Abstract
Purpose
To study the protection offered by empty liposomes alone against acrolein-induced changes in urothelial cell viability and explored uptake of liposomes by primary (rat) urothelial cells.
Methods
Acrolein was used as a means to induce cellular damage and reduce urothelial cellular viability. The effect of acrolein or liposomal treatment on cellular proliferation was studied using BrdU assay. Cytokine release was measured after urothelial cells were exposed to acrolein. Temperature dependent uptake study was carried out for fluorescent labeled liposomes using confocal microscopy.
Results
Liposome pretreatment protected against acrolein-induced decrease in urothelial cell proliferation. Liposomes also significantly affected the acrolein induced cytokine (IFNγ) release offering protection to the urothelial cells against acrolein damage. We also observed a temperature-dependent urothelial uptake of fluorescent-labeled liposomes occurred at 37 °C (but not at 4 °C).
Conclusions
Empty liposomes alone provide a therapeutic efficacy against acrolein induced changes in urothelial cell viability and may be a promising local therapy for bladder diseases. Hence our preliminary evidence provides support for liposome-therapy for urothelial protection and possible repair.
Keywords: urothelium, urinary bladder, liposome, injury, barrier function
Introduction
The urinary bladder urothelium (UT) offers the first line protection against injury or damage due to exposure to various urinary toxins. The UT may be disrupted in various conditions such as with infection, inflammation or following a damaging level of stress. UT damage is likely to affect the underlying tissues that contribute to bladder function. Hence there is interest in developing an agent that can repair and restore the UT barrier against various damaging conditions. The urinary bladder can be exposed to acrolein and its metabolites due to dietary, endogenous, drug induced and environmental sources [1,2]. Acrolein has been shown to cause severe inflammation and damage to the bladder.
Two examples of therapies that may eventually result in acrolein formation, tissue damage and hemorrhagic cystitis are cyclophosphamide treatment (CYP) and radiation [3]. Cyclophosphamide (CYP), an antineoplastic and immunosuppressant, has been used clinically to treat over 200,000 patients per year diagnosed with bladder cancer [4]. Radiation is often used in the management of pelvic malignancies, either as primary or as adjuvant treatment. A degree of bladder involvement is inevitable in the case of pelvic irradiation [5]. CYP is converted to acrolein, which activates urothelial transient receptor potential cation channel (TRPV1 and TRPA1) receptors, causing calcium influx and cytokine gene transcription via the calcineurin dependent nuclear factor of activated T cells (NF-AT) pathway. Radiation can have a similar effect by causing membrane lipid peroxidation, leading to acrolein formation and activating inflammatory pathways [2].
Liposomes (LPs) are biocompatible and biodegradable lipid bilayer vesicles similar to the lipid bilayer cell membranes of living cells. Liposomal biocompatibility and its capability to deliver drugs to UT have been shown in our previously reported studies in both humans and animals [6,7]. We have reported that intravesical empty liposomes treatment in animals and humans offered mucosal protection that may be important in the treatment of interstitial cystitis/bladder pain syndrome (IC/BPS) [6,8]. Liposomes may be taken up by the urothelial cells (biological barrier) to produce the desired pharmacological effect in part via enriching the required therapeutic concentration at the desired target site [3,7]. However, the exact cellular entry mechanism underlying liposome uptake by bladder urothelial cells including an effect on cellular viability remains unclear. It has been shown that the endocytic pathway could be a major contributor to the cellular uptake of liposomes [9]. In the present study we investigated uptake of empty liposomes by urothelial cells and the protection offered by liposomes against acrolein induced urothelial cell injury.
Materials and Methods
All procedures were conducted in accordance with IACUC policies at the University of Pittsburgh.
