Abstract
Background
Patients with sickle cell disease (SCD) can suffer frequent hospital admissions for painful vaso-occlusive crises. Hydroxyurea was approved by the FDA in 1998 to decrease the morbidity of SCD, but nationwide hospitalizations for SCD in the United States (US) since 1998 have not been evaluated. We hypothesized that the availability of hydroxyurea for SCD would be associated with a decrease in hospitalizations for SCD over time.
Objective
To assess trends in hospitalization and length-of-stay in hospital for SCD in the US, 1998 through 2008.
Research Design
Retrospective cohort study of SCD-related hospital discharges in the Nationwide Inpatient Sample of US hospital discharges.
Subjects
All discharges in the Nationwide Inpatient Sample associated with a principal diagnosis of SCD in Blacks, 1998 through 2008.
Measures
Trends in hospitalization rates and average length-of-stay in hospital for SCD.
Results
We found 216 (95% confidence interval (CI), 173.3–258.7) SCD-related hospitalizations per 100,000 US Blacks in 1998 and 178.4 (95% CI 144.2–212.5) in 2008, but no consistent yearly decrease, 1998 through 2008 (p=0.30). Conversely, the length-of-stay in hospital in 1998 was 5.38 days and in 2008 was 5.18 days, an absolute change of 0.2 days and a downward trend that was statistically significant.
Conclusion
Between 1998 and 2008, there was not a steady decrease in hospitalization rates for the population of SCD in the US. On the contrary, there was a decline in length-of-stay in hospital over this time. Hydroxyurea underuse is well documented. Efforts to increase hydroxyurea use may help to reduce hospitalization rates.
Keywords: sickle cell disease, hospitalizations, Nationwide Inpatient Sample, length-of-stay
Introduction
Sickle cell disease (SCD) is the most common inherited disease in Blacks in the United States. Nearly 100,000 people in the United States (US) have SCD (1), the vast majority are Black and an estimated 3% are Hispanic (2). The average life expectancy of homozygous SCD in the US is 42 years for males and 48 for females (3). The manifold complications of SCD include severe anemia, fatigue, renal failure, strokes and excruciating episodes of pain. The hallmark clinical manifestation of SCD is the vaso-occlusive crisis, which represents the most common cause of morbidity and leads to frequent hospital admissions. Hospitalization rates in patients with SCD in the Tennessee Medicaid program, for example, were 7–30 times greater than hospitalization rates of Blacks without SCD in this program (4). The aim of this study was to evaluate the improvement in hospitalizations for SCD in the US over a decade, 1998 through 2008.
SCD is a genetic hematologic disorder in which a single amino acid substitution at the sixth position of the beta-globin chain of hemoglobin (β glu→val) produces an abnormal sickle hemoglobin (Hb S). Under conditions of deoxygenation, Hb S polymerizes into rigid fibers (5) that deform the shape of erythrocytes to a ‘sickle’-shaped cell. Sickle cells cause occlusion of microvasculature and ischemia and infarction of distal tissues and organs. The vast majority of hospitalizations in SCD patients are for vaso-occlusive crises. Cellular events such as endothelial cell activation (6, 7), platelet activation (8) and heightened leukocyte adhesion (9, 10) contribute to in vivo vaso-occlusion in SCD, but polymerization of sickle hemoglobin (Hb S) is the principal event in the molecular pathogenesis of SCD. Fetal hemoglobin (Hb F) inhibits the polymerization of Hb S and ameliorates the effects of SCD (11–17). Hydroxyurea is an anti-metabolite that causes a dramatic increase in Hb F levels in phlebotomized non-human primates (18), and in children and adults with SCD (19, 20). Published in 1995, the Multicenter Study of Hydroxyurea (MSH) enrolled 299 adults (aged 18 years and older) with clinically severe SCD in the US over 21 months, with 152 patients randomized to hydroxyurea. Participants receiving hydroxyurea had a 46% decrease in pain crises per year and a 60% decrease in hospitalization rates (21). A subsequent evaluation of average length of stay (LOS) in hospitalized patients on the MSH trial showed responders to hydroxyurea spent an average of 2 fewer days in hospital over the 21-month study period than patients on placebo (22). Based on the results of the MSH trial, in February 1998 hydroxyurea was approved by the US Food and Drug Administration (FDA) for the treatment of SCD in adults. The 1998 approval of hydroxyurea for SCD is arguably the single most significant advancement made in the management of SCD. Hydroxyurea lessens the frequency of pain crises, acute chest syndrome, need for blood transfusions and hospitalizations. The impact of this on the US population of SCD patients has not been previously explored. We hypothesized that the availability of hydroxyurea for the treatment of SCD would be associated with a significant decrease in hospitalizations for SCD in the US.
The primary objectives of this study were to evaluate the changes in national hospitalization rates and LOS in hospital for SCD in the US. We examined in-patient hospitalizations for a primary diagnosis of SCD, 1998 through 2008.
