Abstract
Adult hippocampal neurogenesis is modulated by perturbations in thyroid hormone status; however the role of specific thyroid hormone receptors (TRs) in this process is not completely understood. We show here that loss of the TRβ gene results in a significant increase in the proliferation of adult hippocampal progenitors, without any change in immature neuron number or in the neuronal and glial differentiation of progenitors. Using the mitotic marker 5′-bromo-2-deoxyuridine (BrdU) or the endogenous cell cycle marker, proliferating cell nuclear antigen (PCNA), we find a significant increase in the number of BrdU- and PCNA-immunopositive cells within the subgranular zone (SGZ) of the dentate gyrus subfield in TRβ−/− mice. Further, we find that TRβ−/− mice exhibit a significant increase in the numbers of NeuroD-positive cells within the SGZ, suggesting that the increased numbers of proliferating progenitors translate into enhanced numbers of neuroblasts. Interestingly, the number of BrdU-positive cells that persist 4 weeks post-BrdU injection is unaltered in TRβ−/− mice, indicating that the enhanced proliferation does not result in increased hippocampal neurogenesis. This is also supported by evidence of no change in the numbers of cells expressing markers of immature neurons such as doublecortin or polysialylated neural cell adhesion molecule. Furthermore, no change is observed in the neuronal or glial differentiation of BrdU-positive cells in the TRβ−/− mice. Taken together, our results provide novel evidence for a role of TRβ in modulating hippocampal progenitor cell division, and implicate this receptor in the effects of thyroid hormone on adult hippocampal neurogenesis.
Keywords: TR, adult neurogenesis, subgranular zone, dentate gyrus, BrdU
Thyroid hormone plays a key role in neuronal development, modulating progenitor cell division, cell cycle exit, migration, fate choice and neuronal maturation [1]. While early-onset hypothyroidism during critical periods of neurodevelopment can result in permanent mental retardation, the effects of adult-onset hypothyroidism on brain function are relatively subtle [2, 3]. Recent studies indicate that thyroid hormone perturbations regulate adult hippocampal neurogenesis, with a decrease in progenitor proliferation, survival and neuronal differentiation reported in adult-onset hypothyroid animals [4-6]. This decline in hippocampal neurogenesis has been implicated in the cognitive and behavioral dysfunction associated with adult-onset hypothyroidism [6]. Thyroid hormone receptors (TRs) TRα and TRβ consist of distinct isoforms (TRα1, TRα2, TRβ1 and TRβ2), that mediate the cellular effects of thyroid hormone [7]. While the TRα1 receptor has been recently implicated in the regulation of post-mitotic hippocampal progenitor survival and differentiation [8], the role of the TRβ isoforms in modulating adult hippocampal neurogenesis is at present unknown.
The TRβ gene is implicated in many of the actions of thyroid hormone within the central nervous system [9]. The expression of the TRβ gene is observed relatively later in brain development, with levels of expression increasing across postnatal development. The TRβ1 and TRβ2 isoforms are identical other than in their N-terminal domains, and differ in their expression patterns [9]. The TRβ1 isoform is widely expressed in the brain [10], whereas a more restricted pattern of expression, in particular within the hypothalamus and pituitary [11-13], is reported for the TRβ2 isoform. Previous studies indicate that both the TRβ1 and TRβ2 isoforms are expressed within the hippocampal neurogenic niche in the adult brain [4]. The major aim of this study was to address the role of the TRβ gene in the regulation of distinct aspects of adult hippocampal neurogenesis, using TRβ−/− mice.