Primary bladder urothelial (UT) cell cultures were prepared as previously described from Harlan Sprague Dawley female (2–4 mo) rats [10]. Urinary bladders were removed and placed in cold MEM (Invitrogen, Carlsbad, CA) with HEPES (2.5 g/l, Sigma, St. Louis, MO) and 1% penicillin/streptomycin/fungizone (Invitrogen). The bladder was cut open and incubated in dispase (4°C, 2.0 mg/ml, Invitrogen). Urothelial cells were gently scraped from the underlying tissue, placed in trypsin (0.25% wt/vol; 5–15 min, Invitrogen) triturated, suspended in MEM containing 10% FBS (Invitrogen) and centrifuged (416g; 15 min). Cells were suspended (CNT-16, CELLnTEC, Bern, Switzerland) and plated on collagen-coated glass coverslips (2 × 105 cells/ml); all cultures were cytokeratin positive, demonstrating epithelial phenotype.
Fluorescent imaging of liposome uptake
Cultured UT cells were incubated with fluorescently tagged liposomes (LP, 2mg/ml in media; N-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-Sphingosine-1-Phosphocholine (C12-NBD Sphingomyelin; green) (Lipella Pharmaceuticals, Inc., Pittsburgh, PA). The UT plasma membrane (PM) was fluorescently labeled with wheat germ agglutinin-Alexa Fluor 555 (Invitrogen, red; 10 min; 37ºC). After fixation (4% paraformaldehyde, 10 min), nuclei were stained using To-Pro-3 (Invitrogen, 642/661, 1:1000 in PBS, 10 min, blue). Images were acquired using a Leica SP5 Confocal and analyzed with Velocity 3D Image Analysis Software (Perkin Elmer, Waltham, MA).
Proliferation Assay
Four groups (all analyzed at 3 DIV) were studied. These included untreated control (C), liposome alone (LS, 2 mg/ml; treated at 1 day in vitro-DIV), acrolein (A, 2 μM; treated at 2 DIV) and liposome pretreatment prior to acrolein (LP+A). Following incubation with liposomes and/or acrolein, the cells were processed for 5-Bromo-2′-deoxy-uridine (BrdU; Roche, Indianapolis, IN) uptake following the manufacturer’s instructions. In brief, cells were incubated in BrdU labeling reagent, fixed (50mM glycine/ethanol), incubated (anti-BrdU primary antibody, 1:50; anti-mouse-Ig-fluorescein secondary antibody, 1:200) and then a blue nuclear dye (DAPI; 4,6-diamidino-2-phenylindole; Invitrogen). The percentage of proliferating cells (green) was calculated by counting the number of BrdU positive/total cells. Using GraphPad Prism 5.0, statistical differences were evaluated with one-way ANOVA followed by Neumann-Keuls multiple comparison post-hoc test, with significance equals p<0.05.
Cytokine; ATP measurements
Using the same experimental approaches as above, for ATP release cells were superfused with HBSS buffer (5 mM KCl, 0.3 mM KH2PO4, 138 mM NaCl, 4 mM NaHCO3, 0.3 mM NaH2PO4, 5.6 mM glucose, 10 mM Hepes, 2 mM CaCl2 and 1 mM MgCl2, pH 7.4) using a peristaltic pump (flow rate 0.4 ml/min) and 100 μl of perfusate collected every 60 seconds. Samples were taken for each experimental group for 15 minutes before and during a control stimulus (50% hypotonic), and for 15 minutes during washout with HBSS. ATP levels were quantified immediately after sample collection using a luciferin-luciferase reagent (ATP Luminescence Kit, Sigma-Aldrich) and bioluminescence measured using a luminometer. IFNγ measurement in supernatant and cellular extracts was determined using SearchLight cytokine array technology (Rat Cytokine 3 Array Aushon Biosystems, Billerica, MA). Using GraphPad Prism 5.0, statistical differences were evaluated with one-way ANOVA followed by Neumann-Keuls multiple comparison post-hoc test, with significance equals p<0.05.
Results
Proliferation assay
Proliferation in various groups was evaluated using BrdU assay. Proliferation of acrolein treated cells was decreased by 56.7% (7.4%±2.2% BrdU positive cells) as compared to control (17.06%±1.4% BrdU positive cells; p<0.05), showing acrolein can significantly decrease urothelial proliferation. Pretreatment with liposomes followed by acrolein treatment showed a 2-fold increase in cell proliferation (15.0%±2.9% BrdU positive cells) as compared to the acrolein treatment. The liposome plus acrolein group proliferation rate approximated that of the control group (Fig. 1).