Methods
Data Sources
Nationwide Inpatient Sample
Hospital discharge abstracts from 1998 to 2008 were obtained from the Nationwide Inpatient Sample (NIS) of the Healthcare Cost Utilization Project (HCUP) maintained by the federal Agency for Healthcare Research and Quality (AHRQ). These data cover the period since the initial FDA approval of hydroxyurea in 1998. The NIS is a stratified probability sample of hospitals selected by region, size and teaching status. The NIS is the largest all-payer inpatient database in the US with data from approximately 8 million hospital discharges from 1,056 hospital located in 42 states during 2008 (23). The large sample of discharges in NIS enables analyses of rare conditions such as SCD. The hospitals included in the NIS can vary from year to year and race data is incomplete in some years. Because the NIS database includes publicly available de-identified data, our study was deemed exempt from review by the institutional review board of the Partners Healthcare System.
US Census Bureau
To correct for population growth in the US over the years of the study, we examined hospitalization rates each year. The reference population was based on annual population estimates of the US Black population provided by the US Census Bureau.
The estimates for 1998 and 1999 were extracted from files of the Monthly Postcensal Resident Population, by single year of age, sex, race, and Hispanic origin July 1998 and July 1999. Equivalent information for years 2000 through 2008 was extracted from files of Annual Estimates of the Black or African American Alone Resident Population by Sex and Age for the US: April 1, 2000 to July 1, 2009.
Study Cohort
The study population included discharges with a principal diagnosis of SCD and patients’ race coded as Black in the NIS database from 1998 to 2008. Discharges were identified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes of SCD (282.41, 282.42, 282.6, 282.60 – 282.64, 282.68 and 282.69), as has been used previously (24). We limited our analyses to Blacks in the NIS to facilitate the calculation of hospitalization rates using population estimates as the denominator, recognizing that over 95% of discharges for SCD in the NIS were among Blacks (3% were among Hispanics and 2% among others). Because the US Census Bureau collection of data on ‘Hispanics’ was inconsistent between Census 1990 and Census 2000 (25), we did not include hospitalizations of Hispanic patients in this study. Discharges associated with sickle cell trait (282.5) or thalassemia (282.49) were excluded from this study.
Outcome Measures
The primary outcomes of the study were the hospitalization rates associated with a principal diagnosis of SCD from 1998 to 2008. The secondary outcome was LOS for patients with a principal diagnosis of SCD over the same time period.
Statistical Analysis
The patient population included Black patients with SCD. Weighted least squares regression was used to model hospitalization rates as a function of time (26), within subgroups of age. Poisson regression was used to model hospital trends in LOS over time, adjusting for age, gender, household income and insurance status.
Our analysis was restricted to Blacks, but 11 of 42 states in the NIS did not report race. Blacks comprise approximately 96% of SCD patients, but we could not determine the proportion of patients in these 11 states who were Black. Thus, in order to include patients from these 11 states in the analyses, we multiply imputed patients' race. We fitted a logistic regression model to data from the states with race observed whose outcome was race (Black, non-Black) and whose predictors were variables available in all states, including the 11 states where race was missing, including patient characteristics (e.g., age, gender, median household income by zip code, hospital length of stay, etc.), hospital characteristics (e.g., region, number of beds, etc.), and the logit of the fraction of Blacks in the state. We imputed missing race 10 times. After imputing race, analyses of each imputed dataset were restricted to Blacks. The 10 imputation results were combined to get a single estimate and 95% confidence interval (CI). Analyses excluding the 11 states with incomplete race data yielded similar results (see supplemental digital content material). SAS (Proc Logisitic and Proc MIanalyze) version 9.2 (SAS Institute Inc., Cary, NC) was used for multiple imputations.
Hospitalization rates and LOS analyses were performed using SAS-callable SUDAAN version 10.0.1 (Research Triangle Institute, Research Triangle Park, NC) to account for the complex design of the NIS including clustering by hospital. To ensure that changes in state inclusion in the NIS did not significantly affect the percentage of Blacks represented each year in the NIS, we confirmed that the Black population in the NIS database each year of the study changed by less than 2 percentage points (13%–14.6%). We report two-sided p-values, 95% confidence intervals and significance tests at the 0.05 level.
Results
In 1998 there were 14,030 unweighted discharges associated with a principal diagnosis of SCD in Blacks in the NIS database, representing 74,344 weighted (actual) discharges in the US. In 2008, there were 14,384 unweighted discharges corresponding to a nationally weighted estimate of 69,845 hospital discharges. All results below, including tables and figures, are weighted (actual) values. The characteristics of the patients in 1998 and 2008 are shown in Table 1.
Table 1.