Adult male wild type and TRβ−/− mice, generated as previously described [14] were used in all experiments in accordance with the guidelines set by the European Community Council Directives (86/609/EEC) and were approved by the regional Swedish and the TIFR Institutional Animal Ethics committees. Animals were maintained on a 12 hour light/ dark cycle with access to food and water ad libitum. We followed two distinct BrdU labeling paradigms to address effects on proliferation (Paradigm 1) and survival/differentiation (Paradigm 2). Paradigm 1: To examine proliferation of adult hippocampal progenitors, TRβ−/− mice and littermate wild type controls received a single intraperitoneal (i.p.) injection of the mitotic marker 5-bromo-2′-deoxyuridine (BrdU, 150 mg/kg body weight; Sigma, USA) and were sacrificed 2 hours later (n = 5/group). Paradigm 2: To assess the survival and neuronal differentiation of hippocampal progenitors, TRβ−/− mice and wild type controls received BrdU (150 mg/kg body weight, once daily for 3 days) and were sacrificed 28 days later (n = 3-5/group). Mice were sacrificed by transcardial perfusion with 4% paraformaldehyde (PFA), and brains were postfixed and cryoprotected in 30% sucrose-PFA. Serial coronal sections (30 μm) through the rostro-caudal extent of the hippocampus were generated using a freezing microtome (Leica, Germany) and sections were processed for BrdU immunohistochemistry as described previously [4]. In brief, following DNA denaturation and acid hydrolysis, sections were incubated overnight with mouse anti-BrdU antibody (1:500, Boehringer Mannheim, USA) followed by exposure to the secondary antibody (biotinylated anti-mouse IgG, 1:500, Vector Laboratories, USA). For immunohistochemical and immunofluorescent detection of endogenous markers of proliferation (proliferating cell nuclear antigen, PCNA), immature neurons (doublecortin - DCX, polysialylated neural cell adhesion molecule - PSA-NCAM, TUC-4) and the neurogenic transcription factor (NeuroD), tissue sections were exposed to specific primary antibodies: (1) mouse anti-PCNA (1:250, Accurate Biochemicals, USA) (2) goat anti-DCX (1:250, Santa Cruz Biotechnology, USA) (3) mouse anti-PSA-NCAM (1:500; kind gift from Prof. T. Seki, Juntendo University, Japan; [15]) (4) rabbit anti-TUC-4 (1:250, Chemicon, USA) (5) goat anti-NeuroD (1:250, Santa-Cruz Biotechnology). Following washes, sections were incubated with the following secondary antibodies: (1) Alexa 488-conjugated donkey anti-mouse (1:250, Molecular Probes, USA) (2) biotinylated anti-goat IgG (1:250, Vector Laboratories) (3) Alexa 488-conjugated donkey anti-rabbit (1:250, Molecular Probes) at room temperature for 3 hours. An avidin-biotin complex (Vector Laboratories) was used for signal amplification of biotinylated secondary antibodies, followed by visualization using diaminobenzidine (Sigma). Sections were mounted in DPX or Vectashield (Vector Laboratories) and viewed using a Zeiss Axioskop (Germany) or Nikon Eclipse 90i (Japan) fluorescence microscope. Using sections from animals treated as per Paradigm 1 (Fig 1A), we sought to address whether specific classes of proliferating hippocampal progenitors are altered in TRβ−/− mice, using triple immunofluorescence labeling. We performed studies to detect colocalization of BrdU and GFAP (glial fibrillary acidic protein) a marker observed in quiescent hippocampal stem cells, and for BrdU with DCX, a marker observed in proliferating neuroblasts. Sections were incubated with a cocktail of primary antibodies: mouse anti-BrdU (1:500, Boehringer Mannheim) with goat anti-DCX (1:250, Santacruz) and rabbit anti-GFAP (1:500, Chemicon) followed by incubation with a cocktail of secondary antibodies: biotinylated anti-mouse IgG (1:250, Vector), Alexa 555-conjugated anti-goat (1:250, Molecular Probes), and Cy5 conjugated anti-rabbit (1:250, Molecular Probes). Using sections from animals treated as per Paradigm 2 (Fig 1D), we carried out triple immunofluorescence labeling to detect the neuronal or glial differentiation of BrdU-positive progenitors. Sections were incubated with a cocktail of primary antibodies: rat anti-BrdU (1:500, Accurate Biochemicals) with mouse anti-NeuN (1:1000, Chemicon) and rabbit anti-GFAP (1:500, Chemicon) followed by incubation with a cocktail of secondary antibodies: biotinylated anti-rat IgG (1:500, Chemicon), Alexa 555-conjugated anti-mouse (1:250, Molecular Probes), and Cy5 conjugated anti-rabbit (1:250, Molecular Probes). Signal amplification for BrdU in triple immunofluorescence experiments was performed by incubation with Alexa 488-conjugated streptavidin (1:500, Molecular Probes). To detect colocalization of BrdU with DCX or GFAP and with NeuN or GFAP, confocal Z-plane (1 μm steps) sectioning was carried out using a Zeiss LSM Exciter confocal microscope (Germany).