Fig 1. Liposomes are protective against acrolein (A)-induced reduction in UT cell proliferation.
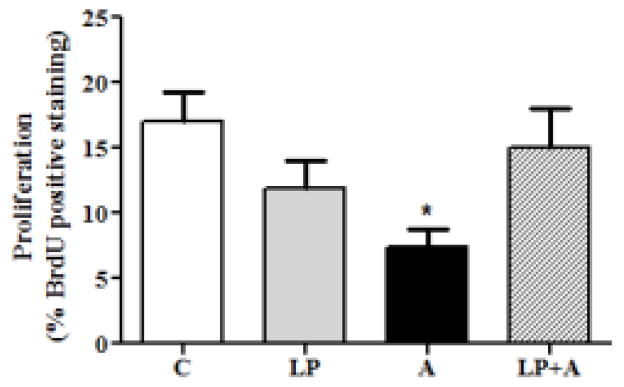
Exposure to acrolein (A) caused a marked reduction (* p<0.05, n=23) in proliferation. Liposomes are protective against A-induced toxicity (LP+A).
Release of Interferon-Gamma (IFNγ); ATP
We examined both IFNγ and ATP levels in the cell culture supernatant in the various groups studied. Acrolein treatment caused a significant release of IFNγ (1.66 fold; 52.7±7.6 pg/ml) as compared to control (p<0.01; 31.8±5.1pg/ml) (Fig. 2). Liposome treatment showed no change in the release of IFNγ in the liposome only group. In a similar manner, hypotonic-evoked ATP release in acrolein-treated cells was elevated (3.5 fold difference) as compared with untreated or liposome-only control. Furthermore, the cells pretreated with liposome followed by acrolein treatment resulted in production and release of ATP as well as IFNγ similar to that of control.
Fig 2. Liposomes alone normalize acrolein-induced UT release of cytokines.
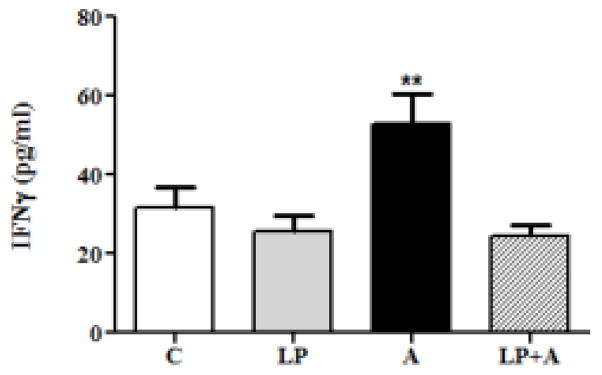
Exposure to acrolein (A) significantly augmented release of the inflammatory cytokine, IFN-γ, from UT cells (** p<0.01; n=8, culture supernatant). Liposome pretreatment normalized (to control values) A-induced increases in IFN-g levels (LP+A).
Endocytotic uptake of liposomes
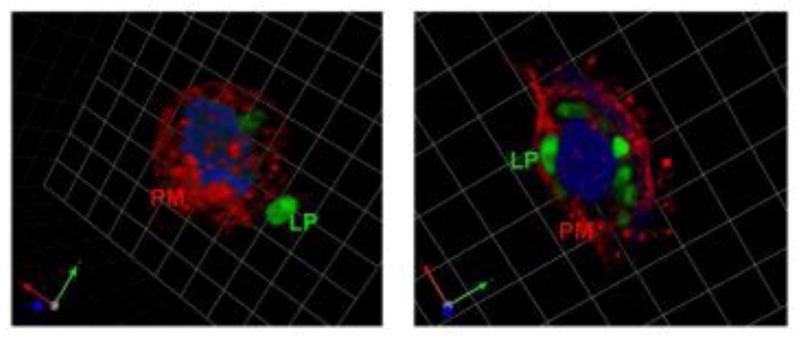
To study the effect of temperature on urothelial liposomal uptake, we incubated urothelial cells with fluorescent-labeled liposomes (2 hrs) at 4°C and 37 °C. Representative confocal microscopy images (incubation of cells at 4°C) revealed a visible extracellular association of liposomes to the external cell surface (plasma membrane) without internalization (Fig. 3, left panel). Increasing the time of incubation (up to 10 hours) did not result in appreciable changes in internalization of liposomes. Further, there was a noticeable lack of internalization of fluorescent-labeled liposomes at 4°C that could be due to depletion of energy and hence limited uptake. Incubation at 37°C showed increased liposomal uptake across the plasma membrane and located near the nucleus (Fig. 3, right panel).
