of our study is that adult animals with a history of ES show enhanced prefrontal 5-HT2 receptor function. Evidence from multiple approaches supports this conclusion. First, 5-HT has atypical dominant excitatory effects, mediated via the 5-HT2A receptor, in a substantial portion of prefrontal layer V neurons of ES animals. Second, 5-HT2-elicited excitatory inward currents in layer V neurons are enhanced in ES animals. Third, the frequency of network events generated in brain slices of PFC and the induction of immediate early gene expression in vivo following 5-HT2 stimulation is significantly increased in ES animals compared with controls. Fourth, cortical 5-HT2-mediated head shake behaviors are potentiated in ES animals. These changes occur without major alterations in the intrinsic excitability of the prefrontal cortex in ES animals. The changes also occur despite unaltered 5-HT2A and 5-HT2C receptor mRNA expression in PFC of ES animals and only a small increase in 5-HT2 receptor binding. Our microarray results provide insight into the molecular changes that accompany the enhanced 5-HT2 responses in ES animals. We show that several genes involved in cellular development, signal transduction, and G-protein signaling are regulated in the PFC following ES. Furthermore, we also find that a component of the gene expression changes in the PFC of ES animals can be reversed by chronic blockade of the 5-HT2 receptor. Together, our results demonstrate enhanced 5-HT2 function in animals with a vulnerability for anxiety and depressive behaviors and motivate a focus on 5-HT2 receptors in the treatment of prodromal psychiatric disorders.
ES animals display aspects of an immature phenotype in prefrontal cortex
Electrophysiological responses to 5-HT in the adult PFC are dominated by its inhibitory receptors under normal circumstances (Gartside et al., 2000; Béïque et al., 2004; Puig et al., 2005), and our data from control animals are consistent with these earlier studies. Strikingly, we find that 5-HT elicits predominant excitatory inward currents via the 5-HT2A receptor in ∼40% of layer V pyramidal neurons of adult ES rats. The exclusive serotonergic excitation of layer V pyramidal neurons through 5-HT2 receptors is typically only seen in the first two postnatal weeks in rodents (Zhang, 2003; Béïque et al., 2004). Our results suggest the retention of an immature response to 5-HT in almost half of the layer V PFC neurons from adult ES animals. The normal developmental period when 5-HT excites layer V pyramidal neurons coincides with a period of extensive cortical maturation, with large increases in dendritic branching and synaptic density (Roth et al., 1991; Zhang, 2003, 2004). It is noteworthy that our ES transcriptome profiling also indicates the enrichment of genes involved in cellular developmental processes that normally exhibit a higher expression during early postnatal development, such as CamKIγ (Sawamura et al., 1996), the catalytic and regulatory domains of calcineurin (Schwartz et al., 2009), and the NR2D NMDA receptor subunit (Monyer et al., 1994). Interestingly, we also observed enhanced expression of neuroligin 1, a postsynaptic cell adhesion molecule that interacts with NMDA receptors at “hot spots” for the establishment of new excitatory synapses (Gerrow et al., 2006; Barrow et al., 2009). Together, our electrophysiological and transcriptome data indicate that early life stress results in an enriched expression of developmental growth pathways and an immature, excitatory 5-HT phenotype in adult PFC.
ES animals exhibit enhanced prefrontal 5-HT2 receptor responses
A history of ES enhances adult excitatory inward currents, network activity, and Arc activation elicited by stimulation with the 5-HT2 agonist DOI. Interestingly, in the absence of 5-HT stimulation there appeared to be little difference in the intrinsic excitability of prefrontal cortex. The changes in the effects of 5-HT2 receptor stimulation occur without a change in 5-HT2A mRNA expression in the PFC of adult animals with a history of ES and with a small increase in 5-HT2 receptor binding. Though such an increase in binding could left shift a concentration response curve under some circumstances (Bourne and von Zastrow, 2007), we found that the 5-HT2A-elicited inward currents in layer V neurons had similar sensitivity to 5-HT in control and ES animals. The induction of calcineurin, which directly participates in the phospholipase Cβ/inositol triphosphate intracellular cascade of 5-HT2 receptors (Day et al., 2002), could contribute to enhanced signaling via 5-HT2 receptors in ES animals. Consistent with the idea that 5-HT2 receptor signaling is changed in ES animals, evidence from previous studies has demonstrated increased levels of Gαq mRNA following ES (Bhansali et al., 2007). We found that a Gαq-mediated (Garcia et al., 2007) head shake behavior induced by DOI is significantly enhanced in ES animals. While the desensitization and G-protein uncoupling of 5-HT2A receptors have been the subject of considerable study (Gray and Roth, 2001), the cellular mechanisms responsible for enhancing the function of 5-HT2A receptors are still not well understood. Recent work indicates that stimulation of corticotrophin-releasing factor (CRF) receptor 1 can sensitize responses to 5-HT2 receptor stimulation and influence receptor recycling (Magalhaes et al., 2010). One can speculate that perturbed CRF levels in ES animals (Plotsky et al., 2005) may influence 5-HT2 receptor responses. Yet, ES animals have reduced CRF receptor 1 expression and binding in prefrontal cortex (Plotsky et al., 2005). Additional studies will be required to understand how the enhanced prefrontal 5-HT2 function arises in ES animals.
