Abstract
PURPOSE
Up to 38% of children with cancer require PICU admission within three years of diagnosis, with reported PICU mortality of 13–27% far exceeding that of the general PICU population. PICU outcomes data for individual cancer types are lacking and may help identify patients at risk for poor clinical outcomes.
METHODS
We performed a retrospective multi-center analysis of 10,365 PICU admissions of cancer patients ≤ 21 years old among 112 PICUs between 1/1/2009 and 6/30/2012. We evaluated the effect of cancer type, age, gender, genetic syndrome, stem cell transplantation, PRISM3 score, infections, and critical care interventions on PICU mortality.
RESULTS
After excluding scheduled perioperative admissions, cancer patients represented 4.2% of all PICU admissions (10,365/246,346), had overall mortality of 6.8% (708/10,365) vs. 2.4% (5,485/230,548) in the general PICU population (RR=2.9, 95% CI 2.7–3.1, p<0.001), and accounted for 11.4% of all PICU deaths (708/6,215). Hematologic cancer patients had greater median PRISM3 score (8 vs 2, p<0.001), rates of sepsis (27% vs 9%, RR=2.9, 95% CI 2.6–3.1, p<0.001), and mortality (9.6% vs 4.5%, RR=2.1, 95% CI 1.8–2.5, p<0.001) compared to solid cancer patients. Among hematologic cancer patients, stem cell transplantation, diagnosis of acute myeloid leukemia, PRISM3 score, and infection were all independently associated with PICU mortality.
CONCLUSIONS
Children with cancer account for 4.2% of PICU admissions and 11.4% of PICU deaths. Hematologic cancer patients have significantly higher admission illness severity, rates of infections, and PICU mortality than solid cancer patients. These data may be useful in risk-stratification for closer monitoring and patient counseling.
INTRODUCTION
Five-year survival for children with cancer now exceeds 83%, credited in large part to advancements in molecular diagnostics, targeted therapies, and vigilant supportive care [1]. Despite these improvements, critical care resources remain crucial for this population, with up to 38% of pediatric cancer patients requiring at least one pediatric intensive care unit (PICU) admission within three years of diagnosis [2, 3]. These admissions are largely for organ dysfunction and infection and reported PICU mortality of 13–27% far exceeds that of the general PICU population [4–13].
The high morbidity and mortality in critically ill pediatric oncology patients stems from both aggressive cancer pathophysiology, which can lead to organ infiltration and immunodeficiency, as well as intensive anti-neoplastic therapies, which can cause systemic toxicity and necessitate invasive vascular access [14–16]. In our experience, these factors cause particular vulnerability in patients with hematologic malignancies, who experience more significant myelosupression and higher rates of mucositis, and for whom life threatening infections can rapidly result in hemodynamic and respiratory failure. Infection risk is further amplified in patients undergoing hematopoietic stem cell transplantation (HSCT), which is often associated with myeloablation and prolonged immune reconstitution.
Accordingly, a number of risk factors for PICU mortality in children with cancer have been identified, including fungal infection, sepsis, elevated Pediatric Risk of Mortality 3 score (PRISM3), use of mechanical ventilation, use of renal replacement therapy (RRT), and history of HSCT [6, 7, 17–22]. However, neither the incidence of these risk factors nor their associations with mortality have been well-described for specific cancer types.
Despite the belief that cancer biology and anti-neoplastic treatment history play a pivotal role in the development and outcome of critical illness, most data published regarding pediatric oncology patients in the PICU have not differentiated outcomes according to cancer type. Previous attempts to investigate these associations have been limited by small sample sizes and heterogeneity within cohorts [8, 14, 21, 23–25]. Therefore the influence of cancer type on infections, need for critical care interventions, and mortality in the PICU remains poorly defined.
In order to fill this gap in knowledge, we aim to (1) describe admission characteristics and rates of infection, critical care interventions, and mortality, and (2) identify factors associated with PICU mortality. We hypothesize that patients with hematologic cancer will have greater admission illness severity, rates of infection, need for critical care interventions, and mortality compared to those with solid cancer.
Better understanding of these relationships may improve risk-stratification, inform goals of care, and identify patients for early interventions. In addition, it may advance our understanding of critical illness in the context of pediatric oncology.
DESIGN AND METHODS
Design
We performed a retrospective multicenter cohort analysis using the Virtual PICU Systems database (VPS, LLC), a collaboration of 112 academic and community PICUs predominantly in the United States. In this database, trained analysts at each site collect patient data from PICU admission to PICU death, transfer, or discharge [26–28]. All PICU admissions are abstracted excluding overflow patients admitted solely due to lack of beds in a general care unit. PICU admission criteria were not standardized between sites. Patient identifiers were unavailable to the study team; therefore, this study was exempt from IRB review.
Patients
We identified 192,956 patients ≤21 years old accounting for a combined 246,346 consecutive PICU admissions between 1/1/2009 and 6/30/2012. Cancer patients were identified through ICD-9 diagnosis codes relating to cancer type, mass or tumor location, and history of chemotherapy or radiation (n=19,993) (Appendix1). We then excluded patients with benign neoplasms or neoplastic syndrome (ie: neurofibromatosis) without documented neoplasm (n=3,011) and patients with no information about histopathology or tumor site (n=444), no information about histopathology but multiple tumor sites (n=378), or multiple unique histopathologies listed without reference to which tumor was active (n=75).
