Summary
Aging is associated with changes in sleep and decline executive functions, such as task-switching and task preparation. Given that sleep affects executive function, age-related changes in executive function may be attributable to changes in sleep. The present study used a sleep detection device to examine whether wake time after sleep onset (WASO) and total sleep time (TST) moderated age differences in task-switching performance and participants' ability to reduce switch costs when given time to prepare. Participants were cognitively healthy (MMSE > 26) younger (n = 54; mean age = 22.9; 67.8% female) and older (n = 45; mean age 62.8; 71.1% female) adults. Using a task-switching paradigm, which manipulated preparation time, we found that smaller global switch costs were associated with lower WASO and longer TST. Greater preparation effects on local switch costs and adoption of a task-set were associated with lower WASO, though this effect was significant only in older adults when stratified by age group. This association was independent of inhibition and working memory abilities. The lack of interactions between sleep and age group indicated that age differences in switch costs were not moderated by better sleep. Our results suggest that young and older adults may benefit similarly from lower WASO and longer TST in overall performance, and individuals with less WASO are more likely to engage preparatory strategies to reduce switch costs and boost task-switching performance.
Keywords: Cognition, task-switching, executive function, sleep continuity, total sleep time, individual differences
Introduction
Advancing age is often characterized by decline in executive function (Kramer et al., 1999, Buckner, 2004). Impairments in executive function may include difficulties in selecting relevant and inhibiting irrelevant information and actions, and in monitoring and updating information (Mayr, 2001). Task-switching, a model paradigm of executive function, is often sensitive to advancing age (Monsell, 2003, Verhaeghen, 2011) beyond general age-related slowing (Wasylyshyn et al., 2011). During task-switching, young adults often exert executive control to adopt a task-set that is maintained with task repetition to improve performance. However, the successful adoption of a task-set sometimes leads to a decrement in performance during task alternations (Kramer et al., 1999, Kray, 2006) reflecting “local switch costs”. While local switch costs are often similar between young and older adults, “global switch costs” in which performance is poorer when coordinating two tasks-sets compared to maintaining a single task-set across a block of trials, are often greater in older adults (Mayr, 2001, Wasylyshyn et al., 2011). Thus, there appears to be age-related decline in maintenance and coordination of two task-sets (Kray and Lindenberger, 2000).
Time to prepare often leads to improved performance and a reduction in switch costs in young adults (Monsell, 2003). This is referred to as the “preparation effect”. The preparation effect reflects the endogenous adoption of a task-set early on to benefit performance. Reduced preparation effects have been identified in older adults (Lawo et al., 2012), suggesting that older adults are less likely to engage preparatory strategies to boost task-switching performance.
Despite findings that task-switching and other executive abilities decline with age, some older adults show very little impairment (Park and Reuter-Lorenz, 2009). This begs the question of which factors contribute to age-related decline in task-switching. There is evidence that factors such as sleep contribute to cognitive abilities. Prior studies have shown that older adults with greater sleep quality, quantity, and continuity perform better on cognitive tasks (Tworoger et al., 2006), particularly executive functions (Blackwell et al., 2006, Nebes et al., 2009). Given that reduced sleep negatively impacts executive functions, including task-switching (Banks and Dinges, 2007, Goel et al., 2009, Couyoumdjian et al., 2010) and decline in sleep continuity and quantity are prevalent with aging (Ohayon et al., 2004, Carskadon and Dement, 2011), these age-related sleep changes may play a role in age-related decline in task-switching abilities (Wilckens et al., 2012).
The goal of the present cross-sectional study was to determine whether aspects of task-switching that change with aging, are associated with objective sleep measures that also change with aging (TST and sleep continuity). We examined whether age differences in task-switching effects were lessened with better sleep. Specifically, our primary aim was to determine whether older adults with greater sleep continuity and quantity (lower WASO and higher TST) engage preparatory strategies to boost performance during task-switching, similar to young adults. We hypothesized older adults with lower WASO and higher TST would demonstrate preparation effects similar to young adults. In terms of global and local switch costs, we expected that WASO and TST would moderate age differences in switch costs, reflecting similar switch costs between young and older adults with lower WASO and higher TST. We expected that this effect would apply particularly to global switch costs and preparation effects, given evidence for associations between age and global switch costs and preparation effects (Wasylyshyn et al., 2011, Lawo et al., 2012). Where sleep was related to switch costs but did not moderate age differences in switch costs, our secondary aim was to examine whether relationships between sleep and switch costs were truly independent of age group. Consistent with the view that older adults with better sleep have better executive function abilities, we hypothesized that within the older group, individuals with lower WASO and higher TST would exhibit lower switch costs with time to prepare, and exhibit greater preparation effects.
