Abstract
Immunity in humans with annual vaccination does not provide effective protection against antigenically distinct strains. As an approach to improve cross-protection in the presence of pre-existing strain-specific immunity, we investigated the efficacy of heterologous and heterosubtypic protection in previously vaccinated mice at earlier times after subsequent immunization with conserved-antigenic target influenza M2 ectodomain (M2e) virus-like particle vaccine (M2e5x VLP). Immunization of mice with H1N1 split vaccine induced virus specific antibodies to homologous influenza virus but did not provide heterosubtypic hemagglutination inhibiting antibody responses and cross-protection. However, subsequent M2e5x VLP immunization induced M2e specific antibody response as well as interferon-γ (IFN-γ) producing cells in systemic and mucosal sites. Upon lethal challenge with H3N2 or H5N1 subtype influenza viruses, subsequently immunized mice with M2e5x VLP were well protected against heterosubtypic influenza viruses. These results provide evidence that non-seasonal immunization with M2e5x VLP, an experimental candidate for universal vaccine, is a promising approach for broadening the cross-protection even in the presence of strain-specific immunity.
Keywords: M2e5x VLPs, Influenza vaccine, pre-existing immunity, Cross protection
1. Introduction
Influenza virus causes respiratory diseases in humans. There are parenterally administered inactivated vaccine and live attenuated influenza vaccine [1]. An inactivated, surfactant-disrupted split type is the most common influenza vaccine [2–3]. However, despite the availability of influenza vaccines, the WHO estimates 3 to 5 million severe illnesses and 250,000 to 500,000 deaths worldwide during annual epidemics [4]. Therefore, current influenza vaccination based on highly variable hemagglutinin (HA) proteins has an intrinsic limitation in inducing cross protective immunity. In addition, there is a risk of a new pandemic such as the emergence of 2009 pandemic H1N1 virus [5–6]. Since 1997, new types of influenza A viruses, H5, H7 and H9 serotypes, have crossed the species barrier from birds to man, resulting in severe human infections on multiple occasions [7–9].
The ion-channel protein M2 has a highly conserved extracellular domain (M2e) which is suggested to be a universal influenza A vaccine target [10]. A molecular construct with M2e tandem repeat (M2e5x) that contains M2e sequences derived from human, swine, and avian influenza viruses was developed in a membrane-anchored form and presented on enveloped VLPs (M2e5x VLP) as a potential universal influenza A vaccine [11–12]. Most pre-clinical studies have been carried out using naïve animals. However, human populations are not immunologically naïve and have a certain level of pre-existing immunity to influenza virus either by annual vaccination or natural infection.
It would be desirable to develop an alternative strategy that avoids seasonality of influenza vaccination and has a potential to significantly improve the cross-protective capacity of existing immunity. In this study, we tested this alternative strategy by immunizing mice early to induce pre-existing immunity and then by subsequent following vaccination of these previously split vaccine-immunized mice with M2e5x VLP.
2. Materials and Methods
2.1. Viruses, vaccine, cell and M2e5x VLPs
The A/California/04/2009 (2009 pandemic H1N1 virus; a gift from Dr. Richard Webby), A/Philippines/2/1982 (H3N2), A/PR/8/34 (H1N1), and reassortant A/Vietnam/1203/2004 (rgH5N1) were propagated as previously described [13]. Purified inactivated viruses were produced by treating formalin at a final concentration of 1:4000 (v/v) as described previously [14]. Commercial human influenza split vaccine (Green Flu-S; Green Cross, Korea) derived from the 2009 pandemic strain of A/California/07/2009 (H1N1) virus was used in this study. M2e5x VLPs that contain a tandem repeat of M2e sequences derived from human (2x), swine (1x), and avian (2x) influenza viruses were produced as previously described [11]. The M2 expressing MDCK cell line was kindly provided by Dr. Andrew Pekosz [15].
2.2. Immunization
For animal experiments, 6- to 8-week-old female BALB/c mice (N=24; Harlan Laboratories) were intramuscularly immunized with 0.6 μg of human split vaccine proteins at weeks 0 and 4 (Fig. 1). Then one group of mice (N=12) was intramuscularly immunized with 10 μg M2e5x VLPs at weeks 8 and 12. The placebo group received PBS as a negative control. Blood samples were collected at 3 weeks after each immunization. All animal experiments presented in this manuscript were approved by Georgia State University IACUC review boards.
Fig. 1. Diagram of experimental protocol.
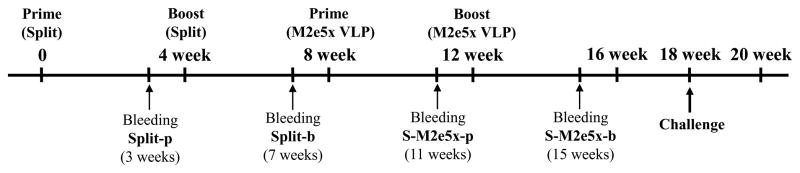
BALB/c mice (N=24) were prime and boost immunized with commercial split vaccine for human use with a 4-week interval. At 4 weeks after split boost immunization, a half of these mice were prime and boost immunized with M2e5x VLP (S-M2e5x, N=12). Sera were collected for antibody responses 3 weeks after each vaccination. In 6 weeks after M2e5x VLP boost immunization, mice were challenged with influenza A viruses, A/Philippines/2/82(H3N2) or A/Vietnam/1203/2004(rgH5N1).
