Abstract
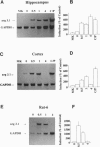
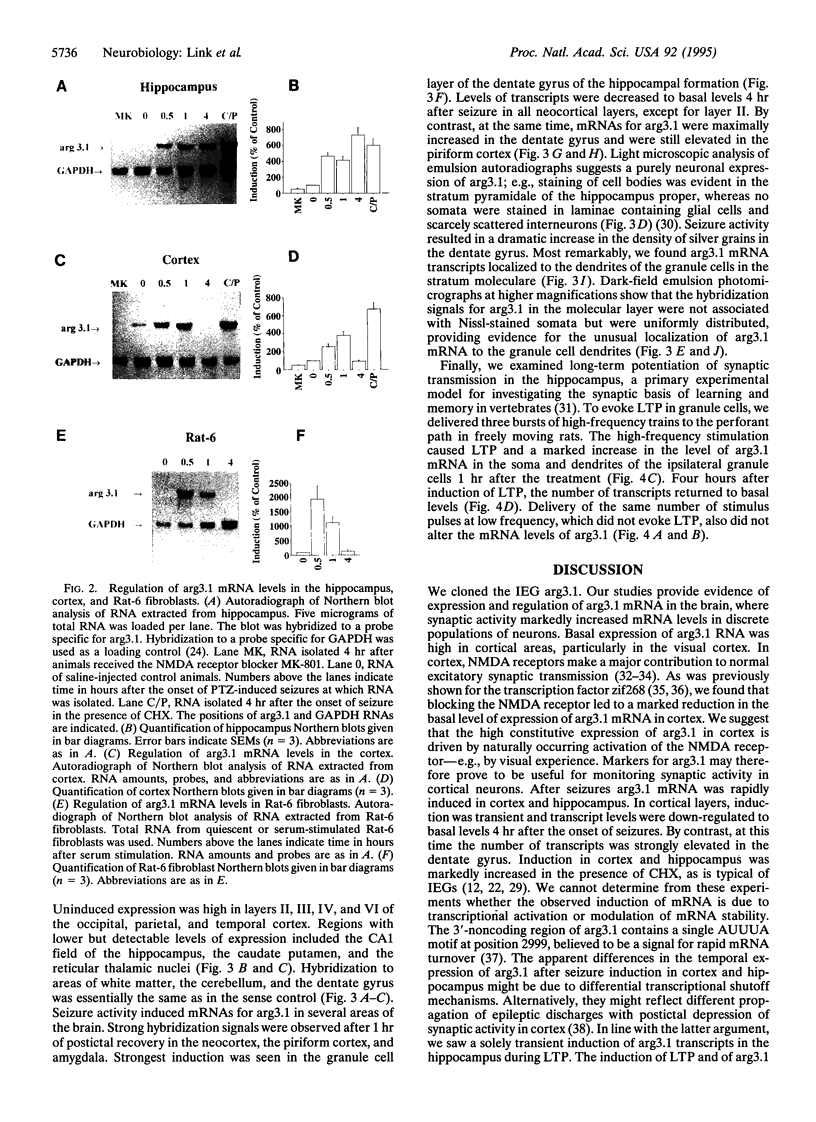
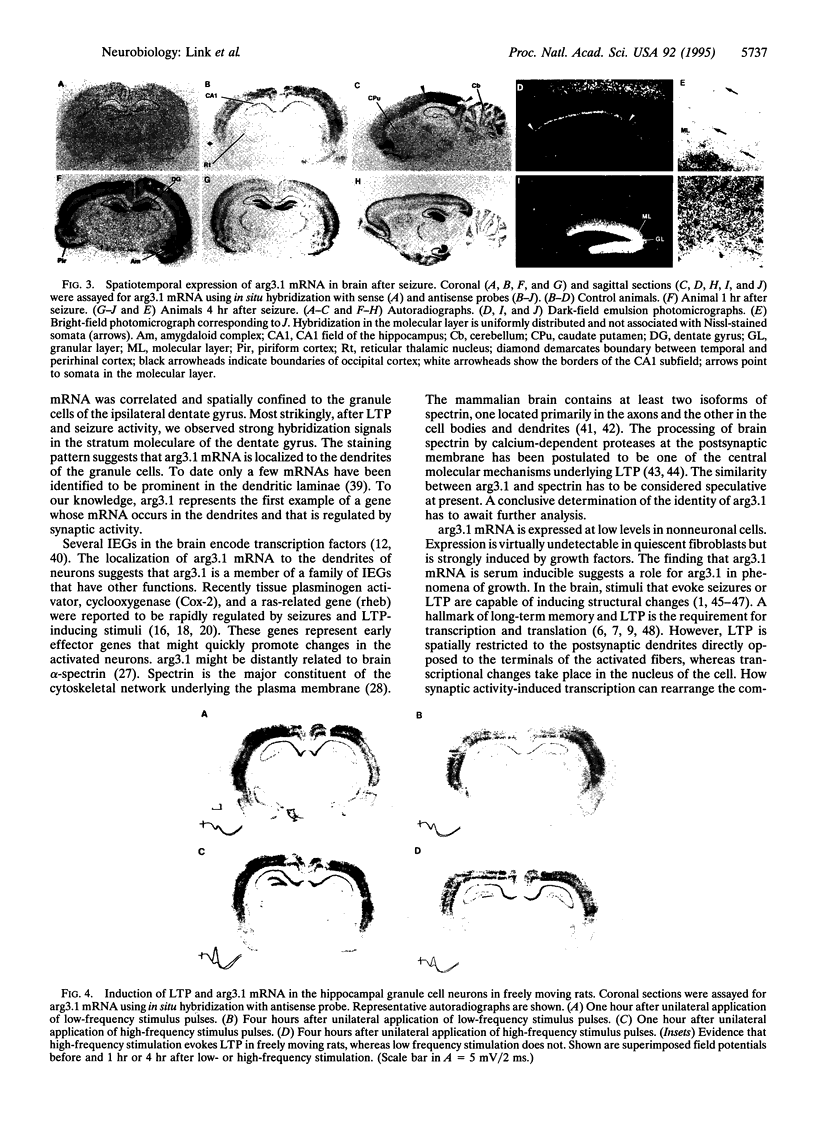
Trans-synaptic activation of gene expression is linked to long-term plastic adaptations in the nervous system. To examine the molecular program induced by synaptic activity, we have employed molecular cloning techniques to identify an immediate early gene that is rapidly induced in the brain. We here report the entire nucleotide sequence of the cDNA, which encodes an open reading frame of 396 amino acids. Within the hippocampus, constitutive expression was low. Basal levels of expression in the cortex were high but can be markedly reduced by blockade of N-methyl-D-aspartate receptors. By contrast, synaptic activity induced by convulsive seizures increased mRNA levels in neurons of the cortex and hippocampus. High-frequency stimulation of the perforant path resulted in long-term potentiation and a spatially confined dramatic increase in the level of mRNA in the granule cells of the ipsilateral dentate gyrus. Transcripts were localized to the soma and to the dendrites of the granule cells. The dendritic localization of the transcripts offers the potential for local synthesis of the protein at activated postsynaptic sites and may underlie synapse-specific modifications during long-term plastic events.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Sommer D., Macdonald-Bravo H., Burckhardt J., Perera J., Bravo R. Complexity of the early genetic response to growth factors in mouse fibroblasts. Mol Cell Biol. 1988 May;8(5):2140–2148. doi: 10.1128/mcb.8.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. H., Kandel E. R. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Represa A. Brief seizure episodes induce long-term potentiation and mossy fibre sprouting in the hippocampus. Trends Neurosci. 1990 Aug;13(8):312–318. doi: 10.1016/0166-2236(90)90135-w. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Tremblay E., Riche D., Ghilini G., Naquet R. Electrographic, clinical and pathological alterations following systemic administration of kainic acid, bicuculline or pentetrazole: metabolic mapping using the deoxyglucose method with special reference to the pathology of epilepsy. Neuroscience. 1981;6(7):1361–1391. doi: 10.1016/0306-4522(81)90193-7. [DOI] [PubMed] [Google Scholar]
- Berridge M. Second messenger dualism in neuromodulation and memory. 1986 Sep 25-Oct 1Nature. 323(6086):294–295. doi: 10.1038/323294a0. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Blumenfeld H., Goelet P., Kandel E. R. Inhibitor of protein synthesis blocks long-term behavioral sensitization in the isolated gill-withdrawal reflex of Aplysia. J Neurobiol. 1989 Jan;20(1):1–9. doi: 10.1002/neu.480200102. [DOI] [PubMed] [Google Scholar]