Black Patients Hospitalized with a Principal Diagnosis of Sickle Cell Disease in the United States, 1998 and 2008.
| 1998 | 2008 | |||||
|---|---|---|---|---|---|---|
| Patients, total No. |
Number | Percent | Number | Percent | p values | |
| 74,344 | 69,845 | |||||
| Gender | 0.66 | |||||
| Male | 35,267 | 47 | 33,547 | 48 | ||
| Female | 39,077 | 53 | 36,298 | 52 | ||
| Age, y | ||||||
| Adults (≥18yrs) | 54,490 | 73 | 55,042 | 79 | 0.11 | |
| Children (<18yrs) | 19,850 | 27 | 14,803 | 21 | ||
| < 5 | 4,760 | 6 | 3,493 | 5 | <0.001 | |
| 5 – 13 | 9,537 | 13 | 6,418 | 9 | ||
| 14 – 17 | 5,553 | 8 | 4,891 | 7 | ||
| 18 – 29 | 26,620 | 36 | 27,586 | 40 | ||
| 30 – 44 | 22,527 | 30 | 19,567 | 28 | ||
| 44 – 64 | 5,203 | 7 | 7,631 | 11 | ||
| 65 + | 139 | 0 | 259 | 0 | ||
| Primary expected payer | 0.05 | |||||
| Medicare | 14,506 | 20 | 16,757 | 24 | ||
| Medicaid | 38,649 | 52 | 35,790 | 51 | ||
| Private/HMO | 16,366 | 22 | 12,578 | 18 | ||
| Self-pay | 2,824 | 4 | 2,954 | 4 | ||
| No charge/other | 1,974 | 3 | 1,644 | 2 | ||
| Hospital Region | 0.55 | |||||
| Northeast | 12,956 | 17 | 15,079 | 22 | ||
| Midwest | 14,374 | 19 | 10,333 | 15 | ||
| South | 41,941 | 56 | 37,855 | 54 | ||
| West | 5,074 | 7 | 6,584 | 9 | ||
| Hospital teaching status | 0.55 | |||||
| Non-Teaching | 21,875 | 30 | 22,641 | 32 | ||
| Teaching | 52,391 | 71 | 47,209 | 68 | ||
Hospitalization Rates
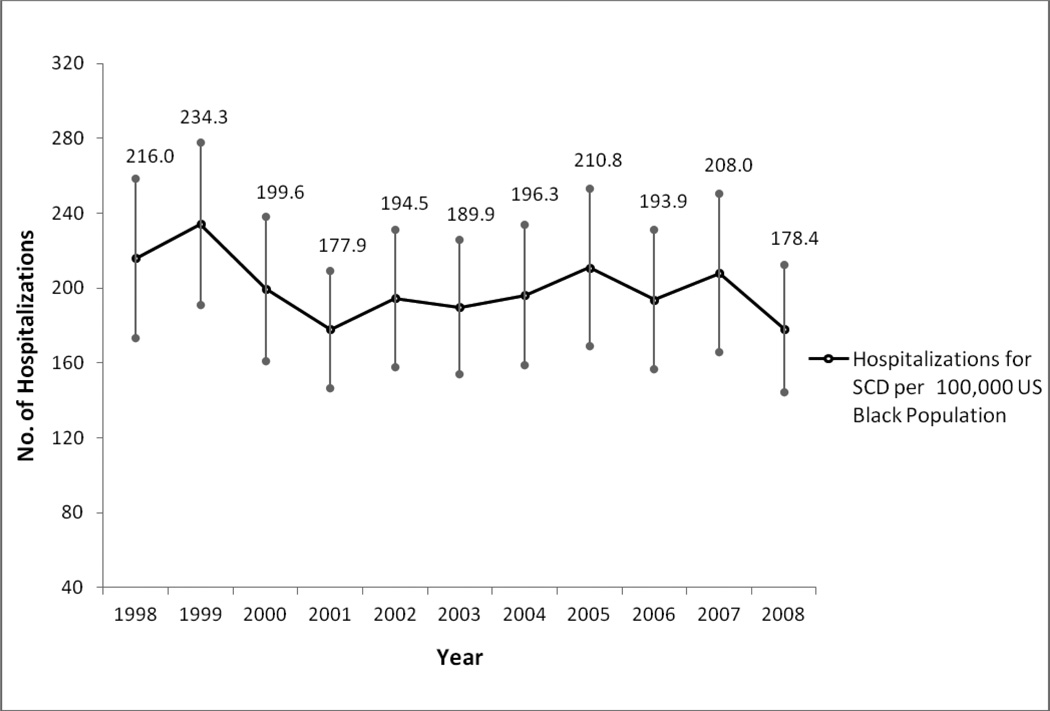
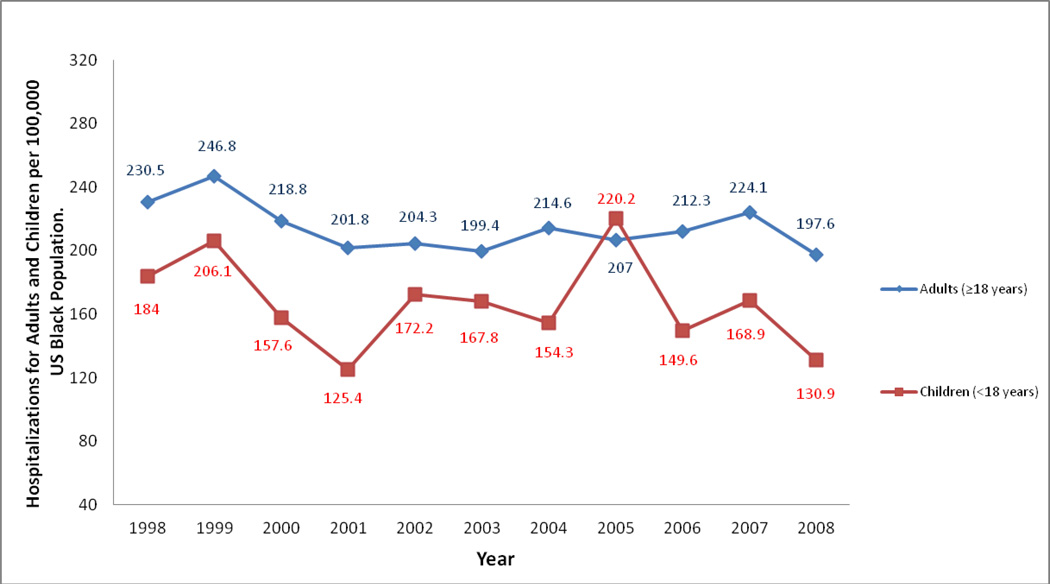
The hospitalization rate for all Black patients with a principal diagnosis of SCD in the US in 1998 was 216.0 (95% CI, 173.3 – 258.7) per 100,000 Blacks in the US population, and in 2008 this rate was 178.4 (95% CI 144.2 – 212.5) but with no statistically significant trend from 1998 to 2008 (p=0.30), see figure 1. The hospitalization rate in adults (≥18 years) in 1998 was 230.5 (95% CI 187.8, 273.2) and in 2008 was 197.5 (95% CI 163.5, 231.6) again with no statistical trend through this period (p=0.30). In children (<18 years) the hospitalization rate was 184.2 (95% CI 119.9, 248.5) in 1998 and 131.0 (95% CI 80.1, 181.8) in 2008 (p=0.34). Though these rates seem strikingly different, there was no consistent downward trend from 1998 through 2008 (Figure 2). Within previously defined age ranges of patients with SCD (24), hospitalization rates varied minimally through the years of study with no statistically significant trends from 1998 to 2008 (See details in Table 2).