Method
Participants
Participants were community-dwelling young adults (ages 21–30) and older adults (ages 55–76) recruited specifically for the research study and were not drawn from a specific clinic sample. Demographic information and participant characteristics for the two age groups are provided in Table 1. Participants took part in the experiment over two sessions spaced one week apart. Informed consent was gained from all participants as approved by the University of Pittsburgh Institutional Review Board. Participants were paid at a rate of $10 per hour for participation in the experiment and $50 for wearing an armband equipped for sleep detection for one week. Exclusion criteria included having depression or currently taking psychiatric medication, dependence on drugs or alcohol, or a diagnosis with a neurodegenerative disease. Inclusion criteria pertinent to the present data included having at least 4 days of data from a sleep detection device, a mini mental state exam (MMSE) score above 26 to rule out evidence for cognitive impairment, and normal or corrected-to-normal vision. Participants included in the present study completed a task-switching paradigm over both sessions of the experiment. Analyses included a total of 54 young adults and 45 older adults. Participants were not excluded based on any self-report or objective sleep measures to capture a wide range of sleep continuity and duration in relation to cognition.
Table 1.
Demographic information, global and local switch costs, WASO and TST averages, standard deviations, and p values for age differences in younger and older participants1.
| Younger (n = 54) | Older (n = 45) | ||
|---|---|---|---|
|
| |||
| % Females | 67.8 | 71.1 | |
| Mean ± SD | Mean ± SD | p value | |
| Age | 22.91 ± 2.32 | 62.82 ± 5.98 | |
| Years of Education | 16.15 ± 1.63 | 15.20 ± 3.16 | 0.06 |
| MMSE | 29.35 ± 0.87 | 28.82 ± 1.02 | 0.007 |
| Delayed Recall | 7.20 ± 1.64 | 5.84 ± 1.95 | < 0.001 |
| Trails A | 21.28 ± 7.27 | 30.14 ± 11.38 | < 0.001 |
| Trails B | 45.35 ± 18.44 | 77.78 ± 37.82 | < 0.001 |
| Digit Symbol Substitution | 46.39 ± 6.39 | 34.13 ± 7.24 | < 0.001 |
| Global Switch Costs | |||
| RT (ms) | 64.28 ± 82.79 | 101.26 ± 118.49 | 0.002 |
| Accuracy | 0.08 ± 0.09 | 0.11 ± 0.14 | 0.063 |
| Local Switch Costs | |||
| RT (ms) | 18.12 ± 44.87 | 31.32 ± 71.06 | 0.053 |
| Accuracy | 0.07 ± 0.10 | 0.06 ± 0.11 | 0.144 |
| WASO (mins) | 48.45 ± 26.46 | 63.43 ± 42.48 | 0.035 |
| TST (mins) | 383.98 ± 57.90 | 362.38 ± 63.11 | 0.079 |
Bold p values = p < 0.05
Procedure
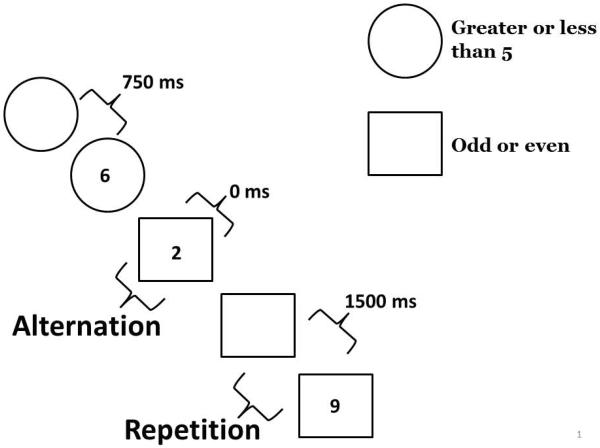
Participants completed the task-switching experiment over a two-day period to reduce the length of any one session and to avoid fatigue. Therefore, half of the experimental trials were collected during session 1 and the other half during session 2. Participants performed a practice block, single task block, and switching blocks at both sessions. All trial types were included in both sessions. In the task-switching procedure the cue-target interval (preparation time) varied on a trial-by-trial basis. Participants were cued on each trial to perform one of two tasks that required judgments about a single-digit number presented on the screen. For one task they judged whether the number was greater than or less than 5 (GL task). In the other task, they judged whether the number was odd or even (OE task). A circle preceding or accompanying the target number cued participants to perform the GL task. A square preceding or accompanying the target number cued participants to perform the OE task. The preparation time was either 0 ms (simultaneous cue and target), 750 ms, or 1500 ms. A practice block comprised of 16 trials of each task and two experimental single task blocks comprised of 32 trials for each task preceded the switching block. Participants performed the GL block followed by the OE block, followed by the switching block. The switching block was comprised of a total of 96 trials: 16 trials of each cue type (GL and OE) for each of the 3 preparation conditions were presented randomly in the switching block with 8 of each correct response type (greater than, less than, odd, even). Given the random presentation of task cues, across participants there were 79–112 task alternations, 78–111 task repetitions and two buffer trials across the two sessions. This task-switching paradigm allowed us to assess global switch costs (task repetition trials within the switching task block relative to performance in the single task block) and local switch costs (task alternation trials relative to task repetition trials within the switching block). We examined the effect of preparation time on both global and local switch costs. Figure 1 illustrates an example sequence of trials in the task-switching procedure.