The remaining 16,085 admissions were categorized as malignancies and sorted as follows. Solid malignancies were subdivided into 7 groups according to the International Classification of Childhood Cancer-3 (ICCC-3) [29]. Eye, epithelial, thyroid, and other solid malignancies were excluded due to low sample size and broad heterogeneity (n=133). Hematologic malignancies were subdivided into 9 groups according to the ICCC-3. Histiocytic disorders were excluded as we found the database could not discriminate between hemophagocytic lymphohistiocytosis and other histiocytoses (n=154). The Not Elsewhere Classified (NEC) category included hematologic malignancies that were rare (ie: monocytic leukemias) or poorly defined at the time of coding. We then excluded scheduled perioperative admissions due to the semielective nature of their admission. Scheduled admission was defined as having ≥12 hours advance notice of PICU admission. Perioperative admission was defined as having had surgery ≤24 hours before or after PICU admission. After exclusions, 10,365 patients were included (eFigure1).
Outcomes
Our outcomes were PICU mortality, infection, and critical care intervention rates. Death, infections, and interventions were included if they occurred at any time between PICU admission and PICU disposition. Gram positive, gram negative, fungal, and viral infections were identified through ICD-9 codes; clinical, radiographic, and microbiologic data in support of these diagnoses were not available. A diagnosis of sepsis included patients with severe sepsis and septic shock, as ICD-9 codes are neither sensitive nor specific in differentiating these [30]. Noninvasive positive pressure ventilation (NIPPV) was defined as CPAP or BiPAP without prior or subsequent endotracheal intubation. Invasive positive pressure ventilation (IPPV) was defined as endotracheal intubation with any mechanical ventilation. RRT was defined as hemodialysis or continuous veno-venous hemofiltration/hemodialysis. Arterial lines were included if placed during that PICU stay and reported as a surrogate for clinical instability; vasoactive medication use was not available. Extra-corporeal membrane oxygenation (ECMO) was defined as any extracorporeal life support. Documentation of infections, IPPV, arterial lines, and ECMO was mandatory at all centers. Due to center agreements, documentation of NIPPV and RRT was optional for 18.4% and 24.4% of respective admissions; analyses of these variables excluded centers not collecting these data.
Covariates
We queried age, gender, presence of a genetic condition, history of HSCT, and PRISM3 score for all patients. All covariates are routinely available on PICU admission; age and gender were chosen for face validity, while the remaining variables have been previously demonstrated to influence mortality in PICU populations [27, 28, 31]. Genetic conditions were defined by any ICD-9 code indicating a structural anomaly (ie: congenital asplenia), chromosomal disorder (ie: Trisomy 21), or defined genetic syndrome (ie: Velocardiofacial Syndrome). PRISM3 raw score was used to reflect admission illness severity and was calculated within the first 12 hours of admission [4, 32]. History of HSCT was documented if deemed relevant by the treating clinician; specific details such as transplant date and type were not available.
Statistics
The distributions of categorical variables were compared with Fisher exact tests and relative risks [33]. Continuous variables were compared with t-tests for normally distributed data and with Wilcoxon rank sum tests otherwise. To analyze the effect of cancer type and our covariates on mortality, we used multivariate generalized estimating equation (GEE) models for binomial outcomes with a logistic link function [34, 35]. These GEE models provides logistic regression while accounting for clustering effects due to variable practices between sites and repeat admissions of the same patient. We examined estimated mortality odds ratios with 95% confidence intervals and 2-tailed p-values based on robust standard error estimates with a nominal significance level of α=0.05.
RESULTS
Overall, children with cancer accounted for 6.5% of all PICU admissions (16,085/246,346) and had 4.6% mortality (730/15,798, eTable1). Scheduled perioperative admissions were 8.1 times more common for solid cancers (5,145/10,863) than for hematologic cancers (288/4935, 95% CI 7.2–9.1, p<0.001) and had 0.4% overall mortality (22/5,433); these low-risk admissions were excluded from further analyses. The remaining cohort accounted for 4.2% of all PICU admissions (10,365/246,346) and is described below.
Cancer Type Affects PICU Admission Characteristics
Patients with hematologic cancer were more likely to have a genetic syndrome (6% vs 3%, RR=2.1, 95% CI 1.7–2.6, p<0.001), more likely to have had a HSCT (14% vs 5%, RR=2.8, 95% CI 2.4–3.2, p<0.001), and had greater admission illness severity (median PRISM3 score 8 vs 2, p<0.001) than patients with solid cancer (eTable2). A breakdown of which patients scored at least one point in the PRISM3 categories of cardiovascular, neurologic, acid-base, chemistry, and hematologic derangements is listed in eTable3.
Cancer Type Affects PICU Mortality
Children with any cancer, solid cancer, or hematologic cancer had mortality of 6.8% (708/10,365), 4.5% (260/5,718), and 9.6% (448/4,647), respectively (eTable2). These represent relative risks of 2.9 (95% CI 2.7–3.1, p<0.001), 1.9 (95% CI 1.7–2.2, p<0.001), and 4.1 (95% CI 3.7–4.4, p<0.001), respectively, when compared to 2.4% mortality for PICU patients without cancer (5,485/230,548). Children with cancer accounted for 11.4% of all PICU deaths (708/6,215). Compared with solid cancer patients, hematologic cancer patients had RR=2.1 (95% CI 1.8–2.5, p<0.001) for mortality in a PICU admission.