Figure 1.
Trends in Hospitalization Rates for Sickle Cell Disease per 100,000 Black Population in the United States, 1998 to 2008. (Trend, p = 0.30)
Figure 2.
Trends in Hospitalization Rates for Sickle Cell Disease in Adults and Children per 100,000 Black Population in the United States, 1998 to 2008. (trend in adults, p = 0.30; trend in children, p = 0.34)
Table 2.
Rates of Hospitalization and Average Length of Stay (LOS) for Black Patients with Sickle Cell Disease in the United States, 1998 through 2008.
| Age, y | Hospitalization Rates per 100,000 US Black population | Average Length of Stay (LOS) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | P value for Trend in Hospital Rates |
LOS, 1998 (days) |
LOS, 2008 (days) |
Absolute change (days) |
P value for Trend in LOS |
|
| Total | 216 | 234.3 | 199.7 | 177.9 | 194.5 | 189.9 | 196.3 | 210.8 | 193.9 | 208 | 178.4 | 0.3 | 5.4 | 5.2 | −0.2 | < 0.001 |
| <18 | 184.2 | 206.3 | 157.5 | 125.4 | 172.5 | 168 | 199.5 | 220.1 | 149.9 | 168.9 | 131 | 0.34 | 4.1 | 3.8 | −0.3 | 0.01 |
| ≥18 | 230.5 | 247 | 219 | 201.7 | 204.3 | 199.5 | 154.3 | 206.9 | 212.4 | 224.2 | 197.5 | 0.3 | 5.8 | 5.6 | −0.2 | 0.29 |
| <5 | 168.4 | 177.6 | 123.9 | 88.6 | 133.5 | 128.1 | 126.5 | 171 | 108.3 | 141.7 | 110 | 0.43 | 3.4 | 3 | −0.4 | < 0.001 |
| 5–13 | 171 | 194 | 155.2 | 120.2 | 170.1 | 155.2 | 137.6 | 210.3 | 143.1 | 153.7 | 118.8 | 0.17 | 4.1 | 3.8 | −0.3 | 0.007 |
| 14–17 | 234 | 268.5 | 203.3 | 181.8 | 223.7 | 242.4 | 221 | 294 | 208.6 | 229 | 179.4 | 0.6 | 4.7 | 4.4 | −0.3 | 0.07 |
| 18–29 | 411 | 433.1 | 370.8 | 346.4 | 352.6 | 344.1 | 379.6 | 387.1 | 390.9 | 411.4 | 362 | 0.77 | 5.8 | 5.6 | −0.2 | 0.3 |
| 30–44 | 271.6 | 309.7 | 288 | 255.5 | 257.5 | 256.2 | 271.3 | 246.2 | 258.2 | 273.8 | 240.1 | 0.21 | 5.8 | 5.7 | −0.1 | 0.97 |
| 45–64 | 86.4 | 79.8 | 72.7 | 77.8 | 83.1 | 80.9 | 84.6 | 82.6 | 87.5 | 96.5 | 86.8 | 0.21 | 5.9 | 5.5 | −0.4 | 0.008 |
| 65+ | 4.9 | 6.9 | 6.1 | 7.7 | 7.6 | 5.5 | 9 | 9.1 | 9.2 | 4.9 | 7.8 | 0.39 | 5.3 | 5.3 | 0 | 0.83 |
Average Length of Stay in Hospital (LOS)
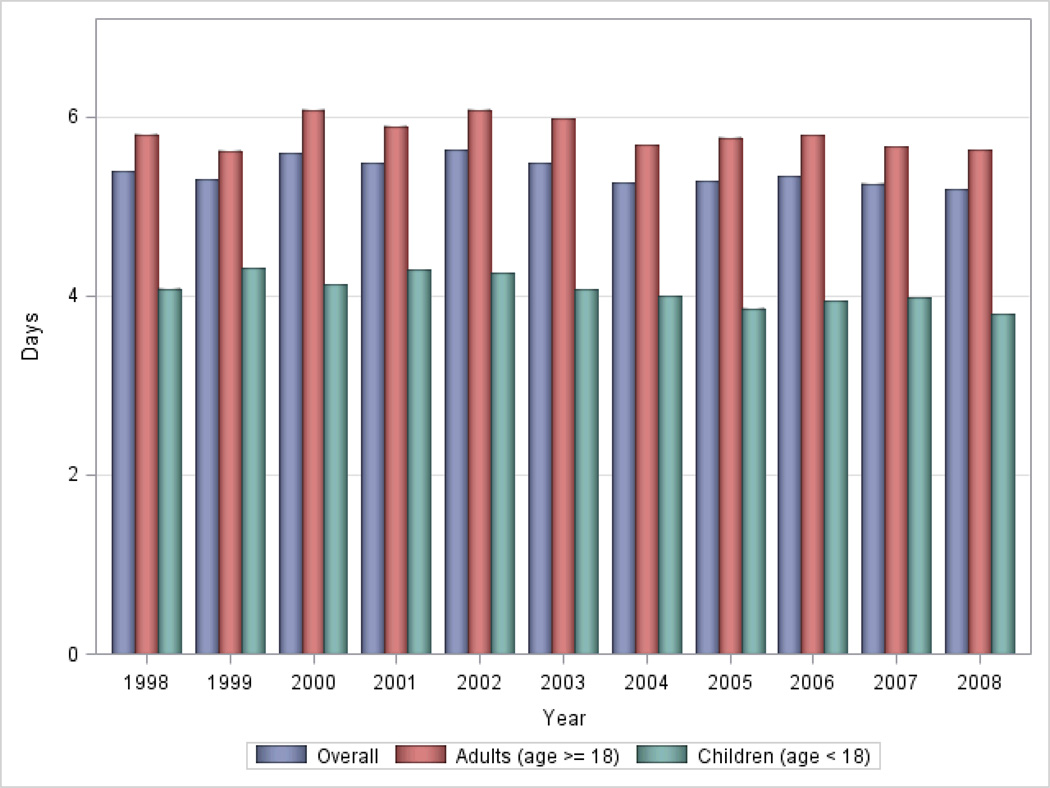