It is interesting to envisage whether the enhanced 5-HT2A responses in ES animals occur in parallel with altered prefrontal 5-HT release. Two scenarios can be envisioned. In the first, waking (Rueter and Jacobs, 1996) and stress-evoked (Kawahara et al., 1993) 5-HT release could result in abnormally high PFC activity by stimulation of 5-HT2A receptors on layer V neurons. Alternatively, if 5-HT2A receptor function was upregulated as homeostatic compensation for low baseline 5-HT levels (Cahir et al., 2007), this change would make the brain particularly vulnerable to abnormal function under conditions of stress when prefrontal 5-HT release is elevated (Kawahara et al., 1993; Yoshioka et al., 1995; Hashimoto et al., 1999; Daniels et al., 2004). However, studies of ES animals have not found consistent differences in baseline 5-HT levels in the PFC (Gartside et al., 2000; Matthews et al., 2001; Daniels et al., 2004; Jezierski et al., 2006). Our finding of increased 5-HT2A-stimulated PFC excitability in ES animals is consistent with clinical evidence showing increased prefrontal activity in patients with affective disorders (Drevets, 2000). Of note, patients suffering from major depression have also been reported to show damage within the PFC (Rajkowska et al., 1999). It is tempting to speculate that early adverse experience may predispose the adult PFC to increased 5-HT2A-mediated hyperexcitability under stressful conditions.
Some changes in gene expression may be compensatory. The normally expressed Kcnh7 potassium channel (Kv 11.3; Erg3) (Saganich et al., 2001) was downregulated in ES animals, and this was accompanied by an upregulation of the relatively rare Kcnh2 potassium channel (Kv11.1; Erg1) (Saganich et al., 2001). This change may decrease one potential source of Gq-mediated excitation, since the upregulated Kcnh2 channel is less sensitive to Gq inhibition than the downregulated Kcnh7 channel (Schledermann et al., 2001). However, such compensation appears somewhat exceptional, since the PFC of adult ES animals exhibits heightened 5HT2-induced inward currents, network activity, immediate early gene expression, and head shake behavior.
Clinical implications and conclusions
In rodents and primates, early life stress increases adult anxiety and depressive behaviors (Sánchez et al., 2001; Newport et al., 2002; Kaffman and Meaney, 2007) and thus serves as a useful model to identify the factors that contribute to the vulnerability of an individual for adult psychopathology. Our results suggest that the enhanced prefrontal 5-HT2A receptor function in adult ES animals may contribute to the vulnerability for anxiety and depressive-like behaviors observed in these animals. Interestingly, postmortem work in humans has shown enhanced 5-HT2 receptor function in affective disorders, as measured by stimulated GTPγS binding in the PFC (Friedman and Wang, 1996). Our microarray analysis in ES animals not only identifies molecular pathways associated with cellular development and signal transduction that accompany the enhanced 5-HT2 function, but also shows changes in genes such as Adrb3 (Stemmelin et al., 2008) and Impa1 (Parthasarathy et al., 2003), which have been implicated in the treatment of mood disorders. Remarkably, these transcriptional alterations are observed >6 weeks following ES, invoking possible epigenetic mechanisms in the long-term maintenance of an altered gene expression pattern (Holmes et al., 2005). Such a persistent expression of an altered phenotype may underlie a life-long vulnerability for emotional dysfunction. Intriguingly, systemic treatment with a 5-HT2 receptor antagonist, a class of molecules that exhibits antidepressant properties (Celada et al., 2004), reverses specific gene expression changes observed in ES animals, including those in Adrb3, Impa1, Kcnh2, and Nlgn1. Previous studies have shown that the regulation by adult stress of gene transcripts in the hippocampus can be blocked by 5-HT2 receptor antagonist treatment (Olsson et al., 1997; Vaidya et al., 1997). Our results indicate that certain transcriptional differences observed many weeks after early life stress can also be reversed with chronic 5-HT2 receptor blockade. This suggests that a component of the transcriptional changes observed in the PFC of ES animals may arise as a consequence of increased 5-HT2 receptor function.