Risk Factors for PICU Mortality Among Solid Cancer Subtypes
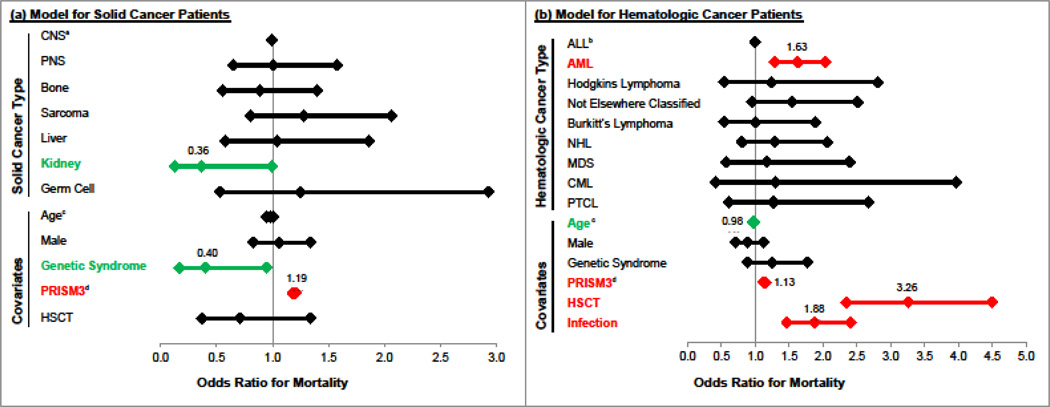
Among children with solid cancer, patients with sarcoma had the highest mortality (7.6%, 25/331). On univariate analysis, only PRISM3 score (RR=1.2, 95% CI 1.17–1.21, p<0.001) was associated with mortality (eTable4). On multivariate analysis, the only solid cancer type independently associated with mortality was kidney cancer, which had OR=0.36 relative to the reference group CNS (95% CI 0.13–0.99, p=0.047). Covariates independently associated with mortality were genetic syndrome (OR=0.40, 95% CI 0.17–0.95, p=0.039) and PRISM3 score (OR=1.19, 95% CI 1.18–1.21, p<0.001) (Figure1, eTable5).
Figure 1. Multivariate Predictors of PICU Mortality.
Model (a) refers to solid cancers only. Model (b) refers to hematologic cancers only. a reference group for solid cancers b reference group for hematologic cancers c OR refers to each additional year of age d OR refers to each additional PRISM3 point. See eTables 5 and 6 for complete model results.
Risk Factors for PICU Mortality Among Hematologic Cancer Subtypes
Among children with hematologic cancer, patients with AML (14.9%, 134/902) and MDS (14.5%, 16/109) had the highest mortality. On univariate analysis, genetic syndrome (RR=1.5, 95% CI 1.1–2.0, p=0.018), HSCT (RR=3.2, 95% CI 2.7–3.8, p<0.001), and increasing PRISM3 score (RR=1.1, 95% CI 1.12–1.16, p<0.001) were associated with mortality (eTable4). On multivariate analysis, AML was independently associated with mortality compared with the reference group ALL (OR=1.66, 95% CI 1.32–2.08, p<0.001). Covariates independently associated with mortality were older age (OR=0.98, 95% CI 0.96–0.99, p=0.023), increasing PRISM3 score (OR=1.14, 95% CI 1.12–1.16, p<0.001), and history of HSCT (OR=3.52, 95% CI 2.53–4.93, p<0.001).
In order to test whether the association of HSCT with mortality could be explained by infections, we tested the associations of both HSCT and infection with mortality in a joint model. In this model, both HSCT (OR=3.26, 95% CI 2.35–4.50, p<0.001) and infection (OR=1.88, 95% CI 1.47–2.41, p<0.001) independently predicted mortality and the effect size of HSCT changed minimally from the prior model, suggesting that infections do not explain the full effect of HSCT on PICU mortality in hematologic cancer admissions (Figure1, eTable6).
Cancer Type Affects PICU Infection Rates
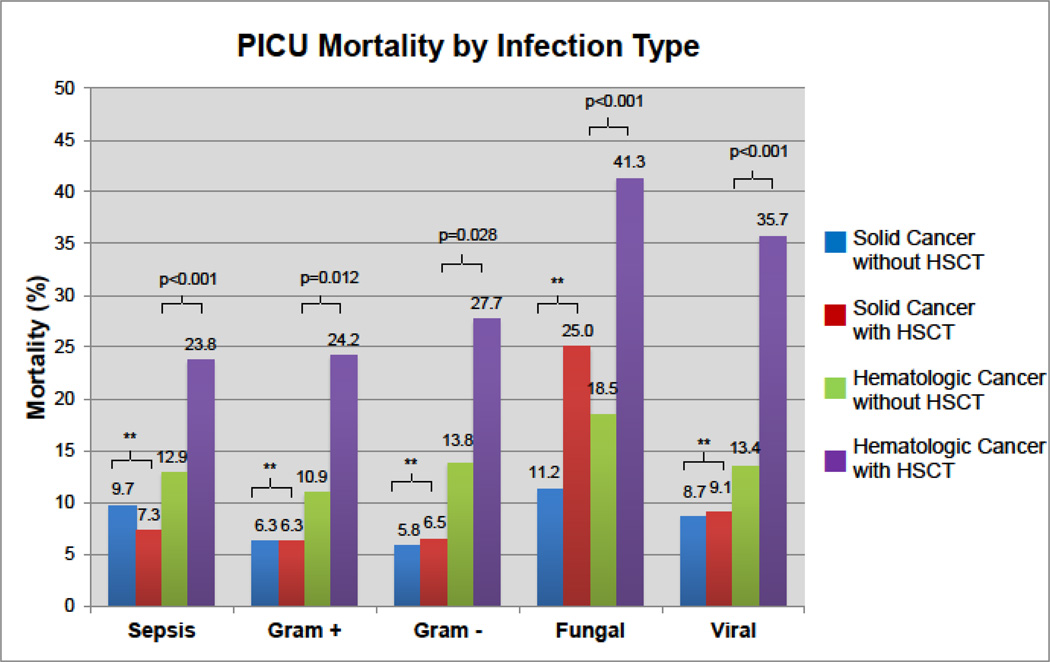
Patients with hematologic cancer were more likely to have sepsis (RR=2.9, 95% CI 2.6–3.1, p<0.001) and gram-positive (RR=2.3, 95% CI 1.9–2.7, p<0.001), gram-negative (RR=1.8, 95% CI 1.5–2.1, p<0.001), fungal (RR=3.3, 95% CI 2.7–4.1, p<0.001), and viral infections (RR=2.7, 95% CI 2.3–3.2, p<0.001) than were patients with solid cancer (Table1). Each infection was associated with increased mortality in hematologic cancers; sepsis, fungal, and viral infections were associated with increased mortality in solid cancers (eTable4). Infected HSCT patients with hematologic cancer had greater mortality than all other groups (Figure2).
