Abstract
Schizophrenia is associated with motivational deficits that interfere with a wide range of goal directed activities. Despite their clinical importance, our current understanding of these motivational impairments is limited. Furthermore, different types of motivational problems are commonly seen among individuals within the broad diagnosis of schizophrenia. The goal of the current study was to examine whether clinically meaningful subgroups could be identified based on approach and avoidance motivational tendencies. We measured these tendencies in 151 individuals with schizophrenia. Although prior studies demonstrate elevated BIS sensitivity in schizophrenia at the overall group level, none have explored various combinations of BIS/BAS sensitivities within this disorder. Cluster analyses yielded five subgroups with different combinations of low, moderate, or high BIS and BAS. The subgroups had interpretable differences in clinically rated negative symptoms and self-reported anhedonia/socio-emotional attitudes, which were not detectable with the more commonly used linear BIS/BAS scores. Two of the subgroups had significantly elevated negative symptoms but different approach/avoidance profiles: one was characterized by markedly low BIS, low BAS and an overall lack of social approach motivation; the other had markedly high BIS but moderate BAS and elevated social avoidance motivation. The two subgroups with relatively good clinical functioning showed patterns of BAS greater than BIS. Our findings indicate there are distinct motivational pathways that can lead to asociality in schizophrenia and highlight the value of considering profiles based on combined patterns of BIS and BAS in schizophrenia.
Keywords: schizophrenia, motivation, behavioral approach and avoidance, social anhedonia, negative symptoms, BIS/BAS
1. Introduction
Schizophrenia is associated with deficits in initiating and persisting in a wide range of goal directed activities in the social, vocational, and independent living realms (Blanchard et al., 2011). Although it is believed that these difficulties stem largely from disturbances in motivation, our understanding of motivational impairments in schizophrenia is limited. In addition, it is well known that schizophrenia is a heterogeneous disorder and the causes of problems in motivation can differ across individuals. For example, some patients show a profound disinterest in social interactions in the apparent absence of loneliness or other negative emotions, while others are interested in social connections but avoid engaging in social activities because of fear of rejection, social anxiety, or concerns about the harmful intentions of others (Horan and Blanchard, 2003; Horan et al., 2006b). Building on affective science models of motivation, we attempted to identify valid subgroups of schizophrenia patients with different motivational profiles.
Across several prominent models of motivation, a basic distinction is made between behavioral approach and behavioral avoidance (Gable and Gosnell, 2013; Gray, 1987; Spielberg et al., 2013). According to J.A. Gray’s model, behavioral approach (i.e. behavioral activation system; BAS) relies on a reward system sensitive to appetitive stimuli and the termination of punishment. Behavioral avoidance (i.e. behavioral inhibition system; BIS), in contrast, is sensitive to aversive stimuli and activated by anxiety, novelty, and innate fear stimuli and is responsible for ceasing or inhibiting behavior. These systems are thought to be relatively independent and to rely on distinct neurobiological substrates (Coan and Allen, 2003; Peterson et al., 2008; Sutton and Davidson, 1997). Gray’s original approach and avoidance model (Gray, 1987) has been extensively studied with the BIS/BAS self-report scales (Carver and Whilte, 1994). On these scales, psychologically healthy people tend to score in the middle for both BIS and BAS sensitivities (Johnson et al., 2003; Mitchell and Nelson-Gray, 2006). However, extreme scores on either scale are associated with various forms of psychopathology. For example, depression is frequently associated with diminished BAS, mania is associated with elevated BAS, and certain anxiety disorders are associated with elevated BIS (Bijttebier et al., 2009; Kasch et al., 2002; Mitchell and Nelson-Gray, 2006).
Although most studies of psychopathology have considered BIS and BAS scores as separate continuous variables, individuals can show different combinations across high, medium, and low levels of both BIS and BAS. According to the joint subsystems hypothesis (Corr, 2001, 2002), the BIS and BAS are conceptualized as interdependent systems and behavioral outcomes are predicted to depend on the strengths of the BIS and BAS systems in relation to each other. Consistent with this hypothesis, initial studies in clinical populations also suggest maladaptive behaviors, such as anxiety and impulsivity, may be better explained with categorical profiles in which BIS or BAS overpowers the other system (Corr, 2002; Nash et al., 2012; Newman et al., 2005).
The BIS/BAS scales have been used in only a few studies of schizophrenia, all of which treated the scales as separate continuous variables. Compared to healthy controls, individuals with schizophrenia report higher BIS sensitivity and no difference in BAS sensitivity (Barch et al., 2008; Horan et al., 2006b; Scholten et al., 2006; Strauss et al., 2011). However, as noted above, schizophrenia is a heterogeneous disorder in which motivational difficulties may reflect different mechanisms, and no studies have explored unique BIS/BAS profiles within this population. Examining BIS and BAS as joint systems within a large schizophrenia sample may help identify sub-groups with distinctive motivational impairments. For example, recent studies suggest that a categorical approach to motivation-related variables, such as negative symptoms, may show greater validity and clinical utility than a continuous approach (Deserno et al., 2013; Strauss and Gold, 2012; Strauss et al., 2013).