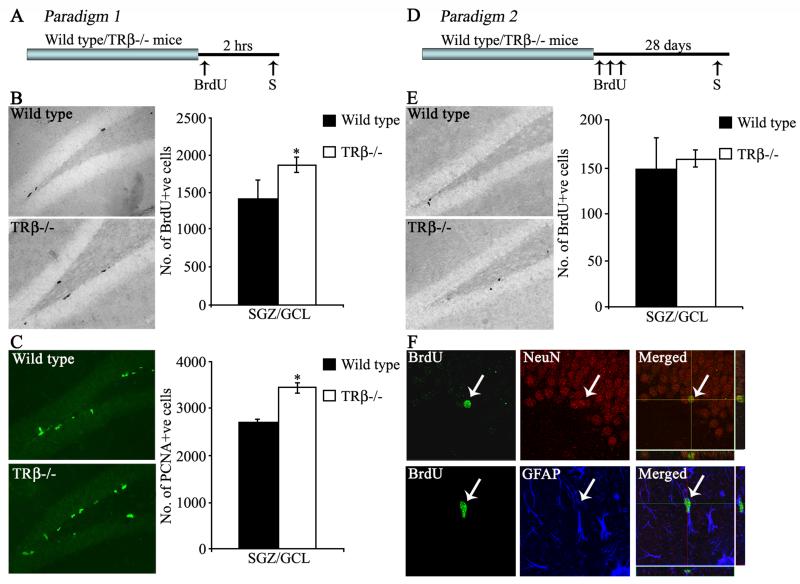
Figure 1. Effect of the loss of TRβ on the proliferation, survival and differentiation of adult hippocampal progenitors.
Shown are schematic representations of the BrdU labeling paradigms to assess effects on proliferation (Paradigm 1, A) and survival/differentiation (Paradigm 2, D) in TRβ−/− mice and littermate wild type controls (S-time point for sacrifice). Representative photomicrographs of BrdU-positive cells in the dentate gyrus of Wild type and TRβ−/− mice in the proliferation (B) and survival (E) paradigms are shown. Representative photomicrographs of PCNA-positive cells (C) in the dentate gyrus of Wild type and TRβ−/− mice are shown. At the 2 hour post-BrdU treatment time point, TRβ−/− mice exhibited a significant increase in both BrdU-positive (B) and PCNA-positive (C) hippocampal progenitors in the subgranular zone (SGZ)/ granule cells layer (GCL) as compared to wild type animals. TRβ−/− mice sacrificed 28 days after the BrdU administration showed no change in the numbers of BrdU-positive progenitors in the SGZ/GCL (E). Shown are representative images of BrdU-, NeuN- and GFAP-positive cells in the dentate gyrus along with merged confocal z-stack images, with arrows indicating a BrdU-positive cell colocalizing with NeuN or GFAP (F). The results are expressed as the mean ± SEM BrdU or PCNA positive cells (n = 5/group). *p<0.05 as compared to Wild type (Student’s t-test).