In conclusion, we demonstrate that a history of early adverse experience enhances adult 5-HT2 responses and excitability in the PFC and results in a lasting alteration in the gene expression of molecular pathways that modulate cellular development, signal transduction, and stress responses. Our studies motivate a focus on the contribution of prefrontal 5-HT2A receptors to the vulnerability for anxiety and depressive disorders.
Footnotes
This research was supported by Wellcome Trust Senior Overseas Fellowship 0408200314133 (V.A.V.), a Tata Institute of Fundamental Research intramural grant (V.A.V.), a National Sciences and Engineering Research Council of Canada Discovery Grant (E.K.L.), the Canadian Foundation for Innovation (E.K.L.), and the Canada Research Chairs Program (E.K.L.). M.B. was supported by a Sarojini Damodaran Fellowship. N.M.G. was supported by a Margaret Santalo fellowship and a Canadian Institute of Health Research Canadian Graduate Scholarship Doctoral Award. We acknowledge Dr. Mohammed Farhan for technical assistance.
References
- Amargós-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, Toth M, Mengod G, Artigas F. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- Barrow SL, Constable JR, Clark E, El-Sabeawy F, McAllister AK, Washbourne P. Neuroligin1: a cell adhesion molecule that recruits PSD-95 and NMDA receptors by distinct mechanisms during synaptogenesis. Neural Dev. 2009;4:17. doi: 10.1186/1749-8104-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béïque JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci. 2004;24:4807–4817. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc Ser B. 1995;57:289–300. [Google Scholar]
- Bhansali P, Dunning J, Singer SE, David L, Schmauss C. Early life stress alters adult serotonin 2C receptor pre-mRNA editing and expression of the α subunit of the heterotrimeric G-protein Gq. J Neurosci. 2007;27:1467–1473. doi: 10.1523/JNEUROSCI.4632-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout AL, Mangelsdorf DJ. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl Recept Signal. 2003;1:e012. doi: 10.1621/nrs.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne HR, von Zastrow M. Drug receptors and pharmacodynamics. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic and clinical pharmacology. Norwalk, CT: Appleton and Lange; 2007. pp. 13–14. [Google Scholar]
- Brawman-Mintzer O, Knapp RG, Nietert PJ. Adjunctive risperidone in generalized anxiety disorder: a double-blind, placebo-controlled study. J Clin Psychiatry. 2005;66:1321–1325. doi: 10.4088/jcp.v66n1016. [DOI] [PubMed] [Google Scholar]
- Burnet PW, Mead A, Eastwood SL, Lacey K, Harrison PJ, Sharp T. Repeated ECS differentially affects rat brain 5-HT1A and 5-HT2A receptor expression. Neuroreport. 1995;6:901–904. doi: 10.1097/00001756-199504190-00019. [DOI] [PubMed] [Google Scholar]
- Cahir M, Ardis T, Reynolds GP, Cooper SJ. Acute and chronic tryptophan depletion differentially regulate central 5-HT1A and 5-HT2A receptor binding in the rat. Psychopharmacology. 2007;190:497–506. doi: 10.1007/s00213-006-0635-5. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig M, Amargós-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- Chung I, Schlichter LC. Native Kv1.3 channels are upregulated by protein kinase C. J Membr Biol. 1997;156:73–85. doi: 10.1007/s002329900189. [DOI] [PubMed] [Google Scholar]
- Daniels WM, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 2004;19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- Day M, Olson PA, Platzer J, Striessnig J, Surmeier DJ. Stimulation of 5-HT2 receptors in prefrontal pyramidal neurons inhibits Cav1.2 L-type Ca2+ currents via a PLCβ/IP3/calcineurin signaling cascade. J Neurophysiol. 2002;87:2490–2504. doi: 10.1152/jn.00843.2001. [DOI] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- D'haenen H, Bossuyt A, Mertens J, Bossuyt-Piron C, Gijsemans M, Kaufman L. SPECT imaging of serotonin2 receptors in depression. Psychiatry Res. 1992;45:227–237. doi: 10.1016/0925-4927(92)90018-y. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Friedman E, Wang HY. Receptor-mediated activation of G proteins is increased in postmortem brains of bipolar affective disorder subjects. J Neurochem. 1996;67:1145–1152. doi: 10.1046/j.1471-4159.1996.67031145.x. [DOI] [PubMed] [Google Scholar]