2.3. Antibody responses and hemagglutinin inhibition (HAI) titer
Virus-specific antibody responses were determined by ELISA using A/California/04/2009 virus as a coating antigen (4μg/ml) and virus-specific IgG isotype antibody responses were determined to homologous influenza virus as previously described [14]. Hemagglutination Inhibition (HAI) titer was determined as described [16].
2.4. Antibody responses to native M2 protein
M2e specific antibody responses were determined by ELISA using synthetic M2e peptides as a coating antigen (4μg/ml) [11]. Also, antibodies recognizing native M2 protein were determined by using cell surface ELISA. Stably M2-expressing MDCK monolayer cells were fixed with 10% buffered formalin and used to determine antibodies binding to M2 expressed on cell surfaces as described [17]. As an additional assay, influenza virus-infected MDCK cells were used to determine serum antibodies binding to M2 expressed on infected cell surfaces. Antibody concentrations were estimated by standard curves prepared using purified mouse antibodies (IgG, IgG1, IgG2a) and horse-radish peroxidase-conjugated goat anti-mouse detecting antibodies of corresponding IgG and isotypes (Southern Biotech, Birmingham, AL). Based on IgG standard curves, the OD values measured in serum samples were converted to concentration values (ng/ml).
2.5. Viral challenge
All immunized mice were challenged with a lethal dose (5xLD50) of A/Philippines/2/82 or rgH5N1 influenza A viruses at 6 weeks after boost immunization with M2e5x VLPs. Mice were monitored daily to record weight changes and mortality.
2.6. Protective efficacy test of immune sera
To test protective efficacy of immune sera in vivo to distinct H1N1 (A/PR/8/34) influenza virus that is antigenically different from 2009 pandemic H1N1 vaccine strain, 2-fold diluted sera were heat-inactivated at 56°C for 30 min and the diluted serum samples were mixed with a lethal dose of influenza virus [11]. Naive mice (N=3, BALB/c) were administered a mixture of influenza virus and sera, and both body weight and survival rates were monitored daily.
2.7. Analysis of bronchoalveolar lavage fluids and lung viral titers
Bronchoalveolar lavage fluids (BALF) were prepared for the analysis of antibody responses and proinflammatory cytokine IL-6 from mice at day 4 after challenge with 5xLD50 of influenza A/Philippines/2/82 (H3N2) virus. Preparations of BALF samples and lung extracts were obtained as described [11]. Ready-Set-Go IL-6 kit (eBioscience) was used to detect cytokine levels in bronchoalveolar fluids (BALF) following the manufacturer’s recommended procedures [13]. Embryonated chicken eggs were inoculated with diluted lung extracts and tested for hemagglutination activity to determine viral titers as described [18].
2.8. Determination of T cell responses
At day 4 post challenge, splenocytes and lung cells were isolated and used to determine T cell responses as described [13]. Interferon (IFN)-γ secreting cell spots were determined on Multi-screen 96 well plates (Millipore) coated with cytokine specific capture antibodies as described [19]. Briefly, 0.5×106 spleen cells or 0.2×106 lung cells per well were cultured with inactivated influenza A/Phil (H3N2), rgH5N1, M2e peptide or M2e5x VLP as an antigenic stimulator (2ug/ml). After 36 h incubation, the spots of IFN-γ secreting T cells were counted using an ELISpot reader.
2.9. Statistical analysis
To determine the statistical significance, a two-tailed Student’s t-test was used when comparing two different conditions. P-values less than 0.05 were considered statistically significant and * or ** were indicated as P-values less than 0.05 or 0.01, respectively.
3. Results
3.2. Subsequent M2e5x VLP vaccination modulates IgG isotype antibodies of pre-existing immunity
In our previous studies, M2e5x VLP was shown to confer effective cross protection against H1, H3, and H5 subtype influenza viruses [11–12]. Here, we investigated whether immunization with M2e5x VLPs would improve the efficacy of cross-protection against influenza A viruses in the presence of pre-existing vaccine immunity. To induce pre-immunity to influenza virus, groups of mice (N=24) were intramuscularly prime and boost immunized with 2009 H1N1 split vaccine (Split) at weeks 0 and 4 (Fig. 1). Levels of virus-specific antibody were significantly increased after boost immunization and maintained for over 8 weeks (Fig. 2A and B). In an attempt to improve the cross-protection in the presence of pre-existing immunity, half of split vaccine immunized mice (N=12) were additionally vaccinated with M2e5x VLP (S-M2e5x) at weeks 8 and 12 (Fig. 1). There was no significant difference in virus-specific total IgG antibody titers between the Split vaccine group and the subsequent M2e5x VLP immunization (S-M2e5x) group (Fig. 2B). These results suggest that additional immunization with M2e5x VLPs did not affect total IgG antibody responses to influenza virus.