All cell counting analysis was performed on coded sections by an experimenter blind to the study code. Quantitation of BrdU-positive cell number in hippocampal sections was carried out using a previously described modified, unbiased stereology protocol [16]. In brief, every 6th hippocampal section was processed for BrdU quantitation (12 sections/animal) and the total number of BrdU-positive cells in the subgranular zone (SGZ)/ granule cell layer (GCL) was estimated by multiplying the total number of BrdU cells counted from every 6th section by the section periodicity (6). Quantitation of DCX, PSA-NCAM, TUC-4 and NeuroD-positive cells was performed in the SGZ (n = 4 sections/animal) and results were expressed as the number of immuno-positive cells per section. To address effects on dendritic complexity, the DCX-positive cells were categorized based on their morphological status as DCX-positive cells (1) with or (2) without tertiary dendrites [17]. To examine BrdU/GFAP/DCX (n = 5 per group) or BrdU/NeuN/GFAP (n = 3 per group) colocalization, the percentage of BrdU-positive cells that were also labeled with specific markers was determined using confocal microscopy. In each animal, 20 - 30 BrdU-positive cells were analyzed for colocalization using Z-plane sectioning with 1 μm steps on a Zeiss LSM Exciter confocal microscope. Results were subjected to statistical analysis using the program Prism (Graphpad, USA). Experiments were analyzed for differences between groups using the unpaired Student’s t-test, with significance determined at p < 0.05.
In order to assess the role of TRβ in the regulation of adult hippocampal neurogenesis, we first addressed the effect of loss of TRβ on adult hippocampal progenitor proliferation (Paradigm 1, Fig 1A). BrdU is incorporated in the S-phase of the cell cycle by proliferative progenitors, and can be detected by BrdU immunohistochemistry in the form of BrdU-immunopositive cell clusters at the border of the GCL and the hilus within the SGZ. Quantitative analysis revealed a significant increase in the number of BrdU-positive cells within the SGZ in TRβ−/− mice compared to wild type animals (Fig 1B). This increase in the proliferation of hippocampal progenitors was also confirmed by immunohistochemistry for the endogenous proliferation marker PCNA, with a significant increase in the number of PCNA immunopositive cells in TRβ−/− mice as compared to wild type animals (Fig 1C). In order to examine whether the loss of TRβ influences the turnover of a specific subset of proliferating hippocampal progenitors, we performed triple immunofluorescence to detect whether the relative distribution of the numbers of BrdU/GFAP double positive progenitors (quiescent hippocampal stem cells) or BrdU/DCX double positive progenitors (cycling neuroblasts) was altered in TRβ−/− mice. No significant difference in the percentage of BrdU-positive progenitors that colocalized with GFAP (Wild type = 7 % ± 0.9, TRβ−/− = 6.25% ± 1.1; n = 5 per group, results are the mean ± SEM) or with DCX (Wild type = 21.3 % ± 0.8, TRβ−/− = 23.5% ± 1.5; n = 5 per group, results are the mean ± SEM) was observed in the TRβ−/− mice. These results indicate that the enhanced proliferation observed in animals that lack TRβ is unlikely to be a consequence of increased proliferation in a particular subset of dividing hippocampal progenitors.
To assess the survival of the hippocampal progenitors, animals were sacrificed 28 days after BrdU administration (Paradigm 2, Fig 1D). Surviving BrdU-positive cells were seen dispersed in the SGZ, and had an ovoid morphology (Fig 1E). We observed that despite the increased number of BrdU-positive progenitors in the SGZ of TRβ−/− mice 2 hours after BrdU administration, the number of BrdU-positive cells that persist 4 weeks later was unaltered in TRβ−/− mice (Fig 1E). BrdU-positive cells in both wild type and TRβ−/− mice showed normal neuronal differentiation, with no significant difference in the percentage of BrdU-positive progenitors that colocalized with the mature neuronal marker NeuN or the glial marker GFAP (BrdU/NeuN: Wild type = 64.3% ± 3.2, TRβ−/− = 70.7% ± 2.4; BrdU/GFAP: Wild type = 18.2% ± 2.8, TRβ−/− = 12.7% ± 0.3; n = 3 per group, results are the mean ± SEM, p>0.05, Student’s t-test, Fig 1F).