Figure 1.
Example sequence of trials in the switching block: The circle cues the participant to prepare to judge whether the number is greater than or less than 5. After 750 ms the number 6 appears and the participant responds “greater”. The next trial, the square and number are presented simultaneously (0 ms preparation time) and the participant responds “even”. The last trial, the square cues the participant to prepare to indicate whether the number is odd or even. The number appears following a 1500 ms preparation time (9) and the participant responds “odd”. The second trial is a task alternation (alternation from circle to square). The third trial is a task repetition trial (square to square).
Participants also completed a battery of computer-based cognitive tasks following the task-switching procedure at session 1. Included in these tasks were a version of the Stroop task with congruent and incongruent trials to assess inhibition, and an N-back task with 1-back and 2-back trials to assess working memory abilities. These tasks were used in the present study to examine whether any preparation-related improvements in task-switching were dependent on working memory and inhibition abilities.
Neuropsychological assessments
Following participation in the task-switching procedure at session 2, participants completed a battery of neuropsychological assessments including MMSE, the digit symbol substitution subset of the Wechsler Adult Intelligence Scale III (Wechsler, 1997), Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Word List Memory test (Morris et al., 1989), and Trail making Tests A and B (Reitan, 1958). Neuropsychological assessment scores are displayed in Table 1. The relationship between sleep measures and neuropsychological and computer-based cognitive tasks are reported elsewhere (Wilckens et al., Unpublished).
Physiological data collection
A sleep detection device (SenseWear™ armband) was used to assess sleep. Participants wore the armband for one week between sessions 1 and 2 of the experiment. The device estimated whether participants were sleeping every 60 seconds based on motion, body axis, heat flux, galvanic skin response, body temperature, and near body temperature (Sunseri et al., 2009). TST reflected the average amount of time in minutes spent sleeping within the nighttime sleep bout. WASO reflected the average number of minutes spent awake following sleep onset within the nighttime sleep bout. The nighttime sleep bout was guided by in and out of bed times each day as indicated in a sleep diary by the participant. WASO values were log transformed for all analyses to correct for non-normality of the distribution.
Statistical analyses
Global and local switch costs were calculated for accuracy and RT on correct trials and used as the dependent variable in the present analyses. Switch costs in contrast to absolute performance ensured that effects of age group were not confounded by general age-related slowing in RT. To assess global switch costs, we subtracted RT and accuracy on repetition trials within the switching block for each preparation condition from performance in the easiest single task block condition (GL single task block). This reflected costs associated with “mixing” tasks relative to performance when maintaining the same task (Monsell, 2003) for each preparation condition. To assess local switch costs, we compared RT and accuracy between task alternation and repetition trials within the switching block for each preparation condition. Global and local switch costs were calculated for each preparation condition to determine how switch costs were reduced as a function of preparation time.
Mixed models analyses were used to assess whether within subject preparation effects on global and local switch costs were related to sleep measures, and whether age differences in switch costs and preparation effects were moderated by sleep. Preparation time was included in the model as a within subjects fixed factor to account for within subject effects of preparation when assessing main effects of sleep. It also allowed us to identify interactions between sleep and preparation to examine whether sleep had an effect on preparatory strategies. A preparation effect was operationalized as a reduction in global RT or accuracy switch costs as a function of preparation time (0 ms > 750 ms > 1500 ms). Age group and sleep (WASO or TST) were included in the models as between subjects fixed factors. Age group was included as a categorical variable given that we were testing two distinct age groups within the same model. Subject was included as a random factor.
We examined both main effects of sleep as well as interactions with age group and preparation. In the case of a significant sleep × age group interaction, we planned to conduct simple slope analyses to assess whether age differences varied at high and low levels of sleep (WASO and TST). We expected that age differences would be smaller in participants with better sleep. Given the distinct age groups, where significant main effects of sleep were found in switch costs across age groups, we stratified analyses by age group. This approach of stratifying by age group for main effects of sleep was used to determine whether main effects of sleep were truly exhibited independently of age group. To avoid issues with multicollinearity separate analyses were performed for WASO and TST (r = −0.44, p < 0.001).
Results
Means and standard deviations for demographic information, sleep measures, and overall performance for both age groups are displayed in Table 1. Mean performance and standard deviations for the full task-switching design are displayed in Table 2 (supporting information). Consistent with prior reports (Wasylyshyn et al., 2011), significant age differences were found in global RT switch costs across preparation times. Unlike previous findings (Wasylyshyn et al., 2011), there was also a significant age difference in local switch cost RT in the 1500 ms preparation condition and a marginally significant age difference in local switch cost RT across preparation conditions. Local switch costs were otherwise not significantly different between young and older adults.