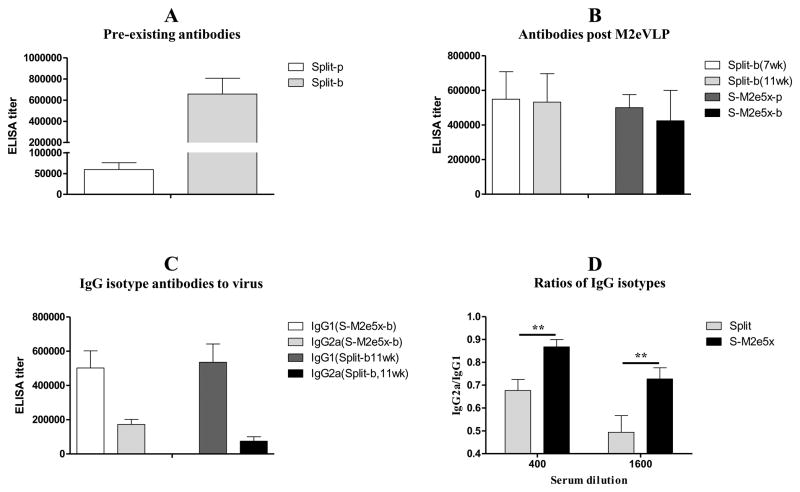
Fig. 2. M2e5x VLP immunization modulates pre-existing IgG isotype antibody to vaccine viral antigen.
(A) Pre-existing IgG antibody responses to virus by earlier vaccine immunization. Split-p: Prime, Split-b: Boost. (B) IgG antibody responses post M2eVLP additional immunization. Split-b(7wk): 7wk after Split-b. Split-b(11wk): 11wk after Split-b. (C) IgG isotype antibody responses to vaccine antigen A/Cali virus. (D) Ratios of IgG2a/IgG1 isotype antibodies to homologous A/Cali virus. Split-b: 7wk after Split-b. BALB/c mice were immunized with split vaccine alone (Split, N=12) or received subsequent additional M2e5x VLP immunization after split vaccine (S-M2e5x, N=12). Sera were collected 3 weeks after each vaccination. The IgG level was detected using homologous influenza A virus, A/California/04/09 (A/Cali, H1N1), as an ELISA coating antigen (2ug/ml). Error bars indicate mean ± SEM.
To further understand types of immune responses, we analyzed IgG isotypes of serum antibodies to vaccine strain (A/California/04/09) with and without M2e5x VLP immunization (Fig. 2C). Virus-specific IgG1 isotype titers were similar between split only and additional M2e5x VLP immunized mice (Fig. 2C). Both groups showed IgG1 as a dominant isotype antibody to vaccine strain (A/California/04/09). In contrast, average levels of IgG2a antibody were increased after additional M2e5x VLP immunization (Fig. 2C). The ratio of IgG2a/IgG1 in additional M2e5x VLP immunized group (IgG2a/IgG1: 0.87 or 0.73) was 27% or 45% higher than that of the split vaccine group (IgG2a/IgG1: 0.68 or 0.50) at 400 or 1,600 serum dilutions, respectively (Fig. 2D). These results provide evidence that M2e5x VLP immunization can impact on types of pre-existing immunity, increasing T helper type 1 (Th1) immune responses such as IgG2a isotype antibody.
3.2. M2e5x VLP immunization does not induce heterosubtypic HAI antibodies
High titers of HAI against the homologous virus A/California/04/2009 were observed regardless of subsequent M2e5x VLP immunization (Fig. 3A). However, serum antibodies from both groups did not show HAI activity to heterosubtypic influenza viruses, A/Philippines/2/82(H3N2) or A/Vietnam/1203/2004(H5N1). These results indicate that influenza split vaccination could induce high HAI titers against homologous virus and subsequent M2e5x VLP immunization did not affect the level of HAI activities.
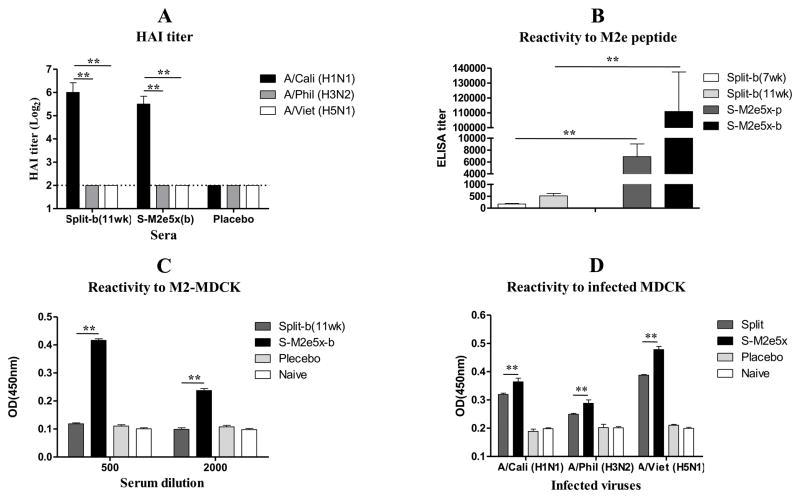
Fig. 3. M2e5x VLP immunization induces M2e-specific antibodies to M2 expressing MDCK cells.