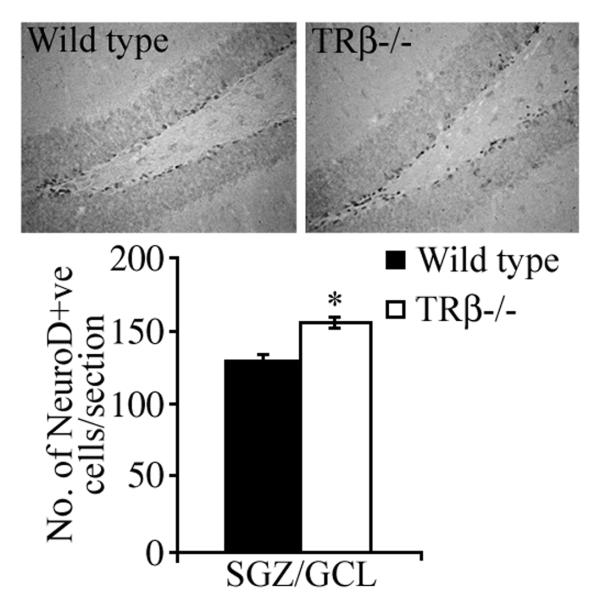
In order to address whether the increased number of BrdU-positive proliferating progenitors persist to become neuroblasts, we examined the numbers of NeuroD-positive cells within the SGZ. The transcription factor NeuroD is intrinsically involved in cell fate specification and is essential for the neuronal differentiation of progenitors in the adult hippocampus [18]. We observed a significant increase in the number of NeuroD-immunopositive cells in the SGZ of TRβ−/− mice as compared to wild type animals (Fig 2).
Figure 2. Effect of the loss of TRβ on the number of NeuroD-positive cells in the dentate gyrus.
Shown are representative photomicrographs of NeuroD-positive cells within the dentate gyrus of wild type and TRβ−/− mice. TRβ−/− mice exhibited a significant increase in the number of NeuroD-positive cells/section within the subgranular zone (SGZ)/ granular cell layer (GCL) as compared to Wild type controls. The results are expressed as the mean ± SEM NeuroD-positive cells/section (n = 5/group). *p<0.05 as compared to Wild type (Student’s t-test).
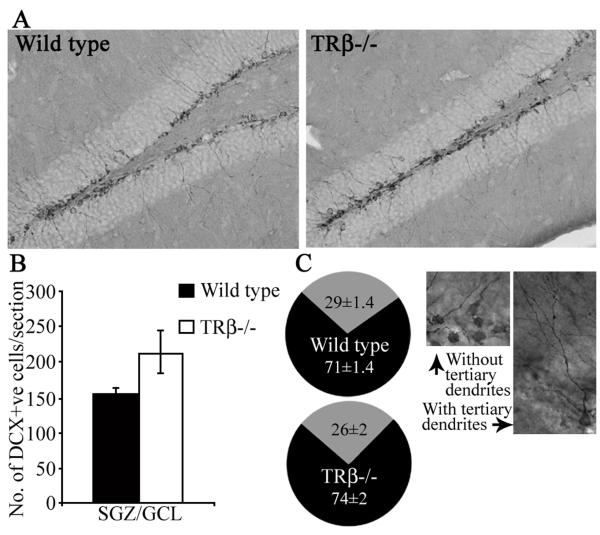
To determine whether the enhanced proliferation and NeuroD-positive cell number translates into increased hippocampal neurogenesis, we examined the numbers of DCX-positive immature neurons within the neurogenic niche of TRβ−/− mice (Fig 3). The number of DCX positive cells in the SGZ of TRβ−/− animals was not significantly different from wild type controls (Fig 3B). However, there was a trend (p = 0.09) towards an increase in the number of DCX-immunopositive cells in TRβ−/− mice. Given that there was no change in the numbers of BrdU-positive cells 28 days after mitotic marker administration, we wanted to further test whether the increased progenitor proliferation and neuroblast number eventually gives rise to more immature neurons. We next examined two more immature neuron markers, PSA-NCAM and TUC-4 in the TRβ−/− mice. There was no significant difference in numbers of PSA-NCAM or TUC-4 immunopositive cells in the neurogenic niche of TRβ−/− mice as compared to wild type controls (PSA-NCAM: Wild type = 149.84 ± 17.3, TRβ−/− = 210.4 ± 33.9; TUC-4: Wild type = 58.4 ± 2.7, TRβ−/− = 63.5 ± 7.2; n = 5 per group, results are the mean ± SEM, p>0.05, Student’s t-test). Taken together these results indicate that loss of the TRβ gene results in enhanced progenitor proliferation, which translates into increased neuroblast number but does not result in enhanced immature neuron formation. To test whether TRβ−/− mice exhibit altered dendritic complexity of newborn neurons we determined the numbers of DCX positive cells that exhibit the presence of complex tertiary dendrites [17] (Fig 3C). We observed no significant difference in the percentage of DCX-positive cells with tertiary dendrites in TRβ−/− mice as compared to wild type controls (Fig 3C).