Fig 3. Representative images depicting fluorescent-labeled liposomes (LP; green) adhering to the plasma membrane (PM; red) as well as internalization within UT cells (which is both temperature and time dependent).
Urothelial cell (UT) in the left panel was incubated at 4ºC (one square=3.47mm) and demonstrates extracellular binding of liposomes. UT cell in the right panel was incubated at 37ºC (one square=6.19 m) and shows intracellular localization of fluorescent-labeled liposomes.
Discussion
The urothelial cells form the specialized lining which covers the luminal surface of the bladder. The urothelium provides the first line of defense by virtue of its tight barrier against most substances found in urine, hence protecting the underlying tissues. This tissue exhibits a variety of cellular functions [11] that include control of permeability and cell-cell communication, and also appears to perform a key role in responding to injury and infection. Studies have shown that the urothelium is also involved in pathological conditions including cancer, detrusor overactivity and bladder pain [12]. In addition, urine itself contains various natural constituents, irritants, infectious agents and toxins [13]. A balance is maintained between the payload of urinary constituents and the urothelial capacity to protect the bladder against the urinary constituents. When the balance is disrupted due to injury or inflammation it can result in passage of toxins into underlying tissues impacting bladder function [11, 14–16]. Acrolein is one such toxin that can cause injury and affect the cellular functions. Hence in the present preliminary study we explored whether empty liposomes offer protection against acrolein-induced changes in urothelial cell viability.
Quantification of a number of bladder carcinogens in normal urothelium and bladder tumor tissue has shown acrolein is present in large levels [17]. Endogenously, acrolein can be produced by lipid peroxidation in biological systems. Amino acid and polyamine catabolism is also a significant source of acrolein [1]. Bladder cancer patients are among the longest cancer survivors [18] and face difficulties due to the adverse effect of treatments including cyclophosphamide and radiation therapy. Hemorrhagic cystitis occurs mainly due to acrolein formation after cyclophosphamide or radiation treatment [2]. Therefore, acrolein induction of cellular (UT) damage is a clinically relevant model for studying mechanisms of underlying bladder urothelial damage and repair.
Applications of nanotechnology in drug delivery are widely reported including intravesical drug delivery for treating urological disorders [19]. Of all the nanomedicine platforms, liposome formulations are the most widely approved by the US Food and Drug Administration for the safe and effective delivery of therapeutics [20]. Liposomal drug delivery has been applied for treatment of cancers and fungal infections [9]. Liposomes have vesicular structures consisting of an aqueous core surrounded by a lipid bilayer [21]. Liposomal formulations are biocompatible, and cause little or no adverse toxic reactions, making liposomal drug delivery attractive for the treatment of bladder diseases [22].
Our preliminary findings support the view that beside a role as a drug carrier, liposomes (LPs) may be of therapeutic benefit in terms of UT protection. The protective effect of LPs against acrolein toxicity may be due in part to forming a protective layer, an anti-inflammatory effect and/or external lipid supplementation to restore the lipid membrane structure. Liposomes may decrease the penetration of acrolein and hence minimize the toxic effect on the cells [6,8]. The symptoms described by patients diagnosed with IC/BPS may be a result of a defective UT barrier. A disruption of the UT in IC/BPS patients may allow access to toxic substances in the urine and thus lead to a cascade of events with associated irritative voiding symptoms and bladder pain [23].