The goal of the current study was to examine whether clinically meaningful subgroups of people with schizophrenia could be identified based on BIS and BAS sensitivities. The validity of motivation-based subgroups was evaluated with respect to clinical symptoms, socio-emotional attitudes, and functional outcomes. We were particularly interested in whether two separable motivation profiles could be distinguished: one rooted in social disinterest and another in active social avoidance.
2. Method
2.1. Participants
Participants included 151 community outpatients diagnosed with schizophrenia (N=131) or schizoaffective disorder (N=20) as determined with the Structured Clinical Interview for DSM-IV (First et al., 1997). Exclusion criteria included mood episode within the past month; substance dependence in the past 6 months; substance abuse in the past month; IQ < 70; history of head injury or neurological disorder. The sample was 57% male with a mean age of 47 (9.5) and average length of illness of 24 (11.5) years. The sample had an average of 12.6 (2.5) years of education, and 13.9 (3.7) years of parental education. Fifty-percent of the sample was African-American, 40% was Caucasian, and 10% was Asian, multi-racial, or other. All participants were receiving antipsychotic medications at clinically determined dosages.
2.2. Procedure
Participants were recruited at four sites as part of a larger study designed to validate a new negative symptom instrument (Kring et al., 2013). After the informed consent process (approved by each site’s Institutional Review Board), participants were administered self-report measures, clinical rating scales, and functional outcome assessments in a fixed order. Interviewers were credentialed for all clinical rating scales with videotaped and in-person co-rated interviews. The current paper presents a secondary analysis to explore the clinical characteristics of subgroups of patients who were classified based on self-reported BIS/BAS sensitivities. To facilitate interpretation, all measures are scaled so that higher scores indicate more severe impairment.
2.3. Measures
2.3.1 Motivation
The Behavioral Inhibition/Behavioral Activation System Scale (BIS/BAS; Carver and Whilte, 1994) is a 24-item self-report measure (items rated 1 – 4) of behavioral avoidance and approach tendencies with established reliability. Sample BIS items include “I feel worried when I think I have done poorly at something,” “Criticism or scolding hurts me quite a bit,” and “I worry about making mistakes.” Sample items from the BAS include “I crave excitement and new sensations,” “I go out of my way to get the things I want,” and “When I see an opportunity for something I like, I get excited right away.” The BAS includes three subscales as well as a total scale.
2.3.2. Anhedonia
The Temporal Experience of Pleasure Scale (TEPS; Gard et al., 2006) is an 18-item Likert-type self-report scale (items rated 1-6) with separate anticipatory and consummatory dispositional pleasure subscales (e.g., “I look forward to a lot of things in my life”). The Social Anhedonia Scale – Brief (SAS; Reise et al., 2011) is a 24-item (dichotomously scored) self-report measure for assessing decreased social pleasure. The SAS includes three subscales that measure distinct aspects of disturbances in social affiliation: Friends not valued measures lack of interest in social connections and diminished effort to initiate and sustain relationships (e.g., “Making new friends isn’t worth the energy it takes”). Aloofness, in contrast, measures the extent to which one finds social interactions aversive and actively avoids them (e.g., “People sometimes think that I am shy when I really just want to be left alone”). The third subscale, Preference for solitude, measures a general preference for activities that do not involve other people (e.g., “I prefer hobbies and leisure activities that do not involve other people”).
2.3.3. Clinical Symptoms
The 24-item Brief Psychiatric Rating Scale (BPRS; Overall and Gorham, 1962) assessed positive and depression symptoms (Kopelowicz et al., 2008). The Clinical Assessment Interview for Negative Symptoms (CAINS; Horan et al., 2011) is a 13-tem instrument that yields two subscales, Motivation and Pleasure (MAP) and Expression, which measure the two primary negative symptom factors.
2.3.4. Functioning
Functional capacity was assessed with the brief version of the UCSD Performance-Based Skills Assessment (UPSA-Brief; Mausbach et al., 2007), which involves performing tasks involving communication and financial skills. A standardized summary score (0 – 50; based on percent correct in each domain) was computed. Current functioning was assessed with the Role Functioning Scale (RFS; McPheeters, 1984) using a semi-structured interview format. The RFS includes separate ratings for Working Productivity, Independent Living, Family Network Relationships, and Social Network Relationships (rated on a scale from 1 – 7). To reduce multiple comparisons we combined Work and Independent Living into a single variable and Family Network and Social Network into a single variable (mean of subscale scores) based on prior studies (e.g., Kee et al., 2003).
2.4. Data analysis
Prior to identifying subgroups, we searched for outliers in the bi-dimensional space of the variables BIS-Total and BAS-Total using Malahanobis distance since cluster analysis can produce small splinter groups if there are extreme observations. Two outliers were identified, but they were not sufficiently extreme to affect the final results so we left them in the analyses. Second, we performed hierarchical cluster analysis on the z-scores for the BIS-Total and BAS-Total using Ward’s method (Ward, 1963). Ward’s method is a bottom-up clustering algorithm that sequentially merges observations or groups of observations at each step while minimizing the growth in the total error sum of squares (i.e., within cluster variance). We chose Ward’s method because studies comparing the ability to recover original group structure across hierarchical clustering methods have shown that Ward’s method performs as well or better (Blashfield, 1976; Hands and Everitt, 1987; Kuiper and Fisher, 1975) and is resistant to the presence of outliers (Milligan, 1996).