Figure 3. Effect of the loss of TRβ on DCX-positive immature neuron number and the morphological maturation of DCX-positive immature neurons in the dentate gyrus.
Shown are representative photomicrographs (A) of DCX-positive cells in the dentate gyrus subfield of Wild type and TRβ−/− mice. Quantitative analysis of DCX-positive cells/section in the subgranular zone (SGZ)/ granular cell layer (GCL) revealed no change in TRβ−/− mice as compared to Wild type controls (B). Pie charts demonstrate that the percentage of DCX-positive cells with (grey) or without (black) tertiary dendrites was not altered in TRβ−/− mice as compared to the Wild type controls (C). Shown are representative photomicrographs of DCX-positive cells with and without tertiary dendrites (C). The results are expressed as the mean ± SEM DCX-positive cells/section (n = 5/group). p>0.05 as compared to Wild type (Student’s t-test).
The present study reveals a novel role for the thyroid receptor TRβ in the regulation of adult hippocampal progenitor proliferation. This increased proliferation observed in TRβ−/− mice, which lack both TRβ1 and TRβ2 isoforms, also translated into increased numbers of NeuroD-positive neuroblasts. Expression of both TRβ1 and TRβ2 isoforms has been previously shown within the adult hippocampal dentate gyrus subfield [4, 10]. However, at present we cannot distinguish between the contributions of the TRβ isoforms to the effects on progenitor turnover. TRβ−/− mice have been previously reported to exhibit both normal hippocampal morphology and hippocampal-dependent spatial learning [14], leading to the suggestion that any neurological deficits in these mutant mice are likely to be subtle and not involve any major change in development. Our results provide the first evidence of such subtle modifications within the adult hippocampal neurogenic niche, with increased proliferation and neuroblast number being observed in TRβ−/− mice.
Prior evidence indicates that adult-onset hypothyroidism results in a decline in hippocampal progenitor proliferation [6]. TRβ−/− mice exhibit increased progenitor cell division, suggesting that TRβ isoforms may normally exert an inhibitory tone on progenitor turnover. Our results implicate the TRβ isoforms in mediating the effects of thyroid hormone on hippocampal progenitor proliferation. It is possible to speculate that an unliganded TRβ aporeceptor inhibits progenitor cell turnover. The evidence that adult hypothyroid animals exhibit decreased progenitor cell division [6], which can be rescued by thyroid hormone replacement, suggests that ligand replacement may alleviate unliganded TRβ repressive effects on progenitor turnover.
