Abstract
Objectives
To examine trends in glucocorticoid (GC) use and dosage among patients diagnosed with rheumatoid arthritis (RA) over time.
Methods
A population-based inception cohort of RA patients diagnosed during 1980–2007 was followed longitudinally through their medical records until death, migration or 12/31/2008. GC start and stop dates were collected along with dosages in prednisone equivalents.
Results
The study population comprised 349 patients (68% female) diagnosed in 1980–1994 and 464 (69% female) diagnosed in 1995–2007, with median followup of 15.3 and 5.7 years, respectively. A higher proportion of patients started GCs in their 1st year of disease in 1995–2007 (68% vs 36%, p<0.001), but the starting dose (mean 8.7 vs 10.3mg, p=0.08) and cumulative dose in the first year of use (mean 1.8g [mean daily dose 4.9mg] vs 2.1g [mean daily dose 5.8mg], p=0.48) were not different. A higher proportion also discontinued GCs in their 1st year of disease in the 1995–2007 cohort (p<0.001). These differences in GC initiation and discontinuation persisted throughout followup. Prevalence of GC use was higher in the 1995–2007 cohort for the1st 3 years of disease.
Conclusion
More patients are starting GCs early in their disease course now compared to previously which is consistent with established treatment guidelines. A higher proportion are also discontinuing GCs, but the proportion of patients on GCs at any given point of disease during the first 4 years is higher now than previously. Despite early addition of a DMARD, some patients may not be able to discontinue GCs over the long term.
INTRODUCTION
With Edward Kendall’s discovery of compound E, Louis Sarrett’s synthesis of ‘cortisone’ and Philip Hench’s successful application to a patient with rheumatoid arthritis (RA), glucocorticoids (GCs) became the ‘wonder drug’ of the 20th century and have held a special place in the management of rheumatoid arthritis ever since (1). Their use is cost-effective and their potent anti-inflammatory properties help provide rapid symptomatic relief. Randomized controlled trials have demonstrated an ability to retard radiographic progression in RA and prevent development of new erosions, thus confirming disease modifying effects when used in low doses (2–4). These gains are maintained only for the duration of therapy and may even be associated with a rebound deterioration of symptoms following withdrawal of treatment.
Unfortunately, while generally regarded as safe with short term use, protracted use of GCs is linked to adverse effects, including osteoporosis, fractures, gastrointestinal bleeding, cataracts, glaucoma, infections and cardiovascular disease, among others. A better understanding of these adversities has changed the status of GCs to ‘best friend, worst enemy’ and despite extensive clinical experience with their use, there remains significant controversy among clinicians with respect to their efficacy, safety, optimal dose and duration of therapy.
In actual clinical practice, the use of GCs in RA treatment is dynamic. It is not only affected by patient and disease related factors, but also physician beliefs, training and preferences (5). High disease activity, severity (evidenced by radiographic joint damage/erosive disease and need for joint surgery) and extra-articular manifestations do necessitate use of GCs, but recent data suggests that up to 25% (5) of patients continue taking GCs despite being in remission or with minimal disease activity. The definition of ‘high dose’ and ‘low dose’ is also highly variable and often depends on individual physician prescribing patterns (6). A standardized nomenclature has been suggested by EULAR with doses of ≤ 7.5 mg prednisone equivalent a day considered low dose, 7.5–30 mg/day considered medium dose and >30 mg/day considered high dose therapy (7). Although, in the past, GCs were merely being used as a temporary ‘bridge’ till disease modifying therapy became effective, a greater recognition of their disease modifying effects has encouraged the use of low dose GC therapy for longer durations with an acceptable adverse effect profile at least in the short to medium term (8).
In the last several decades, a better understanding of RA pathophysiology and disease progression has resulted in several changes to RA therapeutics. A greater emphasis has been placed on early recognition of disease, a treat-to-target approach and aggressive control of disease activity through intensive use of disease modifying anti-rheumatic drugs (DMARDs) and biologic response modifiers (BRM). It is, however, unclear if these practices have affected GC use in RA. We hypothesized that the cumulative use of GC has declined in the recent years.
Our aim in this study was to examine trends in GC use in a population based inception cohort of patients with RA to determine if they have changed with a change in prescribing and treatment practices.
METHODS
Study Population
This retrospective cohort study was approved by the Mayo Clinic Institutional Review Board. It was conducted within the population of Olmsted County, Minnesota using resources of the Rochester Epidemiology Project. This unique system ensures virtually complete ascertainment of all clinically recognized cases of RA among the residents of Olmsted County and provides details of all inpatient and outpatient encounters, including medication use and doses, providing an opportunity to study therapeutic trends in a defined RA population.