The LOS of patients with a principal diagnosis of SCD in 1998 and 2008 were 5.38 days (95% CI 5.07–5.70) and 5.18 days (95% CI 5.0–5.41) respectively, an absolute difference of 0.20 days that was statistically significant (p<0.001). Adjusting for age, gender, median household income and insurance status by Poisson regression, the LOS in all children as a group (age <18 years), and adults aged 45–64 years old showed a decreasing linear trend from 1998 to 2008 (p=0.01 and p<0.01 respectively). See Figure 3; details in Table 2.
Figure 3.
Trends in Hospital Length of Stay (LOS) for Sickle Cell Disease in the United States, 1998 to 2008.
Discussion
Our nationally representative study found inconsistent changes in hospitalization rates for patients with a primary diagnosis of SCD in the US from 1998 to 2008. Though the hospitalization rates in 1998 (216.0) were higher than in 2008 (178.4), there was no steady decline in hospitalization rates through the intervening years. The same was true of hospitalization rates in adults and children examined separately. Conversely, there was a significant decrease in LOS over time. LOS in all patients with SCD decreased by 0.2 days in 2008 compared to 1998, and in children there was a significant decreasing trend through the years, from 4.1 days in 1998 to 3.8 days in 2008.
Since the MSH trial (described above), hospitalizations and LOS have been assessed in other clinical trials in SCD (27–30). They have all shown a decrease in hospitalizations and/or LOS for patients on hydroxyurea, establishing the efficacy of hydroxyurea in closely monitored clinical studies. Also, children on hydroxyurea routinely managed in a tertiary SCD center had decreased hospitalizations compared to patients not on hydroxyurea (31). In contrast, our study of hospitalizations of SCD patients in the US showed no consistent reduction in hospitalization rates over 11 years. Likewise, an earlier retrospective study of hospitalization rates associated with SCD in Maryland between 1995 and 2003 documented an increase in hospitalizations during this time period (32) and a study in Tennessee, showed no trend in hospitalizations for SCD from 1995 to 2002 (4). Thus, in the era of hydroxyurea use in SCD, there has been a reduction in LOS but not a consistent decline in hospitalization rates over time, when viewed at the population level.