Table 1.
PICU Infections by Cancer Type
| N | Sepsis (%) |
Gram(+) (%) |
Gram(−) (%) |
Fungal (%) |
Viral (%) |
||
|---|---|---|---|---|---|---|---|
| SOLID CANCERS | 5718 | 530 (9) | 176 (3) | 221 (4) | 115 (2) | 195 (3) | |
| Central Nervous System | 3502 | 167 (5) | 101 (3) | 106 (3) | 62 (2) | 93 (3) | |
| Peripheral Nervous System | 781 | 111 (14) | 31 (4) | 36 (5) | 14 (2) | 46 (6) | |
| Bone | 437 | 105 (24) | 20 (5) | 24 (5) | 12 (3) | 18 (4) | |
| Sarcoma | 331 | 65 (20) | 6 (2) | 16 (5) | 19 (6) | 9 (3) | |
| Liver | 265 | 33 (12) | 9 (3) | 26 (10) | 3 (1) | 16 (6) | |
| Kidney | 262 | 31 (12) | 6 (3) | 8 (3) | 4 (2) | 13 (5) | |
| Germ Cell Tumor | 140 | 18 (13) | 3 (2) | 5 (4) | 1 (1) | 0 (0) | |
| HEMATOLOGIC CANCERS | 4647 | 1232 (27) | 327 (7) | 322 (7) | 308 (7) | 432 (9) | |
| Acute Lymphoid Leukemia | 2611 | 655 (25) | 147 (6) | 187 (7) | 201 (8) | 266 (10) | |
| Acute Myeloid Leukemia | 902 | 316 (35) | 102 (11) | 80 (9) | 64 (7) | 81 (9) | |
| Hodgkins Lymphoma | 301 | 92 (31) | 20 (7) | 14 (5) | 5 (2) | 20 (7) | |
| Not Elsewhere Classified | 272 | 43 (16) | 19 (7) | 20 (7) | 21 (8) | 19 (7) | |
| Burkitt’s Lymphoma | 171 | 41 (24) | 17 (10) | 6 (4) | 5 (3) | 11 (6) | |
| Non-Hodgkins Lymphoma | 160 | 42 (26) | 7 (4) | 6 (4) | 5 (3) | 8 (5) | |
| Myelodysplastic Syndrome | 109 | 29 (27) | 10 (9) | 9 (8) | 6 (6) | 25 (23) | |
| Chronic Myeloid Leukemia | 62 | 12 (19) | 5 (8) | 0 (0) | 4 (6) | 1 (2) | |
| Peripheral T-Cell Lymphoma | 59 | 2 (3) | 0 (0) | 0 (0) | 1 (2) | 1 (2) | |
| RR 2.9a (2.6–3.1) p<0.001 | RR 2.3a (1.9–2.7) p<0.001 | RR 1.8a (1.5–2.1) p<0.001 | RR 3.3a (2.7–4.1) p<0.001 | RR 2.7a (2.3–3.2) p<0.001 | |||
relative risk with 95% CI for admissions with hematologic cancers, compared to admissions with solid cancers
Figure 2. PICU Mortality by Infection Type.
For each type of infection, hematologic cancer patients with HSCT have greater mortality than those without HSCT. For each type of infection, solid cancer patients with and without HSCT have similar mortality. ** refers to non-significant p-values (>0.05). See Table1 and eTable4 for complete results.
Cancer Type Affects PICU Intervention Rates
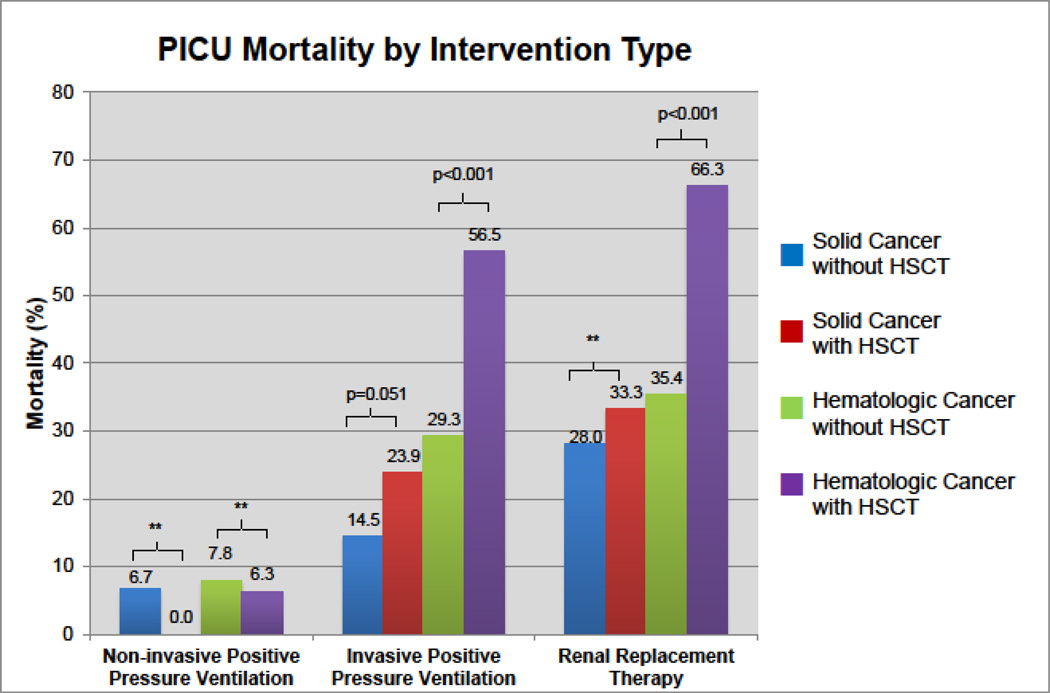
Patients with hematologic cancer were more likely to receive NIPPV without intubation (RR=1.9, 95% CI 1.6–2.3, p<0.001) and RRT (RR=3.6, 95% CI 2.8–4.7, p<0.001) (Table2). They were equally likely to use IPPV (p=0.310) and less likely to use an arterial line (RR=0.85, 95% CI 0.79–0.91, p<0.001). Each intervention was associated with increased mortality in both solid and hematologic cancers. HSCT patients with hematologic cancer who needed IPPV or RRT had greater mortality than all other groups (Figure3). ECMO was used in a total of 36 patients; 28 had a hematologic malignancy (1 with prior HSCT, 14/28 died) and 8 had a solid malignancy (1 with prior HSCT, 4/8 died).