Using this resource, a population-based incidence cohort of patients diagnosed with RA between January 1, 1980, and December 31, 2007, among residents of Olmsted County, Minnesota and ≥18 years of age was assembled. All cases fulfilled the 1987 American College of Rheumatology criteria (ACR) for RA and were followed longitudinally through their complete medical records until death, migration or 12/31/2008.
Data collection
RA disease characteristics assessed included rheumatoid factor (RF) positivity, erythrocyte sedimentation rate (ESR) (at RA incidence and highest during first year after diagnosis) and radiographic erosions. DMARD use was defined as uninterrupted treatment lasting ≥30 days with a particular DMARD (oral or intramuscular gold, sulfasalazine, hydroxychloroquine, azathioprine, D-penicillamine, methotrexate, leflunomide, alkylating agents or BRM).
Definition of glucocorticoid exposure
All generic and trade names of GC medications were used to identify exposures documented in the medical records. Exposures from inpatient, outpatient, emergency department and nursing home encounters were ascertained. Use of oral, intravenous and intramuscular GCs, their doses in prednisone equivalents (categorized as 0–5 mg/day, 6–10 mg/day, 11–15 mg/day, 16–20 mg/day 21+ mg/day), dates of initiation and discontinuation, and duration of therapy were recorded. Intra-articular and inhalational GC use was not abstracted.
Data on RA disease characteristics, use of DMARDs and GCs were collected through medical record review by trained nurse abstractors according to a prespecified and pretested protocol (9). A given dosage of GC was assumed to have continued until it had been discontinued or changed. If the recorded dosage was higher or lower at a subsequent visit, then the date of this change was assumed to have been the date of that subsequent visit. For long intervals between physician visits, the subjects were assumed to have continued taking a given dosage of a GC if it was indicated as a current medication at both the current and former visits, with no documented plan of tapering or other alteration. Changes in the oral GC dosage that remained within a dosage category were not documented.
To assess for interobserver variation in the cumulative GC dose, data were re-abstracted (by JMD) on a random sample of 20 patients and compared against the data collected by the nurse abstractors. A high correlation was noted between the independent observations for GC exposure (r =0.95 for total number of days receiving glucocorticoids; r =0.97 for cumulative glucocorticoid dose) (9).
Comparisons were made between patients diagnosed and treated for RA during the 2 time periods from 1980–1994 vs. 1995–2007 to determine trends in GC use.
Statistical analysis
Descriptive statistics were used to summarize the demographic characteristics of the cohort by time period (1980–1994 vs 1995–2007) with comparisons performed using chi-square and rank-sum tests. Because GC use was collected in categories, the midpoint of each dosage category was used when calculating the cumulative dosage (e.g., for 5–10 mg/day category, 7.5 mg/day). Cumulative incidence of GC initiation and discontinuation was computed with adjustment for competing risk of death (10). The current prevalence of GC use was estimated as the proportion of subjects on GCs from among those under observation during each month of RA duration, adjusted for mortality to avoid survival bias.
RESULTS
Clinical characteristics of patients with RA according to time period of RA incidence are displayed in Table 1. The study population comprised 349 (68% female) patients with incident RA in 1980–1994 and 464 (69% female) in 1995–2007, with a median followup of 15.3 and 5.7 years, respectively. Mean age was 56 years, and 66% patients were rheumatoid factor positive in both cohorts. Patients with incident RA in the 1995–2007 cohort were less likely to be current smokers and had a higher body mass index (BMI; 28.6 vs 26.8, p<0.001) at RA incidence than patients in the 1980–1994 cohort. They also had a lower mean ESR at diagnosis (23.0 vs 27.2 mm/hr, p<0.001) than the 1980–1994 cohort, as well as a lower mean ESR during the first year of diagnosis (30.1 vs 36.2 mm/hr, p<0.001).
Table 1.
Characteristics of the rheumatoid arthritis cohort (1980–2007)
| Variable | 1980–1994 (n=349) |
1995–2007 (n=464) |
p-value |
|---|---|---|---|
| Age, years, mean (SD) | 56.2 (15.9) | 55.6 (15.5) | 0.44 |
| Female, n (%) | 236 (68) | 320 (69) | 0.68 |
| Rheumatoid factor +, n (%) | 231 (66) | 306 (66) | 0.94 |
| BMI at diagnosis, kg/m2, mean (SD) | 26.8 (5.5) | 28.6 (6.2) | <0.001 |
| Smokers, current, n (%) | 98 (28) | 80 (17) | <0.001 |
| ESR at diagnosis, mm/hr, mean (SD) | 27.2 (22.1) | 23.0 (19.0) | 0.01 |
| Highest ESR*, mm/hr, (mean (SD) | 36.2 (26.7) | 30.1 (24.7) | <0.001 |
| Erosions/destructive changes*, n (%) | 85 (24) | 134 (29) | 0.15 |
| DMARDs*, n (%) | 181 (52) | 385 (83) | <0.001 |
| Duration of follow-up, years, mean (SD) | 14.6 (7.2) | 5.9 (3.5) | -- |
During the first year of RA diagnosis
BMI = body mass index; DMARDs = disease modifying antirheumatic drugs; ESR = erythrocyte sedimentation rate
Patients in the 1995–2007 cohort were more likely to have started DMARDs in their first year of disease compared to the 1980–1994 cohort (83% vs. 52%, p<0.001). A higher proportion of patients also started GCs in their first year of disease in the 1995–2007 cohort (68% vs 36%, p<0.001), but the starting dose in prednisone equivalents (mean 8.7 [standard deviation (sd) 5.3] vs 10.3 [sd 7.2] mg, p=0.08) and cumulative dose in the first year of use (mean 1.8 g [sd 1.7g]/mean daily dose 4.9 mg vs 2.1 g [sd 3.4g]/mean daily dose 5.8 mg, p=0.48) were not significantly different compared to the 1980–1994 cohort. There was no apparent trend in the starting dose over time (data not shown).