Ward’s method produces a nested collection of clusterings ranging from n groups (each point by itself) to 1 group (all points together). We used a combination of (i) visual inspection of the dendogram and scatterplots, which show the relative tightness and separation of the resulting clusters, and (ii) clinical interpretability and distinctness of the groups to identify an optimal number of clusters. To examine the stability of the cluster solution we compared the results of Ward’s hierarchical agglomerative method with the standard global partitioning method, K-means (Hartigan, 1975), and evaluated the agreement between the solutions of both methods using the Adjusted Rand index (Hubert and Arabie, 1985).
Finally, we examined the relationship between BIS/BAS and the external variables in two ways. We initially examined the BIS and BAS as separate dimensions and calculated their correlations with external variables. We then used the cluster partition for the BIS/BAS, and conducted ANOVAs and post-hoc comparisons to evaluate differences among the clusters for demographic and external variables.
3. Results
3.1. Cluster analysis
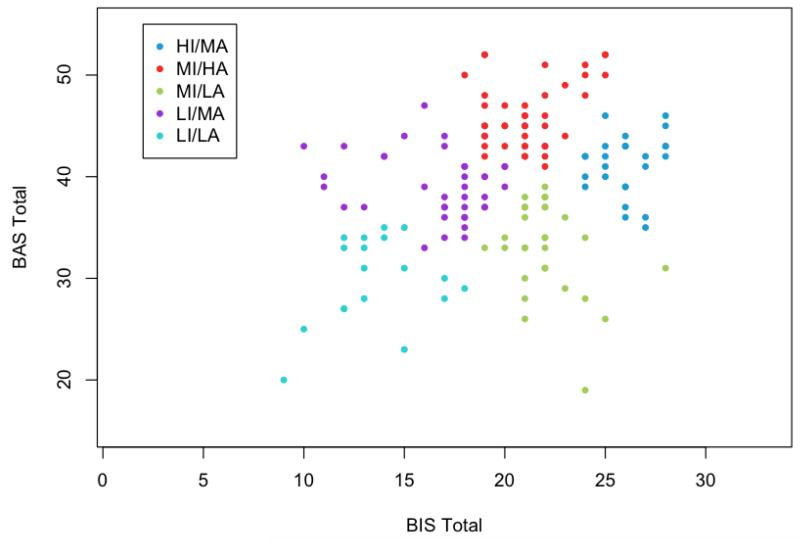
Visual inspection of the dendogram indicated the 5-cluster solution was optimal as that was the point of diminishing returns for improving cluster tightness (total error sum of squares) by including additional groups1, and examination of the scatterplots of the various solutions indicated the 5-cluster solution provided the best separation (Figure 1). Furthermore, the corresponding cluster centroids, which give the average BIS-BAS profile for the group members, were clinically distinct and meaningful. The stability of the 5-cluster solution was supported by strong agreement between the Hierarchical and K-means methods (Adjusted Rand Index = 0.93).
Figure 1.
BIS and BAS score distributions for the five clusters.
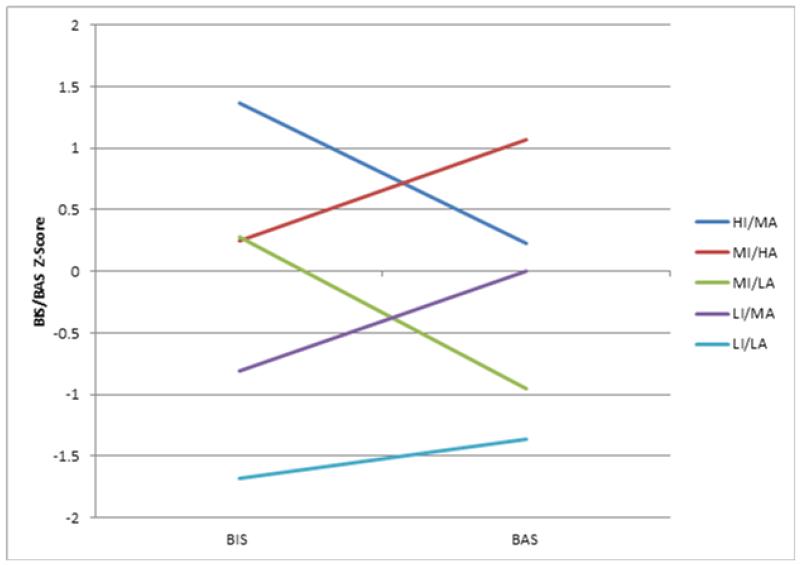
Next we examined the five separable BIS/BAS subgroups for clinical interpretation. For interpreting and labeling the subgroups, we defined “low” as at least 1 standard deviation (SD) below the mean for the entire sample and “high” at least 1 SD above the sample mean (BIS mean = 20.2 (SD = 4.4) and BAS mean = 39.0 (SD = 6.6)). “Moderate” levels of BIS and BAS sensitivity were within 1 SD above or below our sample mean. Our means and standard deviations are consistent with those reported in the schizophrenia literature (Barch et al., 2008; Horan et al., 2006a; Strauss et al., 2011) and the non-psychiatric community (Jorm et al., 1998; Kasch et al., 2002). Thus, we assume the low, moderate, and high BIS/BAS levels would be similarly classified in other samples. We named the subgroups in accordance with the relative BIS and BAS levels, finding distinct combinations of BIS and BAS scores for all subgroups (see Table 1). The labels for the five subgroups are as follows: 1) Low Inhibition/Low Activation (LI/LA; n=15); 2) Low Inhibition/Moderate Activation (LI/MA; n=37); 3) Moderate Inhibition/Low Activation (MI/LA; n=32); 4) Moderate Inhibition/High Activation (MI/HA; n=42); and 5) High Inhibition/Moderate Activation (HI/MA; n=25) (see Figure 2). We compared the subgroups on demographic variables and found no differences in diagnosis, sex, age, or parental education.