Our results indicate that the effects of TRβ isoforms on adult hippocampal neurogenesis appear to be predominantly on progenitor cell division and neuroblast formation, with no change in immature neuron formation in the TRβ−/− mice. This suggests that the enhanced numbers of NeuroD-positive progenitors in TRβ−/− mice do not translate into the formation of more immature neurons. This motivates future studies to address whether loss of TRβ isoforms influences postmitotic neuroblast survival in the hippocampal neurogenic niche. At present, our results do not allow us to rule out an effect of the TRβ isoforms on later stages of hippocampal neurogenesis, as it is possible that these effects may be masked in the mutant mice through compensatory effects of thyroid hormone via the TRα1 receptor.
At present the mechanism that underlies the influence of TRβ on adult hippocampal progenitor mitosis is unclear. While most of the data within the nervous system has implicated the TRβ gene in the maturation aspects of neurodevelopment [19, 20], some insights can be gained into the contribution of TRβ isoforms to cell division based on studies in hepatocarcinoma and breast cancer cells [21]. TRβ1 acts as a metastasis suppressor gene, through suppression of the response of these cancer cells to mitogens such as EGF and IGF-I. TRβ1 reduces the proliferative effects of these mitogens by decreasing receptor expression and by suppressing downstream activation of the ERK and PI3K pathways [21]. It is tempting to speculate that TRβ1 may modulate the proliferation of adult hippocampal progenitors in a similar manner, since EGF is also known to exert strong mitogenic effects in the hippocampal neurogenic niche [22, 23]. While at present the mechanism via which TRβ modulates hippocampal progenitor proliferation is unclear, a point to note is that the TRβ−/− mice exhibit elevated levels of T3, T4 and TSH that could contribute indirectly to influencing proliferative rates of hippocamal progenitors in the TRβ mutants.
In conclusion, our results provide the first evidence of a role for TRβ in the regulation of adult hippocampal progenitor proliferation and neuroblast formation, suggesting that this receptor may contribute to the decline in proliferation reported in adult-onset hypothyroidism. Our results motivate future enquiry into the TRβ isoform responsive target genes that contribute to the modulation of adult hippocampal progenitor cell cycle.
Acknowledgements
This work was supported by intramural funds from TIFR and a Wellcome Trust Senior Overseas Fellowship in Biomedical sciences to VV (04082003114133). Support to BV was obtained from the Swedish Research Council, the Swedish Cancer Society and the Wallenberg Foundations. We are grateful to Professor T. Seki, Juntendo University School of Medicine, Tokyo, for the gift of the PSA-NCAM antibody.
References
- [1].Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab. 2007;3:249–259. doi: 10.1038/ncpendmet0424. [DOI] [PubMed] [Google Scholar]
- [2].Fundaro A. Behavioral modifications in relation to hypothyroidism and hyperthyroidism in adult rats. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:927–940. doi: 10.1016/0278-5846(89)90044-4. [DOI] [PubMed] [Google Scholar]
- [3].Kulikov A, Torrésani J, Jeanningros R. Experimental hypothyroidism increases immobility in rats in the forced swim paradigm. Neurosci Lett. 1997;234:111–114. doi: 10.1016/s0304-3940(97)00664-2. [DOI] [PubMed] [Google Scholar]
- [4].Desouza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA, Vaidya VA. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci. 2005;29:414–426. doi: 10.1016/j.mcn.2005.03.010. [DOI] [PubMed] [Google Scholar]