- Garcia EE, Smith RL, Sanders-Bush E. Role of Gq protein in behavioral effects of the hallucinogenic drug 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane. Neuropharmacology. 2007;52:1671–1677. doi: 10.1016/j.neuropharm.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartside SE, Hajós-Korcsok E, Bagdy E, Hársing LG, Jr, Sharp T, Hajós M. Neurochemical and electrophysiological studies on the functional significance of burst firing in serotonergic neurons. Neuroscience. 2000;98:295–300. doi: 10.1016/s0306-4522(00)00060-9. [DOI] [PubMed] [Google Scholar]
- Gerrow K, Romorini S, Nabi SM, Colicos MA, Sala C, El-Husseini A. A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron. 2006;49:547–562. doi: 10.1016/j.neuron.2006.01.015. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Meana JJ. Heterotrimeric G proteins: insights into the neurobiology of mood disorders. Curr Neuropharmacol. 2006;4:127–138. doi: 10.2174/157015906776359586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT2A receptors by agonists and antagonists. Brain Res Bull. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Inoue T, Koyama T. Effects of conditioned fear stress on serotonin neurotransmission and freezing behavior in rats. Eur J Pharmacol. 1999;378:23–30. doi: 10.1016/s0014-2999(99)00441-0. [DOI] [PubMed] [Google Scholar]
- Holmes A, le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci Biobehav Rev. 2005;29:1335–1346. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Jezierski G, Braun K, Gruss M. Epigenetic modulation of the developing serotonergic neurotransmission in the semi-precocial rodent Octodon degus. Neurochem Int. 2006;48:350–357. doi: 10.1016/j.neuint.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- Kawahara H, Yoshida M, Yokoo H, Nishi M, Tanaka M. Psychological stress increases serotonin release in the rat amygdala and prefrontal cortex assessed by in vivo microdialysis. Neurosci Lett. 1993;162:81–84. doi: 10.1016/0304-3940(93)90565-3. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. Prefrontal cortical network activity: oposite effects of psychedelic hallucinogens and D1/D5 dopamine receptor activation. Neuroscience. 2007;145:900–910. doi: 10.1016/j.neuroscience.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Pei Q, Feldon J, Pryce CR, Harrison PJ. Gene expression in the anterior cingulate cortex and amygdala of adolescent marmoset monkeys following parental separations in infancy. Int J Neuropsychopharmacol. 2009;12:761–772. doi: 10.1017/S1461145708009723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes AC, Holmes KD, Dale LB, Comps-Agrar L, Lee D, Yadav PN, Drysdale L, Poulter MO, Roth BL, Pin JP, Anisman H, Ferguson SS. CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nat Neurosci. 2010;13:622–629. doi: 10.1038/nn.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus RN, McQuade RD, Carson WH, Hennicken D, Fava M, Simon JS, Trivedi MH, Thase ME, Berman RM. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28:156–165. doi: 10.1097/JCP.0b013e31816774f9. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Carpenter LL, McDougle CJ, Price LH. Synergistic action of 5-HT2A antagonists and selective serotonin reuptake inhibitors in neuropsychiatric disorders. Neuropsychopharmacology. 2003;28:402–412. doi: 10.1038/sj.npp.1300057. [DOI] [PubMed] [Google Scholar]