Global Switch Costs
WASO and global switch costs
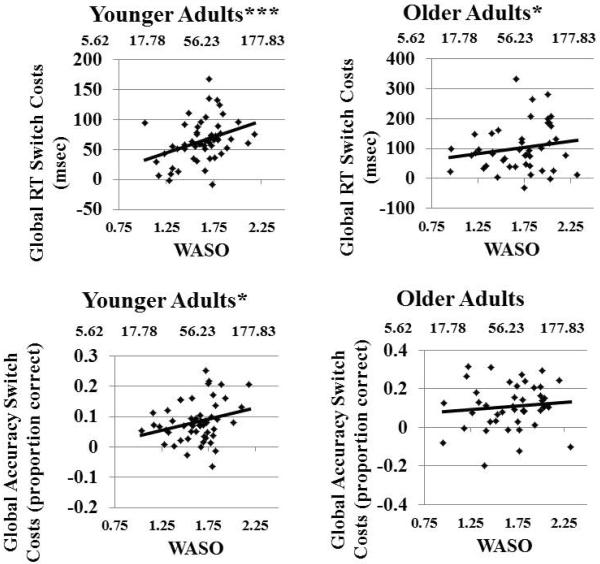
Main effects and interactions involving WASO and global switch costs are displayed in Table 3. There was a significant association between WASO and global RT switch costs and global accuracy switch costs, such that higher WASO was associated with greater switch costs. Given that the WASO × age group interaction did not reach significance, F's < 1, we did not perform follow up simple slope analyses to examine age differences as a function of WASO. However, to confirm that the main effect of WASO was truly an age-independent relationship, we stratified analyses by age group (Table 4), and found that the association between WASO and global switch costs was significant in both the younger group and the older group. Thus, across age groups, there was a significant association between WASO and global RT switch costs. The relationship between WASO and global accuracy switch costs reached significance only in the younger group. There were no significant interactions between WASO and preparation effects on global switch costs. Bivariate relationships between WASO and global RT and accuracy switch costs for young and older adults are displayed in Figure 2.
Table 3.
F values for the main effects of sleep and interactions with age group and preparation for global and local switch costs across both age groups.1
| Global | Local | |||
|---|---|---|---|---|
| RT | Accuracy | RT | Accuracy | |
| Model 1 | ||||
| WASO | 12.98*** | 4.99* | 0.31 | 1.40 |
| Age Group | 17.31*** | 3.29† | 6.41* | 1.75 |
| Preparation | 135.82*** | 25.32*** | 9.9*** | 12.70*** |
| WASO × Age Group | 0.10 | 0.52 | 0.43 | 0.88 |
| WASO × Preparation | 0.25 | 0.23 | 0.38 | 4.61* |
| WASO × Age Group × Preparation | 0.05 | 0.08 | 0.70 | 1.06 |
| Model 2 | ||||
| TST | 7.57** | 6.83** | 1.5 | 0.001 |
| Age Group | 16.45*** | 2.60 | 7.09** | 2.11 |
| Preparation | 134.41*** | 25.23*** | 9.97*** | 12.58*** |
| TST × Age Group | 0.49 | 3.34† | 1.05 | 0.50 |
| TST × Preparation | 0.01 | 0.38 | 0.26 | 0.61 |
| TST × Age Group × Preparation | 0.59 | 0.18 | 1.52 | 0.32 |
p < 0.1 †, p < 0.05 *, p < 0.01 **, p < 0.001 ***, WASO/TST df range (1, 254.47–289.81); Age Group df range (1, 254.47–289.81); Preparation df range (2, 199.01–221.40); WASO/TST × Age Group df range (1, 254.41–288.24); WASO/TST × Preparation df range (2, 201.59–219.70); WASO/TST × Age Group × Preparation df range (2, 198.75–216.08)
Table 4.
F values for the main effects of sleep and interactions with preparation for global and local switch costs separately for the younger and older groups. WASO and TST were analyzed in separate models. Analyses are limited to those justified by a significant main effect or interaction across age groups.1
| Global | Local | |||
|---|---|---|---|---|
| Younger | RT | Accuracy | RT | Accuracy |
| WASO | 15.73*** | 6.68* | -- | -- |
| WASO × Preparation | -- | -- | -- | 0.27 |
| TST | 5.69* | 19.03*** | -- | -- |
| Older | ||||
| WASO | 3.98* | 1.11 | -- | -- |
| WASO × Preparation | -- | -- | -- | 4.91** |
| TST | 3.5† | 0.18 | -- | -- |
p < 0.1 †, p < 0.05 *, p < 0.01 **, p < 0.001 ***; -- denotes analysis not justified by significant effect across age groups; Younger WASO/TST df range (1, 134.58–155.91); Younger Preparation df (2, 111.32); Younger WASO × Preparation df (2, 119.06); Older WASO/TST df range (1, 117.55–129.90); Older Preparation df (2, 86.46); Older WASO × Preparation df (2,88.99).