- Cavazos J. E., Golarai G., Sutula T. P. Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J Neurosci. 1991 Sep;11(9):2795–2803. doi: 10.1523/JNEUROSCI.11-09-02795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Memories of fos. Bioessays. 1987 Dec;7(6):255–258. doi: 10.1002/bies.950070606. [DOI] [PubMed] [Google Scholar]
- Davis H. P., Squire L. R. Protein synthesis and memory: a review. Psychol Bull. 1984 Nov;96(3):518–559. [PubMed] [Google Scholar]
- Feig S., Lipton P. Pairing the cholinergic agonist carbachol with patterned Schaffer collateral stimulation initiates protein synthesis in hippocampal CA1 pyramidal cell dendrites via a muscarinic, NMDA-dependent mechanism. J Neurosci. 1993 Mar;13(3):1010–1021. doi: 10.1523/JNEUROSCI.13-03-01010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K., Sato H., Daw N. The location and function of NMDA receptors in cat and kitten visual cortex. J Neurosci. 1989 Jul;9(7):2443–2454. doi: 10.1523/JNEUROSCI.09-07-02443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U., Krug M., Reymann K. G., Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988 Jun 14;452(1-2):57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Gass P., Herdegen T., Bravo R., Kiessling M. Induction and suppression of immediate early genes in specific rat brain regions by the non-competitive N-methyl-D-aspartate receptor antagonist MK-801. Neuroscience. 1993 Apr;53(3):749–758. doi: 10.1016/0306-4522(93)90621-l. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., Morrell F., deToledo-Morrell L. Increase in the number of axospinous synapses with segmented postsynaptic densities following hippocampal kindling. Brain Res. 1992 Jan 13;569(2):341–347. doi: 10.1016/0006-8993(92)90649-t. [DOI] [PubMed] [Google Scholar]
- Goelet P., Castellucci V. F., Schacher S., Kandel E. R. The long and the short of long-term memory--a molecular framework. 1986 Jul 31-Aug 6Nature. 322(6078):419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Hagihara K., Tsumoto T., Sato H., Hata Y. Actions of excitatory amino acid antagonists on geniculo-cortical transmission in the cat's visual cortex. Exp Brain Res. 1988;69(2):407–416. doi: 10.1007/BF00247586. [DOI] [PubMed] [Google Scholar]
- Kennedy T. E., Kuhl D., Barzilai A., Sweatt J. D., Kandel E. R. Long-term sensitization training in Aplysia leads to an increase in calreticulin, a major presynaptic calcium-binding protein. Neuron. 1992 Dec;9(6):1013–1024. doi: 10.1016/0896-6273(92)90062-i. [DOI] [PubMed] [Google Scholar]