Table 2.
PICU Interventions by Cancer Type
| NIPPVa (%) |
IPPV (%) |
RRTb (%) |
Arterial Line (%) |
||
|---|---|---|---|---|---|
| SOLID CANCERS | 159/4647 (3) | 1403/5718 (25) | 76/4206 (2) | 1386/5718 (24) | |
| Central Nervous System | 81/2845 (3) | 925/3502 (26) | 12/2524 (0.5) | 1053/3502 (30) | |
| Peripheral Nervous System | 15/625 (2) | 159/781 (20) | 22/587 (4) | 100/781 (13) | |
| Bone | 25/349 (7) | 56/437 (13) | 9/320 (3) | 53/437 (12) | |
| Sarcoma | 19/273 (7) | 78/331 (24) | 4/250 (2) | 56/331 (17) | |
| Liver | 7/213 (3) | 80/265 (30) | 5/201 (2) | 39/265 (15) | |
| Kidney | 5/219 (2) | 71/262 (27) | 22/203 (11) | 54/262 (21) | |
| Germ Cell Tumor | 7/123 (6) | 34/140 (24) | 2/121 (2) | 31/140 (22) | |
| HEMATOLOGIC CANCERS | 250/3799 (7) | 1100/4647 (24) | 226/3474 (7) | 957/4647 (21) | |
| Acute Lymphoid Leukemia | 116/2129 (5) | 561/2511 (21) | 126/1967 (6) | 494/2611 (19) | |
| Acute Myeloid Leukemia | 72/720 (10) | 270/902 (30) | 48/641 (7) | 238/902 (26) | |
| Hodgkins Lymphoma | 14/252 (6) | 61/301 (20) | 4/221 (2) | 53/301 (18) | |
| Not Elsewhere Classified | 15/230 (7) | 70/272 (26) | 14/208 (7) | 64/272 (24) | |
| Burkitt’s Lymphoma | 6/141 (4) | 32/171 (19) | 11/133 (8) | 22/171 (13) | |
| Non-Hodgkins Lymphoma | 13/136 (10) | 47/160 (29) | 8/126 (6) | 30/160 (19) | |
| Myelodysplastic Syndrome | 8/94 (9) | 37/109 (34) | 11/94 (12) | 34/109 (31) | |
| Chronic Myeloid Leukemia | 2/49 (4) | 12/62 (19) | 1/39 (3) | 10/62 (16) | |
| Peripheral T-Cell Lymphoma | 4/48 (8) | 10/59 (17) | 3/45 (7) | 12/59 (22) | |
| RR 1.9c (1.6–2.3) p<0.001 | RR 1.0c (0.9–1.03) p=0.310 | RR 3.6c (2.8–4.7) p<0.001 | RR 0.85c (0.79–0.91) p<0.001 | ||
stratified for only centers that document NIPPV and RRT, respectively
relative risk with 95% CI for admissions with hematologic cancers, compared to admissions with solid cancers
Figure 3. PICU Mortality by Intervention Type.
Among those receiving IPPV and RRT, hematologic cancer patients with history of HSCT have greater mortality than those without HSCT. There was a trend towards significance for greater mortality among solid cancer patients with HSCT receiving IPPV compared to solid cancer patients without HSCT. ** refers to non-significant p-values (>0.05). See Table1 and eTable5 for complete results.
DISCUSSION
In this study, after excluding scheduled perioperative patients, we found that children with cancer form 4.2% of PICU admissions (10,365/246,346) and account for 11.4% of all PICU deaths (708/6,215). Children with cancer have a PICU mortality rate of 6.8% (708/10,365) vs 2.4% for non-oncology PICU patients (5,485/230,548). Hematologic cancer patients have significantly greater admission illness severity, infection rates, and mortality compared to solid cancer patients. Diagnosis of AML was the only cancer type associated with mortality independent of age, gender, genetic conditions, PRISM3 score, and HSCT. These findings suggest that cancer type is an important mediator of critical illness in pediatric oncology.
First, the overall PICU mortality rate of 6.8% in our cohort is significantly lower than most reports from the last 20 years (13–27%) [2, 7–9, 13]. While encouraging, this finding should be interpreted with caution due to heterogeneity among published cohorts with regards to both patient and center characteristics, such as cancer types and PICU admission criteria. When we excluded HSCT patients, we found mortality of 5.7% (536/9,420). When we analyzed only patients requiring IPPV, we found PICU mortality of 24% (597/2,503), which is lower than other recent reports of 41–61% [8, 13, 17, 21, 36]. When these admissions requiring IPPV were stratified for patients with and without prior HSCT, we found mortality rates of 49% for those with HSCT (151/306) and 20% for those without HSCT (446/2,197), which are slightly lower than 55% and 25% reported by Tamburro et al [17]. Though these findings could be confounded by differences in patient characteristics, overall these results may suggest lower mortality in the current oncology cohort compared with relatively similar historical cohorts. This may be related to improvements in care such as infection prophylaxis and mechanical ventilation strategies, although this is beyond the scope of our analysis.