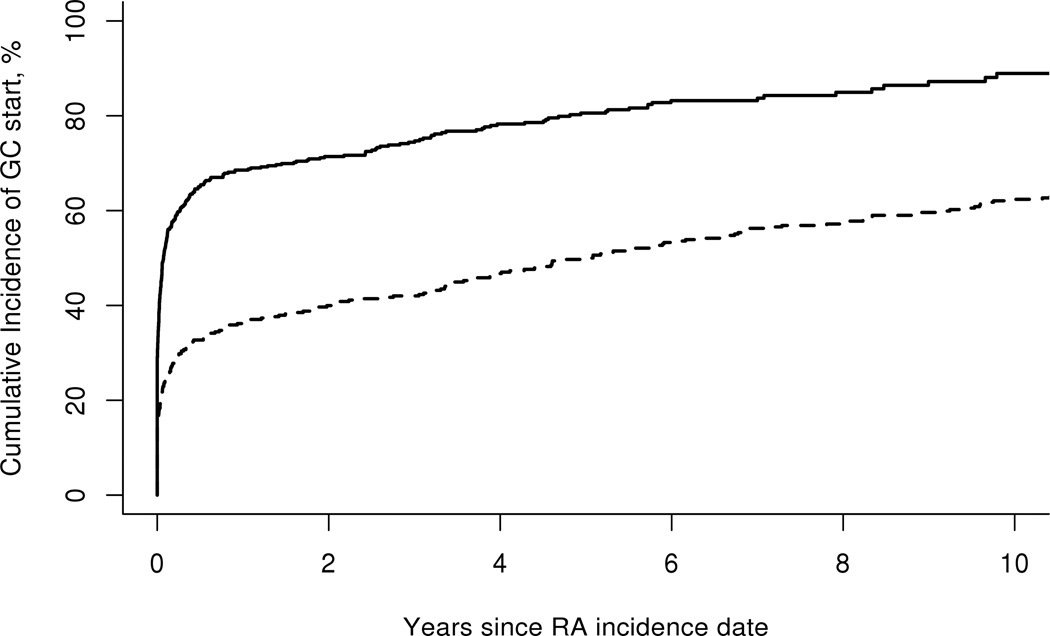
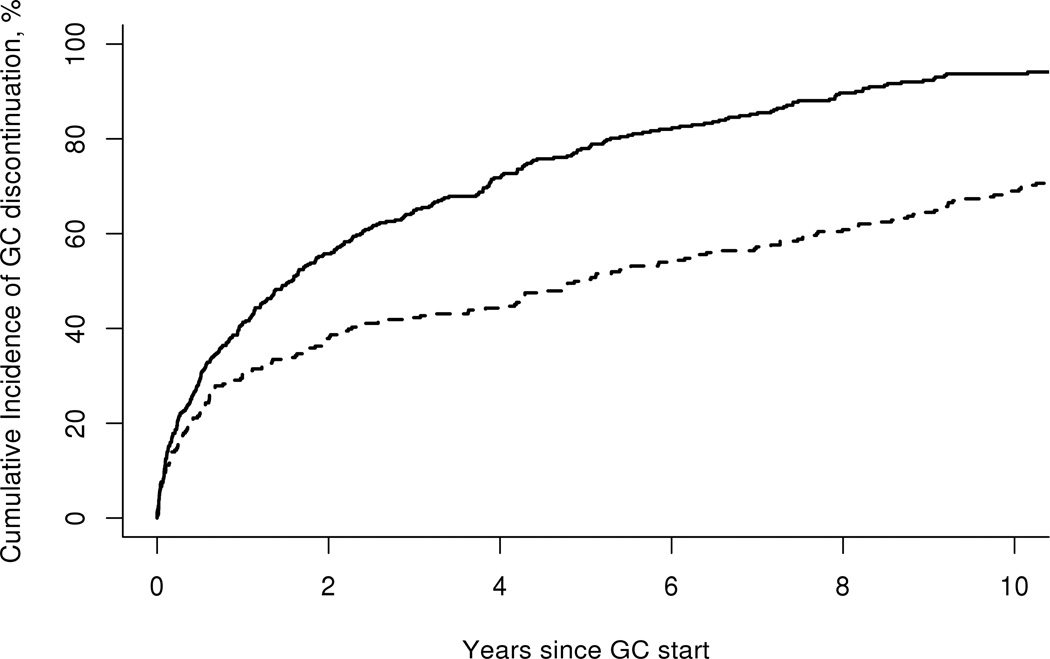
The cumulative incidence (CI) of GC initiation by time period of incident RA is demonstrated in Figure 1. It was higher in the 1995–2007 cohort compared to 1980–1994 cohort for all disease durations studied. Figure 2 illustrates the CI of GC discontinuation (for at least 90 or more days) by time period of incident RA. Rates of GC discontinuation were higher among the 1995–2007 cohort compared to the 1980–1994 cohort for all periods studied since glucocorticoid initiation (p<0.001). When this analysis was restricted to patients who initiated GC within their first year of disease onset, the results were nearly identical.