Recent studies have identified barriers at the patient, provider and systems levels that have adversely impacted hydroxyurea use in SCD. Patients with SCD in the US tend to be of low socioeconomic status and have problems with continuity of health insurance (33), causing difficulty in keeping clinic appointments and obtaining prescribed medications (34). Patient compliance with hydroxyurea can be excellent in controlled SCD studies (30). However, in retrospective observational studies, patient adherence to hydroxyurea was only 35–49% (35). Similarly, 70% of patients with SCD at a university hospital who were appropriate candidates for hydroxyurea were not on the medication (32). This would imply that the full benefit of hydroxyurea use is not being gained by all SCD patients.
Community-based hematologist/oncologists have expressed anxiety about hydroxyurea’s carcinogenic potential, doubts about its effectiveness, perceptions of patient reservations about its adverse effects, and concern about lack of concurrent contraceptive use (36). These concerns about hydroxyurea are in contrast to evidence in studies (21, 29, 37, 38). Classification of hydroxyurea as a chemotherapeutic agent and the need for diligent laboratory monitoring may also make physicians reluctant to administer the drug. Furthermore, physicians who are not well-informed and highly motivated may not escalate patients to the maximum tolerated dose of hydroxyurea, as recommended to induce HbF. Physicians may therefore prematurely conclude that hydroxyurea offered no benefit to a particular patient and discontinue its use.
Hydroxyurea was proven in 1995 to beneficial in SCD. It is perhaps the most significant advancement in the care of individuals with SCD. No change in hospitalization rates in 1998 through 2008 does not equate to no change from a pre-hydroxyurea time. It is possible that there was a decline in hospitalizations from 1995 to 1998 due to hydroxyurea use, though a previous smaller study does not suggest this (32). With the evidence of current hydroxyurea underuse (34, 36), greater impact on hospitalization rates is likely with improved hydroxyurea use.
Increases in hydroxyurea therapy in SCD could reduce hospitalizations and other metrics of morbidity in SCD patients in the US, at the population level. Reduced hospitalizations should also result in decreased healthcare costs. With targeted intervention, significant improvements in medication use are possible (39). Efforts should focus on improving knowledge and awareness of patients and families regarding the evidence-based benefits of hydroxyurea use in SCD, and on educating physicians and other providers in the use of hydroxyurea to manage SCD. Clear guidelines on the indications, dosing, contra-indications and complications of hydroxyurea for specific age groups should also be disseminated to providers. Potential differences in the adoption of effective therapies according to level of specialist involvement seen in other disease states (40, 41), are likely also at play in SCD and can be improved by enhanced collaboration and communication between SCD specialists and primary care providers. This would offset the effects of the limited supply of hematologists in practice (42).
New agents such as histone deacetylase inhibitors, thalidomide derivatives, adenosine receptor agonists and P-selectin inhibitors are currently in Phase I and II trials of SCD. These new agents will benefit from efforts aimed at tackling the current barriers to broader use of hydroxyurea in SCD. Since 2009, the NIH has led an effort to develop a set of evidence-based guidelines for the management of SCD (43). The NIH is also aimed at identifying areas where additional research is needed to guide practice.
Our study had limitations. The NIS database is the largest all-payer inpatient database in the US, but variation in sampled hospitals from year to year contributed to wide confidence intervals around the hospitalization rates. Because race information was incomplete in some states and years, imputations of missing race data were performed and could introduce some statistical imprecision in our results. We analyzed data on Blacks with SCD (>95% of SCD hospitalizations) and may not be able to extrapolate our findings to Hispanic SCD patients in the US. In the absence of a comparison group, it is not possible to attribute the decrease in LOS specifically to the availability of hydroxyurea. Increased use of home health services, changes in hospital and physician reimbursement methods may have favorably influenced LOS for all diseases over the time of the study. Due to the expansion of universal newborn screening and implementation of penicillin prophylaxis, there was an age migration in SCD from pediatrics to adults from 1979 to 2006 (2). However by 1999 and in later years the age migration was minor (2) and was not likely to have affected the population at risk for hospitalizations in the time span of this study. This was a retrospective database study, and the accuracy of diagnosis coding in NIS is unknown, therefore we evaluated SCD hospitalizations making no distinction between hospitalizations for pain crises from other complications of SCD. However only principal diagnoses of SCD were included in this study and SCD patients admitted for unrelated ailments were not included. Finally, we chose to study an 11 year timeframe. Evaluating a longer period of time could reveal more subtle trends that were not detected over our 11 years of evaluation. Despite these limitations, it is unlikely that a substantial reduction in SCD hospitalizations 1998 through 2008 was missed by our analyses.
Conclusion
Our results reflect hospitalizations for a decade after hydroxyurea became standard care. There was a consistent decreasing trend in LOS but no consistent decrease in hospitalization rates. Undoubtedly hydroxyurea was used to varying degrees before 1998, therefore we do not conclude that hydroxyurea has had no impact on hospitalization rates. We however show no steady decrease from 1998 to 2008 and more widespread hydroxyurea use is likely to decrease these hospitalization rates. Studies are needed to evaluate and improve the implementation of hydroxyurea and other evidence-based management approaches in SCD. Specifically, we need to identify ways of broadening access to care that includes access to the appropriate use of hydroxyurea in patients with SCD.