- Konietzko U., Müller C. M. Astrocytic dye coupling in rat hippocampus: topography, developmental onset, and modulation by protein kinase C. Hippocampus. 1994 Jun;4(3):297–306. doi: 10.1002/hipo.450040313. [DOI] [PubMed] [Google Scholar]
- Krug M., Lössner B., Ott T. Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull. 1984 Jul;13(1):39–42. doi: 10.1016/0361-9230(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Kuhl D., Kennedy T. E., Barzilai A., Kandel E. R. Long-term sensitization training in Aplysia leads to an increase in the expression of BiP, the major protein chaperon of the ER. J Cell Biol. 1992 Dec;119(5):1069–1076. doi: 10.1083/jcb.119.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl D., de la Fuente J., Chaturvedi M., Parimoo S., Ryals J., Meyer F., Weissmann C. Reversible silencing of enhancers by sequences derived from the human IFN-alpha promoter. Cell. 1987 Sep 25;50(7):1057–1069. doi: 10.1016/0092-8674(87)90172-3. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Nelson W. J. Erythrocyte and brain forms of spectrin in cerebellum: distinct membrane-cytoskeletal domains in neurons. Science. 1983 Jun 17;220(4603):1295–1296. doi: 10.1126/science.6190228. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Woods C. Biogenesis of the red blood cell membrane-skeleton and the control of erythroid morphogenesis. Annu Rev Cell Biol. 1989;5:427–452. doi: 10.1146/annurev.cb.05.110189.002235. [DOI] [PubMed] [Google Scholar]
- Miller K. D., Chapman B., Stryker M. P. Visual responses in adult cat visual cortex depend on N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5183–5187. doi: 10.1073/pnas.86.13.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Inducible proto-oncogenes of the nervous system: their contribution to transcription factors and neuroplasticity. Prog Brain Res. 1990;86:287–294. doi: 10.1016/s0079-6123(08)63185-4. [DOI] [PubMed] [Google Scholar]
- Nedivi E., Hevroni D., Naot D., Israeli D., Citri Y. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993 Jun 24;363(6431):718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- Nguyen P. V., Abel T., Kandel E. R. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994 Aug 19;265(5175):1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Otani S., Marshall C. J., Tate W. P., Goddard G. V., Abraham W. C. Maintenance of long-term potentiation in rat dentate gyrus requires protein synthesis but not messenger RNA synthesis immediately post-tetanization. Neuroscience. 1989;28(3):519–526. doi: 10.1016/0306-4522(89)90001-8. [DOI] [PubMed] [Google Scholar]