Second, we found that compared with solid cancer patients, hematologic cancer patients had greater illness severity and greater rates of infection, which correlated with greater mortality. This may be due to increased immunodeficiency in hematologic cancers, related to both underlying malignancy as well as more aggressive treatments such as glucocorticoid-based regimens and allogeneic HSCT, although these parameters could not be analyzed in our dataset. Interestingly, our finding of 50% survival in both solid and hematologic cancer patients requiring ECMO is encouraging and consistent with a recent case series suggesting that at least some critically ill children may be suitable candidates for this intervention [37, 38].
Third, we found that although cancer type is a strong univariate predictor of mortality, this effect is largely explained by accounting for age, gender, genetic syndrome, HSCT, and PRISM3 score on multivariate analysis. AML was an exception and was independently associated with mortality even after accounting for the above covariates and infection. This finding suggests that there may be characteristics relevant to underlying AML cancer biology and treatment history that mediate the progression of critical illness. AML cancer biology (which involves high rates of relapse and neutropenia) and treatment history (which typically includes five or more rounds of intensive chemotherapy, including cardiotoxic anthracyclines) may be relevant to the outcome of PICU mortality even after accounting for our covariates [39]. Further investigation into the development and evolution of critical illness in pediatric AML patients should be explored in future studies.
Fourth, we identified a strong influence of HSCT on mortality in patients with hematologic cancer, an effect that was absent among patients with solid cancer. The differential effect of HSCT may be explained by the significantly higher rates of allogeneic transplantation in hematologic cancers, although this was not directly explored. The effect of HSCT may also be mediated by conditioning intensity, donor match, cell processing, and graft versus host disease. Our multivariate analysis suggests that the effect of HSCT on mortality is independent of the presence of infection. Given the large impact of HSCT on the mortality of hematologic cancer patients in the PICU, these additional factors should be explored in future studies.
Fifth, we found that cancer patients represented 6.9% of all PICU admissions, an incidence more than twice as high as a similar 20-center cohort from 15 years prior [2]. Although not analyzed in this study, this finding could be due to increased rates of critical illness in pediatric cancer patients, potentially related to more aggressive anti-neoplastic therapies, or may reflect changing PICU admission criteria among centers and providers, perhaps due to an increased willingness to use critical care resources for children with cancer [11, 12].
Our study is the largest published study of this patient population and has several strengths. Our patient pool represents a broad spectrum of institutions and clinician practice patterns. In addition, the study span is relatively short and contemporary, which reduces the effect of significant practice changes over time on our results.
There are several limitations to our work. We were unable to identify a specific cancer type for a minority of patients, which may reflect either incomplete diagnostic evaluations or database quality. Due to limitations in the database, we were unable to describe the stage/grade of malignancies, details of treatment, and final cause of death. These results may be of limited generalizability to international healthcare systems with different practice patterns; for centers where patients ≥16 years old use adult ICUs, we report age-stratified data for reference (eFigure2, eTable7). Finally, this analysis also does not capture oncology patients who died outside of the PICU.
In conjunction with other valid patient-specific markers of risk, we believe these data may help guide both intensivists and oncologists in risk-stratification for patients, which can be valuable in counseling families regarding goals of care [40, 41]. In addition, they may help inform PICU resource allocation and identify patients that may benefit from closer monitoring and early interventions. These data also provide an opportunity for centers to compare outcomes to a large multi-center contemporary cohort.
CONCLUSIONS
In summary, to our knowledge, this is the largest analysis of pediatric oncology patients requiring intensive care and the first publication to show that different cancers have significantly different admission illness severity, rates of infection, use of critical care interventions, and PICU mortality rates.
Pediatric oncology patients comprise a heterogeneous cohort accounting for 4.2% of all PICU admissions and 11.4% of all PICU deaths. Patients with hematologic cancer are at particularly high risk for infection and PICU mortality. Additional focus on AML and HSCT as mediators of critical illness is warranted. Understanding the pathophysiologic basis for these differences may improve our understanding of critical illness in pediatric oncology patients.
Supplementary Material
Take Home Message.
Children with cancer account for 4.2% of admissions and 11.4% of all deaths among children admitted to the PICU. Children with hematologic cancer have significantly higher admission illness severity, rates of infections, and PICU mortality than children with solid cancer.
Tweet
Children with cancer account for 4.2% of admissions and 11.4% of all deaths among children admitted to the PICU.
ACKNOWLEDGEMENTS
There are no disclosures or conflicts of interest to report. Statistical consultation was provided by John Kornak, PhD, UCSF Department of Biostatistics, and funded by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. VPS data were provided by the VPS, LLC. No endorsement or editorial restriction of the interpretation of these data or opinions of the authors has been implied or stated. Dr. Zinter had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflicts of Interest: None.
Study concept and design: Zinter, Sapru
Acquisition of data: Zinter
Analysis and interpretation of data: Zinter, DuBois, Matthay, Sapru
Drafting of the manuscript: Zinter, Sapru
Critical revision of the manuscript for important intellectual content: Zinter, DuBois, Spicer, Matthay, Sapru
Statistical analysis: Zinter, Sapru
Administrative, technical, or material support: Sapru
Study supervision: DuBois, Matthay, Sapru
Approval of final manuscript: Zinter, DuBois, Spicer, Matthay, Sapru
REFERENCES
- 1.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program Research Data (1973–2011) DCCPS, Surveillance Research Program, Surveillance Systems Branch. 2014 doi: http://www.seer.cancer.gov.