LPs are well tolerated after intravesical administration [6]. Studies in moderate to severe IC/BPS patients have shown that empty LPs are able to decrease the symptoms of urinary frequency and nocturia with efficacy similar to oral pentosan sulfate sodium [6]. There is evidence that LPs can fuse with cells to form a type of molecular film that can promote wound healing. Thus in terms of the urinary bladder, LPs may enhance barrier property of a compromised UT and also increase the resistance to irritant penetration. We have evidence that LPs are protective against potassium chloride/protamine sulfate and acetic acid by decreasing the excitability of irritant-induced activation of afferent nerves and bladder nociceptive pathways [24]. LPs may also stabilize neuronal membranes and decrease hyperexcitability of afferent receptors. Because LPs exhibit a protective effect against acrolein exposure suggests that LPs can adhere and bind to the UT cell membrane. Our findings show that incubation of liposomes at a lower temperature (4°C) did not show evidence of internalization of fluorescent-tagged liposomes however; there appeared to be some adherence or association of the liposome to the urothelial cell membrane (Fig 3). While preliminary, the internalization of fluorescent-labeled liposomes with elevated temperatures suggestions involvement of an endocytic mechanism. We acknowledge limitations in comparing in vitro versus in vivo findings (such as differences in lipid composition of the apical and basolateral membrane domains). However, our in vitro findings are supported by an (unpublished) preliminary in vivo study that revealed a similar observation in rat bladder – plain gold nanoparticles could be localized to the urothelial cell surface, and with increased temperatures liposome encapsulated gold particles were located internalized within the urothelium.
Inflammatory cytokines such as interferon-gamma (IFN-γ) have been shown to be augmented in GI/GU pathology and to play a role in altering epithelial barrier function in a number of tissues [25,26]. Our finding that LP pretreatment decreased the acrolein-induced changes in UT-IFN-γ suggests a protective effect against inflammation. Inflammation or injury may generate free phospholipids from cell membrane at the site of injury that can reduce membrane-damaging mediators and enhance membrane barrier function [27]. In this regards, studies in mice with hypercholesterolemia-induced microvascular and macrovascular endothelial cell dysfunction showed significant improvement and restored function after intravenous phospholipid therapy [28]. In addition, in vitro studies have reported that phospholipid liposomes also have the ability to extract un-esterified cholesterol from cellular membranes that alters both cellular membrane structure and calcium influx [29]. Liposome treatment may also modify the functional properties of cellular membranes thus restoring the lipid content in the plasma membrane of cultured cells [30]. Apart from transmembrane uroplakin proteins, the lipids in the apical membrane of umbrella cells, an uppermost layer of the UT, are also an integral component of the permeability barrier in the bladder [31]. Similar to that occurring in other organs [32], pathology-induced stimulation of lipid synthesis may also occur in the lower urinary tract, which may serve to augment the urothelial barrier function.
Conclusions
While a number of possibilities exist and are worth exploring, the therapeutic efficacy of liposomes may be due to ability to supplement or restore the urothelial membrane. In addition, our findings also suggest an energy dependent endocytotic process is involved in part, in the liposomal uptake across the urothelial membrane. Taken together, our preliminary evidence provides support for liposome-therapy for urothelial protection and possible repair. This is likely to be important in a number of bladder conditions involving epithelial dysfunction caused by chronic infection or disease.
Acknowledgments
This work was supported in part by NIH grants R37 DK54824 and R01 DK57284 (LAB), R01 DK083323 and a Department of Defense Grant (MBC), grants from the Urology Care Foundation Research Scholars Program and the Allergan Foundation (NJ) and the Kidney Imaging Core of the Pittsburgh Center for Kidney Research (P30-DK079307).
Footnotes
Conflict of Interest: The authors declare they have no conflict of interest.