Immune sera were collected from BALB/c mice that were immunized with split vaccine alone (Split, N=12) or additional M2e5x VLP immunization after split vaccine (S-M2e5x, N=12). All sera were collected at 3 week after M2e5x VLP boost. (b) immunization. (A) Hemagglutinin inhibition (HAI) assay. Split-b(11wk): 11wk after Split-b. (B) Reactivity to synthetic M2e peptide. Split-b(7wk): 7wk after Split-b. Split-b(11wk): 11wk after Split-b. (C) Reactivity to M2-expressing MDCK cell. (D) Reactivity to influenza virus-infected MDCK cells. The dotted line denotes the limit of HAI detection. Error bars indicate mean ± SEM.
3.3. M2e5x VLP immunization induces M2e antibody in the presence of pre-existing immunity
In the group of mice additionally primed with M2e5x VLPs after split vaccination, M2e-specific antibody titers to M2e peptide were induced at substantially high levels but not in the split vaccine alone group and, after boost with M2e5x VLPs, M2e antibody titers were further increased by approximately15 fold (Fig. 3B). To determine if M2e5x VLP immunization could induce humoral responses to native M2 protein, immune sera from groups of mice with split vaccination or additional M2e5x VLP immunization were evaluated using M2-expressing MDCK cells. M2e specific IgG antibody levels in M2e5x VLP immunized mice were 4-fold higher than those of the mouse group immunized with split vaccine only (Fig. 3C). M2e reactivity was also evaluated in MDCK cells infected with A/California/04/2009 (H1N1), A/Philippines/2/1982 (H3N2) or rgH5N1. Sera of additional M2e5x VLP-immunized mice showed 15–20% higher IgG antibodies reactive to MDCK cell surface antigens that were infected with heterosubtypic influenza A viruses than those of split vaccine (Fig. 3D). These results suggest that additional immunization with M2e5x VLPs induces antibody responses to M2e antigens as a conserved antigenic target regardless of pre-existing vaccine immunity to influenza virus.
3.4. M2e5x VLP immunization under pre-existing immunity confers improved cross protection
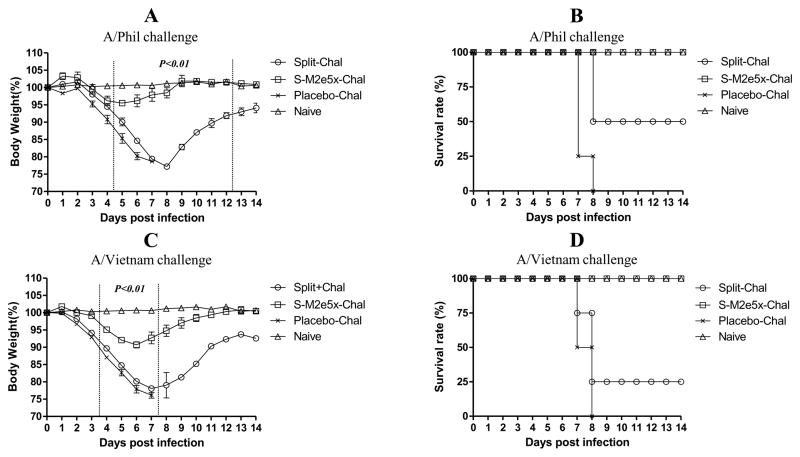
To compare the cross-protective efficacy of additional M2e5x VLP immunization, groups of mice were intranasally challenged with a lethal dose (5xLD50) of heterosubtypic influenza viruses, A/Philippines/2/82(H3N2) or rgH5N1 at week 18 (6 weeks after M2e5x VLP boost immunization) (Fig. 4). Upon A/Philippines/2/82 virus challenge, mice additionally immunized with M2e5x VLP showed only a slight loss (approximately 4%) in body weight, showing 100% protection (S-M2e5x, Fig. 4A and B). In contrast, mice immunized with H1N1 split vaccine alone showed a significant loss of over 20% in body weight as well as a substantial delay in recovery of weight loss (Fig. 4A). The split vaccine group showed 50% protection (Split, Fig. 4B). To further test the breadth of cross protection, rgH5N1 virus challenge was performed. Split vaccine-immunized mice that were subsequently vaccinated with M2e5x VLP showed a slight loss (approximately 9%) in body weight, resulting in 100% protection (Fig. 4C and D). In contrast, immune mice without M2e5x VLP vaccination showed a significant loss of approximately 22%, resulting in only 25% survival protection (Split, Fig. 4C and D). These results demonstrate that subsequent M2e5x VLP immunization under pre-existing vaccine immunity significantly improved cross protection.