Table 1. Descriptives for BIS/BAS raw scores in the five subgroups.
| N | BIS | BAS | BAS-Drive | BAS-Fun Seeking |
BAS- Reward Responsivity |
|
|---|---|---|---|---|---|---|
| Moderate Inhibition/ Low Activation (MI/LA) |
32 | 21.4 (1.8) | 32.7 (4.5) | 9.0 (2.3) | 8.6 (1.8) | 15.1 (2.7) |
| Low Inhibition/ Low Activation (LI/LA) |
15 | 12.8 (1.7) | 30.0 (4.7) | 8.1 (1.8) | 9.1 (2.4) | 12.7 (4.0) |
| Low Inhibition/ Moderate Activation (LI/MA) |
37 | 16.6 (2.7) | 39.1 (3.2) | 10.9 (2.4) | 11.2 (1.8) | 16.9 (1.8) |
| Moderate Inhibition/ High Activation (MI/HA) |
42 | 21.2 (2.0) | 49.1 (3.2) | 13.5 (1.7) | 13.8 (1.8) | 18.8 (1.4) |
| High Inhibition/ Moderate Activation (HI/MA) |
25 | 26.2 (1.4) | 40.6 (3.5) | 11.0 (2.6) | 11.4 (1.9) | 18.1 (1.6) |
Note: Standard deviations are presented in parentheses.
Figure 2.
BIS/BAS Z-score profiles for the five subgroups.
3.2. External variables as a function of BIS/BAS
3.2.1. Correlations
The correlations are shown in Table 2. The BIS linear scale was significantly related to CAINS expression, BPRS depression, and SAS social aloofness. The BAS linear scale was significantly related to TEPS anticipatory and consummatory pleasure, and the SAS friends not valued subscale.2 The magnitudes of the correlations were generally low (i.e., r < 0.3), except for the slightly higher correlations between the TEPS subscales and BAS.
Table 2. Correlations between raw BIS/BAS scores and external variables.
| BIS | BAS | BAS-Drive | BAS-Fun Seeking |
BAS- Reward Responsivity |
|
|---|---|---|---|---|---|
| SAS Social Aloofness | .25** | .04 | .07 | .09 | −.07 |
| SAS Close Friends Not Valued |
−.09 | −.20* | −.15* | −.19* | −.15* |
| SAS Prefers Solitude | .08 | −.14 | −.08 | −.10 | −.14 |
| TEPS Anticipatory | .14 | .43** | .27** | .32** | .41** |
| TEPS Consummatory | .14 | .43** | .25** | .25** | .31** |
| BPRS Positive | −.04 | −.07 | .01 | −.06 | −.10 |
| BPRS Depression | .17* | −.04 | .04 | −.04 | −.09 |
| CAINS Expression | .29** | .15 | .10 | .13 | .12 |
| CAINS MAP | .09 | −.08 | −.06 | −.07 | −.08 |
| RFS Work / Independent Living |
.04 | .00 | −.02 | −.02 | .08 |
| RFS Family / Social Network |
.01 | −.03 | −.07 | .02 | .02 |
| UPSA - B | .15 | .01 | −.04 | −.01 | .07 |
Note:
p < .05
p < .01
3.2.2. Cluster Comparisons
As seen in Table 3, the subgroups showed significant differences on most of the external measures. The exceptions were BPRS positive and depression symptoms, the RFS, and the UPSA-B. Pairwise comparisons (post-hoc LSD) revealed several significant differences between subgroups. To interpret the group differences, we looked for patterns across the variables, which are displayed graphically for symptoms and functioning in Figure 3 and for the anhedonia scales in Figure 4.
Table 3. Z-score means (SD) on external variables for the five BIS/BAS-defined subgroups.