- Martín-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Dalley JW, Matthews C, Tsai TH, Robbins TW. Periodic maternal separation of neonatal rats produces region- and gender-specific effects on biogenic amine content in postmortem adult brain. Synapse. 2001;40:1–10. doi: 10.1002/1098-2396(200104)40:1<1::AID-SYN1020>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Kapur S, Eisfeld B, Brown GM, Houle S, DaSilva J, Wilson AA, Rafi-Tari S, Mayberg HS, Kennedy SH. The effect of paroxetine on 5-HT2A receptors in fepression: an [18F]setoperone PET imaging study. Am J Psychiatry. 2001;158:78–85. doi: 10.1176/appi.ajp.158.1.78. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Nair A, Vadodaria KC, Banerjee SB, Benekareddy M, Dias BG, Duman RS, Vaidya VA. Stressor-specific regulation of distinct brain-derived neurotrophic factor transcripts and cyclic AMP response element-binding protein expression in the postnatal and adult rat hippocampus. Neuropsychopharmacology. 2007;32:1504–1519. doi: 10.1038/sj.npp.1301276. [DOI] [PubMed] [Google Scholar]
- Naughton M, Mulrooney JB, Leonard BE. A review of the role of serotonin receptors in psychiatric disorders. Hum Psychopharmacol. 2000;15:397–415. doi: 10.1002/1099-1077(200008)15:6<397::AID-HUP212>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Stowe ZN, Nemeroff CB. Parental depression: animal models of an adverse life event. Am J Psychiatry. 2002;159:1265–1283. doi: 10.1176/appi.ajp.159.8.1265. [DOI] [PubMed] [Google Scholar]
- Olsson T, Håkansson A, Seckl JR. Ketanserin selectively blocks acute stress-induced changes in NGFI-A and mineralocorticoid receptor gene expression in hippocampal neurons. Neuroscience. 1997;76:441–448. doi: 10.1016/s0306-4522(96)00432-0. [DOI] [PubMed] [Google Scholar]
- Parakh K, Sakhuja A, Bhat U, Ziegelstein RC. Platelet function in patients with depression. South Med J. 2008;101:612–617. doi: 10.1097/SMJ.0b013e318172f732. [DOI] [PubMed] [Google Scholar]
- Parthasarathy LK, Seelan RS, Wilson MA, Vadnal RE, Parthasarathy RN. Regional changes in rat brain inositol monophosphatase 1 (IMPase 1) activity with chronic lithium treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:55–60. doi: 10.1016/s0278-5846(02)00315-9. [DOI] [PubMed] [Google Scholar]
- Pei Q, Lewis L, Sprakes ME, Jones EJ, Grahame-Smith DG, Zetterström TS. Serotonergic regulation of mRNA expression of Arc, an immediate early gene selectively localized at neuronal dendrites. Neuropharmacology. 2000;39:463–470. doi: 10.1016/s0028-3908(99)00148-3. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Preece MA, Dalley JW, Theobald DE, Robbins TW, Reynolds GP. Region specific changes in forebrain 5-hydroxytryptamine1a and 5-hydroxytryptamine2a receptors in isolation-reared rats: an in vitro autoradiography study. Neuroscience. 2004;123:725–732. doi: 10.1016/j.neuroscience.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Price JL. Prefrontal cortical networks related to visceral function and mood. Ann NY Acad Sci. 1999;877:383–396. doi: 10.1111/j.1749-6632.1999.tb09278.x. [DOI] [PubMed] [Google Scholar]
- Puig MV, Artigas F, Celada P. Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: involvement of serotonin and GABA. Cereb Cortex. 2005;15:1–14. doi: 10.1093/cercor/bhh104. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Roth BL, Hamblin MW, Ciaranello RD. Developmental regulation of 5-HT2 and 5-HT1C mRNA and receptor levels. Brain Res Dev Brain Res. 1991;58:51–58. doi: 10.1016/0165-3806(91)90236-c. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Jacobs BL. A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Res. 1996;739:57–69. doi: 10.1016/s0006-8993(96)00809-8. [DOI] [PubMed] [Google Scholar]
- Saganich MJ, Machado E, Rudy B. Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain. J Neurosci. 2001;21:4609–4624. doi: 10.1523/JNEUROSCI.21-13-04609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotonin1A and serotonin2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Sato Y, Yasui-Furukori N, Nakagami T, Saito M, Kaneko S. Augmentation of antidepressants with perospirone for treatment-resistant major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:416–418. doi: 10.1016/j.pnpbp.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Sawamura Y, Sakagami H, Kondo H. Localization of mRNA for Ca2+/calmodulin-dependent protein kinase I in the brain of developing and mature rats. Brain Res. 1996;706:259–266. doi: 10.1016/0006-8993(95)01161-7. [DOI] [PubMed] [Google Scholar]