- 2.Dalton HJ, Slonim AD, Pollack MM. MultiCenter outcome of pediatric oncology patients requiring intensive care. Pediatr Hematol Oncol. 2003;20:643–649. [PubMed] [Google Scholar]
- 3.Rosenman MB, Vik T, Hui SL, Breitfeld PP. Hospital resource utilization in childhood cancer. J Pediatr Hematol Oncol. 2005;27:295–300. doi: 10.1097/01.mph.0000168724.19025.a4. [DOI] [PubMed] [Google Scholar]
- 4.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Tilford JM, Roberson PK, Lensing S, Fiser DH. Differences in pediatric ICU mortality risk over time. Crit Care Med. 1998;26:1737–1743. doi: 10.1097/00003246-199810000-00032. [DOI] [PubMed] [Google Scholar]
- 6.Pound CM, Johnston DL, Armstrong R, Gaboury I, Menon K. The morbidity and mortality of pediatric oncology patients presenting to the intensive care unit with septic shock. Pediatr Blood Cancer. 2008;51:584–588. doi: 10.1002/pbc.21670. [DOI] [PubMed] [Google Scholar]
- 7.Haase R, Lieser U, Kramm C, et al. Management of oncology patients admitted to the paediatric intensive care unit of a general children's hospital - a single center analysis. Klin Padiatr. 2011;223:142–146. doi: 10.1055/s-0031-1275291. [DOI] [PubMed] [Google Scholar]
- 8.Meyer S, Gottschling S, Biran T, Georg T, Ehlayil K, Graf N, Gortner L. Assessing the risk of mortality in paediatric cancer patients admitted to the paediatric intensive care unit: a novel risk score? Eur J Pediatr. 2005;164:563–567. doi: 10.1007/s00431-005-1695-y. [DOI] [PubMed] [Google Scholar]
- 9.Owens C, Mannion D, O'Marcaigh A, Waldron M, Butler K, O'Meara A. Indications for admission, treatment and improved outcome of paediatric haematology/oncology patients admitted to a tertiary paediatric ICU. Ir J Med Sci. 2011;180:85–89. doi: 10.1007/s11845-010-0634-8. 10.1007/s11845-010-0634-8. [DOI] [PubMed] [Google Scholar]
- 10.Hallahan AR, Shaw PJ, Rowell G, O'Connell A, Schell D, Gillis J. Improved outcomes of children with malignancy admitted to a pediatric intensive care unit. Crit Care Med. 2000;28:3718–3721. doi: 10.1097/00003246-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 11.van Veen A, Karstens A, van der Hoek AC, Tibboel D, Hahlen K, van der Voort E. The prognosis of oncologic patients in the pediatric intensive care unit. Intensive Care Med. 1996;22:237–241. doi: 10.1007/BF01712243. [DOI] [PubMed] [Google Scholar]
- 12.Sivan Y, Schwartz PH, Schonfeld T, Cohen IJ, Newth CJ. Outcome of oncology patients in the pediatric intensive care unit. Intensive Care Med. 1991;17:11–15. doi: 10.1007/BF01708402. [DOI] [PubMed] [Google Scholar]
- 13.Ha EJ, Kim S, Jin HS, Bae KW, Lim HJ, Seo JJ, Park SJ. Early changes in SOFA score as a prognostic factor in pediatric oncology patients requiring mechanical ventilatory support. J Pediatr Hematol Oncol. 2010;32:e308–e313. doi: 10.1097/MPH.0b013e3181e51338. [DOI] [PubMed] [Google Scholar]
- 14.Heying R, Schneider DT, Korholz D, Stannigel H, Lemburg P, Gobel U. Efficacy and outcome of intensive care in pediatric oncologic patients. Crit Care Med. 2001;29:2276–2280. doi: 10.1097/00003246-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Heney D, Lewis IJ, Lockwood L, Cohen AT, Bailey CC. The intensive care unit in paediatric oncology. Arch Dis Child. 1992;67:294–298. doi: 10.1136/adc.67.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamburro R. Pediatric cancer patients in clinical trials of sepsis: factors that predispose to sepsis and stratify outcome. Pediatr Crit Care Med. 2005;6:S87–S91. doi: 10.1097/01.PCC.0000161288.00396.49. [DOI] [PubMed] [Google Scholar]
- 17.Tamburro RF, Barfield RC, Shaffer ML, et al. Changes in outcomes (1996–2004) for pediatric oncology and hematopoietic stem cell transplant patients requiring invasive mechanical ventilation. Pediatr Crit Care Med. 2008;9:270–277. doi: 10.1097/PCC.0b013e31816c7260. [DOI] [PubMed] [Google Scholar]
- 18.Fiser RT, West NK, Bush AJ, Sillos EM, Schmidt JE, Tamburro RF. Outcome of severe sepsis in pediatric oncology patients. Pediatr Crit Care Med. 2005;6:531–536. doi: 10.1097/01.pcc.0000165560.90814.59. [DOI] [PubMed] [Google Scholar]
- 19.Baden L, Bensinger W, Casper C, et al. NCCN Clinical Practice Guidelines in Oncology: Prevention and Treatment of Cancer-Related Infections. JNCCN. 2011 doi: 10.6004/jnccn.2016.0093. [DOI] [PubMed] [Google Scholar]
- 20.Haase R, Mathony U, Lieser U, Nagel F, Sitka U, Burdach S. Oncology patients in a pediatric intensive care unit--a 7-year experience. Klin Padiatr. 2003;215:234–240. doi: 10.1055/s-2003-41399. [DOI] [PubMed] [Google Scholar]
- 21.Dursun O, Hazar V, Karasu GT, Uygun V, Tosun O, Yesilipek A. Prognostic factors in pediatric cancer patients admitted to the pediatric intensive care unit. J Pediatr Hematol Oncol. 2009;31:481–484. doi: 10.1097/MPH.0b013e3181a330ef. [DOI] [PubMed] [Google Scholar]