| MI/LA | LI/LA | LI/MA | MI/HA | HI/MA | Test statistic (ANOVA) |
|
|---|---|---|---|---|---|---|
| SAS Social Aloofness | .04 (1.1) | −.30 (1.2)b | −.30 (.85)b | .06 (1.1) | .45 (.72)a | F = 2.60, p = .04 |
| SAS Close Friends Not Valued |
.38 (1.1)a | .44 (1.2)a | −.18 (.85)b | −.27 (.84)b | .07 (1.1) | F = 3.10, p = .02 |
| SAS Prefers Solitude | .25 (1.0)a | .19 (1.2) | −.30 (.96)b | −.19 (.96)b | .35 (1.0)a | F = 2.60, p = .04 |
| TEPS Anticipatory | .41 (1.0)b | .47 (1.3)b | .02 (.80)b | −.66 (.70)a | −.01 (1.0)b | F = 7.70, p<.001 |
| TEPS Consummatory | .17 (1.0)b | .68 (1.0)b | .13 (.86)b | −.57 (.82)a | .17 (1.0)b | F = 6.57, p < .001 |
| BPRS Positive | −.16 (.80) | .24 (1.2) | −.13 (.98) | −.08 (.94) | .24 (1.1) | F = 1.01, p = .41 |
| BPRS Depression | .02 (.84) | −.12 (.97) | −.22(.83)b | −.16(1.0)b | .35(1.3)a | F = 1.47, p = .21 |
| CAINS Expression | −.04 (.87)b | −.36 (1.0)b | −.33(.84)b | .11 (1.1) | .48 (1.1)a | F = 3.15, p =.02 |
| CAINS MAP | .01 (1.0) | .35 (.83)a | −.27(.87)b | −.22 (.98)b | .50 (1.1)a | F = 3.36, p = .01 |
| RFS Work/Independent Living |
−.18 (.97)b | .52 (.85)a | −.10 (1.0)b | −.02 (.95) | .22 (1.1) | F = 1.73, p = .15 |
| RFS Family/Social Network | −.08 (1.1) | .08 (.79) | −.04 (.90) | .09 (1.1) | .10 (1.1) | F = 0.20, p = .94 |
| UPSA-B (Functional Capacity) |
−.06 (1.0) | .12 (1.0) | .10 (.99) | .02 (1.0) | −.04 (1.0) | F = 0.15, p = .96 |
Notes: All measures are scaled such that higher scores indicate greater impairment.
Signify significantly different post-hoc pairwise comparisons with LSD corrections.
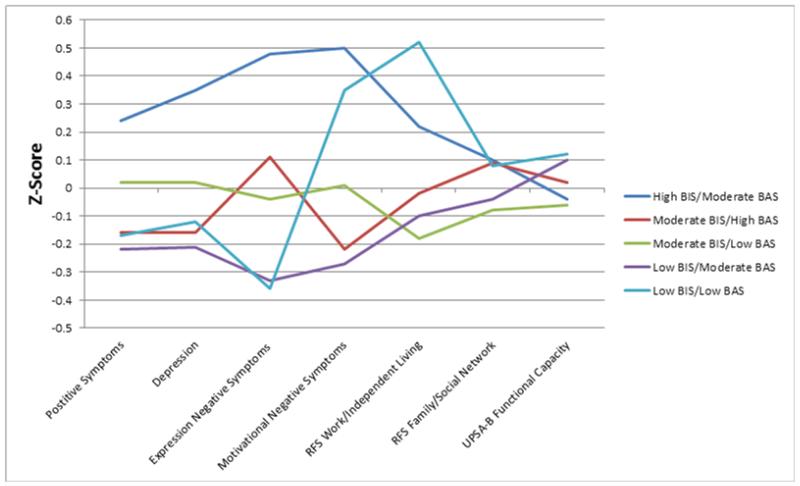
Figure 3.
Symptom and functional outcomes in the five subgroups. Note: Higher z-scores indicate greater impairment.
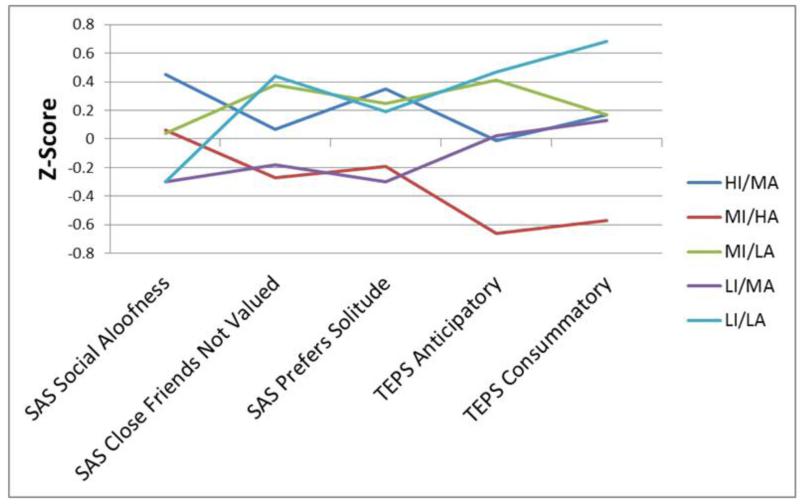
Figure 4.
Scores on the SAS and TEPS subscales in the five subgroups. Note: Higher z-scores indicate greater impairment.
Two groups appeared the most impaired across the variables. First, the HI/MA group was most symptomatic in terms of negative symptoms. It also had the highest scores on SAS social aloofness and preference for solitude subscales. This group showed higher depression symptoms than the LI/MA and MI/HA groups, though this finding must be interpreted cautiously because the omnibus F-test for the BPRS depression subscale was not significant. Second, the LI/LA subgroup had relatively high CAINS MAP negative symptoms, the least amount of anticipatory and consummatory pleasure (TEPS), and the highest SAS close friends not valued score. Examination of Figure 1 shows that this subgroup also had worse impaired RFS work/independent living scores than MI/LA and LI/MA, though this also should be interpreted with caution as the omnibus test was not significant.