Figure 2.
Shorter WASO (better sleep) is associated with smaller global switch costs in RT in younger and older adults, and accuracy in young adults. Log transformed values for WASO are displayed on the lower × axis with corresponding raw WASO values on the upper × axis.
TST and global switch costs
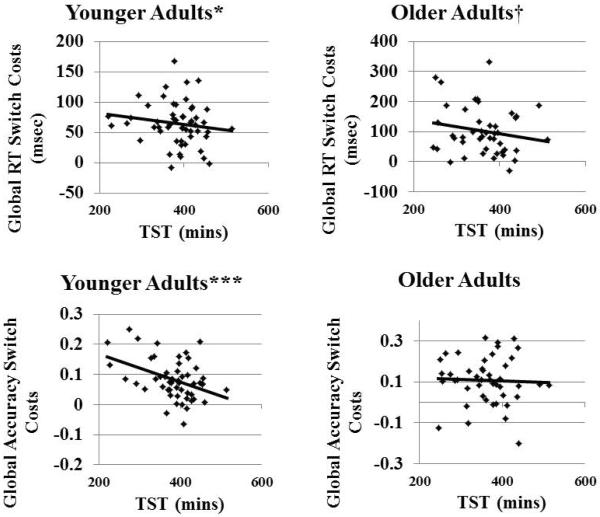
Main effects and interactions involving TST on global switch costs are displayed in Table 3. There were significant associations between TST and global switch costs in RT and global switch costs in accuracy, such that lower TST was associated with greater switch costs. Age stratified analyses revealed that relationships with global RT switch costs were consistent across age groups though marginally significant in the older group (Table 4). The relationship between TST and accuracy was only significant within the younger group, (p = 0.67 in the older group), but the TST × age group interaction was only marginally significant (Table 3). There were no significant interactions involving TST and preparation on global switch costs. Bivariate relationships between TST and global RT and accuracy switch costs for young and older adults are displayed in Figure 3. In summary, smaller global switch costs were associated with lower WASO and higher TST across age groups. However, associations with sleep were more consistent in the younger group.
Figure 3.
In younger adults, longer TST is associated with smaller global switch costs in RT and accuracy. In older adults, longer TST is marginally associated with smaller global switch costs in RT.
Local Switch Costs
WASO and local switch costs
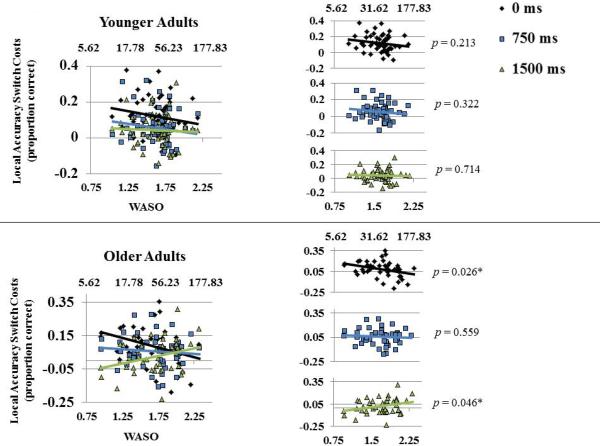
There was a significant preparation × WASO interaction in local accuracy switch costs, reflecting that participants with lower WASO were more likely to benefit from preparation (Table 3). Local accuracy switch costs were largest with 0 ms preparation time, and were reduced with 1500 ms preparation time. This preparation × WASO effect did not interact significantly with age group, so we did not follow up with simple slope analyses. To further examine whether the preparation × WASO interaction was independent of age group, we stratified the mixed model analysis by age group (Table 4). The preparation × WASO effect was only significant in the older group. The preparation effect in the younger group, F(2, 112.67) = 10.34, p < 0.001, was not moderated by WASO. The interaction with preparation in the older group was driven by smaller switch costs associated with higher WASO in the 0 ms condition, F(1, 43) = 5.35, p = 0.026, and smaller switch costs associated with lower WASO in the 1500 ms condition, F(1, 43) = 4.24, p = 0.046 (Figure 4). This interaction is consistent with the view that successful adoption of a task-set can bring about larger switch costs, but given time to prepare, switch costs can be significantly reduced. Given this rationale, lower WASO was associated with successful adoption of a task-set. There were no other significant main effects or interactions involving WASO and local switch costs for RT or accuracy (Table 3).
Figure 4.
Left panel: In the young adult group, preparation effects on local switch costs in accuracy are consistent across levels of WASO (log transformed values on the lower × axis and corresponding raw values on the upper × axis). In the older group, preparation effects are largest at lower levels of WASO (better sleep). Right panel: Bivariate relationships between WASO and local accuracy switch costs and p values separated by preparation condition.
