Abstract
Background
A well accepted definition of frailty includes measurements of physical performance, which may limit its clinical utility.
Study Design
In a cross-sectional study, we compared prevalence and patient characteristics based on a frailty definition that uses self-reported function to the classic performance-based definition and developed a modified self-report–based definition.
Setting & Participants
Prevalent adult patients receiving hemodialysis in 14 centers around San Francisco and Atlanta in 2009–2011.
Index Tests
Self-report–based frailty definition in which a score <75 of the Physical Function (PF) scale of the 36-Item Short Form Health Survey (SF-36) was substituted for gait speed and grip strength in the classic definition; modified self-report definition with optimized PF cut points derived in a development (one-half) cohort and validated in the other half.
Reference Test
Performance-based frailty defined as 3 of the following: weight loss, weakness, exhaustion, low physical activity, and slow gait speed.
Results
387 (53%) were frail based on self-reported function, of whom 209 (29% of the cohort) met the performance-based definition. Only 23 (3%) met the performance-based definition of frailty only. The self-report definition had 90% sensitivity, 64% specificity, 54% positive predictive value (PPV), 93% negative predictive value (NPV), and 72.5% overall accuracy. Intracellular water per kg body weight, serum albumin, prealbumin, and creatinine were highest among non-frail individuals, intermediate among those who were frail by self-report, and lowest among those who were also frail by performance. Age, percentage body fat, and C-reactive protein followed an opposite pattern. The modified self-report definition had better accuracy (84%; 95% CI, 79%–89%) and superior specificity (88%) and PPV (67%).
Limitations
Our study did not address prediction of outcomes.
Conclusions
Patients who meet the self-report but not the performance-based definition of frailty may represent an intermediate phenotype. A modified self-report definition can improve the accuracy of a questionnaire-based method of defining frailty.
Keywords: frailty, physical performance, self-reported function, hemodialysis, physical activity, physical function, end-stage renal disease (ESRD)
Frailty has been recognized as a syndrome resulting from cumulative declines in multiple physiologic systems leading to impaired homeostatic reserve and a decreased capacity to withstand stress.1–3 Frailty is associated with age and comorbidity but is not synonymous with these potential contributors. Identifying frail individuals, who are more vulnerable than robust individuals to adverse health outcomes including falls, disability, hospitalization, institutionalization and death, is of tremendous potential value in designing and implementing interventions to improve outcomes.1
An operational definition of frailty that includes five domains of weight loss, exhaustion, slow walking speed, weak grip, and low physical activity was developed in a cohort of community-dwelling elderly individuals in the Cardiovascular Health Study and is now widely used.1, 2, 4–6 However, it was quickly recognized that a definition that does not require direct measurement of physical performance would offer advantages in many research and clinical settings.7 Consequently, a definition that substituted the Physical Function score of the SF-36 instrument was tested in a cohort of community-dwelling women and found to identify a group of individuals at higher risk for hospitalization and death than their non-frail counterparts.7 Since then, similar definitions of frailty based on self-reported functioning have been applied not only among community-dwelling elders8 but also in patients with chronic kidney disease (CKD)9 and end-stage renal disease (ESRD).10, 11
Patients with ESRD – even those younger than 65 years – have a higher prevalence of frailty than is observed among community-dwelling elders.6, 10–13 Although physical performance is directly correlated with self-reported physical function among patients with ESRD,14 the two methods of characterizing frail individuals are not likely to produce identical results. A recent study compared a performance-based definition of frailty that substituted a timed chair stand test for grip strength to a frailty definition based on self-reported function and found that more patients were identified as frail using self-report.12 However, information on weight loss and leisure-time physical activity was not available in that study. Importantly, the original frailty definition from the Cardiovascular Health Study has never been compared directly to one that substitutes the PF scale of the 36-Item Short Form Health Survey (SF-36) for the performance measures.