In contrast to these two subgroups, the LI/MA and MI/HA subgroups showed generally low levels of severity on all clinical symptom and community functioning measures. Notably, both of these subgroups were characterized by greater approach than avoidance tendencies. The MI/HA subgroup was also characterized by the most anticipatory and consummatory pleasure on the TEPS.
The fifth subgroup, MI/LA, differed the least from the other subgroups in clinical symptoms, consummatory pleasure, and social anhedonia. This group had a relatively high level of variability on BAS among its members (see Figure 1). It shows the same pattern as HI/MA (Figure 2) in that BIS was greater than BAS, but it had relatively lower levels of both BIS and BAS sensitivity.
4. Discussion
This is the first examination of BIS/BAS profiles in schizophrenia. We initially examined BIS and BAS as dimensional variables, and found few clearly interpretable patterns of correlations with external variables. We then identified a well-supported five-cluster solution that classified participants according to different levels of BIS and BAS scores. We found interpretable subgroups in terms of negative symptoms, socio-emotional attitudes, and social anhedonia. The subgroups clearly did not simply reflect differences in overall clinical severity because the five subgroups were equivalent in terms of positive symptoms and functional capacity.
Although prior studies have reported higher BIS in schizophrenia as a group (Barch et al., 2008; Horan et al., 2006a; Strauss et al., 2011), the current findings indicate that it may be informative to consider subgroups with either markedly high or low BIS scores, as both extremes are associated with impaired social motivation. LI/LA and HI/MA showed the most substantial negative symptoms and tended to have the poorest functioning. They showed distinct patterns on BAS and the anhedonia subscales, suggesting these subgroups reflect two types of disturbances in social motivation: HI/MA appears primarily motivated by avoidance tendencies whereas LI/LA is characterized by a lack of approach motivation.
HI/MA demonstrated elevated avoidance motivation. This subgroup reported the highest levels of negative symptoms, as well as the most social aloofness on the SAS. Even though this subgroup appears interested in relationships, they tend to describe them as being more trouble than they are worth (e.g., evoking anxiety and/or fear of rejection), and they avoid interpersonal interactions because they are viewed as aversive. In contrast to pronounced avoidance motivation, LI/LA is marked by low approach motivation, showing the lowest BAS scores and the most impaired anticipatory and consummatory pleasure. This subgroup also endorsed high levels of social anhedonia attributable to not valuing close friends, which reflects diminished interest in people and diminished drive to develop close interpersonal attachments. Interestingly, within the context of the joint subsystems hypothesis, psychopathology is generally associated with one system, either BIS or BAS, overpowering the other. Unlike other examples from psychopathology in which one system dominates, the LI/LA subgroup shows particularly poor clinical and behavioral functioning in the context of similarly diminished BIS and BAS. In this schizophrenia subgroup, poor clinical and community functioning appears to reflect generally diminished motivation of any kind. This profile is similar to definitions of the deficit syndrome in schizophrenia (Kirkpatrick et al., 2001).
Another primary finding is that a profile characterized by relatively higher BAS than BIS may be protective. The MI/HA and LI/MA subgroups stand out with relatively low negative symptoms and better functioning. Although their profiles show many similarities with regard to external variables, they show some notable differences. In particular, MI/HA endorsed high anticipatory pleasure and low motivational negative symptoms. The relatively high BAS sensitivity in MI/HA and LI/MA suggests that they are more motivated by pursuing rewards than avoiding punishments. There is a strong link between approach motivation (Elliot and Thrash, 2002; Nash et al., 2012) and general resilience, and high BAS has been found to longitudinally predict recovery from a depressive episode (Kasch et al., 2002). When considered in the context of the joint systems hypothesis, relatively elevated BAS in schizophrenia appears to override the BIS-driven inhibitory responses and protect against social withdrawal or defeatist beliefs. Additionally, a revised theory incorporates a third system: the fight, flight, freeze system (FFFS) which is responsive to punishment, as the BIS was in the original model (Gray & McNaughton, 2000). In the new model, BIS serves to resolve goal conflicts between the FFFS and BAS when situations include both threat and reward (Bijttebier et al., 2009). It will be useful to conduct future studies on motivation in schizophrenia using this updated theoretical framework.
The current study has several limitations. First, the cross-sectional design prohibits assessment of causal pathways between BIS/BAS sensitivities and the other variables. Although research indicates BIS/BAS scores have high temporal stability in clinical and non-clinical populations (Kasch et al., 2002), BIS/BAS may represent dispositions that contribute to, or result from, the social and motivational impairments in schizophrenia. Second, the patients were chronically ill and it is unclear whether similar results would be found in the early course of illness. Third, all patients were medicated at clinically determined dosages and the impact of medications on the current results is not clear.
Overall, the categorical approach to BIS/BAS subgroups appeared to provide several advantages compared with the conventional approach, which treats BIS and BAS as orthogonal, continuous variables. Continuous BIS and BAS scores showed generally weak and non-significant relationships with self-reported socio-emotional processes and symptoms. In contrast, the BIS/BAS subgroups showed a number of clinically meaningful differences on these variables. The finding that either high or low BIS, in combination with different BAS levels, were both associated with elevated experiential negative symptoms is intriguing and could not have been identified using continuous BIS/BAS scores. The different profiles of socio-emotional traits in these two subgroups (LI/LA, HI/MA) yielded clinically sensible patterns of inter-correlations and they point toward different treatment approaches. Whereas patients with a LI/LA profile might benefit most from interventions focused on behavioral activation and anticipatory pleasure, those in the HI/MA group might benefit more from interventions that address anxiety and self-defeating beliefs that hold them back from engaging in rewarding activities. By identifying meaningful subgroups, the categorical approach used in this paper helps address the vexing issue of heterogeneity in a manner that has clinically useful, more personalized treatment implications for individuals with schizophrenia.