To further understand the relationship between switch costs and WASO, we separated accuracy by task alternation and repetition trials. Across age groups, greater repetition trial accuracy was associated with lower WASO, F(1,283.17) = 4.45, p = 0.036. The association between alternation trial accuracy and WASO was marginally moderated by preparation time, such that participants with lower WASO exhibited a greater preparation effect, F(2, 198.62) = 2.256, p = 0.08 (Figure 5, supporting information). Within the older group, the WASO × preparation interaction was significant in alternation trial accuracy, F(2, 84.12) = 3.57, p = 0.032 and was not evident in repetition trial accuracy, F(2, 88.32) = 0.114, p = 0.89. Thus, greater preparation effects on task alternation accuracy were associated with lower WASO in the older group.
TST and Local Switch Costs
There were no significant associations or interactions involving TST and local switch costs (Table 3). The association between TST and local switch costs was not significant for RT or accuracy. There were no interactions between TST and preparation, and no interactions between TST and age group. The TST × age group × preparation interaction was not significant for RT or accuracy. Given the lack of main effects and interactions involving TST and local switch costs, we did not follow up with age-stratified analyses.
To summarize, greater global RT switch costs were related to higher WASO and lower TST independent of age group. In terms of local switch costs, preparation effects were moderated by WASO. Stratified by age, this interaction was only significant in the older group indicating that given time to prepare, older adults with lower WASO were more likely to reduce local switch costs.
Inhibition and working memory abilities
We reported above one association between sleep (WASO) and preparation. Moderation of this preparation effect by WASO may have been driven by individual differences in inhibitory control and/or working memory abilities. This possibility is consistent with the view that preparation involves inhibition of irrelevant task-sets and maintenance of task-relevant sets in working memory during the preparation interval. To test this account, we assessed whether the preparation × WASO interaction was eliminated after controlling for inhibitory control and working memory abilities. Inhibitory control was operationalized as Stroop inhibition (((incongruent RT-congruent RT) /congruent RT) × 100). Working memory was operationalized as 2-back accuracy, as a measure of high working memory load. We reasoned that if WASO-based differences in preparation were eliminated when controlling for inhibition and working memory, this would suggest that the preparation effect in participants with lower WASO depended on superior inhibition of competing task-sets and maintenance of task-relevant sets. After accounting for working memory and inhibition, the preparation effect interaction with WASO remained significant, F(2, 196.23) = 4.658, p = 0.011. This outcome suggests that the larger preparation effect in participants with lower WASO was not driven by superior inhibitory control or greater working memory abilities. Thus, there was an association between WASO and preparation that was not dependent on inhibition and working memory abilities. Possible sources of this association are discussed below.
Discussion
Global Switch Costs
Across age groups, smaller global switch costs were associated with greater sleep continuity (less WASO) and longer TST. Thus, better global switching performance was associated with longer and more continuous sleep. Although associations between global switch costs and sleep more consistently reached significance in the younger group, particularly for TST, age group did not moderate the relationship between sleep and global switch costs. These findings suggest that WASO and TST are associated with the ability to maintain and coordinate switching tasks and this relationship is independent of age.
The current findings pertaining to TST and global switch costs extend prior work examining sleep and cognition (Blackwell et al., 2006, Nebes et al., 2009, Loerbroks et al., 2010). Some of these studies have found little to no relationship between TST and cognition, especially executive functions (Blackwell et al., 2006, Schmutte et al., 2007, Nebes et al., 2009), and have proposed that sleep continuity is more important for cognition. In contrast, others have demonstrated that long and short TST are associated with poorer cognition (Loerbroks et al., 2010). We attribute our significant associations involving TST to the highly cognitively demanding aspects of the present paradigm and examination of switch costs in relation to TST, conceivably making this paradigm more sensitive to individual differences in TST.
It is noteworthy that associations between WASO and TST and global switch costs were demonstrated in both young and older adults. Although compared to young adults, older adults demonstrate less decrement in cognitive performance in sleep deprivation experiments (Philip et al., 2004, Duffy et al., 2009), these data suggest that both sleep continuity and duration are important in maintaining optimal cognitive abilities in older adulthood.
Local Switch Costs
Findings with local switch costs were more complex than that found with global switch costs. In contrast to the association between sleep and global switch costs, associations between sleep and local switch costs were specific to accuracy and were moderated by preparation. Participants with lower WASO were more likely to exhibit a preparation effect such that switch costs were greatest with no preparation time, but were reduced given time to prepare.