ACTIVE/ADIPOSE (A Cohort To Investigate the Value of Exercise/Analyses Designed to Investigate the Paradox of Obesity and Survival in ESRD) is a cohort study conducted jointly by the US Renal Data System (USRDS) Nutrition and Rehabilitation/Quality of Life Special Studies Centers.15 A major goal of the study was to determine the extent to which patients identified as frail based on criteria that include self-reported function resemble those identified based on direct measures of physical performance. We hypothesized that more patients would be classified as frail by self-report than by directly measured physical performance. We further hypothesized that the original self-report definition, which was derived in a healthy population and which uses a single cutpoint to assign either zero or two points toward the frailty score, would not yield optimal agreement between the performance-based and self-reported measures of frailty. This study aimed to compare the prevalence of frailty using the two standard definitions of frailty, to compare characteristics of patients according to whether they meet these definitions, and to determine whether we could create a modified self-report definition that aligns more closely with the gold standard definition of frailty based on physical performance.
Methods
Study Design and Participants
The ACTIVE/ADIPOSE Study enrolled 771 adult patients receiving maintenance hemodialysis in 14 facilities, seven in the San Francisco Bay Area and seven in the Atlanta, Georgia metropolitan area between June, 2009 and August, 2011.15 Eligible participants included adults on dialysis for at least 3 months who were English- or Spanish-speaking and were able to provide informed consent. The study was approved by the Committee on Human Research at the University of California, San Francisco and the Emory University Institutional Review Board. All participants provided written informed consent.
Study coordinators interviewed participants, abstracted recent clinical and laboratory data from medical records, and measured physical performance and body composition using bioelectrical impedance spectroscopy. Body composition and physical performance testing occurred immediately before a dialysis session on the same day. Patients’ data were also linked to data from the ESRD Medical Evidence Report (Centers for Medicare & Medicaid Services Form 2728) available in the USRDS, from which comorbidity was determined.
Frailty
We ascertained frailty by two definitions, one of which included measures of walking speed and grip strength1 and one of which substituted patients’ self-report of physical function for the performance measures (Table 1). Specifically, the performance-based definition included five domains: weight loss, exhaustion, low physical activity, weak grip and slow walking. Each domain was given a dichotomous score of 0 or 1 based on the following criteria:
Table 1.
Patient characteristics based on performance-based and self-report–based definitions of frailty.
| Component | Physical Performance | Self-reported Function | |
|---|---|---|---|
|
| |||
| Weight loss | Unintentional weight loss of ≥10 lb in past year by patient report | Unintentional weight loss of ≥ 10 pounds in past year by patient report | |
|
| |||
| Exhaustion | Using the CES-D, the following 2 statements are read: (a) I felt that everything I did was an effort; (b) I could not get going. The question is asked, “How often did you feel this way?” 0=rarely or none of the time (<1 day), 1=some or alittle of the time (1–2 days), 2=a moderate amount of the time (3–4 days), or 3=most of the time. Patients answering “2” or “3” to either of these questions are categorized as frail by the exhaustion criterion. | Using the CES-D, the following 2 statements are read: (a) I felt that everything I did was an effort; (b) I could not get going. The question is asked, “How often did you feel this way?” 0=rarely or none of the time (<1 day), 1=some or a little of the time (1–2 days), 2=a moderate amount of the time (3–4 days), or 3=most of the time. Patients answering “2” or “3” to either of these questions are categorized as frail by the exhaustion criterion. | |
|
| |||
| Physical activity | Based on the short version of the Minnesota Leisure Time Activity questionnaire, kilocalories per week expended are calculated using a standardized algorithm; this variable is stratified by sex: those with <383 kcal (men) or <270 kcal (women) of physical activity per week are frail. | Based on the short version of the Minnesota Leisure Time Activity questionnaire, kilocalories per week expended are calculated using a standardized algorithm; this variable is stratified by sex: those with <383 kcal (men) or <270 kcal (women) of physical activity per week are frail. | |
|
| |||
| Walk time | Based on a timed 15-foot walk; this variable is stratified by sex and height:
|
Score <75 on the SF-36 Physical Function scale;* the scale asks participants to report whether they are limited a lot, limited a little, or not limited at all in 10 activities; scores range from 0–100 with higher scores indicating better function. | |
| Men | |||
| Height | Walk time | ||
| ≤173 cm | ≥7 s | ||
| >173 cm | ≥6 s | ||
| Women | |||
| ≤159 cm | ≥7 s | ||
| >159 cm | ≥6 s | ||
|
| |||
| Grip strength | This variable is stratified by sex and BMI:
|
Score <75 on the SF-36 Physical Function scale;* the scale asks participants to report whether they are limited a lot, limited a little, or not limited at all in 10 activities; scores range from 0–100 with higher scores indicating better function | |
| Men | |||
| BMI | Grip strength | ||
| ≤ 24 kg/m2 | ≤29 kg | ||
| 24.1–26 kg/m2 | ≤30 kg | ||
| 26.1–28 kg/m2 | ≤31 kg | ||
| >28 kg/m2 | ≤32 kg | ||
| Women | |||
| ≤23 kg/m2 | ≤17 kg | ||
| 23.1–26 kg/m2 | ≤17.3 kg | ||
| 26.1–29 kg/m2 | ≤18 kg | ||
| > 29 kg/m2 | ≤21 kg | ||
BMI, body mass index; CES-D, Center for Epidemiological Studies Depression Scale; SF-36, 36-Item Short Form Health Survey
Participants receive 2 points if the Physical Function score of the SF-36 is <75. This criterion substitutes for both walk time and grip strength.