Supplementary Material
Acknowledgements
None
Role of funding source
This work was supported in part by NIH grant 1R01MH082890 to Dr. Kring, grants 1R01MH082783 and RO1 MH08272 to Dr. Gur, grant 1R01MH082839 to Dr. Blanchard, and grant 1R01MH082782 to Dr. Horan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental cluster analyses using the three BAS subscales (i.e. Drive, Fun Seeking, and Reward Responsiveness) yielded essentially the same optimal five cluster solution as the primary analyses based on BAS Total scores.
Supplemental bivariate correlational analyses using the three BAS subscales yielded essentially the same pattern of correlations and significance values as the BAS Total score.
Contributors
Authors J.J.B, R.E.G., A.M.K., and W.P.H. designed and conducted the original study, and L.F.R., M.F.G, and W.P.H. conceptualized the secondary analysis. Authors C.A.S. and S.R. conducted the statistical analyses, and authors L.F.R., W.P.H., and M.F.G. wrote the draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest
Dr. Blanchard has received honoraria and travel support from F. Hoffmann-La Roche and Genentech for consulting
Dr. Blanchard has received honoraria and travel support from Merck and Genentech for consulting. The other authors report no competing interests.
Dr. Green has been a consultant to AbbVie, Biogen, DSP, and Roche, and he is on the scientific advisory board of Mnemosyne. He has received research funds from Amgen.
All other authors: None
References
- Barch DM, Yodkovik N, Sypher-Locke H, Hanewinkel M. Intrinsic motivation in schizophrenia: relationships to cognitive function, depression, anxiety, and personality. J. Abnorm. Psychol. 2008;117(4):776–787. doi: 10.1037/a0013944. [DOI] [PubMed] [Google Scholar]
- Bijttebier P, Beck I, Claes L, Vandereycken W. Gray’s Reinforcement Sensitivity Theory as a framework for research on personality-psychopathology associations. Clin. Psychol. Rev. 2009;29(5):421–430. doi: 10.1016/j.cpr.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Kring AM, Horan WP, Gur R. Toward the next generation of negative symptom assessments: the collaboration to advance negative symptom assessment in schizophrenia. Schizophr.Bull. 2011;37(2):291–299. doi: 10.1093/schbul/sbq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blashfield RK. Mixture model tests of cluster analysis: accuracy of four agglomerative hierarchical methods. Psychol. Bull. 1976;83:377–388. [Google Scholar]
- Carver CS, Whilte TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J. Pers. Soc. Psychol. 1994;67:319–333. [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiol. 2003;40(1):106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Corr PJ. Testing problems in J. A. Gray’s personality theory: a commentary on Matthews and Gilliland (1999) Pers. Indiv. Diff. 2001;30:333–352. [Google Scholar]
- Corr PJ. J. A. Gray’s reinforcement sensitivity theory: Tests of the joint subsystems hypothesis of anxiety and impulsivity. Pers. Indiv. Differ. 2002;33(4):511–532. [Google Scholar]
- Deserno L, Boehme R, Heinz A, Schlagenhauf F. Reinforcement learning and dopamine in schizophrenia: dimensions of symptoms or specific features of a disease group? Front. Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot AJ, Thrash TM. Approach-avoidance motivation in personality: approach and avoidance temperaments and goals. J. Pers. Soc. Psychol. 2002;82(5):804–818. doi: 10.1037//0022-3514.82.5.804. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition, Biometrics Research Department. New York State Psychiatric Institute, New York, NY. 1997 [Google Scholar]
- Gable SL, Gosnell CL. Approach and Avoidance Behavior in Interpersonal Relationships. Emot. Rev. 2013;5(3):269–274. [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J. Res. Pers. 2006;40:1086–1102. [Google Scholar]
- Gray JA. The psychology of fear and stress. Cambridge University Press; Cambridge: 1987. [Google Scholar]
- Hands S, Everitt B. A Monte Carlo study of the recovery of cluster structure in binary data by hierarchical clustering techniques. Multivar. Behav. Res. 1987;22:235–243. doi: 10.1207/s15327906mbr2202_6. [DOI] [PubMed] [Google Scholar]
- Hartigan JA. Clustering Algorithms. Wiley, New York: 1975. [Google Scholar]
- Horan WP, Blanchard JJ. Emotional responses to psychosocial stress in schizophrenia: the role of individual differences in affective traits and coping. Schizophr. Res. 2003;60(2-3):271–283. doi: 10.1016/s0920-9964(02)00227-x. [DOI] [PubMed] [Google Scholar]
- Horan WP, Green MF, Kring AM, Nuechterlein KH. Does anhedonia in schizophrenia reflect faulty memory for subjectively experienced emotions? J. Abnorm. Psychol. 2006a;115(3):496–508. doi: 10.1037/0021-843X.115.3.496. [DOI] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr. Bull. 2006b;32:259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ. Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS) Schizophr. Res. 2011;132(2-3):140–145. doi: 10.1016/j.schres.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert L, Arabie P. Comparing partitions. J. Classif. 1985;2(1):193–218. [Google Scholar]