Fig. 4. Improved efficacy of cross protection by M2e5x VLPs immunization after split vaccine.
Groups of mice (N=4) that were immunized with split or split and additional M2e5x VLP were intranasally challenged with a lethal dose (5xLD50) of influenza viruses, A/Philippines/2/82 (H3N2) or rgH5N1, 6 weeks after M2e5x VLP boost immunization. (A) Average body weight changes after A/Philippines/2/82 virus challenge. (B) Survival rates after A/Philippines/2/82 virus challenge. (C) Average body weight changes after rgH5N1 virus challenge. (D) Survival rates after rgH5N1 virus challenge. Error bars indicate mean ± SEM. LD, lethal dose. P value indicates significant difference between groups of split or S-M2e5x immunization.
3.5. M2e antibodies in immune sera play an important role in conferring protection
To further determine the cross-protective role of anti-M2e immune sera, we carried out an in vivo protection assay. Naive mice were infected with a mixture of sera from split, S-M2e5x VLP, or placebo groups and a lethal dose of influenza virus, A/PR/8/34 (A/PR8, H1N1). (Supplementary Fig. 1). Naïve BALB/c mice that were infected with a lethal dose of A/PR8 virus with immune sera of S-M2e5x VLP vaccinated group showed a slight loss (approximately 5%) and then fully recovered to a normal level. However, BALB/c mice that received a lethal dose of A/PR8 virus with immune sera of the split alone group displayed a severe loss (approximately 18%) and a significant delay in weight recovery (Supplementary Fig. 1A). In addition, BALB/c mice that received a lethal dose of A/PR8 virus with sera from the placebo group showed a severe loss of 24% and a significant delay in weight recovery, resulting in 33% protection (Supplementary Fig. 1A and B). These results suggest that immune sera from subsequent M2e5x VLP immunization significantly improve cross protection to distinct H1N1 influenza virus.
3.6. Subsequent M2e5x VLP vaccination contributes to inducing M2e mucosal antibodies
Virus or M2e specific IgG antibody responses in BALF were determined in mice sacrificed at day 4 after viral challenge with H3N2 virus (Fig. 5A and B). Significantly higher levels of IgG antibody responses specific for H1N1 vaccine strain were observed in BALF of both groups of mice with and without additional M2e5x VLP immunization (Fig. 5A). However, significantly high levels of IgG antibody specific for M2e antigen were observed in BALF of mice with additional M2e5x VLP immunization (Fig. 5B). Overall, these results suggest that M2e specific antibodies were effectively induced at mucosal sites in the S-M2e5x group with additional M2e5x VLP immunization upon viral infection.
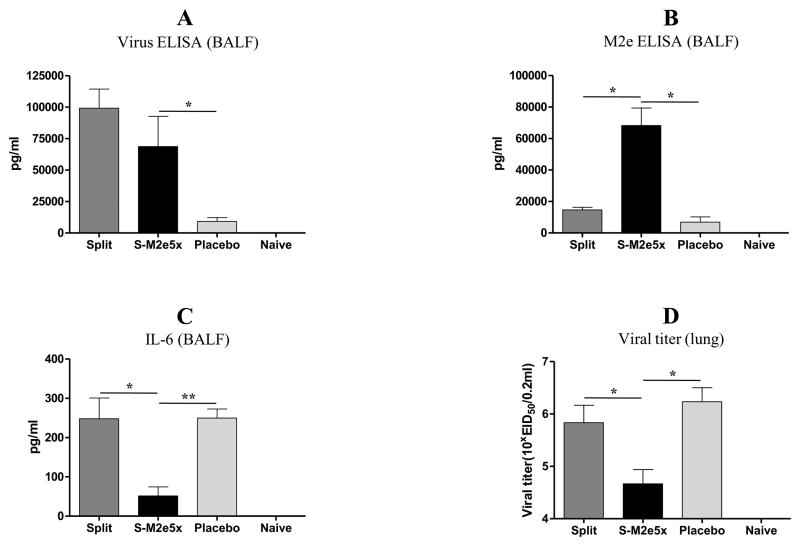
Fig. 5. Mucosal antibody responses, pro-inflammatory cytokine and lung viral titers upon heterosubtypic challenge.
Levels of IgG antibodies to virus or M2e antigens were determined in lung or BALF samples (N=4) at day 4 post-challenge after split or split and additional M2e5x VLP immunization. (A) Virus specific IgG antibody response. (B) M2e specific IgG antibody response. (C) Levels ofIL-6 cytokine in BALF. (D) Lung viral titers. Error bars indicate mean ± SEM. P value indicates significant difference. BALF, bronchoalveolar lavage fluid; pg, picogram.