Weight loss was defined as unintentional weight loss of ≥10 pounds (4.5 kg) in the last year.
Exhaustion was measured by responses to questions about endurance and energy from the Center for Epidemiological Studies Depression Scale (Table 1).16
Low physical activity was ascertained from the short version of the Minnesota Leisure Time Physical Activity Questionnaire, which asks about the frequency and duration of various activities over a two-week time period.17
Weakness was based on measurement of handgrip strength by hand-held dynamometer (JAMAR, Lafayette Instrument Co, Lafayette, IN) immediately prior to a dialysis session. Patients performed three tests with each hand. The mean measurement of the strongest hand was used to determine frailty (Table 1).
Slow walking speed was scored based on a 15-foot timed walk. Patients were asked to walk the course twice at their usual pace immediately prior to a dialysis session, and the faster of the two walking trials was used.
A score of ≥3 points was considered frail.
A self-reported function-based definition was also constructed in which the first three criteria were identical to the performance-based definition. Participants’ scores on the SF-36 Physical Function scale were used to determine frailty in place of the walking test and grip strength measurements (Table 1). The PF scale was administered at the same visit as the physical performance tests and asks participants to report whether they are “not limited at all”, “limited a little”, or “limited a lot” in performing 10 activities and generates a score ranging from 0 to 100, with higher scores indicating better function. Patients with a score <75 were considered to meet the slow walking and weak grip criteria and were given 2 points towards the overall frailty score as previously described.7,10 A score of ≥3 points was considered frail, as for the performance-based definition.
Body Composition
Body mass index (BMI) was calculated using the mean of the last three post-dialysis weights. Whole-body bioelectrical impedance spectroscopy was performed prior to a dialysis session using a device that scans 256 frequencies between 4 and 1000 kHz (SFB7, ImpediMed, San Diego, CA). After participants had been in a supine position for at least 10 minutes, electrodes were placed in a tetra-polar configuration on the hand and foot on the side opposite the dialysis access with proximal and distal electrodes 5 cm apart. Ten measures were performed within a one-minute period. Total body water was estimated using the resistance extrapolated to infinite frequency, and total body fat was calculated by subtracting total body water/0.73 from body weight.18, 19
Laboratory Measures
Results of the most recent routine laboratory testing (within one month) were recorded, and blood was drawn for additional tests within one month of the testing visit. Samples were centrifuged, aliquoted and stored at −80°C. Samples were periodically transported to the core laboratory where they were stored over liquid nitrogen at −196°C until the time of assay measurement. We measured albumin, prealbumin, and C-reactive protein (CRP) concentrations in duplicate on each serum sample using a Beckman Array 360 nephelometer, and mean values were used in analyses.
Activities of Daily Living
Patients were asked if they were unable or needed help from another person to bathe, dress, get in and out of a chair, or walk around their home or apartment. Patients reporting inability to perform any of these independently were considered to have a disability in activities of daily living (ADLs).
Statistical Analyses
Although the performance-based frailty score has been applied to patients missing data on one or two of the components,1 we restricted the analytic cohort to individuals with available data for all components (n=731) in order to avoid differences in frailty status between measures related solely to missing data. Patients were separated into categories based on frailty status by the two measures. We compared characteristics of patients in the four groups using Kruskal-Wallis and Chi square tests with post hoc testing using Mann Whitney tests.