- Qian Z., Gilbert M. E., Colicos M. A., Kandel E. R., Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993 Feb 4;361(6411):453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- Riederer B. M., Zagon I. S., Goodman S. R. Brain spectrin(240/235) and brain spectrin(240/235E): two distinct spectrin subtypes with different locations within mammalian neural cells. J Cell Biol. 1986 Jun;102(6):2088–2097. doi: 10.1083/jcb.102.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Sheng M., Greenberg M. E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990 Apr;4(4):477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- Siman R., Baudry M., Lynch G. Regulation of glutamate receptor binding by the cytoskeletal protein fodrin. Nature. 1985 Jan 17;313(5999):225–228. doi: 10.1038/313225a0. [DOI] [PubMed] [Google Scholar]
- Smirnova T., Laroche S., Errington M. L., Hicks A. A., Bliss T. V., Mallet J. Transsynaptic expression of a presynaptic glutamate receptor during hippocampal long-term potentiation. Science. 1993 Oct 15;262(5132):433–436. doi: 10.1126/science.8105538. [DOI] [PubMed] [Google Scholar]
- Steward O., Banker G. A. Getting the message from the gene to the synapse: sorting and intracellular transport of RNA in neurons. Trends Neurosci. 1992 May;15(5):180–186. doi: 10.1016/0166-2236(92)90170-d. [DOI] [PubMed] [Google Scholar]
- Teyler T. J., Discenna P. Long-term potentiation as a candidate mnemonic device. Brain Res. 1984 Mar;319(1):15–28. doi: 10.1016/0165-0173(84)90027-4. [DOI] [PubMed] [Google Scholar]
- Torre E. R., Steward O. Demonstration of local protein synthesis within dendrites using a new cell culture system that permits the isolation of living axons and dendrites from their cell bodies. J Neurosci. 1992 Mar;12(3):762–772. doi: 10.1523/JNEUROSCI.12-03-00762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw I. Q., Robinson T. E., Schallert T., De Ryck M., Ramirez V. D. Electrical activity of the hippocampus and neocortex in rats depleted of brain dopamine and norepinephrine: relations to behavior and effects of atropine. Exp Neurol. 1978 Dec;62(3):748–767. doi: 10.1016/0014-4886(78)90282-0. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Christy B. A., Nakabeppu Y., Bhat R. V., Cole A. J., Baraban J. M. Constitutive expression of zif268 in neocortex is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5106–5110. doi: 10.1073/pnas.88.12.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K., Andreasson K. I., Kaufmann W. E., Barnes C. A., Worley P. F. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993 Aug;11(2):371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Yamagata K., Sanders L. K., Kaufmann W. E., Yee W., Barnes C. A., Nathans D., Worley P. F. rheb, a growth factor- and synaptic activity-regulated gene, encodes a novel Ras-related protein. J Biol Chem. 1994 Jun 10;269(23):16333–16339. [PubMed] [Google Scholar]