References
- 1.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nirmal J, Tyagi P, Chancellor MB, et al. Development of potential orphan drug therapy of intravesical liposomal tacrolimus for hemorrhagic cystitis due to increased local drug exposure. J Urol. 2013;189:1553–8. doi: 10.1016/j.juro.2012.10.123. [DOI] [PubMed] [Google Scholar]
- 3.Cox PJ. Cyclophosphamide cystitis and bladder cancer. A hypothesis. Eur J Cancer. 1979;15:1071–2. doi: 10.1016/0014-2964(79)90296-2. [DOI] [PubMed] [Google Scholar]
- 4.Grinberg-Funes DJ, Sheldon C, Weiss M. The use of prostaglandin F2 alpha for the prophylaxis of cyclophosphamide induced cystitis in rats. J Urol. 1990;144:1500–4. doi: 10.1016/s0022-5347(17)39786-0. [DOI] [PubMed] [Google Scholar]
- 5.Veerasarn V, Boonnuch W, Kakanaporn C. A phase II study to evaluate WF10 in patients with late hemorrhagic radiation cystitis and prostatitis. Gynecol Oncol. 2006;100:179–84. doi: 10.1016/j.ygyno.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Chuang YC, Lee WC, Lee WC, Chiang PH. Intravesical liposome versus oral pentosan polysulfate for interstitial cystitis/painful bladder syndrome. J Urol. 2009;182:1393–400. doi: 10.1016/j.juro.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Tyagi P, Chancellor M, Yoshimura N, Huang L. Activity of different phospholipids in attenuating hyperactivity in bladder irritation. BJU Int. 2008;101:627–32. doi: 10.1111/j.1464-410X.2007.07334.x. [DOI] [PubMed] [Google Scholar]
- 8.Fraser MO, Chuang Y, Tyagi P, et al. Intravesical liposome administration--a novel treatment for hyperactive bladder in the rat. Urology. 2003;61:656–63. doi: 10.1016/s0090-4295(02)02281-1. [DOI] [PubMed] [Google Scholar]
- 9.Federico C, Morittu VM, Britti D, et al. Gemcitabine-loaded liposomes: rationale, potentialities and future perspectives. Int J Nanomedicine. 2012;7:5423–36. doi: 10.2147/IJN.S34025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chopra B, Barrick SR, Meyers S, et al. Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J Physiol. 2005;562:859–71. doi: 10.1113/jphysiol.2004.071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birder L, Andersson KE. Urothelial Signalling. Physiol Rev. 2013;93:653–80. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashyap M, Kawamorita N, Tyagi V, et al. Down-regulation of nerve growth factor expression in the bladder by antisense oligonucleotides as new treatment for overactive bladder. J Urol. 2013;190:757–64. doi: 10.1016/j.juro.2013.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazzeri M. The physiological function of the urothelium--more than a simple barrier. Urol Int. 2006;76:289–95. doi: 10.1159/000092049. [DOI] [PubMed] [Google Scholar]
- 14.Teichman JM, Moldwin R. The role of the bladder surface in interstitial cystitis/painful bladder syndrome. Can J Urol. 2007;14:3599–607. [PubMed] [Google Scholar]
- 15.Parsons CL, Greenberger M, Gabal L, et al. The role of urinary potassium in the pathogenesis and diagnosis of interstitial cystitis. J Urol. 1998;159:1862–6. doi: 10.1016/S0022-5347(01)63178-1. [DOI] [PubMed] [Google Scholar]
- 16.Kuo YC, Kuo HC. Potential factors that can be used to differentiate between interstitial cystitis/painful bladder syndrome and bladder oversensitivity in women. Int J Clin Pract. 2012;66:146–51. doi: 10.1111/j.1742-1241.2011.02767.x. [DOI] [PubMed] [Google Scholar]