Supplementary Material
Acknowledgments
Funding/Support: This project was supported by discretionary funds of a K12 Harvard Blood Scholars award, Doris Duke Charitable Foundation, Tripplett family donation to SCD Research and Division of Hematology, Brigham and Women’s Hospital, Boston. Dr. Ayanian is supported by the Health Disparities Research Program of Harvard Catalyst/The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers).
References
- 1.Brousseau DC, Panepinto JA, Nimmer M, et al. The number of people with sickle-cell disease in the United States: national and state estimates. American journal of hematology. 2010;85:77–78. doi: 10.1002/ajh.21570. [DOI] [PubMed] [Google Scholar]
- 2.Hassell KL. Population estimates of sickle cell disease in the U.S. American journal of preventive medicine. 2010;38:S512–S521. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. The New England journal of medicine. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 4.Shankar SM, Arbogast PG, Mitchel E, et al. Medical care utilization and mortality in sickle cell disease: a population-based study. American journal of hematology. 2005;80:262–270. doi: 10.1002/ajh.20485. [DOI] [PubMed] [Google Scholar]
- 5.Mozzarelli A, Hofrichter J, Eaton WA. Delay time of hemoglobin S polymerization prevents most cells from sickling in vivo. Science. 1987;237:500–506. doi: 10.1126/science.3603036. [DOI] [PubMed] [Google Scholar]
- 6.Solovey A, Gui L, Key NS, et al. Tissue factor expression by endothelial cells in sickle cell anemia. The Journal of clinical investigation. 1998;101:1899–1904. doi: 10.1172/JCI1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurantsin-Mills J, Ofosu FA, Safa TK, et al. Plasma factor VII and thrombin-antithrombin III levels indicate increased tissue factor activity in sickle cell patients. British journal of haematology. 1992;81:539–544. doi: 10.1111/j.1365-2141.1992.tb02989.x. [DOI] [PubMed] [Google Scholar]
- 8.Villagra J, Shiva S, Hunter LA, et al. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110:2166–2172. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finnegan EM, Turhan A, Golan DE, et al. Adherent leukocytes capture sickle erythrocytes in an in vitro flow model of vaso-occlusion. American journal of hematology. 2007;82:266–275. doi: 10.1002/ajh.20819. [DOI] [PubMed] [Google Scholar]
- 10.Okpala I. Leukocyte adhesion and the pathophysiology of sickle cell disease. Current opinion in hematology. 2006;13:40–44. doi: 10.1097/01.moh.0000190108.62414.06. [DOI] [PubMed] [Google Scholar]
- 11.Watson J. The significance of the paucity of sickle cells in newborn Negro infants. The American journal of the medical sciences. 1948;215:419–423. doi: 10.1097/00000441-194804000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Herman EC, Jr, Conley CL. Hereditary persistence of fetal hemoglobin. A family study. The American journal of medicine. 1960;29:9–17. doi: 10.1016/0002-9343(60)90003-6. [DOI] [PubMed] [Google Scholar]
- 13.Perrine RP, Pembrey ME, John P, et al. Natural history of sickle cell anemia in Saudi Arabs. A study of 270 subjects. Annals of internal medicine. 1978;88:1–6. doi: 10.7326/0003-4819-88-1-1. [DOI] [PubMed] [Google Scholar]
- 14.Hutz MH, Salzano FM, Adams J. Hb F levels, longevity of homozygotes and clinical course of sickle cell anemia in Brazil. American journal of medical genetics. 1983;14:669–676. doi: 10.1002/ajmg.1320140410. [DOI] [PubMed] [Google Scholar]
- 15.Kutlar A, Hattori Y, Bakioglu I, et al. Hematological observations on Arabian SS patients with a homozygosity or heterozygosity for a beta S chromosome with haplotype #31. Hemoglobin. 1985;9:545–557. doi: 10.3109/03630268508997037. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg MA, Husson MA, Bunn HF. Participation of hemoglobins A and F in polymerization of sickle hemoglobin. The Journal of biological chemistry. 1977;252:3414–3421. [PubMed] [Google Scholar]
- 17.Nagel RL, Bookchin RM, Johnson J, et al. Structural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:670–672. doi: 10.1073/pnas.76.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Letvin NL, Linch DC, Beardsley GP, et al. Augmentation of fetal-hemoglobin production in anemic monkeys by hydroxyurea. The New England journal of medicine. 1984;310:869–873. doi: 10.1056/NEJM198404053101401. [DOI] [PubMed] [Google Scholar]
- 19.Platt OS, Orkin SH, Dover G, et al. Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. The Journal of clinical investigation. 1984;74:652–656. doi: 10.1172/JCI111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veith R, Galanello R, Papayannopoulou T, et al. Stimulation of F-cell production in patients with sickle-cell anemia treated with cytarabine or hydroxyurea. The New England journal of medicine. 1985;313:1571–1575. doi: 10.1056/NEJM198512193132503. [DOI] [PubMed] [Google Scholar]