Consistent with the view that better sleep (lower WASO) is associated with successful adoption of a task-set, post-hoc analyses separating task alternation and task repetition trials demonstrated that across preparation times, lower WASO was associated with greater repetition trial accuracy. However, for task alternations, lower accuracy with no time to prepare and higher accuracy with time to prepare were associated with lower WASO. This detriment on task alternations with no preparation time and an increase with preparation time is what occurs when a participant has successfully adopted a task-set (Monsell, 2003). When the participant is then required to switch tasks with no preparation time, task-set reconfiguration is more difficult than a participant who adopts a more diffuse task-set across all trials (Kramer et al., 1999, Kray, 2006). However, with time to prepare, it is easier to disengage from a given task-set and increase performance on the task alternation trial. To that end, lower WASO was associated with adoption of a task-set and use of preparation time to reconfigure the task-set on alternation trials.
While the interaction between WASO, preparation, and age group did not reach significance (p = 0.347), the relationship between preparation and WASO was statistically significant only in the older group. Preparation effects in accuracy switch costs were similar across values of WASO within the younger group. However, given that the interaction with age group was not significant, any differences in age stratified analyses should be interpreted with caution. Nonetheless, the preparation × WASO finding points to the importance of examining in future studies whether cognitive strategies differ as a function of sleep continuity and duration in older adults.
The association between WASO and preparation effects remained significant after controlling for inhibition and working memory abilities, suggesting that the association between sleep and preparation depends on a pathway other than working memory and inhibition. Beyond working memory and inhibition, greater sleep continuity may enhance attentional control, motor and attentional preparation, cognitive flexibility, and possibly motivation (Erickson et al., 2005) leading to a greater preparation effect.
One potential mechanism underlying relationships between switch costs and WASO is that people spend less time in slow-wave sleep, when WASO is higher and their sleep is more fragmented (Bonnet, 1985). Given that the greatest functional brain deactivations relative to wakefulness occur in the prefrontal cortex (PFC) during slow-wave sleep, slow-wave sleep is thought to preferentially benefit the PFC (Werth et al., 1997, Muzur et al., 2002, Dang-Vu et al., 2008). This preferential benefit to PFC function could in turn benefit task-switching and other executive functions (Harrison et al., 2000, Muzur et al., 2002, Wilckens et al., 2012).
There are some limitations to the present study particularly in the use of accelerometer-based sleep measurement. The present study used a SenseWear sleep detection device whereas polysomnography (PSG) is considered the “gold standard” for measuring sleep. While the device has over 90% concordance with PSG in detecting sleep, Sunseri et al. reported approximately 50% concordance with PSG for short periods of wakefulness during sleep. Thus WASO may have been underestimated, and in turn the overall effect of WASO on cognition may have been underestimated in the present study. If WASO was indeed underestimated in the present study, this would suggest that the present results are a conservative estimate of the association between sleep and cognition. Nonetheless, the SenseWear device used here avoids some confounds associated with wrist actigraphy, such as knowing when the device is off the body by collecting additional physiological data (Sunseri et al., 2009). Additionally, participants need not sleep in a laboratory setting or wear electrodes as with PSG. Future studies will benefit from examining whether PSG-measured sleep moderates age differences in cognitive performance. EEG oscillations recorded with PSG during sleep may also shed light on the role of neural network connectivity during sleep in relation to daytime cognitive function. Mander et al. (2013) found that lower slow-wave sleep in older adults was associated with reduced functional connectivity within PFC-hippocampal networks, suggesting that neural synchrony during sleep strengthens connections between the PFC and functionally related brain regions. Along these lines, neuronal synchrony during sleep may also enhance connections within brain networks important for executive functions such as task-switching.
Conclusions
Sleep continuity and sleep duration of both young and older adults were associated with global switch costs. Age differences in task-switching were not diminished in participants with less WASO or longer TST. Nonetheless, older adults' ability to engage preparatory strategies to reduce local switch costs was associated with WASO, suggesting that older adults with greater sleep continuity may be more likely to engage preparatory strategies to benefit performance. These findings beg the question of whether improved sleep could improve cognitive strategies in older adults. These findings have broad public health implications, suggesting that priority should be given to establishing good sleep hygiene in both young and older adults to maintain optimal daytime cognitive function.
Supplementary Material
Acknowledgements
This project was supported by the National Institute of General Medical Sciences (T32GM081760 to K. A. W.) and the National Institute of Mental Health (MH086492 to M.E.W.) at the National Institutes of Health.
The authors thank Afton Kirk, Krupa Patel, Marina Lukac, and Leslie Denlinger for assistance with data collection and scoring.
Footnotes
The authors have no conflicts to disclose.
Author contributions: K.A.W., K.I.E., and M.E.W. designed the study, K.A.W and S.G.W conducted the study, K.A.W and S.G.W analyzed the data. K.A.W wrote the paper with critical comments from K.I.E and M.E.W.