We next sought to optimize the accuracy of the self-reported function-based definition in replicating the performance-based definition of frailty by creating a modified self-report definition. We first randomly divided the cohort into approximately equal development and validation cohorts stratified by performance-based frailty status. Using the development cohort, we determined two cut points in the SF-36 PF scale that optimized the absolute accuracy of the self-reported function-based definition of frailty with respect to the performance-based definition (i.e., the percentage of patients correctly classified as either frail or nonfrail). In other words, rather than choosing a single threshold PF score below which participants earned two points towards the frailty score, we allowed for the possibility of separate thresholds for one point and two points. The thresholds were determined to maximize overall agreement rather than to prioritize the minimization of false positives or false negatives because we had no a priori reason to weigh avoiding false positives or false negatives differently. Because multiple pairs of cut points corresponded to the same maximal level of agreement, we chose the cut points corresponding to the mid-points of the range of values. We repeated the cut point-finding procedures separately for men and women in the development set to determine whether sex-specific cut points improved agreement over using overall cut points.
We then applied the cut points from this development cohort to the validation cohort. We examined overall accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for frailty determined using the cut points we identified (modified self-report definition of frailty) and compared them to those of the definition of frailty derived from the cut point originally developed by Woods et al. (self-report definition of frailty) in a cohort of community-dwelling women (PF<75 = 2 points toward frailty score)7, treating the performance-based measure of frailty as the gold standard.1 All statistical analyses were performed SAS 9.2 (SAS Institute Inc, Cary, NC).
Results
Study Participants
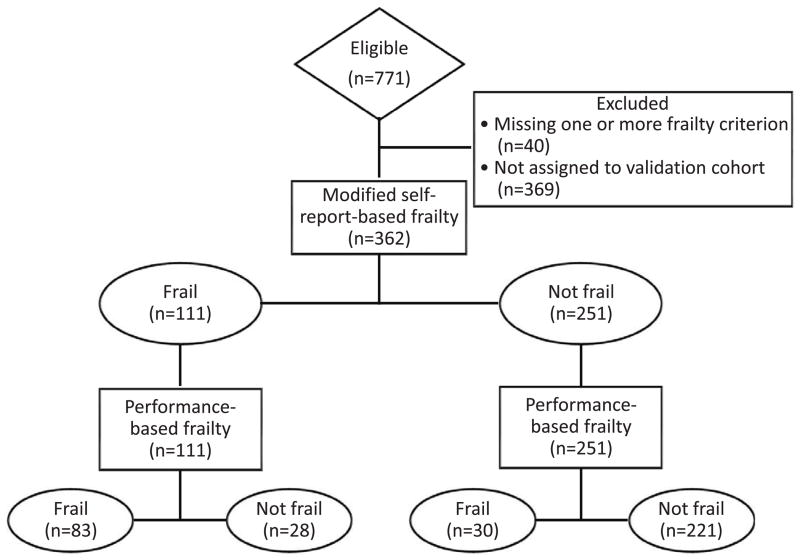
A total of 771 patients were enrolled in ACTIVE/ADIPOSE, of whom 731 (95%) had complete data available for all components of both frailty scores (Figure 1). Patients missing data on frailty were not significantly different based on any of the characteristics in Table 1 except that they had a shorter dialysis vintage (median, 2.1 [interquartile range (IQR), 0.6–5.4] years) and a lower prevalence of hypertension (78%). As expected, self-reported physical function correlated with both walking time (r=0.53) and grip strength (r=0.38; both p<0.001).
Figure 1.
Standards for the Reporting of Diagnostic Accuracy Studies (STARD) flow diagram for the comparison of the self-report definition of frailty with the performance-based definition (gold standard) in the whole study cohort.