3.7. M2e5x VLP vaccination lowers inflammatory cytokine levels and lung viral titers
Over-induction of inflammatory cytokines due to uncontrolled viral replication can cause tissue damage. Cytokines in BALF from infected mice at 4 days after challenge were determined (Fig. 5C and D). High levels of IL-6 were observed in BALF of placebo mice after infection as expected (Fig. 5C). The level of IL-6 in BALF from the split vaccine mice was also significantly higher than that of additional M2e5x VLP immunized mice (S-M2e5x, Fig. 5C). These results support evidence that additional M2e5x VLP immunization under strain-specific pre-existing immunity can improve cross protection by controlling inflammatory cytokine responses. To better assess the protective efficacy against A/Philippines/2/82 (H3N2), lung viral titers were determined at day 4 after challenge (Fig. 5D). The S-M2e5x group with M2e5xVLP immunization showed approximately 10-fold and 30-fold lower lung viral titers compared to those with pre-existing HA immunity alone and naïve-challenge control groups, respectively (Fig. 5D). Therefore, the results indicate that induction of M2e specific immune responses under pre-existing influenza immunity can effectively contribute to controlling viral replication of heterosubtypic challenge virus.
3.8. Subsequent M2e5x VLP immunization enhances IFN-γ secreting T cell responses
Mice were challenged with H3N2 virus at 8 weeks after additional M2e5xVLP immunization and cells from spleen or lung samples were collected at day 4 post-challenge to determine IFN-γ producing cellular responses (Fig. 6). Significant levels of IFN-γ secreting spleen or lung cells were observed in mice immunized with M2e5x VLPs compared to those with split vaccine alone (Fig. 6). Importantly, the group of mice immunized with M2e5x VLPs showed higher levels of IFN-γ secreting cell spots in response to M2e stimulators, M2e peptide or M2e5x VLPs, in both spleen and lung cells as well as to(viral antigens, A/Philippines/82 (H3N2) or rgH5N1, than those of mice immunized with split vaccine alone (Fig. 6A and B). These results provide evidence that additional M2e5x VLP immunization in the presence of pre-existing vaccine immunity induces an effective response of INF-γ secreting T cells specific for M2e or heterosubtypic influenza viral antigens.
Fig. 6. M2e5x VLP immunization after split vaccine enhances IFN-γ producing cellular immune responses.
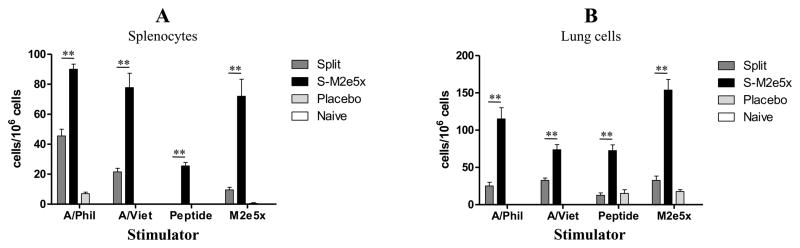
Splenocytes and lung cells were isolated from mice at day 4 post-challenge. IFN-γ secreting cells were determined after antigenic stimulation with A/California /04/09 (H1N1) viral antigen, M2e peptide, or M2e5x VLPs as a stimulator (2μg/ml). (A) IFN-γ in splenocytes. (B) IFN-γ in lung cells. Error bars indicate mean ± SEM. Asterisk indicates significant difference (P<0.01) between split and S-M2e5x groups. IFN, interferon.
4. Discussion
Pre-existing immunity is mainly dependent on host antibody responses to distinct HA protein. However, influenza viruses are continuously evolving by accumulating mutations in the highly variable HA proteins. Most people are susceptible to antigenically drift or pandemic strains despite pre-existing immunity. It would be an ideal goal to develop a highly effective stand-alone universal influenza vaccine. However, protective efficacy of candidate universal vaccines based on conserved antigenic targets is relatively weak despite their broader range of cross protection compared to conventional HA-based influenza vaccines [20–21]. In addition, there are extremely high genetic and antigenic differences among influenza viruses. Due to antigenic diversity of influenza viruses as well as weak protective efficacy of conserved antigenic targets, developing a universal influenza stand-alone vaccine has been a real challenge.