Figure 1.
Cumulative incidence of start of glucocorticoids according to time period of rheumatoid arthritis (RA) incidence - 1980–1994 (dashed line) vs. 1995–2007 (solid line), p<0.001.
Figure 2.
Cumulative incidence of discontinuation of glucocorticoids for at least 90 days according to time period of rheumatoid arthritis incidence: 1980–1994 (dashed line) vs. 1995–2007 (solid line), (p<0.001).
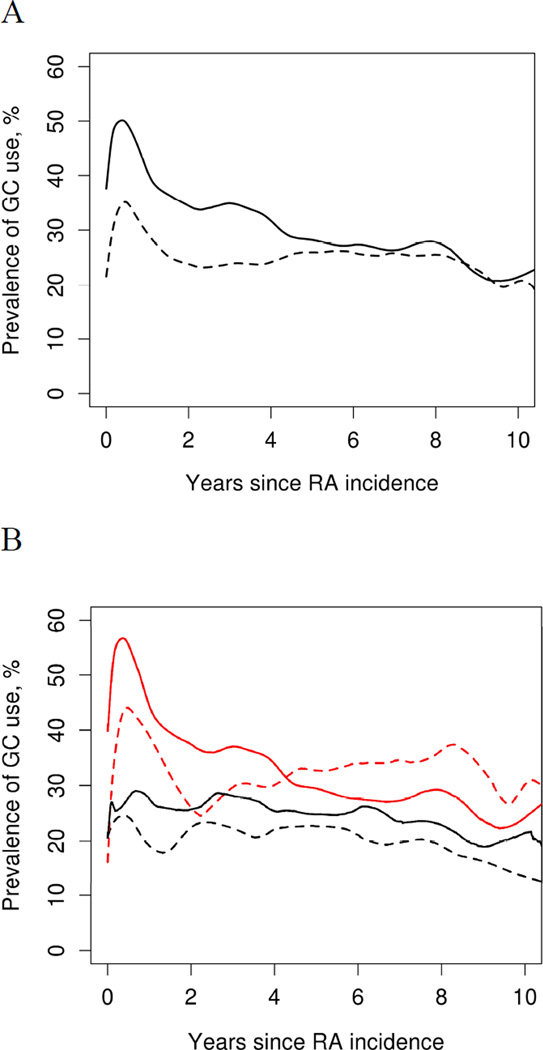
Figure 3 compares current prevalence of GC use by duration of RA among the two incident cohorts. Although use of GCs appears to be higher for the initial 3 years of disease in the 1995–2007 cohort, it approaches that of the 1980–2004 cohort by 4th year of disease (Figure 3A). Furthermore, within each incidence cohort, initiation of a DMARD within the first 6 months of RA did not demonstrate any significant influence on prevalence of GC use (Figure 3B).
Figure 3.
A. Current prevalence of glucocorticoid (GC) use by rheumatoid arthritis (RA) duration, according to time period of RA incidence: 1980–1994 (dashed line; n=394) vs. 1995–2007 (solid line; n=464). B. Patients who started a disease modifying antirheumatic drug in the first 6 months of RA (red line; 1980–94 n=164, 1995–2007 n=356) vs those who did not (black line; 1980–1994 n=185, 1995–2007 n=108).