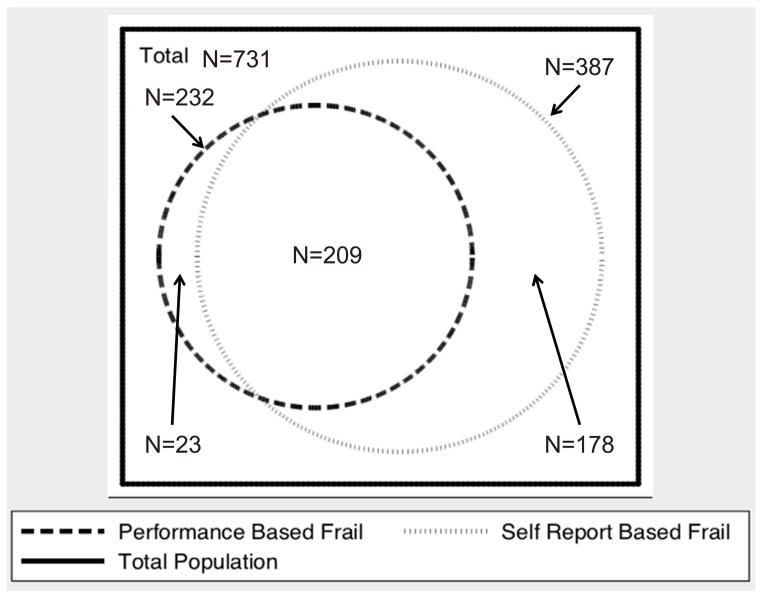
Of the participants with data on frailty, 321 (44%) were not frail by either the performance-based or the self-report based definition. 387 (53%) met the self-report–based definition of frailty, of whom 209 (54% of those frail by self-reported function and 29% of the whole cohort) met the performance-based definition (Figure 2). Only 23 individuals (3% of the cohort) met the performance-based definition of frailty without meeting the definition based on self-reported function. Thus, the overall accuracy of the self-reported function-based definition of frailty for the “gold standard” performance-based definition was 72.5% (530 for whom the definitions agreed/731 patients), with 90% sensitivity and 64% specificity.
Figure 2.
Venn diagram comparing frailty defined by the standard definition using self-reported physical function compared to frailty defined using physical performance measures in the whole cohort (n=731).
Patient Characteristics Based on Performance-Based and Self-Report–Based Definitions
Table 2 shows the characteristics of participants based on frailty status. Patients with diabetes mellitus and heart failure were more likely to be frail, but neither race nor BMI was significantly associated with frailty. Intracellular water per kg of body weight, a surrogate for muscle mass, exhibited a graded pattern and was highest among non-frail individuals, intermediate among those who were frail only by self-reported functioning, and lowest among those who were frail by self-report and performance. A similar pattern was observed for markers of nutritional status, including serum albumin, prealbumin, and creatinine. Age, percentage body fat, and CRP followed an opposite pattern: highest among participants meeting both frailty definitions, intermediate among those meeting only the self-report definition and lowest among the nonfrail, but the small group meeting only the performance-based definition was not intermediate for these characteristics. Physical performance also appeared to show a graded pattern, with both of the groups that were frail by only one definition intermediate between the non-frail group and the group that was frail by both measures. It was particularly notable that the group that was frail only by self-reported function had significantly better PF scores (median, 40; IQR, 30–55) than the group that was also frail by performance measures (median, 25; IQR, 6–45; p<0.001) even though low self-reported function was a prerequisite for inclusion in both groups. However, patients who were frail by physical performance definition only had self-reported function that was better than the other two frail groups and similar to that of the nonfrail group, a finding that is a reflection of the definitions themselves. Patients who were frail only by self-report were approximately twice as likely to report at least one ADL disability as patients who were not frail, and patients who were frail by both definitions were over five times as likely to report an ADL disability (Table 2).
Table 2.
Patient characteristics based on performance-based and self-report–based definitions of frailty.