- [5].Ambrogini P, Cuppini R, Ferri P, Mancini C, Ciaroni S, Voci A, Gerdoni G, Gallo G. Thyroid hormones affect neurogenesis in the dentate gyrus of adult rat. Neuroendocrinology. 2005;81:244–253. doi: 10.1159/000087648. [DOI] [PubMed] [Google Scholar]
- [6].Montero-Pedrazuela A, Venero C, Lavado-Autric R, Fernández-Lamo I, García-Verdugo JM, Bernal J, Guadaño-Ferraz A. Modulation of adult hippocampal neurogenesis by thyroid hormones: implications in depressive-like behavior. Mol Psychiatry. 2006;11:361–371. doi: 10.1038/sj.mp.4001802. [DOI] [PubMed] [Google Scholar]
- [7].Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- [8].Kapoor R, van Hogerlinden M, Wallis K, Ghosh H, Nordstrom K, Vennstrom B, Vaidya VA. Unliganded thyroid hormone receptor {alpha}1 impairs adult hippocampal neurogenesis. FASEB J. 2010;24:4793–4805. doi: 10.1096/fj.10-161802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jones I, Srinivas M, Ng L, Forrest D. The thyroid hormone receptor beta gene: structure and functions in the brain and sensory systems. Thyroid. 2003;13:1057–1068. doi: 10.1089/105072503770867228. [DOI] [PubMed] [Google Scholar]
- [10].Bradley DJ, Towle HC, Young WS., III Spatial and temporal expression of a- and b-thyroid hormone receptor mRNAs, including the b2–subtype, in the developing mammalian nervous system. J Neurosci. 1992;12:2288–2302. doi: 10.1523/JNEUROSCI.12-06-02288.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hodin RA, Lazar MA, Wintman BI, Darling DS, Koenig RJ, Larsen PR, Moore DD, Chin WW. Identification of a thyroid hormone receptor that is pituitary-specific. Science. 1989;244:76–78. doi: 10.1126/science.2539642. [DOI] [PubMed] [Google Scholar]
- [12].Cook CB, Kakucska I, Lechan RM, Koenig RJ. Expression of thyroid hormone receptor beta2 in rat hypothalamus. Endocrinology. 1992;130:1077–1079. doi: 10.1210/endo.130.2.1733708. [DOI] [PubMed] [Google Scholar]
- [13].Lechan RM, Qi Y, Jackson IMD, Mahdavi V. Identification of thyroid hormone receptor isoforms in thyrotropin–releasing hormone neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1994;135:92–100. doi: 10.1210/endo.135.1.7516871. [DOI] [PubMed] [Google Scholar]
- [14].Forrest D, Hanebuth E, Smeyne RJ, Everds N, Stewart CL, Wehner JM, Curran T. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor beta: evidence for tissue-specific modulation of receptor function. EMBO J. 1996;12:3006–3015. [PMC free article] [PubMed] [Google Scholar]
- [15].Seki T, Arai Y. Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J Neurosci. 1993;13:2351–2358. doi: 10.1523/JNEUROSCI.13-06-02351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu M, Pleasure SJ, Collins AE, Noebels JL, Naya FJ, Tsai MJ, Lowenstein DH. Loss of BETA2/NeuroD leads to malformation of the dentate gyrus and epilepsy. Proc Natl Acad Sci U S A. 2000;97:865–870. doi: 10.1073/pnas.97.2.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lebel J, Dussault JH, Puymirat J. Overexpression of the beta 1 thyroid receptor induces differentiation in neuro-2a cells. Proc Natl Acad Sci U S A. 1993;91:2644–2648. doi: 10.1073/pnas.91.7.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Billon N, Tokumoto Y, Forrest D, Raff M. Role of thyroid hormone receptors in timing oligodendrocyte differentiation. Dev Biol. 2001;235:110–120. doi: 10.1006/dbio.2001.0293. [DOI] [PubMed] [Google Scholar]
- [21].Martínez-Iglesias O, Garcia-Silva S, Tenbaum SP, Regadera J, Larcher F, Paramio JM, Vennström B, Aranda A. Thyroid hormone receptor β1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Res. 2009;69:501–509. doi: 10.1158/0008-5472.CAN-08-2198. [DOI] [PubMed] [Google Scholar]
- [22].Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- [23].Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]