DISCUSSION
Since their first use more than 65 years ago (11), GCs have become an indispensable component of all therapeutic regimens utilized for RA management. Physicians treating patients with RA have long recognized the tension between the beneficial and adverse effects of GCs. Their beneficial effects in RA are well recognized (12, 13) in addition to the rapidity of symptom relief and cost effectiveness of treatment. Unfortunately, the symptomatic benefit is often ill sustained once they are discontinued, even in the face of DMARD use (14, 15). In fact, rebound deterioration in RA symptoms has been recognized following withdrawal of GC treatment (16, 17).
The disease modifying properties of GCs have also been much debated. Although a few early trials had suggested disease modifying effects of GCs (13), several other studies showed no convincing evidence for alteration in progression of erosive damage in RA patients receiving GCs (17–20). Kirwan et al revisited this issue more recently (21) and compared prednisolone 7.5 mg/day with placebo in 128 patients with early RA (disease duration <2 y) and reported slowing in radiological progression of disease and of development in new erosions. This study, however, did not control for second line therapy which itself could have provided a disease modifying effect. Thereafter, in a Cochrane review based on data from 15 studies and 1,414 patients with early RA (disease duration up to 2 years), Kirwan et al (3) concluded that there was convincing evidence that GCs given in addition to standard DMARD therapy can substantially reduce rate of erosion progression in RA, and the benefit achieved was over and above that provided by second line therapy. More recently, Bakker et al (2) also confirmed that introducing low dose prednisone 10 mg daily to a methotrexate based treatment strategy for tight control in early RA (<1 year) improves patient outcomes including a decrease in erosive joint damage, improvement in disease activity and reduces physical disability and progression to use of biologics. The 2013 EULAR update on treatment recommendations of RA (22) recommends addition of low dose GC as part of the initial treatment strategy for up to 6 months and tapering as soon as clinically feasible.
This evidence has posed a challenge to the traditional approach of using GCs as ‘bridge therapy’ until disease modifying treatments become effective and may in fact encourage prolonged use, albeit in lower dosages. Although confirmed in early RA, whether the same effects hold true for established RA or longer disease durations of RA (beyond 2 years) remains to be studied. It is also uncertain if these disease modifying benefits seen in early disease affect long term outcomes in RA.
Higher doses and long durations of GC use in RA are associated with multiple toxic effects that include but are not limited to cushingoid facies, hirsutism, cataracts, glaucoma, psychosis, diabetes, hypertension, skin atrophy, peptic ulceration and increased risk of serious infections (23). These side effects are dose dependent, but a few, like cataracts (24), osteoporosis and fractures, may develop at doses as low as 5–7.5 mg/day (25) and have significantly dampened enthusiasm for long term use in RA both at the level of physicians and patients alike. A EULAR task force addressing these issues has developed 10 key propositions related to safe use of systemic GCs in RA, all with an emphasis to reduce the likelihood of long term side effects (26).
GC use has also been recognized as an independent predictor of mortality in RA patients (27, 28). It is however unclear if GCs themselves are the culprit or whether this effect is due to a channelling bias or confounding by indication given that patients with high disease activity and poor prognostic factors tend to have a higher likelihood on being on GCs.
Treatment of RA has significantly changed over the last 30 years. The original ‘step-up’ treatment approach is being replaced by early diagnosis and a ‘treat to target’ approach. With a better understanding of pathophysiological mechanisms in RA, the armamentarium for RA treatment has expanded tremendously. There has been increasing emphasis on early introduction of disease modifying therapy, and availability of newer and more effective DMARDs and BRMs has facilitated this approach (22).
To this point, it has not been known whether these treatment practices have made any difference in GC use over the last several decades has not been studied. This is the first study to our knowledge that describes trends in GC use over 27 years from a single large population based practice of RA patients. We have reported a comparison between the 2 incident cohorts from 1980–1994 and 1995–2007. The results of this study demonstrate a consistently higher rate of GC initiation among RA patients in the more recent incident cohort. This difference persists for all durations of disease since RA incidence. There are several potential explanations for this finding. Among these, there is always a proportion of patients who do not receive treatment with DMARDs. Indeed, the proportion of patients not receiving any DMARDs were noted to have increased from 13% to 18% between 1999–2009 (29). These patients were older, had a longer RA disease duration and had fewer swollen joints. Another study from Germany reported no DMARD use among 13–19% patients from a large longitudinal cohort of RA patients between 1997 and 2007 (30). The reason behind not being on a DMARD is often difficult to discern from retrospective observational studies but may be attributed either to minimal to low disease activity, patient’s personal preference to avoid the risk of side effects or comorbid diseases that prohibit use of immunosuppressants. These patients are often then treated with GCs alone and may continue to remain on low dose GCs in the long term to prevent disease exacerbations. (5). Other factors include physician training, beliefs and experience (31). Patients under the care of generalists or orthopedists are much more likely to be treated with oral or injectable GCs than DMARDs (32). Patients with worse clinical status are more likely to receive GCs, with higher rates of discontinuation among patients with better clinical status (4). As well, there is a lower likelihood of GC use among patients >65 years of age, however if these patients were started on GCs, they were more likely to continue it.