| Variable | Not frail (n=321) | Self-report only (n=178) | Performance only (n=23) | Performance and self-report (n=209) | p-value |
|---|---|---|---|---|---|
| Age (y) | 53.8 ± 14.4 | 57.6 ± 12.1 | 51.6 ± 15.8 | 62.9 ± 13.0 | <0.001 |
| Female Sex | 33.3 | 48.3 | 30.4 | 49.0 | <0.001 |
| Black Race | 61.7 | 68.0 | 69.6 | 56.7 | 0.4 |
| Diabetes mellitus | 36.5 | 50.3 | 54.5 | 62.9 | <0.001 |
| Heart failure | 17.3 | 16.7 | 9.1 | 27.2 | 0.01 |
| CAD | 7.8 | 8.0 | 0 | 12.4 | 0.1 |
| Hypertension | 89.6 | 93.1 | 87.0 | 87.8 | 0.4 |
| Dialysis vintage (y) | 3.7 [1.7–8.3] | 3.3 [1.5–6.0] | 2.3 [1.0–5.7] | 2.8 [1.2–5.6] | 0.007 |
| Serum albumin (g/dL) | 4.1 ± 0.3 | 4.0 ± 0.3 | 4.0 ± 0.6 | 3.9 ± 0.4 | <0.001 |
| Serum creatinine (mg/dL) | 8.8 ± 2.9 | 8.5 ± 2.7 | 9.1 ± 2.8 | 7.4 ± 2.3 | <0.001 |
| Prealbumin (mg/dL) | 31.2 ± 7.4 | 30.2 ± 7.0 | 28.4 ± 7.9 | 27.4 ± 7.6 | <0.001 |
| CRP (mg/L) | 3.1 [1.2–8.0] | 3.8 [1.7–8.9] | 6.9 [2.8–19.9] | 5.1 [1.5–13.0] | 0.005 |
| BMI (kg/m2) | 28.4 ± 6.3 | 29.4 ± 7.4 | 28.0 ± 5.0 | 30.0 ± 8.0 | 0.3 |
| Body fat (%) | 28.5 ± 9.9 | 30.5 ± 9.8 | 25.2 ± 10.5 | 32.1 ± 11.3 | <0.001 |
| ICW/kg body weight | 0.28 ± 0.04 | 0.27 ± 0.04 | 0.29 ± 0.04 | 0.26 ± 0.04 | <0.001 |
| Physical Function score | 85 [65–95] | 40 [30–55] | 85 [75–90) | 25 [6–45] | <0.001 |
| Gait speed (m/s) | 1.0 [0.9–1.2] | 0.9 [0.8–1.0] | 0.8 [0.6–1.1] | 0.6 [0–0.8] | <0.001 |
| Grip strength (kg) | 29.5 ± 11.0 | 27.5 ± 10.9 | 25.5 ± 8.3 | 20.9 ± 8.7 | <0.001 |
| ADL disability | 7.5 | 15.2 | 8.7 | 39.7 | <0.001 |
Note: Values for categorical variables are given as percentages; values for continuous variables are given as mean ± standard deviation or median [interquartile range]. Conversion factor for serum creatinine in mg/dL to μmol/L, ×88.4.
Abbreviations: ADL, activity of daily living; CAD, coronary artery disease; CRP, C-reactive protein; BMI, body mass index; ICW, intracellular water.
Modified Self-Report Definition Optimizing Agreement Between Self-Report–Based and Physical Performance–Based Frailty
In the development data set, we found that a two-threshold score that assigned no points for a PF score >78, 1 point for PF score of13–78, and two points for a score <13 resulted in the best overall accuracy. Performing the optimization procedure separately for men and women did not result in different cut points based on sex.
We applied both the original single threshold of PF<75 (self-report definition) and the two-threshold score produced by our optimization strategy (modified self-report definition) to the validation data set (Figure 3) and computed two indicators for self-reported frailty. Table 3 compares the results for the self-report frailty definition and the modified self-report definition, treating the performance-based frailty classification as the gold standard. Overall accuracy of the modified self-report definition was 84% (95% CI, 79%–89%) (Figure 4), which was substantially better than the overall accuracy of 71% (95% CI, 64%–78%) produced by applying a single threshold at PF <75 (self-report definition). Although the same thresholds optimized accuracy among men and women, accuracy of the modified self-report definition was slightly better for women than men (87% [95% CI, 80%–94%] vs. 82% [95% CI, 76%–88%], respectively), albeit with overlapping 95% CIs. Our strategy of maximizing accuracy produced cut points with superior specificity and PPV and similar NPV compared to the original cut point, at the expense of sensitivity (Table 3).
Figure 3.
Standards for the Reporting of Diagnostic Accuracy Studies (STARD) flow diagram for the comparison of the modified self-report definition of frailty with the performance-based definition (gold standard) in the validation cohort.
Table 3.