- 17.Tang MS, Wang HT, Hu Y, et al. Acrolein induced DNA damage, mutagenicity and effect on DNA repair. Mol Nutr Food Res. 2011;55:1291–300. doi: 10.1002/mnfr.201100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Botteman MF, Pashos CL, Redaelli A, et al. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21:1315–30. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 19.Tyagi P, Hsieh VC, Yoshimura N, et al. Instillation of liposomes vs dimethyl sulfoxide or pentosan polysulphate for reducing bladder hyperactivity. BJU Int. 2009;104:1689–92. doi: 10.1111/j.1464-410X.2009.08673.x. [DOI] [PubMed] [Google Scholar]
- 20.Yang F, Jin C, Jiang Y, et al. Liposome based delivery systems in pancreatic cancer treatment: from bench to bedside. Cancer Treat Rev. 2011;37:633–42. doi: 10.1016/j.ctrv.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Gregoriadis G, Jain S, Papaioannou I, et al. Improving the therapeutic efficacy of peptides and proteins: a role for polysialic acids. Int J Pharm. 2005;300:125–30. doi: 10.1016/j.ijpharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Lee WC, Chuang YC, Lee WC, et al. Safety and dose flexibility clinical evaluation of intravesical liposome in patients with interstitial cystitis or painful bladder syndrome. Kaohsiung J Med Sci. 2011;27:437–40. doi: 10.1016/j.kjms.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Nickel JC, Downey J, Morales A, et al. Relative efficacy of various exogenous glycosaminoglycans in providing a bladder surface permeability barrier. J Urol. 1998;160:612–14. [PubMed] [Google Scholar]
- 24.Fraser MO, Chuang HC, Tyagi P, et al. Intravesical liposome administration- a novel treatment for hyperactive bladder in the rat. Urology. 2003;61:656–63. doi: 10.1016/s0090-4295(02)02281-1. [DOI] [PubMed] [Google Scholar]
- 25.Penrose HM, Marchelletta RR, Krishnan M, et al. Spermidine stimulates T cell protein-tyrosine phosphatase-mediated protection of intestinal epithelial barrier dysfunction. J Biol Chem. 2013;288:32651–62. doi: 10.1074/jbc.M113.475962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smaldone MC, Vodovotz Y, Tyagi V, et al. Multiplex analysis of urinary cytokine levels in rat model of cyclophosphamide-induced cystitis. Urology. 2009;73:421–6. doi: 10.1016/j.urology.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker P, Rye KA, Gamble JR, et al. Phospholipid composition of reconstituted high density lipoproteins influences their ability to inhibit endothelial cell adhesion molecule expression. J Lipid Res. 2000;41:1261–7. [PubMed] [Google Scholar]
- 28.Williams KJ, Scalia R, Mazany KD, et al. Rapid restoration of normal endothelial functions in genetically hyperlipidemic mice by a synthetic mediator of reverse lipid transport. Arterioscler Thromb Vasc Biol. 2000;20:1033–9. doi: 10.1161/01.atv.20.4.1033. [DOI] [PubMed] [Google Scholar]
- 29.Bialecki RA, Tulenko TN, Colucci WS. Cholesterol enrichment increases basal and agonist-stimulated calcium influx in rat vascular smooth muscle cells. J Clin Invest. 1991;88:1894–900. doi: 10.1172/JCI115512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soloviev AI, Stefanov AV, Bazilyuk OV, et al. Phospholipid vesicles (liposomes) restore endothelium-dependent cholinergic relaxation in thoracic aorta from spontaneously hypertensive rats. J Hypertens. 1993;11:623–7. doi: 10.1097/00004872-199306000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Truschel ST, Ruiz WG, Shulman T, et al. Primary uroepithelial cultures. A model system to analyze umbrella cell barrier function. J Biol Chem. 1999;274:15020–9. doi: 10.1074/jbc.274.21.15020. [DOI] [PubMed] [Google Scholar]
- 32.Nonas S, Miller I, Kawkitinarong K, et al. Oxidized phospholipids reduce vascular leak and inflammation in rat model of acute lung injury. Am J Respir Crit Care Med. 2006;173:1130–8. doi: 10.1164/rccm.200511-1737OC. [DOI] [PMC free article] [PubMed] [Google Scholar]