Interestingly, we did not note a significant difference in the mean starting dose of GC (in prednisone equivalents) between the 2 incident cohorts (10.3 vs 8.7 mg). This is in contrast to the report by Pincus et al (8) who noted a decline in mean initial prednisone dose from 10.3 mg/day to 3.6 mg/day between 1980 and 2004. Also, there was no difference in the cumulative GC dose over first year of GC use between the 2 incident cohorts. In a recent study by del Rincón et al (33), an increase in mortality rate was associated with GC use in RA, with a daily threshold dose of 8 mg, at which the number of deaths increased in a dose-dependent manner. It is notable that the initial doses used in our 2 incident cohorts exceeded this threshold, but may have been necessary to treat an acute flare at presentation or bring the disease activity under control rapidly. We did not study associations with mortality in our cohort of patient.
We also observed higher rates of discontinuation in GC use in the more recent incident cohort with the difference persisting among the two time-frames for all durations since GC initiation. This may be a result of increasing use of DMARD. In our study, use of DMARDs in the first year of disease increased significantly from 52% to 83% between 1980–1994 and 1995–2007, possibly facilitating the decline in GC use. This trend is also mirrored in other studies (29).
Although prevalence of GC use appears to be higher for the initial 3 years of disease in the 1995–2007 cohort, it tends to approach that of the 1980–2004 cohort by 4th year of disease (Figure 5A). While not investigated in this study, this finding may be attributable to better awareness of disease modifying effects of GC in low doses and their propensity to diminish radiological progression in early RA. This also appears to be in line with established treatment guidelines (34). Given the risk of side effects in the long term and unclear disease modifying effects in late disease, persistent use of GCs is generally discouraged (35).
Interestingly, our results showed that early initiation of a DMARD within the first 6 months of RA did not have a significant influence on prevalence of GC use in either cohort (Figure 5B). It is possible that this is due to the practice of keeping patients on GCs to provide symptom relief and avoid damage until slow acting DMARD can demonstrate clinical benefit.
The strengths of our study include its population-based design with standardized case ascertainment and use of a comprehensive medical record linkage system (in-patient and out-patient care from all local providers) providing an opportunity to study a large cohort of RA patients through inclusion of 2 successive incidence cohorts with long and complete follow-up of all subjects. We think that results of this study would be generalizable to other population based settings where RA is treated, but the pattern of GC use (doses, durations and mode of administration) is not standardized and could vary greatly between rheumatology practices, institutions and countries.
Study limitations include the fact that this was a retrospective observational study with its inherent biases that relied on clinical information recorded in the patient’s medical records, including GC doses and their initiation and discontinuation dates. Thus, it was based on the assumption that these were in fact recorded correctly. Any significant changes in doses that were not recorded would have been missed. The GC doses were also abstracted as categories rather than individual doses. Also, physician recommendations do not always directly translate into patient compliance and could have affected the results. In some patients, use of intra-articular GCs can be frequent and substantial, even when they are otherwise exposed to minimal amounts of systemic GC. Information on intra-articular use of GC was not collected in this study. Different durations of follow up in the 2 incident cohorts made it difficult to compare cumulative doses long term.
In summary, our findings demonstrate that a higher proportion of patients are starting GCs early in their disease course now compared to previously. Although more patients are also discontinuing GCs now compared to previously, the proportion of patients on GCs at any given time point of disease duration is higher now than previously, at least during the first 4 years of disease. In addition, some patients who start GCs may not be able to discontinue them over the long term, despite the addition of a DMARD early in their disease course. It remains unclear if they remain on high doses due to persistent disease activity or low doses primarily for decreasing risk of flares and obtaining a disease modifying effect, as this was not studied.
Despite changing practice patterns, the starting GC dose and cumulative GC dose during first year of GC use have not remarkably changed between the 2 time periods. We think that GC use in RA may not only be representative of disease severity in RA, but rather an indicator of the secular trend of changing practice patterns. More aggressive use of GCs early in RA may also be representative of a change in goals of treatment from merely controlling disease activity to inducing a state of ‘remission’(36).
SIGNIFICANCE AND INNOVATION.
Earlier and more intensive use of disease modifying therapy have revolutionized rheumatoid arthritis management over the last 30 years, but their effects on glucocorticoid (GC) usage are unknown.
A higher proportion of patients are starting GCs early in their disease course now compared to previously.
Patients that start GCs may not discontinue them over the long term, despite the addition of a disease modifying antirheumatic drug (DMARD) early in their disease course.
Despite changing practice patterns, the starting GC dose and cumulative GC dose during the first year of GC use has not changed between the 2 time periods 1980–1994 vs. 1995–2007.