An alternative approach is to significantly improve the capacity of pre-existing immunity to confer cross protection. In this study, we investigated the cross protective effects of subsequent immunization with M2e5x VLP in the presence of pre-existing immunity to an influenza vaccine strain. We found that additional vaccination with M2e5x VLPs effectively induced M2e-specific humoral and cellular immune responses. Notably, additional M2e5x VLP vaccination conferred enhanced cross protection against the distantly related heterologous A/PR/8/34 (H1N1) and heterosubtypic A/Philippines/2/82 (H3N2) and rgH5N1 influenza A viruses. As expected, mice that were immunized with split vaccine (H1N1) alone earlier times were not well protected against heterologous or heterosubtypic challenge infections as evidenced by significantly lower survival rates as well as severe weight losses. We have observed M2e-immune mediated protection on lowering lung viral loads and inflammatory cytokine IL-6 as well as induction of T cell responses in lungs and spleens after challenge with H1N1 (A/PR/8/34) virus using a C57BL/6 mouse model [22]. These post-challenge data with H1 virus showed a similar pattern that was observed in this study using H3N2 virus post-challenge experiments. Thus, similar results are expected with post-H5N1 virus challenge although post-challenge immune responses and titers remain to be determined. Therefore, additional vaccination with M2e5x VLPs under the condition of pre-existing immunity can improve cross protection of existing immunity to current influenza vaccination. More importantly, M2e5x VLP immunization would not have seasonality and could be administered before or after seasonal vaccination. It is also desirable and possible scenario to have a stock-file of M2e5x VLP vaccines that can be immediately used to vaccinate before the availability of manufacturing new pandemic vaccines. In an effort to enhance M2e antibodies and IFN-γ producing T cell responses, M2e5x VLP that was adjuvanted with AS04 was shown to be more effective than M2e5x VLP alone in conferring protection as well as in inducing recall M2e antibodies and interferon-γ producing CD4 T cell responses in a C57BL/6 mouse model [22]. Therefore, M2e5x VLP vaccines with some adjuvants may have the potential to further improve cross-protection.
A possible mechanism by which M2 antibodies provide protection is that influenza viruses bound to non-neutralizing antibodies are likely to be recognized and removed by opsonophagocytosis via Fc receptors [12, 23–27]. In this study, M2e specific antibody after M2e5x VLP immunization was reactive to M2-expressing MDCK cell or MDCK cells infected with influenza viruses, which suggests that binding antibody to infected cell can mediate opsonophagocytosis. In addition, antibody isotypes to virus are known to be important in antiviral immunity. IgG1 isotype antibodies are likely to be involved in neutralizing homologous virus [24]. In contrast, IgG2a isotype antibodies were reported to play a role in assisting the clearance of virus infected host cells [24]. In this study, average levels of IgG2a antibody response to heterosubtypic influenza viruses were also increased after additional M2e5x VLP immunization with a statistical difference (Supplementary Fig. 2). Also, in line with this result, co-immunization with mix of M2e5x VLP and split vaccines was shown to induce high levels of IgG2a antibody and improved cross protection [27]. Thus, shifting immunoglobulin isotypes to IgG2a antibodies as a result of M2e5x VLP immunization later might have contributed to improving cross protection in addition to the induction of M2e specific antibodies.
These results provide evidence that additional M2e5x VLP vaccination to split vaccine can modify the pattern of host immune responses to the vaccine in a direction of increasing Th1 type immunity. Vaccine specific Th1 immune responses such as an increased level of IFN-γ secreting T cells would contribute to improved cross protection by additional M2e5x VLP vaccination. Therefore, M2e5x VLPs could have a dual role of inducing M2e immunity as well as displaying an adjuvant effect by enhancing and modulating host immune responses to vaccine toward the Th1 responses. The distribution of IgG2a isotype antibodies is likely to be dictated through signaling by Th1 type cytokines [28–29], which is often associated with interferon (IFN)-γ [29] and other Th1 cytokines [30]. As shown in our study, ineffective cross-protection is primarily due to a lack of immunity to conserved targets such as M2e and Th2 biased immune responses by influenza HA-based split vaccination.
In summary, this present study investigated a potential approach to overcome strain-specific pre-existing immunity induced by seasonal vaccination, which may mimic a condition with pre-existing immunity in human population. Subsequent immunization with M2e5x VLP as a potential universal vaccine candidate induced significant levels of M2e antibodies in mice with strain-specific vaccine-induced pre-existing immunity. Following M2e5x VLP immunization induced Th1 type immune responses and conferred significantly improved cross protection to strain-specific immune mice. The results in this study support the concept that candidate universal vaccines can be administered regardless of seasonality of vaccination.
Supplementary Material
Research highlight.
M2e5x VLP immunization induced M2e antibodies in mice with pre-existing immunity.
Subsequent M2e5x VLP immunization primed IFN-γ producing cells.
Subsequent M2e5x VLP immunization improved heterosubtypic cross-protection.