Accuracy of traditional and newly developed self-reported function thresholds for meeting frailty criteria in validation cohort.
| Measure | Traditional* | Modified** | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Overall | Men | Women | Overall | Men | Women | |
|
| ||||||
| Sensitivity | 88 (82 – 94) | 82.5 (73 – 92) | 93 (86 – 100) | 73 (65 – 82) | 65 (53 – 77) | 82 (72 – 92) |
|
| ||||||
| Specificity | 63 (57 – 69) | 69 (62 – 76) | 55 (45 – 65) | 89 (85 – 93) | 88 (83 – 93) | 90 (84 – 96) |
|
| ||||||
| PPV | 52 (45 – 59) | 50 (40 – 60) | 54 (44 – 64) | 75 (67 – 83) | 67 (55 – 80) | 82 (72 – 92) |
|
| ||||||
| NPV | 92 (88 – 96) | 91 (86.2 – 96.5) | 93 (86 – 100) | 88 (84 – 92) | 87 (82 – 92) | 90 (84 – 96) |
|
| ||||||
| Accuracy | 71 (64 – 78) | 73 (65 – 81) | 69 (57 – 81) | 84 (79 – 89) | 82 (76 – 88) | 87 (80 – 94) |
Note: Numbers in parentheses are 95% confidence intervals.
Abbreviations: PPV, positive predictive value; NPV, negative predictive
Physical Function score < 75 = 2 points.
Physical Function score >78 = 0 points; 13–78 = 1 point; <13 = 2 points.
Figure 4.
Venn diagram comparing the new frailty definition using self-reported function and frailty defined using physical performance measures in the validation cohort (n=362). Optimal cutpoints were PF >78 = 0; PF 12–78 = 1; PF <13 = 2.
Discussion
We prospectively applied the “gold standard” performance-based frailty criteria developed by Fried and colleagues1 in the Cardiovascular Health Study and the original self-reported function-based frailty criteria7 in a diverse cohort of patients receiving hemodialysis. We found that the self-reported function-based measure identified a larger number of individuals as frail than the performance-based measure as others have suggested.12 Indeed, approximately half of participants categorized as frail by self-reported function did not meet the definition based on physical performance. We identified thresholds for self-reported function that matched more closely the performance-based frailty definition. This modified self-report definition of frailty performed well in the validation cohort, correctly classifying 84% of patients.
Ultimately, the value of classifying patients according to frailty constructs lies in their ability to accurately predict which patients are at higher risk of adverse outcomes and which patients could benefit from interventions to decrease the risk of adverse events. Our results suggest that self-reported function can be useful in establishing frailty status. First, the traditional cut point for function-based frailty identified nearly all patients who met the performance-based definition of frailty (high sensitivity). Thus, if the goal of frailty assessment is to identify persons with or at high risk for frailty, aiming to miss few if any cases, this approach could be applied. Second, the traditional function-based criterion could be applied as an initial screen, with the performance tests done only among patients who screen positive, thus minimizing the number of physical performance tests needed to identify those meeting the performance-based (“Fried”) frailty criteria.
Another intriguing possibility suggested by our findings is that patients meeting the self-reported function-based frailty definition but not the performance-based measure may constitute a group that is intermediate between non-frail and frail by the traditional performance-based definition. This group had self-reported function and physical performance that were worse than the nonfrail group but better than the group that met both definitions of frailty. In addition, indicators of nutritional status and inflammation in this group were intermediate between the other two groups. In this cross-sectional study, we could not determine whether this group is destined to become frail or is at higher risk for development of frailty, but this possibility can be explored in longitudinal analyses, along with the possibility that this group has a risk of adverse events associated with frailty that is also intermediate between that of nonfrail individuals and those who meet performance-based criteria.
On the other hand, the modified thresholds for self-reported function we identified had 84% overall accuracy in identifying patients who met the performance-based frailty measure. The PPV and NPV were 75% and 88%, respectively, suggesting that frailty could be assessed with reasonable accuracy without the need for performance testing. Although longitudinal studies will be needed to determine whether designation of frailty in this manner would have similar predictive value for mortality and hospitalization as frailty defined using tests of physical performance, results of a recent study showed that the same performance-based definition of frailty used in our study was strongly associated with mortality and hospitalization in a cohort of prevalent patients receiving hemodialysis.6
The strengths of our study include that it was specifically designed to collect the data necessary to define frailty by the original performance-based and self-reported function-based definitions. Thus, no substitutions were needed to compare to other populations, and we were able to directly compare the most commonly utilized performance criteria for frailty to the most commonly used self-reported function-based criterion. Second, the study was conducted in two large metropolitan areas, and the ACTIVE/ADIPOSE population was diverse in terms of age, sex, race, ethnicity, and vintage. Third, we had complete data on the five frailty domains in nearly all participants, abrogating the need for imputation and limiting misclassification of participants with missing data in one or two domains as nonfrail. Finally, our cohort was large enough to allow us to develop our modified self-reported function cut points in a development cohort and then test them in a separate validation cohort.