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health, NIAMS (R01AR46849), National Institute on Aging (AG034676) and National Center for Advancing Translational Science (NCATS) (CTSA Grant Number UL1 TR000135). The sponsors of these grants did not have any involvement with the study design, data collection, analysis and interpretation of data, in the writing of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Disclosure of Interests
None of the authors have any relevant financial or other conflicts of interests to disclose relevant to this manuscript.
BIBLIOGRAPHY
- 1.Hillier SG. Diamonds are forever: the cortisone legacy. J Endocrinol. 2007;195(1):1–6. doi: 10.1677/JOE-07-0309. [DOI] [PubMed] [Google Scholar]
- 2.Bakker MF, Jacobs JW, Welsing PM, Verstappen SM, Tekstra J, Ton E, et al. Low-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trial. Ann Intern Med. 2012;156(5):329–339. doi: 10.7326/0003-4819-156-5-201203060-00004. [DOI] [PubMed] [Google Scholar]
- 3.Kirwan JR, Bijlsma JW, Boers M, Shea BJ. Effects of glucocorticoids on radiological progression in rheumatoid arthritis. Cochrane Database Syst Rev. 2007;(1) doi: 10.1002/14651858.CD006356. CD006356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Everdingen AA, Jacobs JW, Siewertsz Van Reesema DR, Bijlsma JW. Low-dose prednisone therapy for patients with early active rheumatoid arthritis: clinical efficacy, disease-modifying properties, and side effects: a randomized, double-blind, placebo-controlled clinical trial. Ann Intern Med. 2002;136(1):1–12. doi: 10.7326/0003-4819-136-1-200201010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Caplan L, Wolfe F, Russell AS, Michaud K. Corticosteroid use in rheumatoid arthritis: prevalence, predictors, correlates, and outcomes. J Rheumatol. 2007;34(4):696–705. [PubMed] [Google Scholar]
- 6.Criswell LA, Henke CJ. What explains the variation among rheumatologists in their use of prednisone and second line agents for the treatment of rheumatoid arthritis? J Rheumatol. 1995;22(5):829–835. [PubMed] [Google Scholar]
- 7.Buttgereit F, da Silva JA, Boers M, Burmester GR, Cutolo M, Jacobs J, et al. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology. Ann Rheum Dis. 2002;61(8):718–722. doi: 10.1136/ard.61.8.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pincus T, Sokka T, Castrejon I, Cutolo M. Decline of mean initial prednisone dosage from 10.3 to 3.6 mg/day to treat rheumatoid arthritis between 1980 and 2004 in one clinical setting, with long-term effectiveness of dosages less than 5 mg/day. Arthritis Care Res (Hoboken) 2013;65(5):729–736. doi: 10.1002/acr.21899. [DOI] [PubMed] [Google Scholar]
- 9.Davis JM, 3rd, Maradit Kremers H, Crowson CS, Nicola PJ, Ballman KV, Therneau TM, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2007;56(3):820–830. doi: 10.1002/art.22418. [DOI] [PubMed] [Google Scholar]
- 10.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Hench PS, Kendall EC, et al. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin. 1949;24(8):181–197. [PubMed] [Google Scholar]
- 12.EMPIRE Rheumatism Council; multi-centre controlled trial comparing cortisone acetate and acetyl salicylic acid in the long-term treatment of rheumatoid arthritis; results up to one year. Ann Rheum Dis. 1955;14(4):353–370. doi: 10.1136/ard.14.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.EMPIRE Rheumatism Council: multi-centre controlled trial comparing cortisone acetate and acetyl salicylic acid in the long-term treatment of rheumatoid arthritis; results of three years' treatment. Ann Rheum Dis. 1957;16(3):277–289. doi: 10.1136/ard.16.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gotzsche PC, Johansen HK. Meta-analysis of short-term low dose prednisolone versus placebo and non-steroidal anti-inflammatory drugs in rheumatoid arthritis. Bmj. 1998;316(7134):811–818. doi: 10.1136/bmj.316.7134.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saag KG, Criswell LA, Sems KM, Nettleman MD, Kolluri S. Low-dose corticosteroids in rheumatoid arthritis. A meta-analysis of their moderate-term effectiveness. Arthritis Rheum. 1996;39(11):1818–1825. doi: 10.1002/art.1780391107. [DOI] [PubMed] [Google Scholar]
- 16.Harris ED, Jr, Emkey RD, Nichols JE, Newberg A. Low dose prednisone therapy in rheumatoid arthritis: a double blind study. J Rheumatol. 1983;10(5):713–721. [PubMed] [Google Scholar]