Acknowledgments
This work was supported by NIH/NIAID grants AI105170 (S.M.K.) and AI093772 (S.M.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nicholson KG, Tyrrell DA, Harrison P, Potter CW, Jennings R, Clark A, et al. Clinical studies of monovalent inactivated whole virus and subunit A/USSR/77 (H1N1) vaccine: serological responses and clinical reactions. J Biol Stand. 1979;7:123–36. doi: 10.1016/s0092-1157(79)80044-x. [DOI] [PubMed] [Google Scholar]
- 2.Stephenson I, Nicholson KG, Gluck R, Mischler R, Newman RW, Palache AM, et al. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: phase I randomised trial. Lancet. 2003;362:1959–66. doi: 10.1016/S0140-6736(03)15014-3. [DOI] [PubMed] [Google Scholar]
- 3.Cox JC, Coulter AR. Adjuvants--a classification and review of their modes of action. Vaccine. 1997;15:248–56. doi: 10.1016/s0264-410x(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 4.Stohr K. Preventing and treating influenza. BMJ. 2003;326:1223–4. doi: 10.1136/bmj.326.7401.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 6.Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–5. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 7.Peiris M, Yuen KY, Leung CW, Chan KH, Ip PL, Lai RW, et al. Human infection with influenza H9N2. Lancet. 1999;354:916–7. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- 8.Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A. 2004;101:1356–61. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong SS, Yuen KY. Avian influenza virus infections in humans. Chest. 2006;129:156–68. doi: 10.1378/chest.129.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin J, Zhang J, Dong X, Fang H, Chen J, Su N, et al. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet. 2006;368:991–7. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- 11.Kim MC, Song JM, OE, Kwon YM, Lee YJ, Compans RW, et al. Virus-like Particles Containing Multiple M2 Extracellular Domains Confer Improved Cross-protection Against Various Subtypes of Influenza Virus. Mol Ther. 2013;21:485–92. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MC, Lee JS, Kwon YM, OE, Lee YJ, Choi JG, et al. Multiple heterologous M2 extracellular domains presented on virus-like particles confer broader and stronger M2 immunity than live influenza A virus infection. Antiviral research. 2013;99:328–35. doi: 10.1016/j.antiviral.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, et al. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology. 2010;405:165–75. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol. 2008;82:1350–9. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grantham ML, Stewart SM, Lalime EN, Pekosz A. Tyrosines in the influenza A virus M2 protein cytoplasmic tail are critical for production of infectious virus particles. J Virol. 2010;84:8765–76. doi: 10.1128/JVI.00853-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan FS, Yoo DG, Song JM, Clements JD, Compans RW, Kang SM. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J Virol. 2009;83:4489–97. doi: 10.1128/JVI.02035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song JM, Wang BZ, Park KM, Van Rooijen N, Quan FS, Kim MC, et al. Influenza virus-like particles containing M2 induce broadly cross protective immunity. PLoS One. 2011;6:e14538. doi: 10.1371/journal.pone.0014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen HS, Wang SP. Mouse adaptation of the Asian influenza virus. J Infect Dis. 1959;105:9–17. doi: 10.1093/infdis/105.1.9. [DOI] [PubMed] [Google Scholar]
- 19.Song JM, Van Rooijen N, Bozja J, Compans RW, Kang SM. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc Natl Acad Sci U S A. 2011;108:757–61. doi: 10.1073/pnas.1012199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bush RM, Bender CA, Subbarao K, Cox NJ, Fitch WM. Predicting the evolution of human influenza A. Science. 1999;286:1921–5. doi: 10.1126/science.286.5446.1921. [DOI] [PubMed] [Google Scholar]
- 21.Plotkin JB, Dushoff J. Codon bias and frequency-dependent selection on the hemagglutinin epitopes of influenza A virus. Proc Natl Acad Sci U S A. 2003;100:7152–7. doi: 10.1073/pnas.1132114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YN, Kim MC, Lee YT, Hwang HS, Cho MK, Lee JS, et al. AS04-adjuvanted virus-like particles containing multiple M2 extracellular domains of influenza virus confer improved protection. Vaccine. 2014;32:4578–85. doi: 10.1016/j.vaccine.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001;166:7381–8. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 24.Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, et al. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol. 2006;13:981–90. doi: 10.1128/CVI.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mozdzanowska K, Feng J, Eid M, Zharikova D, Gerhard W. Enhancement of neutralizing activity of influenza virus-specific antibodies by serum components. Virology. 2006;352:418–26. doi: 10.1016/j.virol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol. 2007;81:3487–94. doi: 10.1128/JVI.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim MC, Lee YN, Ko EJ, Lee JS, Kwon YM, Hwang HS, et al. Supplementation of Influenza Split Vaccines with Conserved M2 Ectodomains Overcomes Strain-Specificity and Provides Long-term Cross Protection. Mol Ther. 2014 doi: 10.1038/mt.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran TM, Park H, Fernandez-Sesma A, Schulman JL. Th2 responses to inactivated influenza virus can Be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J Infect Dis. 1999;180:579–85. doi: 10.1086/314952. [DOI] [PubMed] [Google Scholar]
- 29.Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, et al. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–8. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 30.Vanderplasschen A, Markine-Goriaynoff N, Lomonte P, Suzuki M, Hiraoka N, Yeh JC, et al. A multipotential beta-1,6-N-acetylglucosaminyl-transferase is encoded by bovine herpesvirus type 4. Proc Natl Acad Sci U S A. 2000;97:5756–61. doi: 10.1073/pnas.100058897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.