- 21.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. The New England journal of medicine. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 22.Ballas SK, Bauserman RL, McCarthy WF, et al. Hydroxyurea and Acute Painful Crises in Sickle Cell Anemia: Effects on Hospital Length of Stay and Opioid Utilization During Hospitalization, Outpatient Acute Care Contacts, and at Home. J Pain Symptom Manage. 2010 doi: 10.1016/j.jpainsymman.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NIS. HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). 1998–2008. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Available at: www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed] [Google Scholar]
- 24.Steiner CA, Miller JL. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): 2006. Sickle Cell Disease Patients in U.S. Hospitals, 2004: Statistical Brief #21. [Google Scholar]
- 25.Cresce AR, Schmidley AD, Ramirez RR. Identification of Hispanic Ethnicity in Census 2000: Analysis of Data Quality for the Question on Hispanic Origin. In: Commerce USDo, editor. Washington, DC: United States Census Bureau; [Google Scholar]
- 26.Koch G, Stokes M. Annotated Computer Applications of Weighted Least Squares Methods of Illustrative Analyses of Examples Involving Health Survey Data. In: Statistics USNCfH, editor. 1979. [Google Scholar]
- 27.Ferster A, Tahriri P, Vermylen C, et al. Five years of experience with hydroxyurea in children and young adults with sickle cell disease. Blood. 2001;97:3628–3632. doi: 10.1182/blood.v97.11.3628. [DOI] [PubMed] [Google Scholar]
- 28.Braga LB, Ferreira AC, Guimaraes M, et al. Clinical and laboratory effects of hydroxyurea in children and adolescents with sickle cell anemia: a Portuguese hospital study. Hemoglobin. 2005;29:171–180. doi: 10.1081/hem-200066299. [DOI] [PubMed] [Google Scholar]
- 29.Voskaridou E, Christoulas D, Bilalis A, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS) Blood. 2010;115:2354–2363. doi: 10.1182/blood-2009-05-221333. [DOI] [PubMed] [Google Scholar]
- 30.Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011;377:1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nottage KA, Hankins JS, Smeltzer M, et al. Hydroxyurea use and hospitalization trends in a comprehensive pediatric sickle cell program. PloS one. 2013;8:e72077. doi: 10.1371/journal.pone.0072077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanzkron S, Haywood C, Jr, Segal JB, et al. Hospitalization rates and costs of care of patients with sickle-cell anemia in the state of Maryland in the era of hydroxyurea. American journal of hematology. 2006;81:927–932. doi: 10.1002/ajh.20703. [DOI] [PubMed] [Google Scholar]
- 33.Raphael JL, Dietrich CL, Whitmire D, et al. Healthcare utilization and expenditures for low income children with sickle cell disease. Pediatric blood & cancer. 2009;52:263–267. doi: 10.1002/pbc.21781. [DOI] [PubMed] [Google Scholar]
- 34.Thornburg CD, Calatroni A, Telen M, et al. Adherence to hydroxyurea therapy in children with sickle cell anemia. The Journal of pediatrics. 2010;156:415–419. doi: 10.1016/j.jpeds.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Candrilli SD, O'Brien SH, Ware RE, et al. Hydroxyurea adherence and associated outcomes among Medicaid enrollees with sickle cell disease. American journal of hematology. 2011;86:273–277. doi: 10.1002/ajh.21968. [DOI] [PubMed] [Google Scholar]
- 36.Zumberg MS, Reddy S, Boyette RL, et al. Hydroxyurea therapy for sickle cell disease in community-based practices: a survey of Florida and North Carolina hematologists/oncologists. American journal of hematology. 2005;79:107–113. doi: 10.1002/ajh.20353. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. American journal of hematology. 2010;85:403–408. doi: 10.1002/ajh.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballas SK, McCarthy WF, Guo N, et al. Exposure to hydroxyurea and pregnancy outcomes in patients with sickle cell anemia. Journal of the National Medical Association. 2009;101:1046–1051. doi: 10.1016/s0027-9684(15)31072-5. [DOI] [PubMed] [Google Scholar]
- 39.Stafford RS, Monti V, Furberg CD, et al. Long-term and short-term changes in antihypertensive prescribing by office-based physicians in the United States. Hypertension. 2006;48:213–218. doi: 10.1161/01.HYP.0000229653.73128.b6. [DOI] [PubMed] [Google Scholar]
- 40.Ayanian JZ, Hauptman PJ, Guadagnoli E, et al. Knowledge and practices of generalist and specialist physicians regarding drug therapy for acute myocardial infarction. The New England journal of medicine. 1994;331:1136–1142. doi: 10.1056/NEJM199410273311707. [DOI] [PubMed] [Google Scholar]
- 41.Hirth RA, Fendrick AM, Chernew ME. Specialist and generalist physicians' adoption of antibiotic therapy to eradicate Helicobacter pylori infection. Medical care. 1996;34:1199–1204. doi: 10.1097/00005650-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Todd RF, 3rd, Gitlin SD, Burns LJ, et al. Subspeciality training in hematology and oncology, 2003: results of a survey of training program directors conducted by the American Society of Hematology. Blood. 2004;103:4383–4388. doi: 10.1182/blood-2003-11-3986. [DOI] [PubMed] [Google Scholar]
- 43.What the NHLBI Has Done Up To Now. 2012 Available at: www.nhlbi.nih.gov/guidelines/scd/progress.htm. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.