References
- Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, Hillier TA, Fink HA, Stone KL. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. Effect of Sleep Disruption on Sleep. Sleep. 1985;8:1. doi: 10.1093/sleep/8.1.11. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. Normal Human Sleep: An Overview. In: Kryger MH, et al., editors. Principles and practice of sleep medicine. W. B. Saunders; St. Louis: 2011. [Google Scholar]
- Couyoumdjian A, Sdoia S, Tempesta D, Curcio G, Rastellini E, L DEG, Ferrara M. The effects of sleep and sleep deprivation on task-switching performance. J Sleep Res. 2010;19:64–70. doi: 10.1111/j.1365-2869.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Schabus M, Desseilles M, Albouy G, Boly M, Darsaud A, Gais S, Rauchs G, Sterpenich V, Vandewalle G, Carrier J, Moonen G, Balteau E, Degueldre C, Luxen A, Phillips C, Maquet P. Spontaneous neural activity during human slow wave sleep. PNAS. 2008;105:15160–15165. doi: 10.1073/pnas.0801819105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Willson HJ, Wang W, Czeisler CA. Healthy older adults better tolerate sleep deprivation than young adults. J Am Geriatr Soc. 2009;57:1245–1251. doi: 10.1111/j.1532-5415.2009.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, Kramer AF. Neural correlates of dual-task performance after minimizing task-preparation. Neuroimage. 2005;28:967–979. doi: 10.1016/j.neuroimage.2005.06.047. [DOI] [PubMed] [Google Scholar]
- Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–339. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison Y, Horne JA, Rothwell A. Prefrontal neuropsychological effects of sleep deprivation in young adults--a model for healthy aging? Sleep. 2000;23:1067–1073. [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Gopher D. Task coordination and aging: explorations of executive control processes in the task switching paradigm. Acta Psychologica. 1999;101:339–378. doi: 10.1016/s0001-6918(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Kray J. Task-set switching under cue-based versus memory-based switching conditions in younger and older adults. Brain Res. 2006;1105:83–92. doi: 10.1016/j.brainres.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Kray J, Lindenberger U. Adult age differences in task switching. Psychol aging. 2000;15:126–147. doi: 10.1037//0882-7974.15.1.126. [DOI] [PubMed] [Google Scholar]
- Lawo V, Philipp AM, Schuch S, Koch I. The role of task preparation and task inhibition in age-related task-switching deficits. Psychol aging. 2012;27:1130–1137. doi: 10.1037/a0027455. [DOI] [PubMed] [Google Scholar]
- Loerbroks A, Debling D, Amelang M, Sturmer T. Nocturnal sleep duration and cognitive impairment in a population-based study of older adults. Int J Geriatr Psychiatry. 2010;25:100–109. doi: 10.1002/gps.2305. [DOI] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16:357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr U. Age differences in the selection of mental sets: the role of inhibition, stimulus ambiguity, and response-set overlap. Psychol aging. 2001;16:96–109. doi: 10.1037/0882-7974.16.1.96. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends Cogn Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends Cogn Sci. 2002;6:475–481. doi: 10.1016/s1364-6613(02)01992-7. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64:180–187. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annual review of psychology. 2009;60:173. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip P, Taillard J, Sagaspe P, Valtat C, Sanchez-Ortuno M, Moore N, Charles A, Bioulac B. Age, performance and sleep deprivation. J Sleep Res. 2004;13:105–110. doi: 10.1111/j.1365-2869.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- Reitan Validity of the trail making test as an indicator of organic brain disease. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Schmutte T, Harris S, Levin R, Zweig R, Katz M, Lipton R. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: results from the Bronx aging study. Behavioral sleep medicine. 2007;5:39–56. doi: 10.1207/s15402010bsm0501_3. [DOI] [PubMed] [Google Scholar]
- Sunseri M, Liden C, Farringdon J, Pelletier R, Safier S, Stivoric J, Teller A, Vishnubhatla S. The SenseWear armband as a Sleep Detection Device. 2009. pp. 1–9. [Google Scholar]
- Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Disease & Associated Disorders. 2006;20:41–48. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P. Aging and executive control: reports of a demise greatly exaggerated. Current Directions in Psychological Science. 2011;20:174–180. doi: 10.1177/0963721411408772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyshyn C, Verhaeghen P, Sliwinski MJ. Aging and task switching: a meta-analysis. Psychol aging. 2011;26:15–20. doi: 10.1037/a0020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Administration and scoring manual. The Psychological Corporation; San Antonio: 1997. Wechsler Adult Intelligence Scale. WAIS-III. [Google Scholar]
- Werth E, Achermann P, Borbely AA. Fronto-occipital EEG power gradients in human sleep. J Sleep Res. 1997;6:102–112. doi: 10.1046/j.1365-2869.1997.d01-36.x. [DOI] [PubMed] [Google Scholar]
- Wilckens KA, Erickson KI, Wheeler ME. Age-related decline in controlled retrieval: The role of the PFC and sleep. Neural Plast. 2012;2012 doi: 10.1155/2012/624795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens KA, Woo SG, Kirk AR, Erickson KI, Wheeler ME. The role of sleep continuity and total sleep time in executive function across the adult lifespan. Unpublished. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.