- Johnson S, Turner RJ, Iwata N. BIS/BAS Levels and Psychiatric Disorder: An Epidemiological Study. J. Psychopathol. Behav. 2003;25(1):25–36. [Google Scholar]
- Jorm AF, Christensen H, Henderson AS, Jacomb PA, Korten AE, Rodgers B. Using the BIS/BAS scales to measure behavioural inhibition and behavioural activation: Factor structure, validity and norms in a large community sample. Personal. Indiv. Diff. 1998;26(1):49–58. [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. J. Abnorm. Psychol. 2002:589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- Kee KS, Green MF, Mintz J, Brekke JS. Is emotional processing a predictor of functional outcome in schizophrenia? Schizophr. Bull. 2003;29:487–497. doi: 10.1093/oxfordjournals.schbul.a007021. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT., Jr. A separate disease within the syndrome of schizophrenia. Arch. Gen. Psychiat. 2001;58(2):165–171. doi: 10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- Kopelowicz A, Ventura J, Liberman RP, Mintz J. Consistency of Brief Psychiatric Rating Scale factor structure across a broad spectrum of schizophrenia patients. Psychopathology. 2008;41(2):77–84. doi: 10.1159/000111551. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): Final development and validation. Am. J. Psychiat. 2013;170:165–172. doi: 10.1176/appi.ajp.2012.12010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper FK, Fisher L. A Monte Carlo comparison of six clustering procedures. Biometrics. 1975;31:777–783. [Google Scholar]
- Mausbach BT, Harvey PD, Goldman SR, Jeste DV, Patterson TL. Development of a brief scale of everyday functioning in persons with serious mental illness. Schizophr. Bull. 2007;33(6):1364–1372. doi: 10.1093/schbul/sbm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPheeters HL. Statewide mental health outcome evaluation: a perspective of two southern states. Community Ment. Hlt. J. 1984;20(1):44–55. doi: 10.1007/BF00754103. [DOI] [PubMed] [Google Scholar]
- Milligan GW, De Soete D. Clustering validation: results and implication for applied analyses. In: Arabie P, Hubert LJ, editors. Clustering and Classification. World Scientific Publications; River Edge, pp.: 1996. pp. 346–347. [Google Scholar]
- Mitchell JT, Nelson-Gray RO. Attention-Deficit/Hyperactivity Disorder symptoms in adults: Relationship to Gray’s Behavioral Approach System. Personal. Indiv. Diff. 2006;40(4):749–760. [Google Scholar]
- Nash K, Inzlicht M, McGregor I. Approach-related left prefrontal EEG asymmetry predicts muted error-related negativity. Biol. Psychol. 2012;91(1):96–102. doi: 10.1016/j.biopsycho.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Newman JP, MacCoon DG, Vaughn LJ, Sadeh N. Validating a distinction between primary and secondary psychopathy with measures of Gray’s BIS and BAS constructs. J. Abnorm. Psychol. 2005;114(2):319–323. doi: 10.1037/0021-843X.114.2.319. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. THE BRIEF PSYCHIATRIC RATING SCALE. Psychol. Rep. 1962;10(3):799–812. [Google Scholar]
- Peterson CK, Gable P, Harmon-Jones E. Asymmetrical frontal ERPs, emotion, and behavioral approach/inhibition sensitivity. Soc. Neurosci. 2008;3(2):113–124. doi: 10.1080/17470910701612736. [DOI] [PubMed] [Google Scholar]
- Reise SP, Horan WP, Blanchard JJ. The challenges of fitting an item response theory model to the Social Anhedonia Scale. J. Pers. Assess. 2011;93(3):213–224. doi: 10.1080/00223891.2011.558868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten MRM, van Honk J, Aleman A, Kahn RS. Behavioral inhibition system (BIS), Behavioral activation system (BAS) and schizophrenia: Relationship with psychopathology and physiology. J. Psychiat. Res. 2006;40(7):638–645. doi: 10.1016/j.jpsychires.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Spielberg JM, Heller W, Miller GA. Hierarchical brain networks active in approach and avoidance goal pursuit. Front. Hum. Neurosci. 2013;7(284) doi: 10.3389/fnhum.2013.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am. J. Psychiatr. 2012;169(4):364–373. doi: 10.1176/appi.ajp.2011.11030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Horan WP, Kirkpatrick B, Fischer BA, Keller WR, Miski P, Buchanan RW, Green MF, Carpenter WT., Jr. Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J. Psychiat. Res. 2013;47(6):783–790. doi: 10.1016/j.jpsychires.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Wilbur RC, Warren KR, August SM, Gold JM. Anticipatory vs. consummatory pleasure: what is the nature of hedonic deficits in schizophrenia? Psychiat. Res. 2011;187(1-2):36–41. doi: 10.1016/j.psychres.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal Brain Asymmetry: A Biological Substrate of the Behavioral Approach and Inhibition Systems. Psychol. Sci. 1997;8(3):204–210. [Google Scholar]
- Ward JH. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963;58:234–244. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.