- Schledermann W, Wulfsen I, Schwarz JR, Bauer CK. Modulation of rat erg1, erg2, erg3 and HERG K+ currents by thyrotropin-releasing hormone in anterior pituitary cells via the native signal cascade. J Physiol. 2001;532:143–163. doi: 10.1111/j.1469-7793.2001.0143g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N, Schohl A, Ruthazer ES. Neural activity regulates Synaptic properties and dendritic structure in vivo through calcineurin/NFAT signaling. Neuron. 2009;62:655–669. doi: 10.1016/j.neuron.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Sanders-Bush E, Manier DH, Lewis DA. Elevated 5-HT2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience. 2009;158:1406–1415. doi: 10.1016/j.neuroscience.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmelin J, Cohen C, Terranova JP, Lopez-Grancha M, Pichat P, Bergis O, Decobert M, Santucci V, Françon D, Alonso R, Stahl SM, Keane P, Avenet P, Scatton B, le Fur G, Griebel G. Stimulation of the β3-adrenoceptor as a novel treatment strategy for anxiety and depressive disorders. Neuropsychopharmacology. 2008;33:574–587. doi: 10.1038/sj.npp.1301424. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res. 2003;37:357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Temple MD, Worley PF, Steward O. Visualizing changes in circuit activity resulting from denervation and reinnervation using immediate early gene expression. J Neurosci. 2003;23:2779–2788. doi: 10.1523/JNEUROSCI.23-07-02779.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VA, Marek GJ, Aghajanian GK, Duman RS. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci. 1997;17:2785–2795. doi: 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber ET, Andrade R. Htr2a gene and 5-HT2A receptor expression in the cerebral cortex studied using genetically modified mice. Front Neurosci. 2010 doi: 10.3389/fnins.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, González-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- Wright IK, Garratt JC, Marsden CA. Effects of a selective 5-HT2 agonist, DOI, on 5-HT neuronal firing in the dorsal raphe nucleus and 5-HT release and metabolism in the frontal cortex. Br J Pharmacol. 1990;99:221–222. doi: 10.1111/j.1476-5381.1990.tb14683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatham LN, Liddle PF, Dennie J, Shiah IS, Adam MJ, Lane CJ, Lam RW, Ruth TJ. Decrease in brain serotonin2 receptor binding in patients with major depression following desipramine treatment: a positron emission tomography study with fluorine-18-labeled setoperone. Arch Gen Psychiatry. 1999;56:705–711. doi: 10.1001/archpsyc.56.8.705. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Liddle PF, Shiah IS, Scarrow G, Lam RW, Adam MJ, Zis AP, Ruth TJ. Brain serotonin2 receptors in major depression: a positron emission tomography study. Arch Gen Psychiatry. 2000;57:850–858. doi: 10.1001/archpsyc.57.9.850. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Matsumoto M, Togashi H, Saito H. Effects of conditioned fear stress on 5-HT release in the rat prefrontal cortex. Pharmacol Biochem Behav. 1995;51:515–519. doi: 10.1016/0091-3057(95)00045-x. [DOI] [PubMed] [Google Scholar]
- Zanardi R, Artigas F, Moresco R, Colombo C, Messa C, Gobbo C, Smeraldi E, Fazio F. Increased 5-hydroxytryptamine-2 receptor binding in the frontal cortex of depressed patients responding to paroxetine treatment: a positron emission tomography scan study. J Clin Psychopharmacol. 2001;21:53–58. doi: 10.1097/00004714-200102000-00010. [DOI] [PubMed] [Google Scholar]
- Zhang ZW. Serotonin induces tonic firing in layer V pyramidal neurons of rat prefrontal cortex during postnatal development. J Neurosci. 2003;23:3373–3384. doi: 10.1523/JNEUROSCI.23-08-03373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZW. Maturation of layer V pyramidal neurons in the rat prefrontal cortex: intrinsic properties and synaptic function. J Neurophysiol. 2004;91:1171–1182. doi: 10.1152/jn.00855.2003. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol. 1999;82:2989–2999. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]