There are several relevant limitations to this study as well. While the sample size was larger than other studies in the ESRD population in which performance-based frailty definitions were employed, a larger sample size might have allowed us to further optimize agreement between self-report–based and performance-based definitions of frailty. In addition, the racial/ethnic distribution of our cohort does not match that of the general dialysis population. Thus, although we did not find an association of race with frailty, these results will need to be confirmed in other cohorts. The principal limitation relates to the study’s cross-sectional design. Serial determinations of frailty by both performance-based measures and self-report would inform the field about the trajectory of frailty and allow determination of the extent to which different definitions of frailty predict adverse outcomes.
In conclusion, over half of the patients in our cohort met at least one definition of frailty, similar to results of previous reports,10, 11 and approximately twice as many patients were frail when a definition based on self-reported function was employed compared to a performance-based definition. Patients who were frail based on self-reported function were less robust than those who were not frail and may represent an intermediate phenotype. Modified thresholds of self-reported function can improve the accuracy of this method of defining frailty relative to performance-based frailty. Ultimately, identifying interventions that might lessen the impact of frailty or attenuate the trajectory of frailty toward disability and loss of functional independence would be of great value to the ESRD program.
Acknowledgments
Support: This work was supported by the National Institutes of Health (contract N01-DK-0005 to Dr Johansen and K23 DK093584 to Dr Dalrymple. The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: research idea and study design: KLJ, GMC, GAK; data acquisition: KLJ, GAK, CD; data analysis/interpretation: KLJ, BG, JK, LD, CD, GMC, GAK; statistical analysis: KLJ, JK, BG, GMC, LSD; supervision or mentorship: KLJ, GMC, GAK. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. KLJ takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Med Sci. 2001;56A(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Morley JE, Vellas B, Abellan van Kan G, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol Med Sci. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Jentof AJ, Baeyns JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report ofthe European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the Women’s Health and Aging Studies. J Gerontol Med Sci. 2006;61A(3):262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 6.McAdams-Demarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53(8):1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 8.Barreto PS, Greig C, Ferrandez AM. Detecting and categorizing frailty status in older adults using a self-report screening instrument. Arch Gerontol Geriatr. 2012;54(3):e249–e254. doi: 10.1016/j.archger.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Wilhelm-Leen ER, Hall Y, Tamura MK, Chertow GM. Frailty and chronic kidney disease: The third national health and nutrition evaluation survey. Am J Med. 2009;122(7):664–671. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18(11):2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 11.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172(14):1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Painter P, Kuskowski M. A closer look at fraitly in ESRD: getting the measure right. Hemodial Int. 2013;17(1):41–49. doi: 10.1111/j.1542-4758.2012.00719.x. [DOI] [PubMed] [Google Scholar]
- 13.Johansen KL, Dalrymple L, Delgado C, et al. Association between body composition and frailty among prevalent hemodialysis patients: a USRDS special study. J Am Soc Nephrol. 2014;25(2):381–389. doi: 10.1681/ASN.2013040431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansen KL, Painter P, Kent-Braun JA, et al. Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int. 2001;59(3):1121–1127. doi: 10.1046/j.1523-1755.2001.0590031121.x. [DOI] [PubMed] [Google Scholar]
- 15.Renal Data System US: USRDS 2011 Annual Data Report. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 16.Orme J, Reis J, Herz E. Factorial and discriminate validity of the Center for Epidemiological Studies depression (CES-D) scale. J Clin Psychol. 1986;42(1):28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 17.Taylor HL, Jacobs DR, Schuker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure-time physical activities. J Chronic Dis. 1978;31(12):741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 18.Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis - part I: review of principles and methods. Clin Nutr. 2004;23(5):1226–1243. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Moissl UM, Wabel P, Chamney PW, et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas. 2006;27(9):921–923. doi: 10.1088/0967-3334/27/9/012. [DOI] [PubMed] [Google Scholar]