- 17.van Gestel AM, Laan RF, Haagsma CJ, van de Putte LB, van Riel PL. Oral steroids as bridge therapy in rheumatoid arthritis patients starting with parenteral gold. A randomized double-blind placebo-controlled trial. Br J Rheumatol. 1995;34(4):347–351. doi: 10.1093/rheumatology/34.4.347. [DOI] [PubMed] [Google Scholar]
- 18.Hansen TM, Dickmeiss E, Jans H, Ingemann Hansen T, Ingeman-Nielsen M, Lorenzen I. Combination of methylprednisolone pulse therapy and remission inducing drugs in rheumatoid arthritis. Ann Rheum Dis. 1987;46(4):290–295. doi: 10.1136/ard.46.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen TM, Kryger P, Elling H, Haar D, Kreutzfeldt M, Ingeman-Nielsen MW, et al. Double blind placebo controlled trial of pulse treatment with methylprednisolone combined with disease modifying drugs in rheumatoid arthritis. Bmj. 1990;301(6746):268–270. doi: 10.1136/bmj.301.6746.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liebling MR, Leib E, McLaughlin K, Blocka K, Furst DE, Nyman K, et al. Pulse methylprednisolone in rheumatoid arthritis: a double-blind cross-over trial. Ann Intern Med. 1981;94(1):21–26. doi: 10.7326/0003-4819-94-1-21. [DOI] [PubMed] [Google Scholar]
- 21.Kirwan JR. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. The Arthritis and Rheumatism Council Low-Dose Glucocorticoid Study Group. N Engl J Med. 1995;333(3):142–146. doi: 10.1056/NEJM199507203330302. [DOI] [PubMed] [Google Scholar]
- 22.Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bollet AJ, Black R, Bunim JJ. Major undesirable side-effects resulting from prednisolone and prednisone. J Am Med Assoc. 1955;158(6):459–463. doi: 10.1001/jama.1955.02960060017005. [DOI] [PubMed] [Google Scholar]
- 24.Huscher D, Thiele K, Gromnica-Ihle E, Hein G, Demary W, Dreher R, et al. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. 2009;68(7):1119–1124. doi: 10.1136/ard.2008.092163. [DOI] [PubMed] [Google Scholar]
- 25.Saag KG, Koehnke R, Caldwell JR, Brasington R, Burmeister LF, Zimmerman B, et al. Low dose long-term corticosteroid therapy in rheumatoid arthritis: an analysis of serious adverse events. Am J Med. 1994;96(2):115–123. doi: 10.1016/0002-9343(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 26.Hoes JN, Jacobs JW, Boers M, Boumpas D, Buttgereit F, Caeyers N, et al. EULAR evidence-based recommendations on the management of systemic glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis. 2007;66(12):1560–1567. doi: 10.1136/ard.2007.072157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leigh JP, Fries JF. Mortality predictors among 263 patients with rheumatoid arthritis. J Rheumatol. 1991;18(9):1307–1312. [PubMed] [Google Scholar]
- 28.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37(4):481–494. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 29.Kim SC, Yelin E, Tonner C, Solomon DH. Changes in use of disease modifying anti-rheumatic drugs for rheumatoid Arthritis in the U.S. for the period 1983–2009. Arthritis Care Res (Hoboken) 2013 doi: 10.1002/acr.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziegler S, Huscher D, Karberg K, Krause A, Wassenberg S, Zink A. Trends in treatment and outcomes of rheumatoid arthritis in Germany 1997–2007: results from the National Database of the German Collaborative Arthritis Centres. Ann Rheum Dis. 2010;69(10):1803–1808. doi: 10.1136/ard.2009.122101. [DOI] [PubMed] [Google Scholar]
- 31.Criswell LA, Such CL, Yelin EH. Differences in the use of second-line agents and prednisone for treatment of rheumatoid arthritis by rheumatologists and non-rheumatologists. J Rheumatol. 1997;24(12):2283–2290. [PubMed] [Google Scholar]
- 32.Roussy JP, Bessette L, Rahme E, Bernatsky S, Legare J, Lachaine J. Rheumatoid arthritis pharmacotherapy and predictors of disease-modifying anti-rheumatic drug initiation in the early years of biologic use in Quebec, Canada. Rheumatol Int. 2013 doi: 10.1007/s00296-013-2828-7. [DOI] [PubMed] [Google Scholar]
- 33.del Rincon I, Battafarano DF, Restrepo JF, Erikson JM, Escalante A. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):264–272. doi: 10.1002/art.38210. [DOI] [PubMed] [Google Scholar]
- 34.Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509. doi: 10.1136/annrheumdis-2013-204573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smolen JS, Landewe R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69(6):964–975. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bijlsma JW, Hoes JN, Van Everdingen AA, Verstappen SM, Jacobs JW. Are glucocorticoids DMARDs? Ann N Y Acad Sci. 2006;1069:268–274. doi: 10.1196/annals.1351.025. [DOI] [PubMed] [Google Scholar]