- 22.Vogiatzi L, Ilia S, Sideri G, et al. Invasive candidiasis in pediatric intensive care in Greece: a nationwide study. Intensive Care Med. 2013;39:2188–2195. doi: 10.1007/s00134-013-3057-y. [DOI] [PubMed] [Google Scholar]
- 23.Ben Abraham R, Toren A, Ono N, Weinbroum AA, Vardi A, Barzilay Z, Paret G. Predictors of outcome in the pediatric intensive care units of children with malignancies. J Pediatr Hematol Oncol. 2002;24:23–26. doi: 10.1097/00043426-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Akhtar N, Fadoo Z, Panju S, Haque A. Outcome and prognostic factors seen in pediatric oncology patients admitted in PICU of a developing country. Indian J Pediatr. 2011;78:969–972. doi: 10.1007/s12098-011-0391-3. [DOI] [PubMed] [Google Scholar]
- 25.Singer K, Subbaiah P, Hutchinson R, Odetola F, Shanley TP. Clinical course of sepsis in children with acute leukemia admitted to the pediatric intensive care unit. Pediatr Crit Care Med. 2011;12:649–654. doi: 10.1097/PCC.0b013e31821927f1. [DOI] [PubMed] [Google Scholar]
- 26.Gupta P, Green JW, Tang X, et al. Comparison of high-frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. JAMA Pediatr. 2014;168:243–249. doi: 10.1001/jamapediatrics.2013.4463. [DOI] [PubMed] [Google Scholar]
- 27.Typpo KV, Petersen NJ, Petersen LA, Mariscalco MM. Children with chronic illness return to their baseline functional status after organ dysfunction on the first day of admission in the pediatric intensive care unit. J Pediatr. 2010;157:108–113.e1. doi: 10.1016/j.jpeds.2009.12.029. 10.1016/j.jpeds.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Typpo KV, Petersen NJ, Hallman DM, Markovitz BP, Mariscalco MM. Day 1 multiple organ dysfunction syndrome is associated with poor functional outcome and mortality in the pediatric intensive care unit. Pediatr Crit Care Med. 2009;10:562–570. doi: 10.1097/PCC.0b013e3181a64be1. 10.1097/PCC.0b013e3181a64be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer. 2005;103:1457–1467. doi: 10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 30.Weiss SL, Parker B, Bullock ME, Swartz S, Price C, Wainwright MS, Goodman DM. Defining pediatric sepsis by different criteria: discrepancies in populations and implications for clinical practice. Pediatr Crit Care Med. 2012;13:e219–e226. doi: 10.1097/PCC.0b013e31823c98da. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein B, Giroir B, Randolph A International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 32.Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. 1988;16:1110–1116. doi: 10.1097/00003246-198811000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Ludbrook J, Dudley H. Issues in biomedical statistics: analysing 2 × 2 tables of frequencies. Aust N Z J Surg. 1994;64:780–787. doi: 10.1111/j.1445-2197.1994.tb04539.x. [DOI] [PubMed] [Google Scholar]
- 34.Diggle PJ, Heagerty P, Liang K, Zeger SL. Analysis of Longitudinal Data. Oxford Statistical Science Series; 2002. [Google Scholar]
- 35.Hardin J, Hilbe J. Generalized Estimating Equations. London: Chapman and Hall/CRC; 2003. [Google Scholar]
- 36.Pancera CF, Hayashi M, Fregnani JH, Negri EM, Deheinzelin D, de Camargo B. Noninvasive ventilation in immunocompromised pediatric patients: eight years of experience in a pediatric oncology intensive care unit. J Pediatr Hematol Oncol. 2008;30:533–538. doi: 10.1097/MPH.0b013e3181754198. 10.1097/MPH.0b013e3181754198. [DOI] [PubMed] [Google Scholar]
- 37.Gow KW, Heiss KF, Wulkan ML, et al. Extracorporeal life support for support of children with malignancy and respiratory or cardiac failure: The extracorporeal life support experience. Crit Care Med. 2009;37:1308–1316. doi: 10.1097/CCM.0b013e31819cf01a. [DOI] [PubMed] [Google Scholar]
- 38.Di Nardo M, Locatelli F, Palmer K, et al. Extracorporeal membrane oxygenation in pediatric recipients of hematopoietic stem cell transplantation: an updated analysis of the Extracorporeal Life Support Organization experience. Intensive Care Med. 2014;40:754–756. doi: 10.1007/s00134-014-3240-9. [DOI] [PubMed] [Google Scholar]
- 39.Margolin JF. Molecular diagnosis and risk-adjusted therapy in pediatric hematologic malignancies: a primer for pediatricians. Eur J Pediatr. 2011;170:419–425. doi: 10.1007/s00431-011-1424-7. [DOI] [PubMed] [Google Scholar]
- 40.Demaret P, Pettersen G, Hubert P, Teira P, Emeriaud G. The critically-ill pediatric hematooncology patient: epidemiology, management, and strategy of transfer to the pediatric intensive care unit. Ann Intensive Care. 2012;2:14. doi: 10.1186/2110-5820-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piastra M, Fognani G, Franceschi A ICARO Italian Network For Intensive Care In Pediatric Oncology. Pediatric Intensive Care Unit admission criteria for haemato-oncological patients: a basis for clinical guidelines implementation. Pediatr Rep. 2011;3:e13. doi: 10.4081/pr.2011.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.